Abstract
Because of the changing demographics of hepatitis C, screening for viral hepatitis should be done routinely in all pregnant woman. This process should include linkage to care. The best elimination strategy would incorporate universal screening for hepatitis C in all individuals over the age of 18.
Abbreviations
- ALT
alanine aminotransferase
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HCVab
HCV antibody
- HIV
human immunodeficiency virus
The discovery and development of therapies for hepatitis C virus (HCV) remains one of the greatest success stories in modern medicine with ambitious goals being set by multiple international and national health organizations for elimination of viral hepatitis as a major public health threat.
In the United States, screening for hepatitis C has been based on risk behaviors (e.g., intravenous drug use), risk exposures (e.g., recipients of blood transfusions prior to 1992) and other conditions, including unexplained elevated alanine aminotransferase (ALT) levels. HCV antibody (HCVAb) testing for hepatitis C for those born between 1945 and 1965 was recommended in 2012 to augment merely risk‐based screening; however, adherence to this recommendation has remained suboptimal to date.1 From 2011‐2014, commercial laboratory data have indicated a 22% increase in national rates of HCV detection among women of childbearing age with a 68% increase in the rate of infants born to HCV‐infected mothers; in addition, those under the age of 40 now have the highest rates of new hepatitis C infections.2, 3
The report by Kim et al. in this month’s issue of Hepatology Communications is an important snapshot of the progress being made yet shows the challenges we still face to ensure that all individuals at risk for hepatitis C are appropriately screened and linked to care. The authors assessed HCV screening and infection rates of reproductive age women (aged 15‐44) within 12 clinics in the San Francisco Health Network via electronic medical records with a subgroup analysis of those with a history of pregnancy. These results were compared with the screening rates for hepatitis B virus (HBV) and human immunodeficiency virus (HIV), in which higher screening rates exist due to established recommendations from the Centers for Disease Control (CDC) and other organizations.
In the total cohort, 7,406 (38.4%) received HCVAb testing, 206 (2.8%) demonstrated detectable HCVAb, 177 demonstrated viremia, and only 41 of 177 individuals received HCV therapy. Those with a history of pregnancy were screened at a higher rate (615 of 1,017, 60.5%), of whom 16 of 615 demonstrated positive HCVAb, 10 of 16 had detectable HCV RNA, and 6 of 10 received HCV treatment. Those with higher ALT levels, prior HBsAg testing, and HIV co‐infection were more likely to be screened for hepatitis C. Latina race was most common in this cohort, and Latinas and Asians were screened for HCV infection at lower rates than whites or blacks. Four factors predicted HCV infection (HCVAb positivity): older age, white race, higher ALT level, and HBsAb positivity.
The screening rates reported by Kim et al., while suboptimal, do demonstrate significant improvement compared with other recent reports of risk‐based screening in pregnant women. One report demonstrated low screening rates in pregnant women receiving care in an urban obstetrical practice, noting an overall 7% screening rate in pregnant women with a low level of screening (28 of 78) even in those with any risk factor for HCV.4 Higher screening rates did occur in those with traditional risk factors for HCV, including intravenous drug use and HIV infection, but risk‐based screening alone was insufficient to identify all incident cases of HCV.
Current screening guidelines by obstetrical societies and the CDC recommend risk‐based screening for pregnant women.5 However, after a recent update, American Association for the Study of Liver Diseases/Infectious Diseases Society of America guidelines now recommend that all pregnant women be screened for HCV (https://www.hcvguidelines.org/unique-populations/pregnancy) in addition to the current screening that occurs during pregnancy for HBV, HIV, and syphilis. High rates of screening for HIV and HBV were reported by Kim et al., which suggests the importance of implementation of guidelines to promote screening in appropriate populations. Furthermore, a recent modeling study has reported that universal antenatal screening for HCV was cost‐effective across a broad range of eligibility scenarios, even when HCV prevalence dropped below 0.1%.6
The challenges of improving the cascade of care in those diagnosed with HCV are also demonstrated in this report. In the overall cohort, high rates of reflex testing to HCV RNA were demonstrated, yet still over 75% of individuals did not receive treatment for HCV. The most important step in the cascade of care is to screen appropriate populations for HCV with HCVAb testing and to maximize the opportunity to correctly identify those who require therapy. To accomplish this, there should be reflex testing for HCV RNA for any HCVAb positive test. Although genotyping for HCV is currently being done in the United States, with the introduction of pan‐genotypic therapies, this step may be eliminated in order to make elimination strategies more efficient, and recent reports have proposed steps such as this for HCV elimination.7 In this report by Kim et al., 60% of individuals with a pregnancy history with detectable HCV RNA received treatment. A preliminary report suggested that it is feasible to treat pregnant women with direct‐acting antiviral (DAA) therapy, although this strategy will require further study to determine whether it is more advantageous (and safe) to treat during pregnancy or to provide therapy postpartum.8 Regardless, if HCV infection is identified during pregnancy, there should be an established pathway for linkage to therapy, as many HCV‐infected individuals will be maximally engaged in the health care system during this period (Fig. 1).
Figure 1.
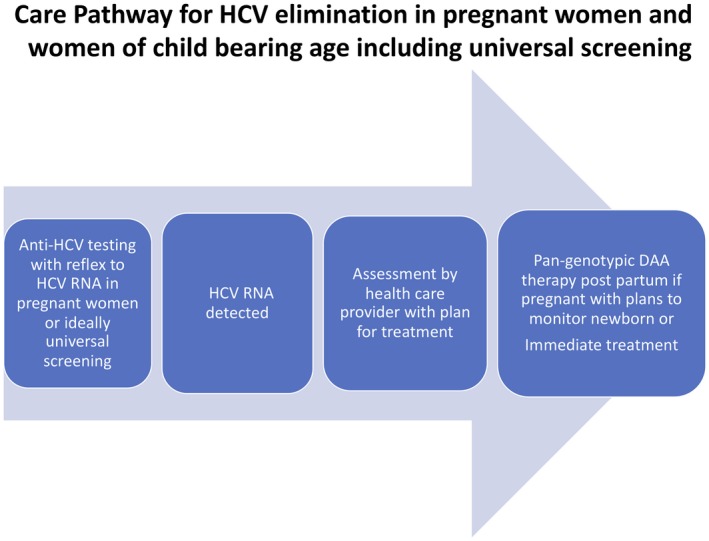
Care pathway for HCV elimination in pregnant women and women of child‐bearing age, including universal screening.
The prevalence of HCV in the cohort of reproductive‐age women was statistically similar to the prevalence of those with a history of pregnancy, with the highest testing rates and highest positive HCVAb rates occurring in the cohorts aged 30‐44. Although the overall age at which women are giving birth has increased in the United States, there is such heterogenous distribution across the United States of race, ethnicity, economic status, access to health care, and education that the most effective screening strategy is universal screening. A recent publication explored multiple simulated strategies including birth cohort testing (born between 1945 and 1965) and testing those over the ages of 40, 30, and 18 years. Screening those age 18 or older led to the greatest case identification (256,000 additional infected persons) and cure rates (280,000 additional cures) at the lowest cost per quality‐adjusted life year (QALY) ($28,000/QALY).9 Given that the incidence of HCV is rising most rapidly in those under the age of 40, this strategy would be most likely to capture the greatest number of undiagnosed HCV‐infected individuals, many of whom may be perceived not to have risk factors for HCV or have limited engagement with health care providers. Incorporation of testing algorithms including electronic health record alerts may also help increase screening rates in pregnant women and other populations, including reproductive‐age women.
In summary, the article by Kim et al. informs us that screening for HCV does occur in women with a history of pregnancy and in all those of reproductive age, but there is room for improvement. Over time, it is hoped that there will be broad consensus for universal screening in pregnant women, which will help identify both mothers and infants who require monitoring. In fact, screening during pregnancy with a plan to link to care will be an important elimination approach to build momentum to achieve ambitious public health goals as promoted by the World Health Organization and others. Universal screening with universal access to evaluation and treatment of HCV is the strategy that could allow the elimination of HCV in the United States. This would complete the success story of HCV and would improve quality of life and survival by preventing downstream effects such as progression of liver disease, thus allowing resources to be directed to the many other health care challenges that we face.
Potential conflict of interest: Dr. Kwo owns stock in Durect. He advises for Dova, Eisai, Shionogi, Conatus, Merck, Surrozen, and Edigene. He advises and received grants from BMS, Gilead, and AbbVie. He received grants from Assembly, Allergan, and La Jolla. He is on the DSMB for Janssen.
See Article on Page 1183.
References
- 1. Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost‐effectiveness of birth‐cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Int Med 2012;156:263‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenberg ES, Rosenthal EM, Hall EW, Barker L, Hofmeister MG, Sullivan PS, et al. Prevalence of hepatitis C virus infection in US states and the District of Columbia, 2013 to 2016. JAMA Network Open 2018;1:e186371‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011‐2014. MMWR Morb Mortal Wkly Rep 2016;65:705‐710. [DOI] [PubMed] [Google Scholar]
- 4. Boudova S, Mark K, El‐Kamary SS, editors. Risk‐based hepatitis C screening in pregnancy is less reliable than universal screening: a retrospective chart review Open Forum Infectious Diseases. Oxford University Press US; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hughes BL, Page CM, Kuller JA, Medicine SfM‐F . Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol 2017;217:B2‐B12. [DOI] [PubMed] [Google Scholar]
- 6. Chaillon A, Rand EB, Reau N, Martin NK. Cost‐effectiveness of universal hepatitis C virus screening of pregnant women in the United States. Clin Infect Dis 2019. 10.1093/cid/ciz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dieterich DT, Ahn J, Bacon B, Bernstein D, Bourlière M, Flamm S, et al. A simplified algorithm for the management of hepatitis C infection. Gastroenterol Hepatol 2019;15:S3. [PMC free article] [PubMed] [Google Scholar]
- 8. Chappell C, editor. A phase‐1 study of ledipasvir/sofosbuvir in pregnant women with hepatitis C virus In Proceedings of the 26th Conference on Retroviruses and Opportunistic Infections, Seattle, WA; 2019. [Google Scholar]
- 9. Barocas JA, Tasillo A, Eftekhari Yazdi G, Wang J, Vellozzi C, Hariri S, et al. Population‐level outcomes and cost‐effectiveness of expanding the recommendation for age‐based hepatitis C testing in the United States. Clin Infect Dis 2018;67:549‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]


