Abstract
Objectives
Compared with normal fetuses, fetuses with hypoplastic left heart syndrome (HLHS) have smaller brain volumes and are at higher risk of brain injury, possibly due to diminished cerebral blood flow and oxygen content. By increasing cerebral oxygen delivery, maternal hyperoxygenation (MH) might improve brain development and reduce the risk of brain injury in these fetuses. This study investigated whether gestational age and baseline cerebrovascular resistance affect the response to MH in fetuses with HLHS.
Methods
The study population comprised 43 fetuses with HLHS or HLHS variant referred for fetal echocardiography between January 2004 and September 2008. Middle cerebral artery (MCA) pulsatility index (PI), a surrogate measure of cerebrovascular resistance, was assessed between 20 and 41 weeks’ gestation at baseline in room air (RA) and after 10 min of MH. Z-scores of MCA-PI were generated. A mixed-effects model was used to determine whether change in MCA-PI depends upon gestational age and baseline MCA-PI.
Results
In RA and following MH, MCA-PI demonstrated a curvilinear relationship with gestational age in fetuses with HLHS, peaking at around 28 weeks and then falling more steeply near term. MCA-PI Z-score declined in a linear manner, such that it was 1.4 SD below that in normal fetuses at 38 weeks. Increase in MCA-PI Z-score after MH was first seen at ≥ 28 weeks. A baseline MCA-PI Z-score ≤ −0.96 was predictive of an increase in cerebrovascular resistance in response to MH.
Conclusion
In fetuses with HLHS, MCA-PI first increases in response to MH at ≥ 28 weeks’ gestation. A baseline MCA-PI Z-score ≤ −0.96 predicts an increase in cerebrovascular resistance in response to MH. These results may have implications for clinical trials utilizing MH as a neuroprotective agent.
Keywords: congenital heart disease, fetal echocardiography, maternal hyperoxygenation, neurodevelopmental outcome
INTRODUCTION
Fetuses with congenital heart disease are at risk for postnatal adverse neurodevelopmental outcome. Despite advances in surgical and intensive care unit management, neurodevelopmental outcomes for individuals with hypoplastic left heart syndrome (HLHS) have remained unchanged over the past 20 years1,2. Compared with normal fetuses, fetuses with congenital heart disease have smaller brain volumes at birth, delayed maturation of the brain3–8 and are at higher risk of structural brain abnormalities9. In addition, studies have demonstrated that neonates with congenital heart disease have diminished preoperative cerebral blood flow10 and a higher rate of periventricular leukomalacia (PVL) at birth, with significant increases in PVL observed after open heart surgery11–13. All of these differences may be secondary to diminished cerebral oxygen delivery to the brain3. Previous studies have shown that maternal hyperoxygenation (MH) increases fetal partial pressure of oxygen in the umbilical artery and vein, as well as umbilical arterial oxygen saturation14–17. Therefore, we hypothesize that MH would increase cerebral oxygen delivery by increasing the partial pressure of oxygen within the fetus. As a neuroprotective agent, it may ameliorate some of the adverse metabolic events occurring in the fetal brain as a consequence of chronic hypoxemia that lead to white matter injury, delayed maturation and diminished brain volume.
The use of MH as a neuroprotective agent has not been investigated to date. In the third trimester, multiple complex processes, including expansion of the white matter, cortical folding and myelination, occur within the fetal brain18. In HLHS, we and other investigators have demonstrated previously that cerebrovascular resistance, as assessed via the Doppler-derived middle cerebral artery (MCA) pulsatility index (PI), is lower than in normal fetuses19–23, particularly in the third trimester22. This may occur in an autoregulatory manner to support the increased metabolic demands of the brain during this critical period of brain development in the third trimester of pregnancy18. In this observational study, we investigated MCA-PI in room air (RA) and after 10 min of MH in fetuses with HLHS. We aimed to determine how gestational age (GA) and/or baseline MCA-PI in RA might affect the response of cerebrovascular resistance to MH in fetuses with HLHS.
METHODS
The study population consisted of fetuses referred for echocardiography at our institution between January 2004 and September 2008 with HLHS (mitral stenosis/atresia and aortic stenosis/atresia) or HLHS variant (double outlet right ventricle with mitral and aortic stenosis/atresia or severely unbalanced atrioventricular canal with aortic stenosis/atresia). Subjects had been enrolled in a prospective observational study investigating the utility of MH to predict the need for urgent intervention on the atrial septum at birth in fetuses with HLHS or HLHS variant. All fetuses underwent serial echocardiography between 21 and 40 weeks’ gestation according to an institutional review board approved protocol (IRB-2004-10-3948). Fetuses were scanned over the course of gestation according to a three-phase research protocol (baseline RA, MH and recovery in RA), as described previously21. During MH, mothers were administered 8 L of oxygen, delivered via a non-rebreather mask, which effectively provides a FiO2 of 60%, for at least 10 min prior to obtaining the MCA Doppler signal21.
All fetuses subsequently underwent a single ventricle palliation in the postnatal period. All mothers provided informed consent for their and their fetus’ participation.
Inclusion criteria were singleton fetus with HLHS or HLHS variant, no extracardiac anatomical abnormality and normal umbilical artery Doppler flow patterns. Fetuses of mothers with diabetes mellitus, thyroid disorders or pre-eclampsia were excluded. Cerebrovascular resistance was assessed via the Doppler-derived MCA-PI, calculated as: (peak systolic velocity – end-diastolic velocity)/time-averaged mean velocity. MCA Doppler flow pattern was sampled with the mother in RA and after 10 min of MH. For each echocardiographic parameter, three measurements were made and averaged. All echocardiographic measurements were made using Syngo Dynamics v. 9 cardiovascular software package (Siemens Medical Solutions, Ann Arbor, MI, USA). Normal data were used to generate Z-scores for MCA-PI at baseline in RA and after 10 min of MH24.
Statistical analysis
Continuous baseline data were summarized using medians and interquartile range (IQR). MCA-PI in RA and after 10 min of MH, both the measured value and the Z-score, were explored graphically as a function of GA. Depending on the results of the models, the results were presented graphically using either a loess smoothing method to allow for non-linearity in the association, or a linear model.
The association between GA and MCA-PI was quantified using a mixed-effects model. Both the original values and the Z-score in either RA (ZRA) or after 10 min MH (ZMH) were considered. The mixed-effects model takes into account the potential correlation between repeated measurements in some subjects. For each outcome variable, two models were considered, one that included a cubic spline to allow curvature in the association between variables and one that allowed a simple linear association. The simpler linear model was used unless a likelihood ratio (LR) test rejected the linear model in favor of the curvilinear model. The P-value is reported for the LR test if the cubic spline was chosen, or the hypothesis test of a zero slope if the linear model was chosen.
The change in MCA-PI Z-score after 10 min of MH compared with in RA (ΔZ) was explored and analyzed as a function of either GA or baseline Z-score in RA.
Finally, modeling of ΔZ as a function of two predictors, the baseline Z-score in RA and/or GA was considered using eight different models. Four models included the predictors individually, modelled as a linear function or as a spline. The other four models included both predictors in all possible combinations of either linear terms or splines. The Bayesian information criterion (BIC) was used to suggest a ‘best’ model. Lastly, a receiver – operating characteristics (ROC) curve was determined based on an outcome of whether ΔZ was positive (an increase in MCA-PI Z-score after 10 of min MH). The results for the models with the smallest BIC are reported. A 95% exact binomial confidence interval is reported for the original proportion of observations demonstrating a positive response to MH.
The type-I error rate was set to 0.05 throughout and all tests were two-sided. The analysis was carried out using statistical computing in R v. 3.3.0 (2016) using packages nlme, and pROC (R Core Team, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
RESULTS
The study population comprised 43 fetal subjects. Of these, 38 had one or more measurements both at baseline in RA and following 10 min of MH. A total of 28 subjects had measurements at a single visit, six at two visits, eight at three visits and one at four visits. Median GA derived from fetal ultrasound measurement at enrollment was 28.7 (IQR, 25.5 – 32.6.) weeks and that based on last menstrual period (LMP) was 28.1 (IQR, 25.8 – 33.6) weeks. LMP-based GA was used for analysis as these data were complete for all subjects. At the first visit, median MCA-PI in RA was 1.76 (IQR, 1.59 – 1.98) and median MCA-PI Z-score (ZRA) was −0.57 (IQR, −1.06 to 0.02). Due to technical issues conducting the measurements, two observations of MCA-PI in RA were missing and 10 observations of MCA-PI after 10 min of MH were missing. Figure 1a,c demonstrates MCA-PI in fetuses with HLHS in RA, both the raw values and the Z-score (ZRA), as a function of GA. In RA, the trend in the raw values showed significant curvature (P = 0.001, LR test, curvilinear vs linear model) with somewhat lower values at lower GA and a pronounced decline at higher GA. For the standardized values, mean ZRA declined in a linear fashion as a function of GA (P < 0.001, test of slope = 0). The equation describing the mean decline is:
Figure 1.
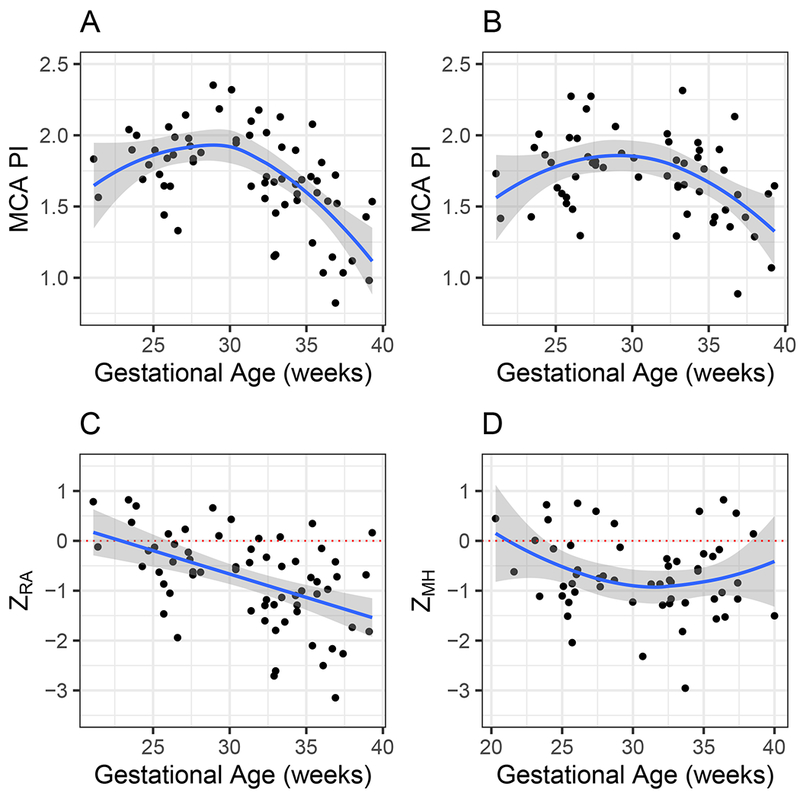
Middle cerebral artery (MCA) pulsatility index (PI) as function of gestational age in 43 fetuses with hypoplastic left heart syndrome (HLHS) or HLHS variant, in either room air (a,c) or after maternal hyperoxygenation (b,d), as raw values (a,b) and as Z-scores (ZRA and ZMH, respectively) (c,d). Lines indicate either loess smooth (a,b,d) or linear (c) model. Choice of model displayed was based on likelihood ratio test. Gray area is 95% CI. Dotted line indicates value of 0, representing expected MCA-PI Z-score for normally developing infants in room air.
In this equation, GA is ‘centered’ at week 28, so that a fetus with a GA of 28 has a value of 0 for the slope and the mean ZRA at 28 weeks is the intercept, a value of −0.47 (95% CI, −0.73 to −0.22, test of intercept = 0, P = 0.001). At a linear change in mean ZRA of −0.096 per week (95% CI, −0.13 to −0.06, test of slope = 0, P < 0.001), mean ZRA in fetuses with HLHS at 38 weeks is expected to be −1.44 (95% CI, −1.76 to −1.11), or almost 1.5 SD lower than expected for normal fetuses. The data confirm that MCA-PI in fetuses with HLHS is considerably lower than that of normally developing fetuses, and that the severity of the deficit increases with fetal age. Figure 1b,d shows MCA-PI in fetuses with HLHS after 10 min of MH, both the raw value and the Z-score (ZMH) as a function of GA. In MH, the raw values again showed significant curvature (P = 0.002, LR test of curvilinear vs linear model), with somewhat lower values at younger GA and a more pronounced decline at older GA. ZMH was also curvilinear (P = 0.029). Using the model, mean ZMH at 28 weeks was −0.76 (95% CI, −1.16 to −0.36). At 38 weeks, mean ZMH was estimated to be −0.70 (95% CI, −1.18 to −0.23). These values remain significantly lower than those of normally developing fetuses.
Figure 2 shows the change in Z-score for MCA-PI, ΔZ, as a function of either GA or baseline ZRA. As a function of GA, mean ΔZ shows a linear increase of 0.079 per week (test of slope = 0, P = 0.001). Mean ΔZ demonstrated a linear increase of 0.48 units per unit decrease in ZRA (P < 0.001, test of slope = 0). After 10 min of MH, the equation describing mean ΔZ is:
Figure 2.
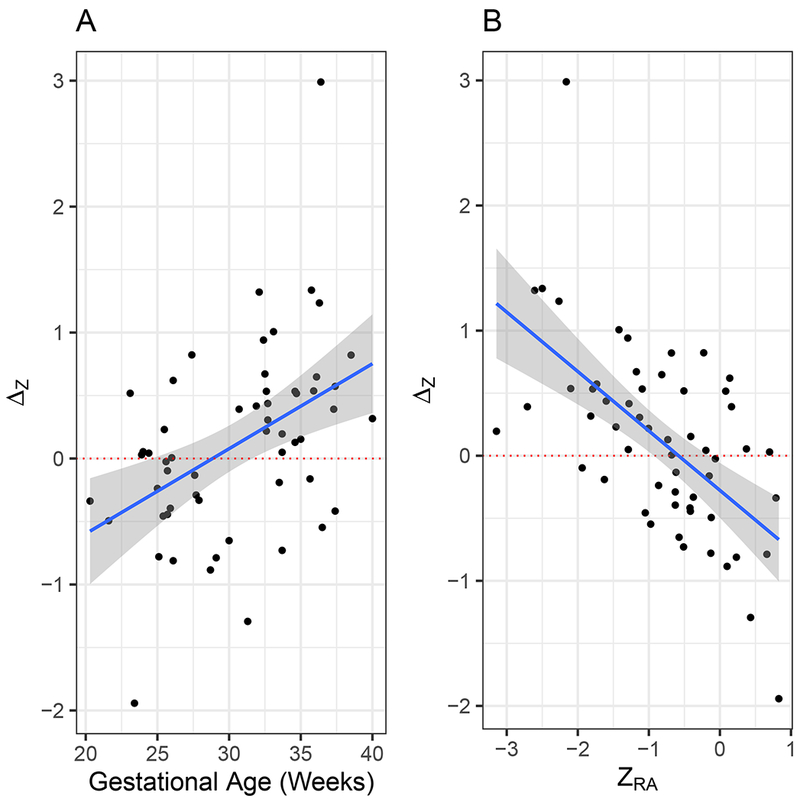
Change in middle cerebral artery (MCA) pulsatility index (PI) Z-score (ΔZ) from in room air to after maternal hyperoxygenation in 43 fetuses with hypoplastic left heart syndrome (HLHS) or HLHS variant, as function of: (a) gestational age and (b) MCA-PI Z-score in room air (ZRA). Solid lines are linear fit. Dotted line indicates ΔZ of zero. Shading represents 95% CI of linear fit.
We further explored whether ZRA alone, GA alone or a combination of the two variables would best explain the variation in ΔZ (Table 1). A model with ZRA alone was suggested, based on the smallest BIC. The BIC for this model was slightly smaller than for the model with both ZRA and GA and the term for GA did not achieve statistical significance (P = 0.13). Considering the change in MCA-PI, this result suggests that GA adds only a small amount of information beyond that provided by ZRA.
Table 1.
Linear models for change in middle cerebral artery pulsatility index Z-score (ΔZ) from room air (ZRA) to after maternal hyperoxygenation in 43 fetuses with hypoplastic left heart syndrome (HLHS) or HLHS variant
| Model Term | Model Selection Criteria | |||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | ZRA | GA-285 | ||||||
| Model | Mean± SE6 | P1 | Mean ± SE6 | P1 | Mean ± SE6 | P1 | BIC2 | AUC3 |
| ZRA | −0.28 ± 0.11 | 0.018 | −0.48 ± 0.09 | <0.001 | NA | NA4 | 117.8 | 0.756 |
| GA | −0.09 ± 0.11 | 0.42 | NA4 | NA4 | 0.079 ± 0.018 | 0.001 | 128.2 | 0.725 |
| ZRA + GA | −0.29 ± 0.11 | 0.013 | −0.39 ± 0.10 | 0.002 | 0.030 ± 0.019 | 0.13 | 119.3 | 0.777 |
P-values given for test of null hypothesis of model parameter having value of 0.
BIC, Bayesian information criterion;
AUC, area under the receiver – operating characteristics curve;
NA, not applicable;
GA, gestational age;
SE, standard error.
Using the model incorporating ZRA only, Table 2 shows the estimated values of ΔZ expected for different values of ZRA. Solving the model equation indicates that a ZRA of−0.58 would yield a mean ΔZ of 0. Values below −0.58 on average would yield an improvement in ΔZ, and values ≤ −0.96 would yield a 95% CI that does not include 0. Lastly, we considered an increase in ΔZ (> 0 or < 0) as a binary outcome and constructed a ROC curve based on each model. In the original dataset, the proportion of observations with a positive ΔZ was 59% (95% CI, 45 – 72%). The AUC for the model incorporating ZRA only was 0.756, just slightly smaller than the AUC of 0.777 for that incorporating ZRA and GA. This result suggests that the baseline ZRA has prognostic significance with respect to determining which subjects may respond to brief MH. It further confirms that fetal age has minimal prognostic significance beyond the information contained in ZRA.
Table 2.
Predicted value of change in middle cerebral artery pulsatility index Z-score (ΔZ) from room air (ZRA) to after maternal hyperoxygenation in fetuses with hypoplastic left heart syndrome (HLHS) or HLHS variant
| ZRA | ΔZ(95% CI) |
|---|---|
| 0 | −0.28 (−0.50 to −0.05) |
| −0.58 | 0.00 (−0.20 to 0.20) |
| −0.96 | 0.18 (0.001 to 0.38)1 |
| −1.0 | 0.20 (0.02 to 0.39)1 |
Lower limit of 95% CI exceeds 0 by at least 0.001.
DISCUSSION
Concordant with previous investigations19–23, the current study demonstrated low cerebrovascular resistance in fetuses with HLHS, particularly in the third trimester22. In addition, cerebrovascular resistance, as assessed via MCA-PI, demonstrates a curvilinear relationship with MH in fetuses with HLHS similar to in fetuses with normal cardiovascular anatomy24. In both RA and after 10 min of MH, MCA-PI initially increases in the second trimester, peaks at around 25 – 30 weeks’ gestation, and then falls steeply near term. In contrast, MCA-PI Z-score declines sharply in a linear fashion over the course of gestation, such that the mean MCA-PI Z-score for fetuses with HLHS is 1.4 standard deviations below normal values at 38 weeks’ gestation.
As shown in Figure 2, MCA-PI first increases in response to MH at 28 weeks’ gestation (intercept crossing 0 at this GA), suggesting that MH is more likely to be effective in increasing cerebrovascular resistance at or beyond 28 weeks’ gestation. Our modeling suggests that, in fact, GA is largely a proxy for baseline MCA-PI. GA and MCA-PI in RA are correlated (Figure 1) and, individually, each is a predictor of improvement in MCA-PI response to MH (Figure 2). However, when both variables are included in the model, the dominant variable is clearly MCA-PI in RA.
We also identified which fetuses with HLHS would be the best candidates in which to increase cerebrovascular resistance using short term MH. Fetuses with MCA-PI Z-scores lower than −0.58 tend to show positive increases in MCA-PI following MH. For those with MCA-PI Z-scores ≤ −0.96, the 95% CI excludes zero; in these fetuses, there is stronger evidence of a positive effect of MH. We hypothesize that an increase in MCA-PI toward normal values may correlate with better neurological values. In advance of this analysis, we proposed 28 weeks’ gestation as the starting point for a future MH trial using chronic MH. While using the Z-score might yield better precision with respect to predicting increases in cerebrovascular resistance in response to MH, it is not practical to bring pregnant mothers repeatedly into the clinic to assess fetal Z-scores. The results of this study suggest that fetuses older than 28 weeks are more likely to have lower Z-scores and potentially benefit from MH. Use of gestational age to guide patient inclusion criteria for clinical trials may be implemented more readily than use of strict criteria based on Z-scores alone.
Whether increases or decreases in cerebrovascular resistance in response to MH are helpful or harmful to neurodevelopmental outcome remains to be determined. In normal fetuses, some studies have demonstrated an increase in cerebrovascular resistance in response to MH25, while others have demonstrated a decrease26. Therefore, given these conflicting results, it is difficult to make any direct comparisons between HLHS and normal fetuses with regard to these findings. However, we do not believe that the increase seen in response to MH is due to run-off into other vascular beds, since we demonstrated previously no change in umbilical artery PI in response to MH in fetuses with HLHS21. Similarly, Simchen et al. demonstrated no change in umbilical or uterine artery PI in response to MH in normal fetuses26. It is conceivable that the increase in cerebrovascular resistance in the third trimester could be due to run-off into the pulmonary vascular bed, since pulmonary vascular resistance decreases in the third trimester in HLHS fetuses with an unobstructed atrial septum21. However, if diminished pulmonary vascular resistance were responsible for this increase in cerebrovascular resistance, then one would expect that cerebrovascular resistance would increase for all fetuses with HLHS in the third trimester. However, Figure 2 demonstrates a heterogeneous response to MH in fetuses with HLHS. Those with MCA-PI Z-scores > −0.58 tended to have lower increases in cerebrovascular resistance in response to MH, even in the late third trimester, while fetuses with MCA-PI Z-scores < −0.58 tended to have higher increases in cerebrovascular resistance in response to MH, with the most dramatic increases seen for those with MCA-PI Z-score < −0.96. We hypothesize that HLHS fetuses with inadequate oxygen delivery to the brain undergo vasodilation of the cerebrovasculature in order to augment oxygen and nutritional delivery. Metabolic demands of the fetal brain are hypothesized to be the greatest during the third trimester since myelination, cortical folding and white matter development18 occur at this time. Therefore, this might explain why changes in cerebrovascular resistance are most pronounced in the third trimester and do not occur earlier in gestation when oxygen delivery may be sufficient for fetal brain development. Autoregulatory compensation, however, is not complete, with multiple studies demonstrating smaller head circumferences27–29, greater brain immaturity5–8 and increased risk of periventricular luekomalacia12,13 in fetuses with HLHS compared with in normal fetuses. These data are consistent with the hypothesis that, as cerebral oxygen demand increases during the third trimester, oxygen delivery in fetuses with HLHS may be insufficient to meet this demand. Therefore, chronic MH therapy may serve as a useful means to increase oxygen delivery to the fetal brain, thereby improving the maturity and growth of the brain, as well as potentially protecting against the development of periventricular leukomalacia. However, we note that this study investigated only acute changes in cerebrovascular resistance with MH therapy. Whether HLHS fetuses would respond similarly to chronic MH remains to be determined.
We considered possible explanations for why the greatest increase in MCA-PI in response to MH was observed in HLHS fetuses with the lowest MCA-PI at baseline. We hypothesize that HLHS fetuses with higher MCA-PI Z-scores have adequate cerebrovascular oxygen and nutrient delivery to the fetal brain. These fetuses have no need to augment oxygen delivery to the brain. However, fetuses with inadequate cerebral oxygen delivery may be maximally vasodilated in order to increase oxygen delivery to the brain. In response to MH, cerebrovascular resistance increases toward normal values as a consequence of improved cerebral oxygen content of the blood. We hypothesize that this increase in cerebrovascular resistance in response to MH could allow for a safer transition to neonatal circulation and enhanced neuroprotection.
Prospective trials are needed to investigate the utility of chronic MH as a neuroprotective agent. As we move towards innovative research into the utility of MH as a neuroprotective agent in fetuses with HLHS, our study may prove valuable for the design of future studies. Importantly, we note little improvement in cerebrovascular resistance for HLHS fetuses with baseline MCA-PI Z-scores > −0.58 or prior to 28 weeks’ gestation.
ACKNOWLEDGMENTS
Funding for M.P. was provided in part by U54 HD086984. Funding for A.S. was provided in part by a National Institutes of Health training grant (T32-HL007915).
REFERENCES
- 1.Forbess JM, Visconti KJ, Bellinger DC, Jonas RA. Neurodevelopmental outcomes in children after the fontan operation. Circulation 2001; 104: I27–I132. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar-Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: The single ventricle reconstruction trial. Circulation 2012; 125:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, Hickey E, Miller S, Seed M. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015; 131:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, Knirsch W, Latal B; Heart and Brain Research Group. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr 2015; 167: 1259–1263. e1. [DOI] [PubMed] [Google Scholar]
- 5.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009; 137:529–536;discussion 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010; 121:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007; 357:1928–1938. [DOI] [PubMed] [Google Scholar]
- 8.Sethi V, Tabbutt S, Dimitropoulos A, Harris KC, Chau V, Poskitt K, Campbell A, Azakie A, Xu D, Barkovich AJ, Miller SP, McQuillen PS. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res 2013; 73: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brossard-Racine M, du Plessis AJ, Vezina G, Robertson R, Bulas D, Evangelou IE, Donofrio M, Freeman D, Limperopoulos C. Prevalence and spectrum of in utero structural brain abnormalities in fetuses with complex congenital heart disease. AJNR Am J Neuroradiol 2014; 35: 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, Tabbutt S, Durning SM, Shera DM, Gaynor JW, Spray TL, Clancy RR, Zimmerman RA, Detre JA. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg 2004; 128 841–849. [DOI] [PubMed] [Google Scholar]
- 11.Brossard-Racine M, du Plessis A, Vezina G, Robertson R, Donofrio M, Tworetzky W, Limperopoulos C. Brain injury in neonates with complex congenital heart disease: What is the predictive value of MRI in the fetal period? AJNR Am J Neuroradiol 2016; 37: 1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004;127:692–704. [DOI] [PubMed] [Google Scholar]
- 13.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002. 106:I109–I114. [PubMed] [Google Scholar]
- 14.Huch A, Huch R, Schneider H, Rooth G. Continuous transcutaneous monitoring of fetal oxygen tension during labour. Br J Obstet Gynaecol 1977; 84 (Suppl 1): 1–39. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaides KH, Campbell S, Bradley RJ, Bilardo CM, Soothill PW, Gibb D.Maternal oxygen therapy for intrauterine growth retardation. Lancet 1987;1: 942–945. [DOI] [PubMed] [Google Scholar]
- 16.Parpaglioni R, Capogna G, Celleno D, Fusco P. Intraoperative fetal oxygen saturation during Caesarean section: general anaesthesia using sevoflurane with either 100% oxygen or 50% nitrous oxide in oxygen. Eur J Anaesthesiol 2002; 19: 115–118. [DOI] [PubMed] [Google Scholar]
- 17.Willcourt RJ, King JC, Queenan JT. Maternal oxygenation administration and the fetal transcutaneous PO2. Am J Obstet Gynecol 1983, 146:714–715. [DOI] [PubMed] [Google Scholar]
- 18.Clouchoux C, Kudelski D, Gholipour A, Warfield SK, Viseur S, Bouyssi-Kobar M, Mari JL, Evans AC, du Plessis AJ, Limperopoulos C. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct 2012; 217: 127–139. [DOI] [PubMed] [Google Scholar]
- 19.Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol 2003; 24: 436–443. [DOI] [PubMed] [Google Scholar]
- 20.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol 2005; 25: 32–36. [DOI] [PubMed] [Google Scholar]
- 21.Szwast A, Tian Z, McCann M, Donaghue D, Rychik J. Vasoreactive response to maternal hyperoxygenation in the fetus with hypoplastic left heart syndrome. Circ Cardiovasc Imaging 2010; 3: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szwast A, Tian Z, McCann M, Soffer D, Rychik J. Comparative analysis of cerebrovascular resistance in fetuses with single-ventricle congenital heart disease. Ultrasound Obstet Gynecol 2012; 40: 62–67. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto Y, Khoo NS, Brooks PA, Savard W, Hirose A, Hornberger LK. Severe left heart obstruction with retrograde arch flow importantly influences fetal cerebral and placental blood flow. Ultrasound Obstet Gynecol 2013; 42: 294–299. [DOI] [PubMed] [Google Scholar]
- 24.Ebbing C, Rasmussen S, Kiserud T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol 2007; 30: 287–296. [DOI] [PubMed] [Google Scholar]
- 25.Almstrom H, Sonesson SE. Doppler echocardiographic assessment of fetal blood flow redistribution during maternal hyperoxygenation. Ultrasound Obstet Gynecol 1996; 8: 256–261. [DOI] [PubMed] [Google Scholar]
- 26.Simchen MJ, Tesler J, Azami T, Preiss D, Fedorko L, Goldszmidz E, Fisher J, Kingdom J, Slorach C, Hornberger LK. Effects of maternal hyperoxia with and without normocapnia in uteroplacental and fetal Doppler studies. Ultrasound Obstet Gynecol 2005; 26: 495–499. [DOI] [PubMed] [Google Scholar]
- 27.Hinton RB, Andelfinger G, Sekar P, Hinton AC, Gendron RL, Michelfelder EC, Robitaille Y, Benson DW. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res 2008; 64: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzar S, Nair AK, Pai MG, Al-Khusaiby SM. Head size at birth in neonates with transposition of great arteries and hypoplastic left heart syndrome. Saudi Med J. 2005; 26: 453–456. [PubMed] [Google Scholar]
- 29.Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol 1996; 143:505–513. [DOI] [PubMed] [Google Scholar]


