Abstract
Coordination of oxytocin (OT) activity and partner interactions is important for the facilitation and maintenance of monogamous pair bonds. We used coppery titi monkeys (Callicebus cupreus) to identify the effects of male aggressive temperament on OT activity, affiliative partner-directed behaviors, aggressive partner-directed behaviors, anxiety-related behaviors, and hormone-behavior interactions. We used a mirror technique, simulating an intruder in the home territory of pairs to elicit behavioral responses, and quantified behaviors using an established ethogram. Plasma concentrations of OT were quantified using enzyme immunoassay. We used general linear mixed models to predict 1) percent change in OT as a function of aggression score, and 2) percent change in behaviors as a function of aggression, OT, and OT by aggression interactions. Males who display high levels of partner-directed aggression (High-aggressive) exhibited a significant drop in OT concentration (pg/ml) relative to control when exposed to the front of the mirror (t = −2.20, P = 0.04). High-aggressive males spent significantly less time in contact with their mates (t = −2.26, P = 0.04) and lip-smacked less (t = −2.32, P = 0.03) relative to control. We also saw a trend toward an interaction effect between OT and proximity such that High-aggressive males displaying a negative percent change in OT exhibited a smaller percent increase social proximity (t = 1.96, P = 0.07). Males exhibiting a decrease in OT also trended towards back-arching and tail-lashing less in response to the mirror (t = 1.82, P = 0.09). To our knowledge, this is the first empirical study to examine interactions between OT and temperament in adult monogamous primates. Future studies should incorporate measures of pair-mate interactions and early-life experience to further understand variation in responses to social stressors in monogamous primates and their effects on pair bonding.
Keywords: oxytocin, pair bonding, temperament, anxiety, affiliation, aggression
Graphical Abstract
Introduction
While 90% of birds are considered monogamous, fewer than 5% of mammals follow this reproductive strategy (De Boer, van Buel, & Ter Horst, 2012; Kleiman, 1977; Lukas & Clutton-Brock, 2013; Numan, 2015). Pair bonding involves preference for a familiar partner, distress upon separation, and the partner’s ability to buffer stress (Anzenberger, 1988; Fernandez-Duque, Mason, & Mendoza, 1997; Mendoza & Mason, 1986a). The socially monogamous prairie vole (Microtus ochrogaster) has been the model organism for understanding the neurobiological mechanisms underlying monogamy (Bales et al., 2017; Young, Gobrogge, Liu, & Wang, 2011). Prairie vole studies have demonstrated that both arginine-vasopressin (AVP) and oxytocin (OT) are necessary for facilitating and maintaining monogamy in both males and females (Cho, DeVries, Williams, & Carter, 1999; Ross et al., 2009). Endogenous OT that is released centrally in both males (Borrow & Cameron, 2012) and females (Ross et al., 2009) during mating is particularly important for facilitating individual recognition and persistent selective attraction (Numan & Young, 2016), two mechanisms essential for the facilitation and maintenance of social monogamy. Although this rodent work has informed much of our understanding of the biological basis of mammalian pair-bonding, it is likely that the neural circuitry underlying primate monogamy differs from that of monogamous rodents, given primates’ greater dependence on visual stimuli and differences in OT receptor (OTR) distribution (Bales et al., 2017; Freeman et al., 2014; Freeman & Young, 2016).
While mating and the associated dopamine release (Young et al., 2011) strengthens the neural circuitry underpinning monogamy, relationship quality and physiology also have bidirectional effects on each other which can strengthen pair bonds (Cohen & Wills, 1985). Men and women who report having strong, supportive relationships have higher baseline levels of plasma OT and show a greater increase in OT after spending time in warm, emotional, and physical contact with their partners compared to unsupportive pairs (Grewen, Girdler, Amico, & Light, 2005). The rise in OT may exert anxiolytic effects, dampening the hypothalamic-pituitary-adrenal (HPA)-axis stress response in non-humans (Bakermans-Kranenburg & Van Ijzendoorn, 2013; Gobrogge & Wang, 2015) and humans alike (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Kirsch et al., 2005). Central OT release may also selectively inhibit aggressive responses and upregulate prosocial behaviors towards familiar pair-mates and offspring (Olff et al., 2013). For monogamous primates such as titi monkeys (Callicebus spp.), affiliative behaviors such as grooming, tail-twining, coordinated vocalizations, and maintenance of close physical proximity all function to maintain pair bonds (Fernandez-Duque, Valeggia, & Mason, 2000; Mason, 1966). These affiliative interactions between pair-mates may impact neuropeptide levels in a way that is similar to hormone-behavior interactions observed in humans and act to reinforce monogamous pair bonds.
In conjunction with affiliative behaviors, evolved agonistic behaviors such as mate-guarding have been hypothesized to help maintain pair bonds in monogamous species (Fisher-Phelps et al., 2016). In the wild, extra-pair copulations occasionally occur in titi monkeys, and males have been observed restraining their mates to prevent females from mating with other males (Mason, 1966; Spence-Aizenberg, Di Fiore, & Fernandez-Duque, 2016; Van Belle, Fernandez-Duque, & Di Fiore, 2016). Based on results from live-intruder tests, males are more responsive to perceived same-sex competitors than females, displaying aggressive behaviors such as tail-lashing and back-arching (Cubicciotti & Mason, 1978; Fernandez-Duque et al., 2000; Mendoza & Mason, 1986a). Mate-guarding could lead to additional partner-directed agonistic behaviors such as pair-mate restraint. Studies have demonstrated that low OT is correlated with increased intermale aggression in rodents (Nelson & Trainor, 2007; Todeschin et al., 2009; Veenema, Bredewald, & Neumann, 2007; Winslow, Shapiro, Carter, & Insel, 1993; Winslow et al., 2000) and aggression in humans (Siever, 2008). Low endogenous OT may also be associated with HPA-axis activity. In marmosets (Callithrix jacchus), blockade of OT receptors during a social stress task results in elevated urinary cortisol and reduced time spent in proximity to a social partner (Cavanaugh, Carp, Rock, & French, 2016). Interactions between testosterone and OT have also been found to predict context-specific behaviors, with high testosterone and low OT predicting antagonistic aggression, and high OT and low testosterone predicting affiliation and intimacy (Crespi, 2016; Van Anders, Goldey, & Kuo, 2011; Winslow & Insel, 1991). A dysregulation of normal OT functioning may therefore result in atypical social interactions, such as decreased affiliation, increased partner-directed aggression, and increased anxiety.
The physiological mechanisms (e.g. testosterone, progesterone, cortisol, DHEA, vasopressin, GABA, serotonin, oxytocin) underlying agonistic interspecific interactions has been robustly studied in non-monogamous macaque species (Macaca spp.; de Almeida, Cabral, & Narvaes, 2015; de Jong & Neumann, 2017). Aggression has also been studied in non-monogamous New World primates (Saguinus spp., Buchanan-Smith & Jordan, 1992; Epple, 1978; Epple, 1990; French & Inglette, 1989; French & Snowdon, 1981). Several studies have also measured the behavioral correlates of aggression in monogamous titi monkeys (Callicebus spp.; Cubicciotti & Mason, 1978; Fernandez-Duque, Valeggia & Mason, 2000). Fewer studies, however, have examined the physiological mechanisms underlying agonistic interactions in monogamous primates. Upon exposure to an unfamiliar conspecific, pair-bonded titi monkeys (Callicebus spp.) exhibit an elevated heart-rate (Anzenberger, Mendoza & Mason, 1986; Cubicciotti & Mason, 1976; Cubicciotti, Mendoza & Mason, 1986), increased plasma cortisol concentrations (Maninger et al., 2017; Mendoza & Mason, 1986a), and increased plasma testosterone (Maninger et al., 2017). Very few studies have examined the interaction between OT and aggression in pair-bonding primates. Intranasal manipulations of OT in black-tufted marmosets (Callithrix penicillata) had no effect on aggressive behaviors (Smith, Ågmo, Birnie, & French, 2010). One study that measured OT following exposure to a perceived social threat in coppery titi monkeys (Callicebus cupreus) found no statistically significant correlation between plasma OT and jealousy (Maninger et al., 2017). Another titi monkey study found no overall change in plasma OT in response to a perceived intruder, but a positive correlation between plasma OT and specific behavioral correlates of aggression (Fisher-Phelps et al., 2016).
Macaques do not form adult pair bonds, and brain OTR distribution in rhesus macaques (Macaca mulatta) is highly restricted (Freeman, Inoue, Smith, Goodman, & Young, 2014). Monogamous coppery titi monkeys exhibit a more widespread distribution of brain OTR with particularly dense binding in the hippocampal formation and presubiculum (Freeman et al., 2014). Given the importance of OT in the facilitation and maintenance of monogamous bonds, it is essential to understand the interplay between excessive aggression and the neurobiological mechanisms that are pivotal to the formation and maintenance of monogamy in primates.
We used coppery titi monkeys, a socially monogamous New World monkey, to analyze the effects of aggressive temperament on endogenous neuropeptide concentrations and behavioral responses to a simulated intruder in adult males. Titi monkeys display quintessential aspects of social monogamy, including pair bonding (Bales et al., 2017), living in pairs or small family units (Fuentes, 1998), biparental care (Kinzey, Rosenberger, Heisler, Prowse, & Trilling, 1977; Mason, 1966), and coordination of territorial displays (Anzenberger, Mendoza, & Mason, 1986a; Fuentes, 1998; Robinson, Wright, & Kinzey, 1987). We compared males who display high levels of partner-directed aggression (High-aggressive) to those who display low levels of partner-directed aggression (Low-aggressive). We hypothesized that, when confronted with a social stressor, High-aggressive males would exhibit: 1) lower baseline levels of OT than Low-aggressive males (Nelson & Trainor, 2007; Siever, 2008) and 2) no rise in OT in response to a perceived intruder while in the presence of their partner (Fisher-Phelps et al., 2016; Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). With regards to changes in behavior in response to a perceived intruder, we hypothesized that observer ratings of aggression would be 1) negatively correlated with partner-directed affiliative behaviors, 2) positively correlated with acts of aggression, and 3) positively correlated with anxiety-related behaviors (Cavanaugh, Carp, Rock, & French, 2016). The results of this study will advance our understanding of the relationship between the endogenous OT system and aggression in a monogamous primate.
Methods
Selection of Subjects and Housing
We used 20 pairs (N = 20 adult males from 20 separate pairs) of adult coppery titi monkeys housed at the California National Primate Research Center (CNPRC). All subjects were housed with their pair-mate and any offspring. Families were housed in either 1.2m × 1.2 m × 2.1 m stainless steel cages or 1.2m × 1.2m × 1.8m cages (based on where they were located in the facility), which included four horizontal perches extending the width of the cage at varying heights, an enrichment perch located beside the front door of the cage, a food bowl and two Lixit® water dispensers for continued access to water. Animals were fed twice daily with a diet of New World monkey chow, rice cereal, carrots, apples, raisins and bananas. Animals were kept on a 12-hour light, 12-hour dark cycle (lights on at 0600 hour and off at 1800 hour). Room temperatures were maintained at approximately 21°C. For more details regarding animal husbandry, please refer to Tardif et al. (2006) and Mendoza and Mason (1986a). This study complied with IACUC protocols, legal requirements of the United States, and the policies of the American Society of Primatologists on ethical treatment of primates.
In general, titi monkeys are very affiliative with their pair-mates and only show aggression towards unfamiliar conspecifics (Mendoza & Mason, 1986a). Within our colony, however, we have noted males who display levels of partner-directed aggression that are typically higher than those usually observed in our colony. For our study, we used several methods to identify these males who act aggressively towards their mates. First, we have full health information on all animals throughout their lifetime and were able to identify males who display high levels of partner-directed aggression based on records of pair fighting and partner-inflicted trauma. We also surveyed members of the lab who were familiar with the adult males in our colony, asking them to rate males’ levels of aggression. For a previous study conducted in 2015 (Fontaine, 2015), five lab members rated all males in our colony as displaying high, medium or low levels of aggression. “Low aggression” was scored as a 1 and defined as either no aggression or only some food-related aggression during the first month of pairing. “Medium aggression” was scored as a 2 and was defined as persistent food-related aggression after the first month of pairing. “High aggression” was scored as a 3 and defined as high frequency of food and non-food related aggression that resulted in injury or separation of the pair. Scores for each male were averaged and rounded to the nearest whole number. A total of 4 lab members rated males with 82.0% agreement on scores. The raters had from 3 to 7 years of experience with the titi monkey colony. These previous rankings were also used to identify potential subjects for the present study.
In order to further tease apart variation in aggression displayed by males in our colony, we developed a Likert-type scale of 0–4 for aggression for the present study. A male who is given a score of 0 has never shown any aggression towards his partner. A male who scores a 1 is generally non-aggressive, showed some food aggression early in the pairing, but no longer displays food aggression. A score of 2 represents minor fighting unrelated to food that is short-lasting and has not been known to escalate to chasing or fighting. A male who scores a 3 shows persistent food aggression throughout the pairing that sometimes results in injury. A male who scores a 4 shows persistent non-food-related aggression that escalates to chasing and sometimes injury. A total of 4 lab members rated males using this new scale. These individuals had been members of the lab for 9 months to 12 years at the time of the study. There was unanimous agreement for males who were given a score of 0, 3, or 4. There was some variability between males who were given a score of 1 or 2, and so these ratings were averaged across raters and rounded to the nearest whole number. Overall, there was an 82.75% agreement on scores for males. If a rater was unsure what score to assign a male, he/she left the score blank and we relied on health records and notes from current daily interactions with the titi monkeys to determine a male’s aggression score.
Ultimately, we grouped males who were given a score of 0, 1 and 2 (N=10) into our low aggression (Low-aggressive) category because these behaviors tend to be more common occurrences and almost never lead to partner-inflicted injuries. Males who were given a score of 3 or 4 (N=10) were combined for our high aggression (High-aggressive) category because these behaviors indicate more persistent forms of aggression that are less common in our colony and sometimes result in partner-inflicted injuries. Very aggressive males who repeatedly injure their partners are separated from their partners. These males are either reunited with their mates and moved to a less densely populated space or paired with a new mate. In our colony, we had a total of two highly aggressive males who were ineligible for this study due to increased risk of injury during testing. At the time of testing, there were no significant differences between High-aggressive and Low-aggressive males with regards to male’s average age, weight, length of time paired with current partner, and age difference between pair-mates (Table 1).
Table 1.
Comparison of demographic information between males that show high levels of partner-directed aggression (High-aggressive; N = 10) and males that display low levels of partner-directed aggression (Low-aggressive; N = 10). Age, weight, length of time paired with pair-mate, and difference in age between pair-mates did not significantly differ between groups (P > 0.05). Findings based on a Welch’s T-test. All tests were two-tailed and the significance threshold was set at .05.
| Low-aggressive | High-aggressive | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | df | t | P | |
| Age | 10.30 | 1.15 | 9.44 | 0.93 | 36.49 | 0.58 | 0.56 |
| Weight | 1.35 | 0.07 | 1.30 | 0.05 | 16.08 | 0.55 | 0.59 |
| Pairing Length | 3.75 | 0.88 | 2.23 | 0.50 | 30.26 | 1.50 | 0.14 |
| Age Difference | 3.64 | 1.33 | 4.21 | 0.81 | 14.84 | −0.37 | 0.72 |
Mirror Exposure and Behavioral Assessment
We used a validated mirror technique (Fisher-Phelps et al., 2016) to simulate the introduction of a same-sex stranger to the test subject and his pair-mate. In the wild, titi monkey pairs work together to defend their territories from intruders (Mason, 1966). A mirror serves as an ecologically relevant stimulus that allows us to assess behavioral and physiological responses to a perceived social stressor. Fisher-Phelps and colleagues (2016) found that titi monkeys reacted to the front side of the mirror in ways that indicated they did not recognize themselves in the mirror. Titi monkeys also reacted to their reflection similarly to how they would react to a live unfamiliar conspecific (Mendoza & Mason, 1986a). Titi monkeys did not habituate to the mirror after being tested twice per week for 5 weeks in the validation study (Fisher-Phelps et al., 2016).
On the day of testing, a 33 × 22 cm mirror was placed on top of a movable cart (82.6 cm in height) and moved in front of the subject’s homecage. The mirror was placed approximately 16.5 cm in front of the enrichment perch so the male would see his reflection (as well as that of his mate). Offspring over 6 months of age were temporarily removed from the homecage for the duration of the mirror test (no subjects had infants ≤ 6 months of age). Titi monkey parents do not exhibit any behavioral or physiological changes upon separation from their offspring (Mendoza, 2017; Mendoza, Capitanio, & Mason, 2000; Mendoza & Mason, 1986b); therefore, removal offspring is not expected to influence OT and behavioral outcomes. Each session lasted 5 minutes, with either the non-reflective back of the mirror showing (control condition), or the reflective front of the mirror showing (experimental condition). For the present study, a total of 2 exposures (1 Experimental, 1 Control) were completed, with 2 weeks between each exposure from 4/29/2016 to 3/10/2017. All tests were conducted between 1200 and 1330 hours. We counter-balanced the order of testing and randomized which testing condition the subject experienced first. During each session, subjects were filmed, and behaviors were later scored using Behavior Tracker 1.5 (www.behaviortracker.com) using an established ethogram (Table 2). Videos were coded by one observer. We used a two-step validation process. First, to achieve >95% inter-rater reliability, two observers scored behaviors for three separation mirror test sessions. The second observer was an experience graduate student who was already validated on scoring behaviors for the mirror study. After the observers achieved >95% agreement on all three sessions, the first observer then scored three new sessions three times. The observer was considered validated when they achieved >95% agreement three time in a row for all three mirror test sessions.
Table 2.
Stimulus Exposure Ethogram
| Behavior | Definition |
|---|---|
| Affiliative Social Interactions | |
| Social Proximity1 | Male comes within arm’s reach of female |
| Passive Contact1 | Passive physical contact between male and a pair-mate that does not include tail-twining |
| Lip-smacking2 | Male repeatedly and rapidly opens and closes mouth |
| Tail-twining1 | Male sitting side by side with female, with tails wrapped around each other for at least one rotation |
| Mount/Sex2 | Male mounts female or attempts/succeeds to copulate |
| Aggressive Social Interactions | |
| Male back-arching/tail-lashing2 | Male arches back (as in a frightened cat), the subject may also have his arm and trunk lifted off the perch; Male repetitively swings whole tail from side to side (area greater than 40°, usually sign of agitation); the two can co-occur or occur separately |
| Male restraining2 | Male forcibly restrains female by placing hands on her shoulders |
| Male aggression2 | Male displays aggression to female (e.g. biting, hitting, grabbing) |
| Anxiety-related Interactions | |
| Male leave2 | Male breaks contact/proximity with female |
| Male locomotion1 | Male moves one body length or more |
Scored as duration (in seconds)
Scored as frequency (count)
Blood Collection and Processing
To establish control OT levels, we conducted femoral blood draws after the control condition. We also conducted blood draws following exposure to the front of the mirror to measure changes from control. Two samples were collected from each subject using 1-cc syringes pretreated with heparin. Subjects underwent normal handling conditions (capture in a transport cage, hand captured and manually restrained), and samples were collected 5 minutes after initial exposure to the mirror to capture rapid OT responses (Fisher-Phelps et al., 2016). The average time of blood collection from entrance to the cage to completion of blood draw was 158 ± 11 seconds (sec; mean ± standard error), and the average time between removing a monkey from the transport box until blood draw was 104 ± 11 sec. Following blood collection, samples were immediately placed on ice, centrifuged at 1,610 x g at 4°C, and the plasma extracted and stored at −80°C until assay.
We analyzed OT concentrations (pg/ml) using enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY) with samples diluted at 1:8. Chemical validation of parallelism and quantitative recovery was reported previously (Bales, Kramer, Hostetler, Capitanio, & Mendoza, 2005). Since that time, we have used this assay in a number of studies in titi monkeys, which have provided biological validation for this species. This includes a rise in OT during social distress (Hinde et al., 2016), a biological response which has also been found in prairie voles (Grippo et al., 2007) and humans (Taylor, Saphire-Bernstein, & Seeman, 2010). In addition, we found higher levels of OT in response to serotonin 1a receptor agonism (Larke, Maninger, Ragen, Mendoza, & Bales, 2016), as has previously been found in rodents (Jorgensen, Riis, Knigge, Kjaer, & Warberg, 2003; Osei-Owusu, James, Crane, & Scrogin, 2005).
There is significant controversy over processing of samples for OT enzyme immunoassay, principally whether to extract the samples or not (McCullough, Churchland, & Mendez, 2013). Levels in unextracted samples are significantly higher and more variable than levels in extracted samples, and it is possible that this difference is due to OT fragments or other processing of the molecule, which may or may not be biologically active. However, when OT was measured by a novel but robust nano-liquid chromatography-mass spectrometry platform, the levels were “startlingly high” and in the range of unextracted, not extracted samples (Brandtzaeg et al., 2016). These authors suggest that extraction of samples results in the discard of all but a small amount of free OT that is not bound to plasma proteins. For this study and our other studies, we have therefore elected to continue to use unextracted samples, but acknowledge the significant disagreement over this topic in the literature.
Inter-assay c.v. was 10.8% and intra-assay c.v. was 2.34 ± 0.27%. Sensitivity of the assay is 15 pg/ml with a range of 15.6 −1000 pg/ml.
Data Analysis
We were interested in understanding how male temperament affects males’ physiological and behavioral responses to a perceived intruder. To focus on males’ response to the mirror, all dependent variables were converted to percent change from the control condition to the experimental condition within each individual. This percent change value allowed us to compare the amount and directionality of change for each dependent variable for High-aggressive and Low-aggressive males. All analyses were completed using the percent change value for all dependent variables (for a summary of mean values for dependent variables in the raw metric, see Supplementary Table 1). For all percent change variables, we performed a Shapiro-Wilk test of normality and examined skewness and kurtosis (Royston, 1983). Variables that were non-normally distributed were transformed as necessary.
Physiological responses to mirror exposure.
Using the lme4 package (Bates, Maechler, Bolker, & Walker, 2015) in R Statistical Software (version 3.2.2, R Core Development Team, 2015), we used general linear mixed models (LMM) to model percent change in OT concentration from the control to the mirror condition as a function of aggression category (fixed effect) and whether males saw the front of the mirror first or second (random, repeated measures, factor). Based on the findings from Fisher-Phelps et al. (2016), we did not expect the order in which the mirror was presented to the subject to significantly predict behavior.
Behavioral responses to mirror exposure.
We also used LMM to model percent change in each behavioral measure from the control to the mirror condition. Given the interrelatedness of physiology and behavior (Cohen & Wills, 1985), models examining percent change in behavioral responses to the mirror relative to control included percent change in OT as a fixed effect. Full models included the fixed effect of aggression category (Low-aggressive or High-aggressive), percent change in OT (fixed effect), percent change in OT by aggression category interaction (fixed effect), and whether males saw the front of the mirror first or second (random, repeated measures, factor). Out of the 10 behaviors measured in our ethogram (Table 2), 3 behaviors occurred too infrequently for analysis (percent change in tail-twining, mounting, and aggression). Therefore, we constructed a total of 7 LMM to analyze the effect of male aggression, percent change in OT, and their interaction on behavioral outcomes.
For all 8 models (1 for percent change in OT, 7 for percent change in behavior), we used backwards selection, removing non-significant fixed effects, to determine the most parsimonious model for each outcome measure (Bentler & Mooijaart, 1989). When determining the model of best fit, we conducted a loglikelihood ratio test to compare the fit of the full model to that of the more parsimonious models (Vuong, 1989). Because we have a relatively small sample size (N = 20), we also relied on Akaike information criterion (AIC) comparison to determine which model to compare to the null model (Akaike, 1974; West, Taylor, & Wu, 2012). This comparison was used to determine whether removal of the aggression by percent change in OT interaction effect and the main effect of percent change in OT improved model fit. If the model was significantly improved by removing the interaction effect and the main effect of percent change in OT, then we removed those predictors from our final model. We chose to include aggression score in our final model, even if it did not significantly predict behavior, because this is a key component of our a priori model constructed based on prior knowledge of the biological mechanisms underlying physiological and behavioral responses in other species (Burnham & Anderson, 2002; Guthery, Brennan, Peterson, & Lusk, 2005).
When aggression score did significantly predict a behavior, we also conducted a loglikelihood ratio test to compare the fit of the full model to that of a null model where we removed aggression score as a predictor of percent change in behavior from control to mirror condition (Vuong, 1989). Because we had strong a priori hypotheses regarding relations between outcome measures and our predictors, we did not use post-hoc corrections for multiple comparisons. All tests were two-tailed and the significance threshold was set at .05.
Results
Physiological responses to mirror exposure
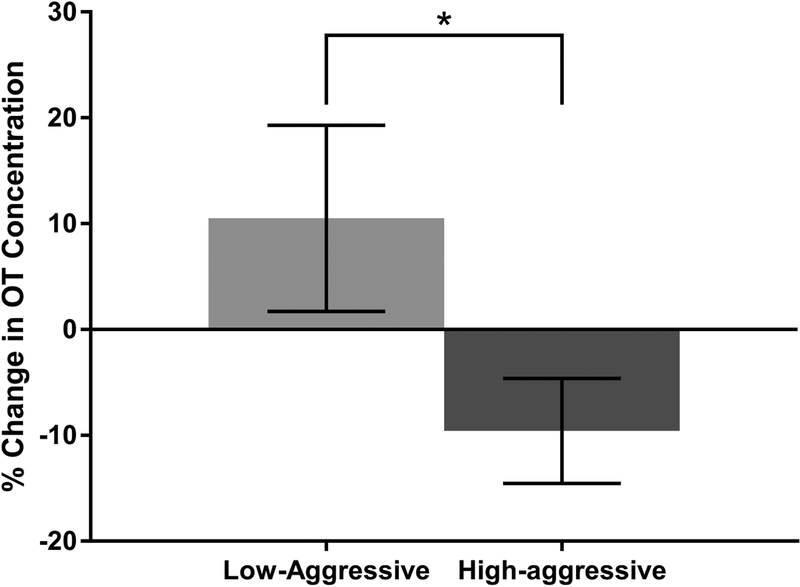
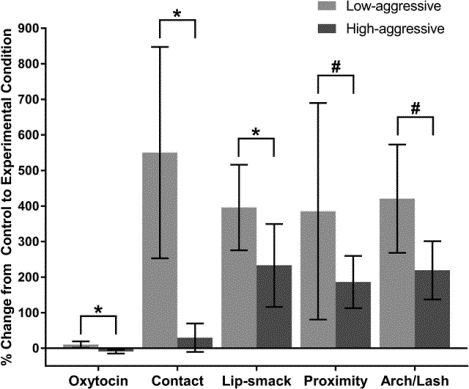
For males in the Low-aggressive category, when exposed to the front of the mirror, OT concentrations increased 11 ± 9% (mean ± standard error) relative to the control condition. OT concentrations dropped 10 ± 5% for High-aggressive males (Figure 1). The percent change OT concentration from the control to the mirror condition was normally distributed (W = 0.98, P = 0.93); therefore, no transformations were necessary. Aggression category did significantly predict percent change in OT response to the mirror (Table 3A; LMM: β = −0.22, SE = 0.10, t = −2.20, P = 0.04), indicating that being a High-aggressive male significantly predicted a decrease in OT concentration relative to control. Our model that included aggression score as a predictor fit significantly better than the null model based on a loglikelihood ratio test (Supplementary Table 2A; X2 = 3.98, df = 1, P = 0.05).
Figure 1.
Percent change in endogenous OT release in response to the front of the mirror (experimental condition) relative to responses to the back of the mirror (control condition). Comparisons were made between males who show high levels of partner-directed aggression (High-aggressive; N = 10) and males who display low levels of aggression (Low-aggressive; N = 10). Percent change in OT concentration was significantly lower for High-aggressive males (LMM: β = −0.22, SE = 0.10, t = −2.20, P = 0.04). Error bars represent standard error of the mean.
* indicates a significant (P < 0.05) main effect of aggression
Table 3.
Parameter estimates from general linear mixed models (LMM) for percent change in oxytocin (OT) concentration and percent change in behavioral measures (proximity, contact, lip-smacking, back-arching/tail-lashing, restraining, movement, and breaking affiliation) from control to mirror condition predicted by males who display high levels of partner-directed aggression (High-aggressive; N = 10) relative to those who display low levels of partner-directed aggression (Low-aggressive). For all behaviors, models included male’s aggression score (High-aggressive; N = 10), percent change in OT concentration from the control condition to the mirror condition (OT), and an interaction between aggression and percent change in OT (High-aggressive*OT). The dependent variables percent change in proximity, contact, lip-smacking, arch/lash, and restrain were log transformed; therefore, estimates are interpreted percent change in the behavior increasing 100*Estimate percent for every one unit increase in the independent variable (High-aggressive, OT, and High-aggressive*OT). The dependent variables percent change in OT concentration, movement, and break affiliation were not transformed. Estimates for percent change in OT concentration relative to baseline is 12 ± 9% for Low-aggressive males and 12 ± 9% - 22 ± 10% for High-aggressive males. Estimates for percent change in movement from the control to mirror condition is equal to 178 ± 46% for Low-aggressive males and 178 ± 46% - 112 ± 65% for males in the High-aggressive category. Estimates for percent change in breaking affiliative contact is equal to 53 ± 35% for Low-aggressive males and 53 ± 35% + 8 ± 50% for High-aggressive males.
| β | SE | t | P | |
|---|---|---|---|---|
| 3A: Oxytocin | ||||
| Intercept | 0.12 | 0.09 | 1.35 | 0.32 |
| High-aggressive | −0.22 | 0.1 | −2.2 | 0.04 |
| 3B: Contact | ||||
| Intercept | 1 | 0.47 | 2.13 | 0.17 |
| High-aggressive | −1.35 | 0.6 | −2.26 | 0.04 |
| OT | −1.91 | 1.27 | −1.51 | 0.15 |
| 3C: Lip-smacking | ||||
| Intercept | 1.55 | 0.3 | 5.2 | < 0.01 |
| High-aggressive | −1.02 | 0.44 | −2.32 | 0.03 |
| OT | −1.31 | 0.93 | −1.4 | 0.18 |
| 3D: Proximity | ||||
| Intercept | 0.19 | 0.69 | 0.28 | 0.81 |
| High-aggressive | 1.03 | 0.75 | 1.38 | 0.19 |
| OT | −0.19 | 1.72 | −0.11 | 0.91 |
| High-aggressive*OT | 6.8 | 3.48 | 1.96 | 0.07 |
| 3E: Restrain | ||||
| Intercept | 0.582 | 0.26 | 2.237 | 0.038 |
| High-aggressive | −0.38 | 0.368 | −1.034 | 0.315 |
| 3F: Back-Arch/ Tail-Lash | ||||
| Intercept | 1.35 | 0.35 | 3.88 | < 0.01 |
| High-aggressive | −0.3 | 0.52 | −0.58 | 0.57 |
| OT | −1.42 | 1.23 | −1.16 | 0.26 |
| High-aggressive*OT | 4.53 | 2.5 | 1.82 | 0.09 |
| 3G: Movement | ||||
| Intercept | 1.78 | 0.46 | 3.88 | < 0.01 |
| High-aggressive | −1.12 | 0.65 | −1.72 | 0.1 |
| 3H: Break Affiliation | ||||
| Intercept | 0.53 | 0.35 | 1.51 | 0.15 |
| High-aggressive | 0.08 | 0.5 | 0.15 | 0.88 |
Behavioral responses to mirror exposure
Affiliative partner-directed responses to the mirror.
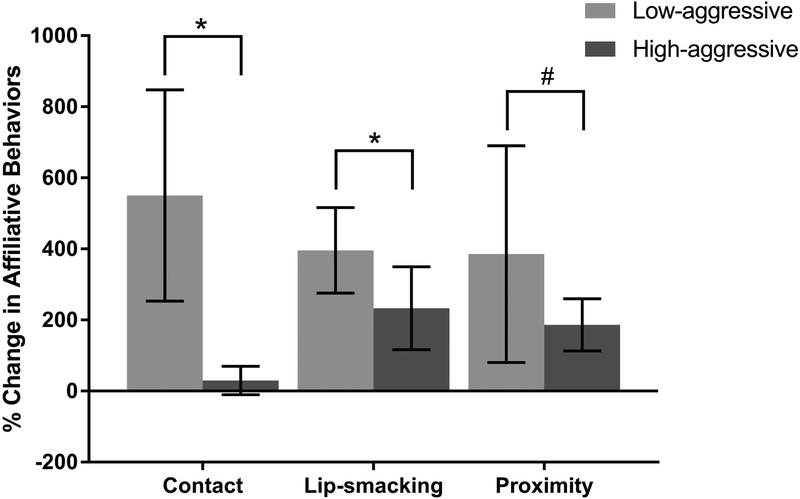
Percent change in time spent in social contact, frequency of lip-smacking, and time spent in social proximity were used to measure change in amount in time spent in affiliative contact with the subject’s pair-mate from the control to the mirror condition. Males in the Low-aggressive category increased time spent in social contact with their mate by 550 ± 30% relative to the control condition. Conversely, High-aggressive males only spent 30 ± 40% more time in contact with their mates in the mirror condition relative to control (Figure 2). Percent change in social contact was positively skewed (skewness = 2.23 ± 2.18, P < 0.001); therefore, a log transformation was used to achieve normal distribution for percent change in social contact (W = 0.93, P = 0.13). There was a significant main effect of aggression on percent change in social contact (Table 3B; LMM: β = −1.35, SE = 0.60, t = −2.26, P = 0.04), indicating that High-aggressive subjects displayed a smaller percent change in time in contact with their partner compared to Low-aggressive males. The best fitting model included both the main effect of percent change in OT and aggression score (Supplementary Table 2B). A loglikelihood ratio test suggested this model fit better than the null model (Supplementary Table 2C; X2 = 5.89, df = 2, P = 0.05).
Figure 2.
Percent change in affiliative partner-directed behaviors in response to the front of the mirror (experimental condition) relative to responses to the back of the mirror (control condition). Comparisons were made between males who show high levels of partner-directed aggression (High-aggressive; N = 10) and males who display low levels of aggression (Low-aggressive; N = 10). High-aggressive males show a smaller percent increase in contact with partners (t = −2.26, P = 0.04) and a smaller percent increase in lip-smacking (t = - 2.32, P = 0.03) when exposed to the front of the mirror. Percent change in time spent in social proximity bouts of lip-smacking did not significantly differ between High-aggressive and Low-aggressive males. Percent change in time spent in social proximity was trending towards being lower for High-aggressive males when percent change in OT concentration from control was negative (t = 1.96, P = 0.07). Error bars represent standard error of the mean.
# indicates a trend (P ≤ 0.10) in interaction effect between percent change in OT and aggression
* indicates a significant (P < 0.05) main effect of aggression
An examination of percent change in lip-smacking in the mirror condition relative to the control condition revealed that Low-aggressive males increased lip-smacking 396 ± 120% while High-aggressive males increased lip-smacking 233 ± 117% (Figure 2). Percent change in lip-smacking was positively skewed (skewness = 1.51 ± 1.47, P < 0.01) but normally distributed following a log transformation (W = 0.97, P = 0.72). There was a significant main effect of aggression (Table 3C; LMM: β = −1.02, SE = 0.44, t = −2.32, P= 0.03), demonstrating, compared to Low-aggressive males, High-aggressive males increased lip-smacking significantly less than Low-aggressive males in the mirror condition relative to the control condition. As with contact, the model including the main effect of percent change in OT and aggression fit better than our more parsimonious model based on a loglikelihood ratio test (Supplementary Table 2D). A loglikelihood ratio test suggested this model was trending towards a better fit than the null model (Supplementary Table 2E; X2 = 5.70, df = 2, P = 0.06).
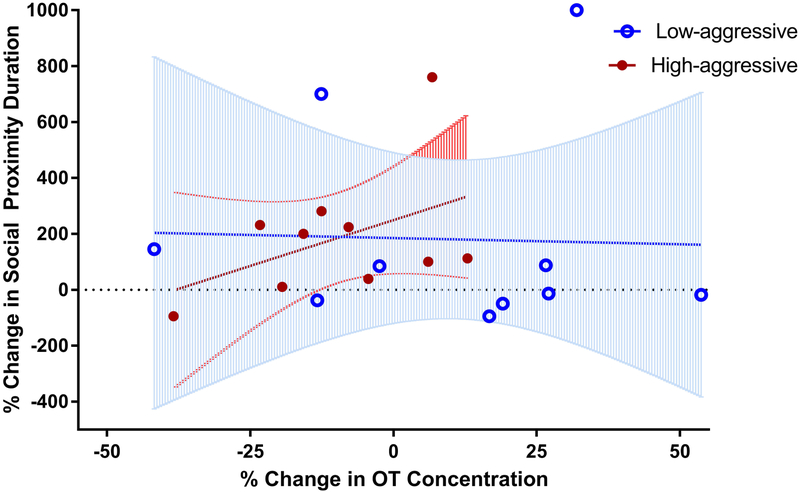
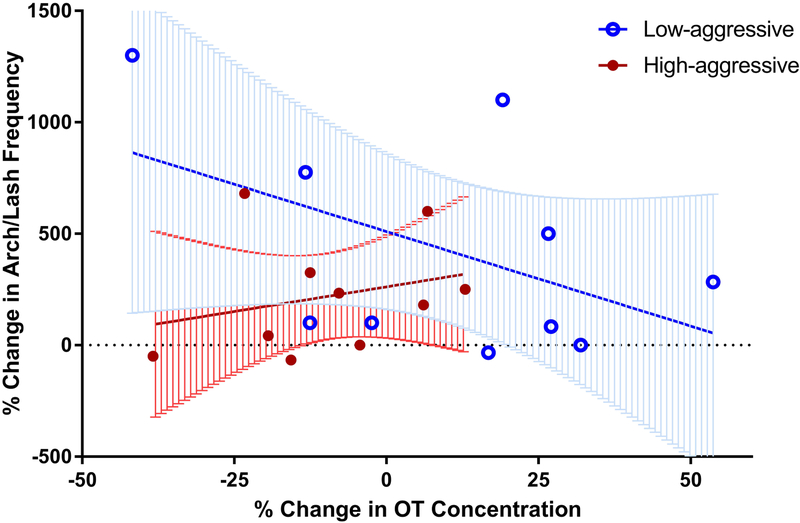
Low-aggressive males spent 385 ± 305% while High-aggressive males spent 186 ± 74% more time in proximity to their mates relative to the control condition (Figure 2). Percent change in social proximity in the mirror condition relative to control was not normally distributed (W = 0.81, P = 0.001); therefore, we used a log transformation on this outcome variable (W = 0.94, P = 0.20). There was a non-significant trend towards an interaction between change in OT relative to the control condition and male aggression score (Figure 3; Table 3D; LMM: β = 6.80, SE = 3.48, t = 1.96, P = 0.07). This finding indicates that High-aggressive subjects trended towards spending less time in close proximity to their partner when their percent change in OT concentration from the control to experimental condition was negative. The full model with the interaction effect fit slightly better than the more parsimonious models that did not include the interaction effect, based on a comparison of AIC and a loglikelihood ratio test (Supplementary Table 2F). This model did not fit better than the null model based a log likelihood ratio test (Supplementary Table 2G; X2 = 3.90, df = 3, P = 0.27).
Figure 3.
Percent change in time spent in social proximity to the pair-mate in response to the front of the mirror (experimental condition) relative to responses to the back of the mirror (control condition). Comparisons were made between males who show high levels of partner-directed aggression (High-aggressive; N = 10) and males who display low levels of aggression (Low-aggressive; N = 10). Percent change in time spent in social proximity was trending towards being smaller for High-aggressive males when percent change in OT concentration (pg/ml) from control was negative (t = 1.96, P = 0.07).
Percent change in time spent tail-twining and frequency of mounting were also measures of affiliation; however, these behaviors occurred too infrequently for analysis. In the control condition, one Low-aggressive male spent 3 seconds tail-twining with his mate and one High-aggressive male spent 23 seconds tail-twining; however, neither male tail-twined when exposed to the front of the mirror. Another Low-aggressive male did not tail-twine with his mate during the control condition but spent 84 seconds tail-twining in the experimental condition. In the control condition, one High-aggressive male mounted his mate, but he did not mount her when exposed to the front of the mirror. No other males mounted their mates during testing.
Agonistic partner-directed responses to the mirror.
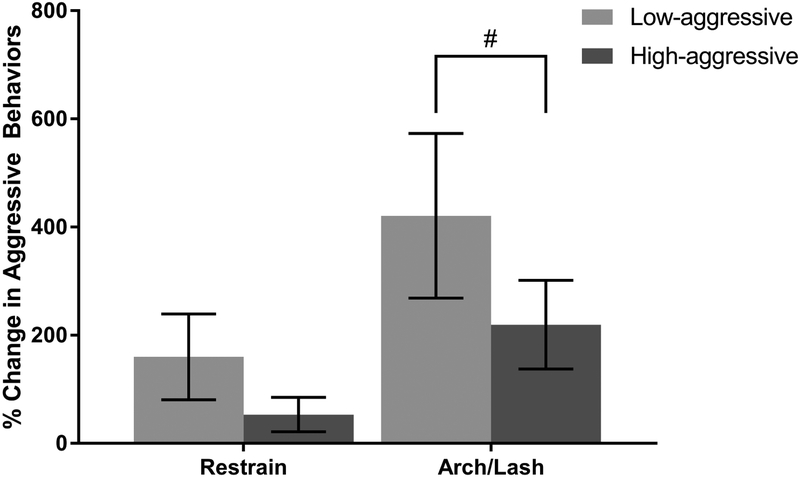
Percent change in frequency of pair-mate restraint, back-arching and tail-lashing displays (arching/lashing), and bouts of partner-directed aggression (e.g. biting, grabbing, hitting) were measures of the amount of change in agonistic partner-directed responses to the mirror relative to the control condition. Low-aggressive males restrained their pair-mates 160 ± 80% more relative to the control condition, while High-aggressive males showed an 82 ± 53% increase in pair-mate restraint relative to control (Figure 4). Restraining behavior was positively skewed (skewness = 1.71 ± 1.68, P < 0.001) so we conducted a log transformation to achieve normality (W = 0.94, P = 0.27). Based on an AIC comparison and a loglikelihood ratio test, including the main effect of percent change in OT and the interaction between percent change in OT and aggression score did not improve model fit (Supplementary Table 2H); therefore, our final model only included aggression score as a fixed effect and the order in which a subject was exposed to the mirror and control condition as a random effect. Aggression did not significantly predict percent change in restraining from control (Table 3E; LMM: β = −0.38, SE = 0.37, t = −1.03, P = 0.32).
Figure 4.
Percent change in aggressive partner-directed behaviors in response to the front of the mirror (experimental condition) relative to responses to the back of the mirror (control condition). Comparisons were made between males who exhibit high levels of partner-directed aggression (High-aggressive; N = 10) and males who display low aggression (Low-aggressive; N = 10). Percent change in pair-mate restraining behavior (restrain) did not significantly differ between High-aggressive and Low-aggressive males. Percent change in back-arching and tail-lashing bouts (arch/lash) was trending towards being smaller for High-aggressive males when percent change in OT concentration from control was negative (t = 1.82, P = 0.09). Error bars represent standard error of the mean.
# indicates a trend (P ≤ 0.10) in interaction effect between percent change in OT and aggression
Arching/lashing behaviors increased 421 ± 152% for Low-aggressive males and 219 ± 82% for High-aggressive males (Figure 4). Based on a Shapiro-Wilk test of normality, arching/lashing was non-normally distributed (W = 0.85, P = 0.006); however, arching/lashing was normally distributed following a log transformation (W = 0.97, P = 0.83). The interaction between OT and aggression score was trending towards significantly predicting changes in arching/lashing in response to the mirror relative to control (Figure 5; Table 3F; LMM: β = 4.53, SE = 2.5, t = 1.82, P = 0.09). High-aggressive males with a negative percent change in OT from control trended towards exhibiting less back-arching and tail-lashing displays when exposed to the front of the mirror. The full model showed significantly improved fit compared to the more parsimonious models that did not include the interaction term or the main effect of OT (Supplementary Table 2I). The full model did not fit significantly better than the null model, though, based on a loglikelihood ratio test (Supplementary Table 2J; X2 = 4.82, df = 3, P = 0.19).
Figure 5.
Percent change in frequency of back-arching and tail-lashing (arch/lash) displays in response to the front of the mirror (experimental condition) relative to responses to the back of the mirror (control condition). Comparisons were made between males who show high levels of partner-directed aggression (High-aggressive; N = 10) and males who display low levels of aggression (Low-aggressive; N = 10). Percent change in arch/lash displays was trending towards being smaller for High-aggressive males when change in OT concentration from control was negative (t = 1.82, P = 0.09).
Acts of aggression occurred too infrequently for analysis, and only occurred in the experimental condition. One High-aggressive male displayed aggression towards his partner twice. One High-aggressive and one Low-aggressive male each displayed one act of partner-directed aggression in response to exposure to the front of the mirror. None of these instances of aggression lead to injury. No other males exhibited acts of aggression during testing.
Anxiety-related responses to the mirror.
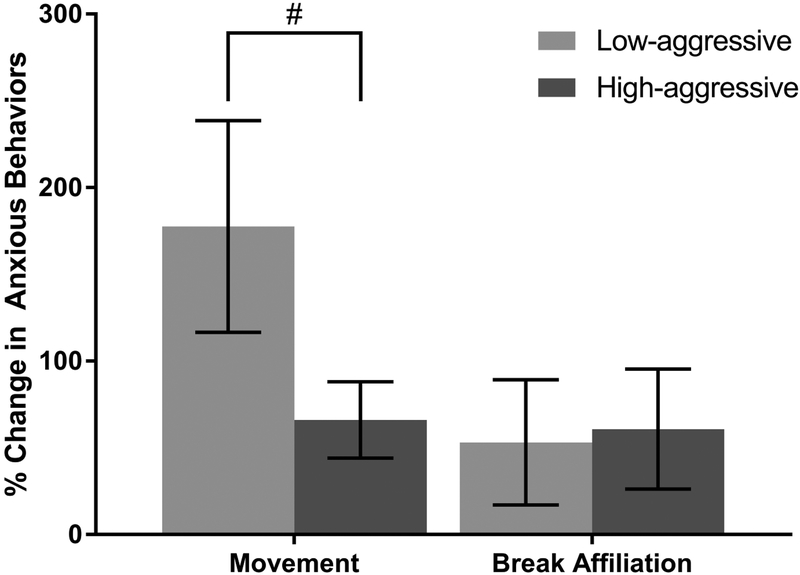
Percent change in the amount of time a male spent moving during the mirror test represented a measure of anxiety-like behaviors in response to a perceived social stressor (Capitanio, Mason, Mendoza, Del Rosso, & Roberts, 2006; Ragen, Freeman, Laredo, Mendoza, & Bales, 2015). Percent change in number of times a male broke affiliative contact with his mate during the test also measured anxiety-like behavior (Amaral, 2002; Barros, Major, Huston, & Tomaz, 2008; Steimer, 2002). Low-aggressive males increased time spent moving during the mirror condition relative to the control condition 178 ± 61%, whereas High-aggressive males displayed a 66 ± 22% increase in locomotion duration (Figure 6). Percent change in movement relative to the control condition was very slightly non-normally distributed, based on the Shapiro-Wilk test of normality (W = 0.90, P = 0.04); however, there was no significant skew (skewness = 0.67 ± 0.66, P > 0.05) or kurtosis (kurtosis = −0.82 ± −0.42, P > 0.05). Therefore, we decided not to transform this outcome variable. While there was no significant main effect of OT or interaction effect of aggression and OT (Supplementary Table 2K), there was a non-significant trend towards locomotion being predicted by aggressive temperament (Table 3G; LMM: β = −1.12, SE = 0.65, t = −1.72, P = 0.10). High-aggressive males trended towards a lower percent increase in moving around the homecage compared to Low-aggressive males when exposed to the front of the mirror relative to control. A loglikelihood ratio test suggest a non-significant trend towards better fit for the full model compared to the null model (Supplementary Table 2L; X2 = 3.05, df = 1, P = 0.08).
Figure 6.
Percent change in anxiety-like behaviors in response to the front of the mirror (experimental condition) relative to responses to the back of the mirror (control condition). Comparisons were made between males who show high levels of partner-directed aggression (High-aggressive; N = 10) and males who display low levels of aggression (Low-aggressive; N = 10). Percent change in times a male broke affiliative contact with his partner (break affiliation) did not significantly differ between High-aggressive and Low-aggressive males. Percent change in time spent locomoting during the test (movement) was trending towards being smaller for High-aggressive males (t = −1.72, P = 0.10). Error bars represent standard error of the mean.
# indicates a trend (P ≤ 0.10) towards a main effect of aggression
Males in the Low-aggressive group increased the number of times they broke affiliative contact with their mate relative to the control condition by 53 ± 36% while High-aggressive males increased leaving their mates 61 ± 35% more relative to the control condition when exposed to the front of the mirror (Figure 6). The measure for percent change in males breaking affiliative contact with their mate relative to the control condition was non-normally distributed (W = 0.87, P = 0.01); however, there was no significant skewness (skewness = 0.77 ± 0.75, P > 0.05) or kurtosis (kurtosis = −0.69 ± −0.35, p > 0.05). A log transformation did not result in normal distribution (W = 0.92, P = 0.09); therefore, we decided to not transform this variable. Neither the interaction between percent change in OT and aggression, nor the main effect of percent change in OT relative to control predicted males breaking affiliative contact with their mate (Supplementary Table 2M); therefore, these fixed effects were removed from the final model. Aggression was also not a significant predictor of percent change in males breaking affiliative contact with pair-mates relative to the control condition (LMM: β = 0.08, SE = 0.50, t = 0.15, P = 0.88).
Discussion
We used captive coppery titi monkeys to explore the effects of male aggressive temperament on physiological and behavior responses to a perceived social stressor (a mirror). Based on human (Fries et al., 2005), rodent (Veenema, 2012), and primate research with non-monogamous species such as macaques (de Jong & Neumann, 2017; Veenema, 2009), we expected control levels of OT to be lower in High-aggressive males. OT also acts as an anxiolytic and rises in response to social threat (Bakermans-Kranenburg & Van Ijzendoorn, 2013; Gobrogge & Wang, 2015); however, aggression is typically negatively correlated with OT response to stressors (Veenema et al., 2007; Winslow et al., 1993; Winslow et al., 2000). Given the degree of conservation of OT and OTR across species (Gimpl & Fahrenholz, 2001), we hypothesized that OT concentrations would rise in response to social stress in Low-aggressive males, whereas High-aggressive monogamous male primates would not exhibit the typical anxiolytic rise in OT concentration relative to control when exposed to the front of the mirror. We did find that OT concentrations rose for Low-aggressive males and decreased relative to the control condition for High-aggressive males, and this difference was statistically significant.
These differences in physiological responses to the mirror may be explained in part by the observed differences in partner interactions during the test. In the present study, we hypothesized that High-aggressive males would exhibit lower levels of partner-directed affiliative behaviors relative to control when exposed to the front of the mirror. Low-aggressive males spent significantly more time in contact with their mates in response to the mirror and lip-smacked more than High-aggressive males in the mirror condition relative to the control condition. These findings suggest that High-aggressive males, which displayed a decrease in endogenous OT release in response to a perceived social stressor, spend significantly less time in affiliation with their partners in a challenging social context.
Pair-mates can act as buffers for stressors, and bidirectionally influence changes in neuroendocrine concentrations and behavior (Cohen & Wills, 1985; Heinrichs et al., 2003). In humans, everyday interactions between men and women in long-term relationships influence their positive and negative behaviors towards each other (Gable, Reis, & Downey, 2003). Variation in amount of time spent in affiliative contact with partners also impacts hormone concentrations in humans (Grewen et al., 2005). Differences in relationship quality and levels of attachment anxiety can also differentially impact couples’ physiology and behavior (Diamond, Hicks, & Otter-Henderson, 2008).
Grewen and colleagues (2005) found that both men and women who report having strong, supportive relationships had higher control levels of OT and showed a greater increase in OT after spending time in warm, emotional, and physical contact with their partners compared to pairs who did not report high levels of support. Our observed differences in percent change in time spent in social contact for Low-aggressive and High-aggressive males may have been driven by differences in pair-mate interactions initiated by females. If the neurobiological mechanisms underlying responses to warmth and physical contact in titi monkeys are similar to those observed in humans (Grewen et al., 2005), then this increase in time spent in contact with pair-mates may have driven the observed rise in OT concentrations in Low-aggressive males in response to the mirror.
While we only observed one instance of mating during testing, mating induces endogenous OT release (Borrow & Cameron, 2012; Ross et al., 2009; Waldherr & Neumann, 2007). Other affiliative behaviors like tail-twining and sitting in warm physical contact may have similar anti-anxiety effects which may be related increased in OT concentrations in Low-aggressive males upon exposure to a perceived intruder (Walker, Trotter, Swaney, Marshall, & Mcglone, 2017). Hormone-behavior interactions are highly bidirectional (Cohen & Wills, 1985); therefore, it is likely that affiliative behaviors and OT responses exert a synergistic effect on each other.
We hypothesized that High-aggressive males would display more agonistic partner-directed responses when exposed to the front of the mirror. Temperament did not significantly predict changes in frequency of displays of pair-mate restraint from control. For displays of back-arching and tail-lashing, there was a non-significant trend towards an interaction effect between aggression and percent change in OT concentration from control such that High-aggressive males with a greater decrease OT concentration relative to the control condition displayed a greater decrease in arching/lashing. While this non-significant trend describes the interaction effect for the entire group of males, it is important to note that there was variability in percent change in OT. Seven High-aggressive males showed a negative percent change in OT in response to the mirror, while three High-aggressive males showed a positive percent change in OT in response to a perceived intruder. Conversely, four Low-aggressive males exhibited a negative percent change in OT in response to the mirror, while six Low-aggressive males exhibited a positive percent change in OT in response to the mirror. The non-significant trend towards an interaction between percent change in OT and aggression score may be driven by the seven High-aggressive males displaying a decrease in OT in response to a perceived social threat.
Back-arching and tail-lashing are two of the coordinated activities titi monkeys display to guard territories and visually demonstrate their pair bond (Anzenberger et al., 1986; Fuentes, 1998; Robinson et al., 1987). Low-aggressive males increased back-arching and tail-lashing displays slightly more than High-aggressive males when faced with a perceived intruder in their homecage. High-aggressive males exhibited a non-significant trend towards showing a smaller percent change in arching/lashing when they exhibited an endogenous decrease in OT in response to the social threat. Conversely, Low-aggressive males exhibited a non-significant trend towards a smaller percent change in arching/lashing when they exhibited a positive percent change in OT in response to the mirror. These trends were likely non-significant due to variability in percent change in OT in response to the mirror. As previously mentioned, seven High-aggressive males and four Low-aggressive males exhibited a negative percent change in OT in response to the mirror.
A rise in OT may underlie coordinated displays between pair bonded primates. During testing, when one partner started back-arching and tail-lashing, the other would join in the display. This behavior may therefore reflect greater coordination between partners, rather than greater agonistic partner-directed behaviors.
Of the four instances of aggression (e.g. biting, hitting, grabbing) observed during the mirror test, three were from High-aggressive males, and one was from a Low-aggressive male. To display other agonistic partner-directed behaviors such as pair-mate restraint, females must let their partners sit close enough to grab them. As demonstrated by our measures of affiliative interactions, High-aggressive males spent significantly less time in contact with their mates; therefore, they had fewer chances to display aggressive partner-directed behaviors during this test. During testing, we observed females actively avoiding High-aggressive males. While our current ethogram does not measure this exchange of male approach and female avoid, this would be an interesting dyadic interaction to model to better elucidate females’ responses to their aggressive partners.
Our fourth hypothesis was that, compared to Low-aggressive males, High-aggressive males would display more anxiety-related responses to a perceived social stressor, measured by locomotion (Capitanio et al., 2006; Ragen et al., 2015) and breaking affiliative contact with a pair-mate (Amaral, 2002; Barros et al., 2008; Steimer, 2002). We found no statistically significant differences between Low-aggressive and High-aggressive males in the percent change in breaking affiliative contact with pair-mates relative to the control condition. High-aggressive males displayed a slightly smaller percent increase in time moving around their homecage relative to the control condition compared to Low-aggressive males, but this difference was not statistically significant.
Locomotion, particularly pacing, is a behavioral correlate for anxiety in non-human primates (Capitanio et al., 2006; Ragen et al., 2015). Differences in increases in time spent locomoting during the mirror test may further explain the trends in affiliative and aggressive partner-directed behaviors observed in our study. In humans, OT mediates fear and stress in response to social stimuli (Heinrichs et al., 2003; Kirsch et al., 2005). In a monogamous primate species like titi monkeys, if a male were displaying anxiety-like behaviors, then female pair-mates may have begun initiating greater amounts of affiliative behaviors, such as social contact, to reinforce their pair bond (Fernandez-Duque et al., 2000; Mason, 1966). During this test, when one partner was pacing, we often observed their pair-mate approach and lip-smack. Affiliative behaviors may buffer partners from stressors (Kikusui, Winslow, & Mori, 2006) and may be initiated more in times of social stress. To test this theory, we would need to identify which partner initiated social contact and whether contact was initiated more frequently following bouts of pacing.
The dysregulation of the OT system detected in our High-aggressive males may be related to the observed alterations in a suite of behaviors (e.g. anxiety-like behaviors, affiliative interactions, aggressive partner-directed responses) in response to a social stressor; however, from this experiment it is not possible to infer causality. Aggressive human adolescents exhibit a blunted OT and cortisol response in response to social stressors (Fragkaki, Cima, & Granic, 2018). These findings suggest that aggressive adolescents may not perceive a stressful situation as such and therefore may not exhibit the expected rise in OT in response to stress. In response to a perceived social stressor, our High-aggressive males increased pacing less than Low-aggressive males and exhibited a decrease in endogenous OT release, suggesting these males may similarly have exhibited a lowered stress response compared to Low-aggressive males. Future studies should measure cortisol levels as well as OT levels to examine the interactions between HPA-axis activity and the endogenous OT system. Given the opposing effects of OT and testosterone on aggression (Crespi, 2016; Van Anders et al., 2011; Winslow & Insel, 1991), future studies should also measure testosterone levels to further understand the physiological mechanisms underlying partner-directed aggression in monogamous primates.
Conclusions.
This mirror technique serves as a valuable tool for examining individual differences in response to social stressors in adult titi monkeys. All subjects demonstrated a robust response to the mirror, exhibiting increases in behaviors relative to control conditions ranging from a 30% (male increasing time in contact with partner) to a 550% (contact) increase. Low-aggressive males exhibited a rise in OT in response to social threat, whereas circulating levels of OT decreased for High-aggressive males when exposed to the front of the mirror. Males which display abnormally high levels of partner-directed aggression spent less time in affiliative contact with their mates and lip-smacked less relative to control when exposed to the front of the mirror. We also saw non-significant trend toward decreases in time spent in social proximity and frequency of arching/lashing bouts for High-aggressive males as their change in OT concentration from control decreased. To our knowledge, this is the first empirical study to demonstrate differences in behavioral and physiological responses to a social stressor in adult monogamous primates based on variations in aggressive temperament. Future studies should incorporate measures of dyadic physiological and behavioral interactions between partners as well as measures of early-life experience to further understand differences in responses to social stressors in monogamous primates.
Supplementary Material
Acknowledgements
We would like to thank the following for their invaluable assistance: Dr. John Capitanio, Dr. Sara Freeman, Dr. Emily Rothwell, Dr. Tamara Weinstein, Dr. Becky Larke, Paul-Michael Sosa, Rocio Arias Del Razo, Leana Goetze, Liz Sahagun, Alexandra Castro and several UC Davis undergraduate interns. We would also like to thank the CNPRC veterinary staff. Funding was provided by the American Society of Primatologists small grant, the Good Nature Institute; NIH grant HD017998; the University of California, Davis Department of Psychology and California National Primate Research Center grant OD011107. We confirm compliance with animal care regulations and national laws.
Footnotes
Disclosure
The authors report no conflict of interests in this work.
References
- Akaike H (1974). A new look at the statistical model identification. IEEE transactions on automatic control, 19(6), 716–723. [Google Scholar]
- Amaral DG (2002). The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biological psychiatry, 51(1), 11–17. [DOI] [PubMed] [Google Scholar]
- Anzenberger G (1988). The pairbond in the titi monkey (Callicebus moloch): intrinsic versus extrinsic contributions of pairmates. Folia Primatologica, 50, 188–203. [DOI] [PubMed] [Google Scholar]
- Anzenberger G, Mendoza SP, & Mason WA (1986). Comparative studies of social behavior in Callicebus and Saimiri: Behavioral and physiological responses of established pairs to unfamiliar pairs. American Journal of Primatology, 11(1), 37–51. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2013). Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational psychiatry, 3(5), e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Arias del Razo R, Conklin QA, Hartman S, Mayer HS, Rogers FD, Simmons TC, Smith LK, Williams A, Williams DR, Witczak LR, & Wright EC (2017). Titi Monkeys as a Novel Non-Human Primate Model for the Neurobiology of Pair Bonding. The Yale Journal of Biology and Medicine, 90(3), 373–387. [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Hostetler CM, Capitanio JP, & Mendoza SP (2005). Validation of oxytocin and vasopressin blood assay for primates: what can blood tell us? American Journal of Primatology, 66, 73. [Google Scholar]
- Barros M, Maior RS, Huston JP, & Tomaz C (2008). Predatory stress as an experimental strategy to measure fear and anxiety-related behaviors in non-human primates. Reviews in the Neurosciences, 19(2–3), 157–170. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar]
- Bentler PM, & Mooijaart AB (1989). Choice of structural model via parsimony: a rationale based on precision. Psychological bulletin, 106(2), 315–317. [DOI] [PubMed] [Google Scholar]
- Borrow AP, & Cameron NM (2012). The role of oxytocin in mating and pregnancy. Hormones and Behavior, 61(3), 266–276. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip FK, MacLean EL, Gesquiere LR, Leknes S, Lundanes E, & Wilson SR (2016). Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Scientific Reports, 6, 31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Smith HM, & Jordan TR (1992). An experimental investigation of the pair bond in the callitrichid monkey, Saguinus labiatus. International journal of primatology, 13(1), 51–72. [Google Scholar]
- Burnham KP, & Anderson DR (2002). Model Selection and Multi-model Inference: A Practical Information-Theoretic Approach (2nd Ed.). Springer-Verlag, New York, NY, USA. [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, & Roberts JA (2006). Nursery rearing and biobehavioral organization In Nursery rearing of nonhuman primates in the 21st century (pp. 191–214). Springer, Boston, MA. [Google Scholar]
- Cavanaugh J, Carp SB, Rock CM, & French JA (2016). Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psych neuroendocrinology, 66, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, & Carter CS (1999). The effects of oxytocin and vasopressin on partner preference in male and female prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 133, 1071–1079. [DOI] [PubMed] [Google Scholar]
- Cohen S, & Wills TA (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98(2), 310–357. [PubMed] [Google Scholar]
- Crespi BJ (2016). Oxytocin, testosterone, and human social cognition. Biological Reviews, 91(2), 390–408. [DOI] [PubMed] [Google Scholar]
- Cubicciotti D III, & Mason WA (1976). Comparative studies of social behavior in Callicebus and Saimiri: Male-female emotional attachments. Behavioral Biology, 16(2), 185–197. [DOI] [PubMed] [Google Scholar]
- Cubicciotti DD, & Mason WA (1978). Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behavioral Ecology and Sociobiology, 3(3), 311–322. [Google Scholar]
- Cubicciotti DD, Mendoza SP, Mason WA, & Sassenrath EN (1986). Differences between Saimiri sciureus and Callicebus moloch in physiological responsiveness: Implications for behavior. Journal of comparative psychology, 100(4), 385. [PubMed] [Google Scholar]
- de Almeida RMM, Cabral JCC, & Narvaes R (2015). Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiology & behavior, 143, 121–135. [DOI] [PubMed] [Google Scholar]
- De Boer A, Van Buel EM, & Ter Horst GJ (2012). Love is more than just a kiss: a neurobiological perspective on love and affection. Neuroscience, 201, 114–124. [DOI] [PubMed] [Google Scholar]
- de Jong TR, & Neumann ID (2017). Oxytocin and aggression. Current topics in behavioral neurosciences, 1–18. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, & Otter-Henderson KD (2008). Every time you go away: changes in affect, behavior, and physiology associated with travel-related separations from romantic partners. Journal of Personality and Social Psychology, 95(2), 385. [DOI] [PubMed] [Google Scholar]
- Epple G (1978). Lack of effects of castration on scent marking, displays, and aggression in a South American primate (Saguinus fuscicollis). Hormones and behavior, 11(2), 139–150. [DOI] [PubMed] [Google Scholar]
- Epple G (1990). Sex differences in partner preference in mated pairs of saddle-back tamarins (Saguinus fuscicollis). Behavioral Ecology and Sociobiology, 27(6), 455–459. [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP (1997). Effects of separation on responses to mates and strangers in the monogamous titi monkey. American Journal of Primatology, 43, 225–237. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, & Mason WA (2000). Effects of pair-bond and social context on male-female interactions in captive titi monkeys (Callicebus moloch, Primates: Cebidae). Ethology, 106(12), 1067–1082. [Google Scholar]
- Fisher-Phelps ML, Mendoza SP, Serna S, Griffin LL, Schaefer TJ, Jarcho MR, Rage BJ, Goetze LR, & Bales KL (2016). Laboratory simulations of mate-guarding as a component of the pair-bond in male titi monkeys, Callicebus cupreus. American Journal of Primatology. doi: 10.1002/ajp.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine C (2015). Aggressiveness in male titi monkeys (Callicebus cupreus). Ecole Nationale Veterinaire de Toulouse (unpublished master’s thesis). [Google Scholar]
- Fragkaki I, Cima M, & Granic I (2018). The Role of Trauma in the Hormonal Interplay of Cortisol, Testosterone, and Oxytocin in Adolescent Aggression. Psychoneuroendocrinology, 88, 24–37. [DOI] [PubMed] [Google Scholar]
- Freeman SM, & Young LJ (2016). Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of neuroendocrinology, 28(4). doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, & Young LJ (2014). The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology, 45, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, & Young LJ (2014). Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience, 273, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, & Inglett BJ (1989). Female-female aggression and male indifference in response to unfamiliar intruders in lion tamarins. Animal Behaviour, 37, 487–497. [Google Scholar]
- French JA, & Snowdon CT (1981). Sexual dimorphism in responses to unfamiliar intruders in the tamarin, Saguinus oedipus. Animal Behaviour, 29(3), 822–829. [Google Scholar]
- Fries ABW, Ziegler TE, Kurian JR, Jacoris S, & Pollak SD (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America, 102(47), 17237–17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A (1998). Re-evaluating primate monogamy. American Anthropologist, 100(4), 890–907. [Google Scholar]
- Gable SL, Reis HT, & Downey G (2003). He said, she said a quasi-signal detection analysis of daily interactions between close relationship partners. Psychological Science, 14(2), 100–105. [DOI] [PubMed] [Google Scholar]
- Gimpl G, & Fahrenholz F (2001). The oxytocin receptor system: structure, function, and regulation. Physiological reviews, 81(2), 629–683. [DOI] [PubMed] [Google Scholar]
- Gobrogge K, & Wang Z (2015). Neuropeptidergic regulation of pair-bonding and stress buffering: lessons from voles. Hormones and behavior, 76, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, & Light KC (2005). Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine, 67(4), 531–538. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, & Carter CS (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology, 32(8–10), 966–980. doi: S0306–4530(07)00167–9 [pii]; 10.1016/j.psyneuen.2007.07.004 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthery FS, Brennan LA, Peterson MJ, & Lusk JJ (2005). Invited paper: Information theory in wildlife science: critique and viewpoint. Journal of Wildlife Management, 69(2), 457–465. [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, & Ehlert U (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398. [DOI] [PubMed] [Google Scholar]
- Hinde K, Muth C, Maninger N, Ragen BJ, Larke RH, Jarcho MR, Mendoza SP, Mason WA, Ferrer E, Cherry SR, Fisher-Phelps ML, & Bales KL (2016). Challenges to the pair bond: neural and hormonal effects of separation and reunion in a monogamous primate. Frontiers in Behavioral Neuroscience, 10, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen H, Riis M, Knigge U, Kjaer A, & Warberg J (2003). Serotonin receptors involved in vasopressin and oxytocin secretion. Journal of Neuroendocrinology, 15, 242–249. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: relief from stress and anxiety. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 361(1476), 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzey WG, Rosenberger AL, Heisler PS, Prowse DL, & Trilling JS (1977). A preliminary field investigation of the yellow handed titi monkey, Callicebus torquatus, in northern Peru. Primates, 18(1), 159–181. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, & Meyer-Lindenberg A (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG (1977). Monogamy in mammals. The Quarterly review of biology, 52(1), 39–69. [DOI] [PubMed] [Google Scholar]
- Larke RH, Maninger N, Ragen BJ, Mendoza SP, & Bales KL (2016). Serotonin 1A agonism decreases affiliative behavior in pair-bonded titi monkeys. Hormones and Behavior, 86, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, & Clutton-Brock TH (2013). The evolution of social monogamy in mammals. Science, 341(6145), 526–530. [DOI] [PubMed] [Google Scholar]
- Maninger N, Mendoza S, Williams D, Mason W, Cherry S, Rowland D, Schaefer T, & Bales KL (2017). Imaging, Behavior and Endocrine Analysis of “Jealousy” in a Monogamous Primate. Frontiers in Ecology and Evolution, 5, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA (1966). Social organization of the South American monkey, Callicebus moloch: a preliminary report. Tulane Studies in Zoology, 13, 23–28. [Google Scholar]
- McCullough ME, Churchland PS, & Mendez AJ (2013). Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neuroscience & Biobehavioral Reviews, 37, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Mendoza SP (2017). Social Stress: concepts, assumptions, and animal models In Pfaff DW & Joels M (Eds.), Hormones, Brain, and Behavior (3rd ed.; pp. 261–284). Oxford, United Kingdom: Academic Press. [Google Scholar]
- Mendoza SP & Mason WA (1986a). Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology & Behavior, 38, 795–801. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, & Mason WA (1986b). Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch). Animal Behaviour, 34(5), 1336–1347. [Google Scholar]
- Mendoza SP, Capitanio JP, & Mason WA (2000). Chronic social stress: studies in non-human primates. Biology of animal stress: Basic principles and implications for animal welfare, 227–247. [Google Scholar]
- Nelson RJ, & Trainor BC (2007). Neural mechanisms of aggression. Nature Reviews Neuroscience, 8(7), 536. [DOI] [PubMed] [Google Scholar]
- Numan M (2015). Neurobiology of social behavior: toward an understating of the prosocial and antisocial brain. London, England: Elsevier: Academic Press. [Google Scholar]
- Numan M, & Young LJ (2016). Neural mechanisms of mother–infant bonding and pair bonding: similarities, differences, and broader implications. Hormones and Behavior, 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz B, Yee JR, & van Zuiden M (2013). The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38(9), 1883–1894. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, James A, Crane J, & Scrogin KE (2005). 5-Hydroxytriptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. Journal of Pharmacology and Experimental Therapeutics, 313, 1324–1330. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Ragen BJ, Freeman SM, Laredo SA, Mendoza SP, & Bales KL (2015). μ and κ opioid receptor distribution in the monogamous titi monkey (Callicebus cupreus): Implications for social behavior and endocrine functioning. Neuroscience, 290, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JG, Wright PC, & Kinzey WG (1987). Monogamous cebids and their relatives: intergroup calls and spacing. Primate Societies, 44–53. [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, & Young LJ (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. The Journal of Neuroscience, 29(5), 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston JP (1983). Some techniques for assessing multivarate normality based on the Shapiro-Wilk W. Applied Statistics, 32(2), 121–133. [Google Scholar]
- Siever LJ (2008). Neurobiology of aggression and violence. American Journal of Psychiatry, 165(4), 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, & French JA (2010). Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and behavior, 57(2), 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence-Aizenberg A, Di Fiore A, & Fernandez-Duque E (2016). Social monogamy, male–female relationships, and biparental care in wild titi monkeys (Callicebus discolor). Primates, 57(1), 103–112. [DOI] [PubMed] [Google Scholar]
- Steimer T (2002). The biology of fear-and anxiety-related behaviors. Dialogues in clinical neuroscience, 4(3), 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Moeller EL, Abbott D, Schultz-Darken N, Mendoza S, Mason W, Bourgeois S, & Ruiz J (2006) Preparing New World monkeys for laboratory research. ILAR Journal, 47(4), 307–315. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, & Seeman TE (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science, 21, 3–7. [DOI] [PubMed] [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MHV, Aranda BCC, Jacobs S, Fernandes MC, Ribeiro MFM, Sanvitto GL, & Lucion AB (2009). Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Hormones and behavior, 56(1), 93–100. [DOI] [PubMed] [Google Scholar]
- Van Anders SM, Goldey KL, & Kuo PX (2011). The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology, 36(9), 1265–1275. [DOI] [PubMed] [Google Scholar]
- Van Belle S, Fernandez-Duque E, & Di Fiore A (2016). Demography and life history of wild red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador: A 12-year study. American journal of primatology, 78(2), 204–215. [DOI] [PubMed] [Google Scholar]
- Veenema AH (2009). Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: What can we learn from animal models? Frontiers in Neuroendocrinology, 30, 497–518. [DOI] [PubMed] [Google Scholar]
- Veenema AH (2012). Toward understanding how early-life social experiences alter oxytocin-and vasopressin-regulated social behaviors. Hormones and behavior, 61(3), 304–312. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, & Neumann ID (2007). Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology, 32(5), 437–450. [DOI] [PubMed] [Google Scholar]
- Vuong QH (1989). Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica: Journal of the Econometric Society, 307–333. [Google Scholar]
- Waldherr M, & Neumann ID (2007). Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proceedings of the National Academy of Sciences, 104(42), 16681–16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S, Trotter P, Swaney W, Marshall A, & Mcglone F (2017). C-tactile afferents: Cutaneous mediators of oxytocin release during affiliative tactile interactions?. Neuropeptides, 64, 27–38. [DOI] [PubMed] [Google Scholar]
- West SG, Taylor AB, & Wu W (2012). Model fit and model selection in structural equation modeling In Hoyle RH (Ed.), Handbook of structural equation modeling (pp. 209–231). [Google Scholar]
- Winslow JT, & Insel TR (1991). Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. Journal of Neuroscience, 11(7), 2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, & Insel TR (2000). Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Hormones and Behavior, 37(2), 145–155. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro L, Carter CS, & Insel TR (1993). Oxytocin and complex social behavior: Species comparisons. Psychopharmacology Bulletin. [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, & Wang Z (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Frontiers in neuroendocrinology, 32(1), 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.