Abstract
Autism is a brain disorder characterized by social impairments. Progress in understanding autism has been hindered by difficulty in obtaining brain-relevant tissues (eg, cerebrospinal fluid [CSF]) by which to identify markers of disease and targets for treatment. Here, we overcome this barrier by providing evidence that mean CSF concentration of the “social” neuropeptide arginine vasopressin (AVP) is lower in children with autism versus controls. CSF AVP concentration also significantly differentiates individual cases from controls and is associated with greater social symptom severity in children with autism. These findings indicate that AVP may be a promising CSF marker of autism’s social deficits.
Autism is a neurodevelopmental disorder characterized by social impairments and the presence of restricted, repetitive behaviors.1 Autism is male-biased in prevalence (4:1, male:female) and impacts 1 in 59 US children.2 Although autism’s disease biology remains poorly understood, it has been hypothesized that oxytocin (OXT) and/or arginine vasopressin (AVP) may be involved.3 These neuropeptides are key regulators of social behavior in mammals, and targeted disruption of these pathways induces social deficits in multiple species.4 These collective findings suggest that disturbances in brain OXT and/or AVP signaling may be related to the presence and severity of social impairments in people with autism.
Evaluating a role for these brain neuropeptides in autism has been hindered, however, by the extraordinary difficulty in obtaining brain-relevant samples (eg, cerebrospinal fluid [CSF]) from children for research purposes. Here, we surmount this challenge by assembling the largest autism cohort to date in which to evaluate CSF neuropeptide concentrations. We first tested whether children with and without autism show different CSF neuropeptide concentrations at a group level. We then tested whether CSF neuropeptide concentration distinguishes individual autism cases from controls and predicts individual symptom severity for core autism features, particularly social impairments. We also explored whether there is evidence for sex-specific autism disease biology (as AVP may more selectively regulate social functioning in male mammals, whereas OXT may more selectively regulate social functioning in female mammals5).
Subjects and Methods
Participants
Study protocols were approved by institutional review boards (ie, Stanford University, University of California, San Francisco [UCSF], and National Institute of Mental Health [NIMH]) at each site, and participants were recruited as previously described.6–9 Parents and/or legal guardians provided written informed consent (and children provided assent, based on age and developmental level) prior to initiating study procedures. Participants were required to be English speaking and willing to provide CSF for research analysis. Children with autism were required to meet Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision criteria for autistic disorder,10 which was confirmed with research diagnostic methods (ie, Autism Diagnostic Interview-Revised11 and Autism Diagnostic Observation Schedule [ADOS]).12 Control children were required to be diagnosed with or worked up for a medical problem (other than autism) that necessitated lumbar puncture. All participants were free of severe mental disorders (eg, schizophrenia, schizoaffective disorder, bipolar disorder) as determined by an extensive neurological evaluation in children with autism and by a medical chart review in control children.
CSF Collection, Case-Control Matching, and Neuropeptide Quantification
CSF was collected from the lumbar region, flash-frozen, and stored at −80 °C until quantification. Children with autism who had sufficient available CSF sample volumes from the NIMH site were matched with control children from the Stanford and UCSF sites 1:1 on sex and within a 1-year band on age (age range = 1.5–9 years; combined cohort, N = 72; see Tables 1 and 2 for participant characteristics). CSF AVP and OXT concentrations were quantified at Stanford University using enzyme immunoassays (Enzo Life Sciences, Farmingdale, NY).6
TABLE 1.
Participant Characteristics for Children with Autism and Control Children
| Group | n | Sex | Ethnicity | Age, yr | CSF Collection Time, h:min:s | ||
|---|---|---|---|---|---|---|---|
| Female | Male | Caucasian | Other | ||||
| Autism | 36 | 12 | 24 | 27 | 9 | 4.72 ± 0.27 | 10:55:08 am ± 0:09:45 |
| Control | 36 | 12 | 24 | 19 | 17 | 4.66 ± 0.32 | 10:37:17 am ± 0:23:33 |
Fisher exact test was used to test whether the distribution of individuals to different groups differed by sex and ethnicity. For age and blood collection time, differences between groups were tested using a simple 1-way general linear model (values are reported as mean ± standard error). No significant differences were observed between children with autism and control children.
CSF = cerebrospinal fluid.
TABLE 2.
Behavioral and Symptom Severity Information for Children with Autism
| Behavioral Functioning | Mean | SD |
|---|---|---|
| Nonverbal developmental quotienta | 53.57 | 17.1 |
| Vineland-II Adaptive Behavior Composite Score | 64.28 | 8.64 |
| ADOS Symptom Severity Scores | Median | Range |
| Social Affect Calibrated Severity Score | 7 | 4–10 |
| Restricted and Repetitive Behaviors Calibrated Severity Score | 9 | 5–10 |
| Total Calibrated Severity Score | 7 | 4–10 |
Nonverbal developmental quotient (ratio of age equivalents to chronological age) was calculated for nonverbal cognitive ability, from either the Mullen Scales of Early Learning or the Differential Ability Scales, based on age and ability level.
ADOS = Autism Diagnostic Observation Schedule; SD = standard deviation.
Statistical Analyses
Data were analyzed using JMP Pro 13 and SAS 9.4. We used least-squares general linear models (LS-GLM) to test for group differences in CSF AVP and/or OXT concentrations. We used logistic regression models, implemented as restricted maximum likelihood generalized linear models, to test whether CSF AVP and/or OXT concentrations accurately differentiated cases from controls at an individual (rather than group) level. A post hoc contrast was used to perform an equivalence test to confirm that we were sufficiently powered to detect a meaningful effect size in OXT concentrations. Finally, we used LS-GLM to test whether CSF AVP and/or OXT concentrations predicted individual symptom severity in the autism group. We first examined overall symptom severity using the ADOS-Calibrated Severity Score (CSS)13; secondary analyses were then conducted on the Social Affect (SA)-CSS and the Restricted and Repetitive Behaviors (RRB)-CSS, with critical alpha set at 0.025 to account for multiple testing within this instrument. Participant age, CSF sample collection time, ethnicity, and sex were included as control variables in all models. Interaction terms were included and then removed when nonsignificant, following best practice.14 To meet the underlying assumptions of the analytical methods,14,15 homogeneity of variance, normality of error, and linearity were confirmed post hoc14 and CSF AVP and OXT concentrations were log-transformed in all analyses.
Results
Mean CSF AVP concentration was significantly lower in the autism versus control group (F1, 66 = 14.20, p = 0.0004, regression coefficient β1 ± standard error [SE] = −0.09005 ± 0.02390; autism vs control = 66%, 95% confidence interval = 53–82%; see Fig 1). No evidence for a CSF AVP concentration-by-sex interaction was found (F1, 65 = 0.0521, p = 0.8202). CSF OXT concentration did not differ by group (F1, 66 = 0.4498, p = 0.5048). At an individual level, CSF AVP concentration also significantly differentiated cases from controls (likelihood ratio [LR] chi-square = 15.14, p < 0.0001). This relationship was observed in both males and females, as there was no evidence for a CSF AVP concentration-by-sex interaction (LR = 0.7279, p = 0.3936). This effect was also specific to AVP, as CSF OXT concentration did not predict autism likelihood in these same individuals (LR = 0.5200, p = 0.4710). An equivalence test showed that the effect size for CSF OXT concentration was significantly lower than that for CSF AVP concentration (LR = 6.93, p = 0.0085; ie, there was sufficient power to detect a biologically meaningful effect size for CSF OXT in this study).
FIGURE 1:
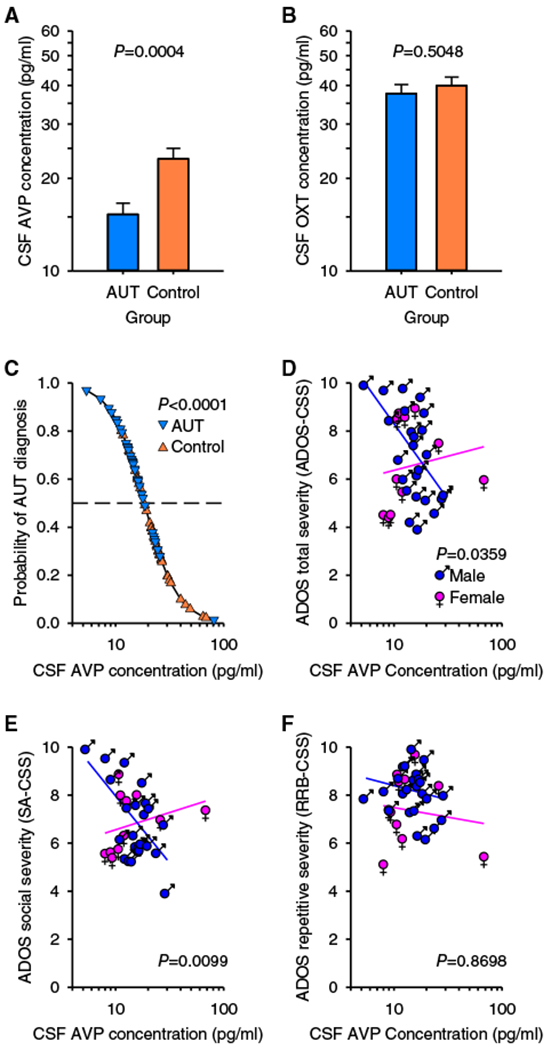
Cerebrospinal fluid (CSF) arginine vasopressin (AVP) concentration differs between children with and without autism (AUT), predicts AUT diagnosis, and predicts symptom severity. (A) CSF AVP concentration is lower in children with AUT (n = 36) compared to control children (n = 36), whereas (B) CSF oxytocin (OXT) concentration does not differ between groups. Data are plotted as least-squares means ± standard error (SE; ie, corrected for the other variables in the analysis). (C) The effect of CSF AVP concentration on predicted (line) and observed (symbols) group is plotted, corrected for the other variables in the analysis. Children with AUT plotted above, and control children plotted beneath, the dashed line (which represents 50% probability) are correctly classified. Specifically, across the range of observed CSF AVP concentrations, the likelihood of AUT increased over 1,000-fold, corresponding to nearly a 500-fold increase in risk with each 10-fold decrease in CSF AVP concentration (range odds ratio = 1,080, unit odds ratio = 494, β1 ± SE = −6.202 ± 1.898). (D) CSF AVP concentration predicts Autism Diagnostic Observation Schedule (ADOS)–Calibrated Severity Score (CSS) in male but not in female children with AUT. (E) CSF AVP concentration predicts Social Affect (SA)-CSS, in male but not in female children with AUT. (F) CSF AVP concentration does not predict Restricted and Repetitive Behaviors (RRB)-CSS in children with AUT of either sex. Data are plotted as residuals from the least-squares line (ie, both data and the regression line are corrected for the other variables in the analysis). The significance of the interaction (ie, the difference between the slope of the lines) is shown.
At an individual level, CSF AVP concentration significantly predicted overall symptom severity in a sex-dependent manner (F1, 27 = 4.878, p = 0.0359), whereby lower CSF AVP concentration predicted greater symptom severity in males (F1, 27 = 6.221, p = 0.0190, β1, 1 ± SE = −5.091 ± 2.041), but not in females (F1, 27 = 0.2346, p = 0.6320, β1, 2 ± SE = 0.9526 ± 0.1.967), as measured by the ADOS-CSS. Further investigation revealed that this effect was specific to the social domain (F1, 27 = 7.708, p = 0.0099), whereby lower CSF AVP concentration predicted greater social impairments as measured by higher SA-CSS in males (F1, 27 = 8.771, p = 0.0063, β1, 1 ± SE = −5.604 ± 1.892) but not in females (F1, 27 = 0.6229, p = 0.4369, β1,2 ± SE = −1.439 ± 1.823; see Fig 1). In contrast, CSF AVP concentration did not predict severity scores for RRB-CSS (F1, 27 = 0.0274, p = 0.8698). Finally, no effect of CSF OXT concentration on any ADOS measure was found (p > 0.05 for all tests).
Discussion
This study implicated CSF AVP concentration in disease presence and symptom severity in children with autism. To date, autism biomarker research has been largely restricted to peripheral tissue, such as blood, due to its ease in collection. However, these measures are less representative of brain biochemistry than CSF. We observed no difference in blood AVP concentrations between children with and without autism in a prior study.9 Results of the present investigation highlight the value of performing CSF-focused biomarker research in autism, and parallel ongoing research in other brain disorders (ie, dementia, multiple sclerosis), which have benefited tremendously from CSF biomarker discovery efforts.16,17
Although AVP and OXT are nearly structurally identical peptides and likely evolved due to duplication of a common ancestral gene,18 only CSF AVP concentration was identified as a driver of group classification. Additionally, the relationship between CSF AVP concentration and symptom severity was only evident in males. A unique aspect of AVP physiology that may explain these results is that AVP can exert sexually dimorphic behavioral effects.5 Specifically, AVP preferentially regulates mating behavior, pair bond formation, and parental care in male versus female mammals.19 Given autism’s male-biased prevalence,2 brain AVP signaling deficits may be particularly relevant to understanding the male-biased risk for, and symptom presentation in, autism.
These biomarker findings are also of interest because there are currently no laboratory-based diagnostic tests to detect autism. However, the accuracy of low CSF AVP concentration to correctly identify autism cases in this study, although robust, was imperfect, as n = 8 children with autism (5 male, 3 female) and n = 9 control children (7 male, 2 female) were misclassified by the analysis. Given that autism is a clinically heterogeneous disorder and that CSF AVP concentration did not predict RRB-CSS in children with autism, it is likely that other as yet unidentified CSF biomarkers may contribute additional explanatory power and could be incorporated into a multi-dimensional panel by which to detect autism with even higher accuracy.
There has been intense interest in OXT as a therapeutic agent for autism. Although the present CSF study does not support a global need for OXT “replacement” in this disorder (if anything, it supports one for AVP), it is nevertheless possible that a subset of people with autism may benefit from OXT treatment. Rodent models of human syndromes with high autism penetrance20,21 have implicated diminished hypothalamic OXT-producing cell numbers in the models’ social deficits, which can be rescued with OXT treatment.20,22 We have also previously shown that idiopathic autism patients with the lowest blood OXT concentrations benefit the most from OXT treatment.23 These collective findings underscore the complexity in detecting and treating idiopathic and syndromic forms of autism, and the urgent need for biomarker-stratified neuropeptide treatment trials in this highly heterogeneous patient population.
There are several limitations to this study. First, the control group was a sample of convenience due to ethical concern in obtaining CSF from children solely for research purposes. Moreover, although the controls did not have autism, they had either unknown conditions necessitating CSF analysis (eg, severe headache, unexplained changes in mental status, rule-out meningitis) or serious medical conditions (eg, pseudotumor cerebri, survivors of blood cancers on maintenance chemotherapy). Evaluation of CSF AVP concentrations in infants at familial risk for autism (some of whom would develop typically), and in adults with autism (age-matched to controls capable of consenting to CSF collection), would enable comparison with healthy participants. Second, given the extreme difficulty in obtaining CSF samples from children, control CSF was collected at Stanford and UCSF hospitals across multiple services and clinics. In contrast, autism CSF was collected in a more structured manner at NIMH. Accordingly, there were differences in clinical sampling conditions: fasting status, fluid replacement, and anesthesia (general vs local) were variable across controls, whereas children with autism were required to be fasted and then underwent general anesthesia and fluid replacement before lumbar puncture. The impact of these differences on the neuropeptide measures is unknown. It seems unlikely that these methodologic differences are responsible for the observed differences in patient groups, however, as we recently observed significantly lower CSF AVP concentrations in socially impaired versus socially competent monkeys undergoing identical CSF collection procedures, and in a small pilot study in which children with autism were not necessarily fasted and did not undergo pre-lumbar puncture fluid replacement.6
In conclusion, this study showed that CSF AVP concentration differentiates cases from controls and is associated with symptom severity in children with autism. Additional research is needed to determine whether low CSF AVP concentration is specific to autism (as opposed to a more general “signature” of altered brain development), and whether low CSF AVP concentration is a disease marker in children at risk for developing autism prior to symptom onset. Given that there is longstanding evidence that AVP administration enhances social functioning in mammals,24 therapeutic enhancement of brain AVP signaling may also hold promise for ameliorating social deficits in autism patients.
Acknowledgment
This research was supported by the NIMH Intramural Research Program (ZIA MH002914, NCT00298246), the Mosbacher Family Fund for Autism Research, Stanford’s Bio-X Program, the Weston Havens Foundation, Stanford’s Child Health Research Institute, the Katherine D. McCormick Fund, and the Yani Calmidis Memorial Fund for Autism Research.
We thank R. Sumiyoshi, L. Jackson, T. Trujillo, J. Moss, D. Carson, S. Hyde, N. Mohsin, K. Hornbeak, B. Fregeau, S. Berquist, M. Strehlow, S. Cheshier, and S. Hannah for helping with this study; and the patients and their families for participating in it.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Diagnostic and statistical manual of mental disorders. 5th ed Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 2.Baio J, Wiggins L, Christensen DL, et al. Prevalence of Autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry 1999;45:145–157. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell HK. Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist 2017:1073858417708284. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci 2009;31:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker KJ, Garner JP, Oztan O, et al. Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci Transl Med 2018;10 pii: eaam9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoffner J, Trommer B, Thurm A, et al. CSF concentrations of 5-methyltetrahydrofolate in a cohort of young children with autism. Neurology 2016;86:2258–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardo CA, Farmer CA, Thurm A, et al. Serum and cerebrospinal fluid immune mediators in children with autistic disorder: a longitudinal study. Mol Autism 2017;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson DS, Garner JP, Hyde SA, et al. Arginine vasopressin is a blood-based biomarker of social functioning in children with autism. PLoS One 2015;10:e0132224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 11.Le Couteur A, Lord C, Rutter M. The autism diagnostic interview-revised (ADI-R). Los Angeles, CA: Western Psychological Services, 2003. [Google Scholar]
- 12.Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule (ADOS). Los Angeles, CA: Western Psychological Services, 1999. [Google Scholar]
- 13.Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord 2014;44:2400–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grafen A, Hails R. Modern statistics for the life sciences. Oxford, UK: Oxford University Press, 2002. [Google Scholar]
- 15.Paul DA. Logistic regression using the SAS system: theory and application. Cary, NC: SAS Institute, 1999. [Google Scholar]
- 16.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016;15:673–684. [DOI] [PubMed] [Google Scholar]
- 17.Fitzner B, Hecker M, Zettl UK. Molecular biomarkers in cerebrospinal fluid of multiple sclerosis patients. Autoimmun Rev 2015; 14:903–913. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008;322:900–904. [DOI] [PubMed] [Google Scholar]
- 19.Young LJ. Being human: love: neuroscience reveals all. Nature 2009; 457:148. [DOI] [PubMed] [Google Scholar]
- 20.Penagarikano O, Lazaro MT, Lu X- H, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med 2015;7:271ra8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis SM, Sagar A, Levin-Decanini T, et al. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res 2014;1580:199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meziane H, Schaller F, Bauer S, et al. An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol Psychiatry 2015;78:85–94. [DOI] [PubMed] [Google Scholar]
- 23.Parker KJ, Oztan O, Libove RA, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci U S A 2017;114:8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in microtus pennsylvanicus (meadow voles). Horm Behav 2001;39:285–294. [DOI] [PubMed] [Google Scholar]


