Abstract
Cognitive impairment associated with aging has emerged as one of the major public health challenges of our time. Although Alzheimer’s disease is the leading cause of clinically diagnosed dementia in Western countries, cognitive impairment of vascular etiology is the second most common cause and may be the predominant one in East Asia. Furthermore, alterations of the large and small cerebral vasculature, including those affecting the microcirculation of the subcortical white matter, are key contributors to the clinical expression of cognitive dysfunction caused by other pathologies, including Alzheimer’s disease. This scientific expert panel provides a critical appraisal of the epidemiology, pathobiology, neuropathology, and neuroimaging of vascular cognitive impairment and dementia, and of current diagnostic and therapeutic approaches. Unresolved issues are also examined to shed light on new basic and clinical research avenues that may lead to mitigating one of the most devastating human conditions.
Keywords: Alzheimer’s disease, cerebral blood flow, cognitive dysfunction, small vessel disease, stroke
Dementia is characterized by a progressive and unrelenting deterioration of mental capacity that inevitably compromises independent living. Advancing age is the main risk factor, and due to the aging of the world population and lack of effective treatments, the number of affected individuals, estimated at 50 million worldwide in 2018, is anticipated to triple by 2050 at a cost approaching $4 trillion (1). The anticipated “dementia epidemic” has spurred world leaders to develop national plans to deal with its devastating socioeconomic impact (2) and increase research funding to accelerate therapeutic development (3).
Alterations in cerebral blood vessels have long been implicated in age-related cognitive impairment. Alois Alzheimer, best known for identifying the condition now called Alzheimer’s disease (AD), was a strong proponent of the concept that the dementia of the elderly was due to cerebrovascular insufficiency (4). The idea was that hardening of the arteries impaired the ability of cerebral blood vessels to relax and to adjust the delivery of blood to the metabolic needs of the brain, causing hypoperfusion, neuronal death, and dementia. This view prevailed well into the 1970s, when measurement of cerebral blood flow (CBF) demonstrated that cerebral blood vessels were able to increase CBF in cognitively impaired individuals (5), casting doubt on the dominant hypothesis of global vasoparalysis and vascular insufficiency. Around the same time, the concept of multi-infarct dementia was introduced, which proposed that cerebrovascular disease causes dementia through multiple brain infarcts (6) and proved to be another argument against the global ischemia concept. Multi-infarct dementia implied that if strokes are preventable, so should be cognitive impairment due to cerebral infarcts, suggesting that some dementias could be treated or prevented. Sub-sequently, the term vascular dementia (VaD) was felt to be too restrictive, failing to capture the entire spectrum of cognitive alterations caused by vascular factors, and the term vascular cognitive impairment (VCI) was proposed (7) and eventually widely adopted (8). The most severe form of VCI is VaD.
With advances in the understanding of the molecular pathology of AD in the 1990s (9), the term dementia became synonymous with AD, and the cognitive impact of vascular pathology was over-looked compared with neurodegenerative pathology (e.g., amyloid plaques and neurofibrillary tangles). More recently, a wealth of epidemiological, clinical-pathological, and basic science observations has led to a reappraisal of the role of vascular factors in cognitive impairment (10), and have identified vascular dysfunction and damage as critical components of the pathophysiology of late-life dementia including AD (11). This state-of-the-art review provides an up-to-date assessment of the role of vascular factors in cognitive health and their clinical manifestations, epidemiology, pathobiology, imaging correlates, and neuropathology. It also examines the current state of prevention and management, and the challenges and opportunities for future research and clinical developments (Central Illustration).
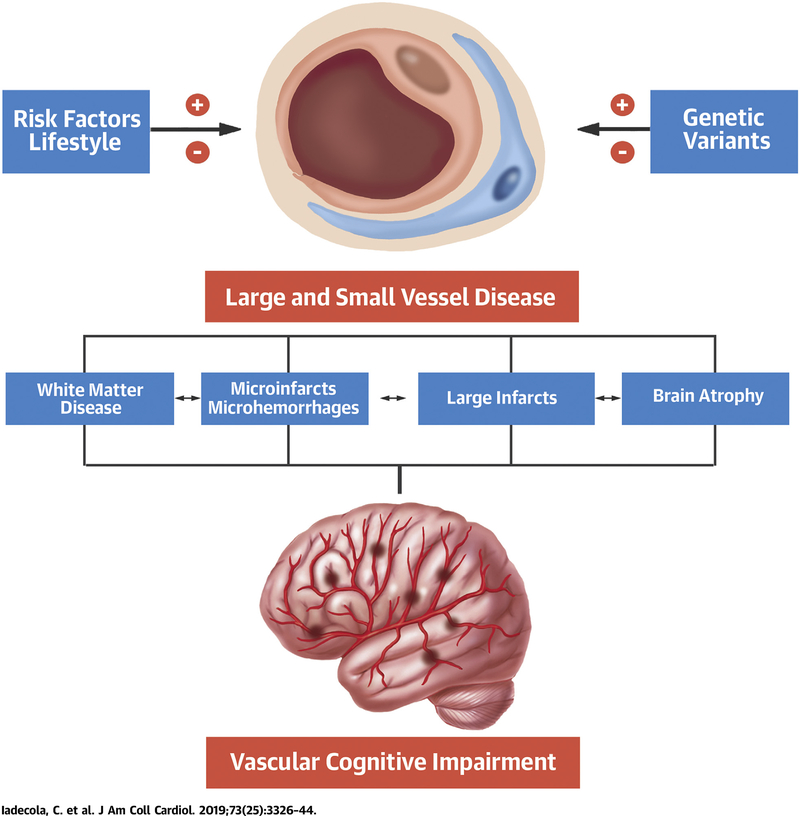
CENTRAL ILLUSTRATION. Vascular Cognitive Impairment and Dementia.
Risk factors and lifestyle, as well as genetic variants, can either promote (+) or stave off (−) damage to large and small cerebral blood vessels, which, in turn, leads to neuropathological changes that result in vascular cognitive impairment.
CLINICAL FEATURES
Dementia refers to a decline in mental ability severe enough to interfere with daily life. The recently published Vascular Impairment of Cognition Classification Consensus Study (VICCCS) guideline defines major VCI (VaD) as clinically significant deficits in at least 1 cognitive domain that are of sufficient severity to cause a severe disruption of (instrumental) activities of daily living (12). The second requirement for mild VCI or major VCI (VaD) is imaging evidence for cerebrovascular disease (Table 1). This new definition evolved from the American Heart Association/American Stroke Association (8) and National Institute of Neurological Disorders and Stroke-Canadian Stroke Network (13) consensus statements, and aligns with revised terminology in DSM-V, which distinguishes between major and minor neurocognitive disorders.
TABLE 1. VICCCS Guidelines for VaD.
|
Shown here are key elements of the guidelines. For further explanations see the text and Skrobot et al. (12).
ADL = activities of daily living; IADL = instrumental activities of daily living; MRI = magnetic resonance imaging; VaD = vascular dementia; VCI = vascular cognitive impairment; VICCCS = Vascular Impairment of Cognition Classification Consensus Study.
CLASSIFICATION.
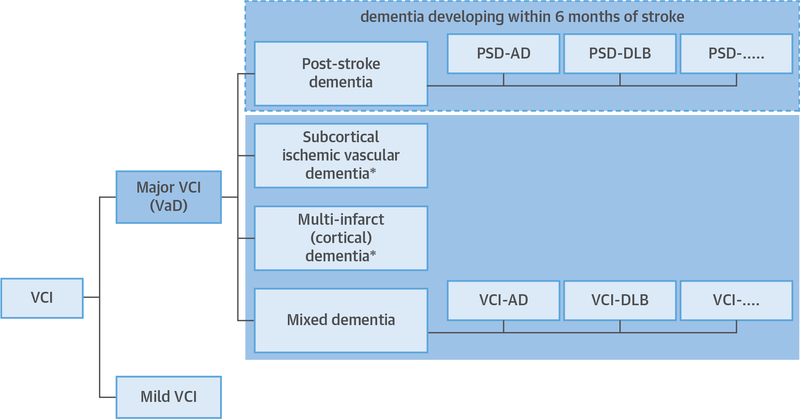
According to the VICCCS, VaD can be classified into 4 major subtypes: 1) post-stroke dementia (PSD), defined as dementia manifesting within 6 months after a stroke; 2) subcortical ischemic vascular dementia (SIVaD); 3) multi-infarct (cortical) dementia; and 4) mixed dementia (Figure 1) (12). By convention, patients with evidence for mixed pathologies (e.g., vascular and AD) are further labeled to specify the presumed predominant cause of dementia (e.g., VaD-AD or AD-VaD) (Figure 1). For a diagnosis of VaD or mild VCI, the new VICCCS guideline generally requires magnetic resonance imaging (MRI) and evidence of vascular lesions that qualify for one of the major diagnostic subtypes (Table 1).
FIGURE 1. Classification of VCI.
Diagnostic classification for major vascular cognitive impairment (VCI) (major VCI = vascular dementia [VaD]). The 6-month temporal basis for cognitive decline after stroke (dashed box) differentiates post-stroke dementia (PSD) from other forms of VaD. *Patients who also have evidence for comorbid pathology representing an established nonvascular cause of dementia such as Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) are classified as mixed dementia. Mild VCI refers to impairment in at least 1 cognitive domain and mild to no impairment in instrumental activities of daily living or activities of daily living (independent of the motor/sensory sequelae of the vascular event). Data from Skrobot et al. (12).
COGNITIVE DOMAINS AFFECTED AND CLINICAL EVALUATION.
The profile and temporal evolution of cognitive deficits in minor VCI and VaD is variable. Cognitive decline may develop gradually, stepwise, or through a combination of both. Current diagnostic criteria no longer require the presence of memory impairment, which is more characteristic of AD (12,13). A common finding in patients with vascular brain lesions are impairments in executive function and processing speed. Executive function comprises a number of processes for initiation, planning, decision making, hypothesis generation, cognitive flexibility, and judgment. Another common finding in patients with VCI is deficits in delayed recall of word lists and visual content (14).
In clinical practice, cognitive assessment should include the following 5 core domains: executive function, attention, memory, language, and visuo-spatial function (12). Information on other domains, such as learning, social cognition, and neuropsychiatry, may help delineating the cognitive syndrome, but is not required for diagnosing VaD. The 5 core domains are covered by the “60-minute” and “30-minute” protocols proposed by the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network VCI harmonization standards (13). These protocols are widely used and supported by the VICCCS guidelines (12).
In certain settings, a detailed cognitive testing is neither feasible nor meaningful, for example, patients with severe depression, severe dementia, or acute stroke. Under such conditions, brief cognitive testing by a validated screening instrument offers a first overview and reference for future investigation. Detailed testing can then be deferred, usually by 3 to 6 months, if clinically appropriate. A suitable instrument for cognitive screening in patients with vascular disease is the Montreal Cognitive Assessment (MoCA), a 1-page 30-point test that can be administered in about 10 min. The MoCA includes items on all of the above core domains, is available in multiple languages, and has been validated in multiple settings including stroke (15). A low MoCA score in the acute phase after stroke is predictive of several adverse long-term outcomes including cognitive impairment, thus supporting routine use of the MoCA in stroke patients (16). However, there are barriers to cognitive testing in the immediate post-stroke period even by screening instruments (17). Up to 20% of hospitalized stroke patients develop delirium, a condition characterized by decreased attention and disturbed consciousness or disorganized thinking, which develops over a short time period and fluctuates during the course of the day (18). Assessment tools for delirium include the delirium rating scale and the confusion assessment method (18). Another diagnostic problem in VCI is aphasia, since it interferes with cognitive testing. However, if there is documented evidence of normal cognitive function before the vascular event that caused aphasia, a diagnosis of “probable VCI” can be made. Instruments for the assessment of premorbid cognitive status include the Informant Questionnaire for Cognitive Decline in the Elderly (19) and the AD8 Screening Interview (20), which should be completed by an informant.
Neuroimaging of VaD should assess the following core measures (12,13): 1) brain atrophy including estimates of general atrophy, ventricular size, and medial temporal lobe atrophy; 2) white matter hyperintensities (WMH) using semiquantitative scales; 3) infarction (number, size stratified for large [>1 cm] and small [3 to 10 mm] infarcts, and location); and 4) hemorrhage (number, size stratified for large [>1 cm] and small [<1 cm] hemorrhages, and location) (see also the Imaging section). Additional measures, such as volumetric readouts for WMH and infarcts, can be added but are not required for clinical diagnosis.
HEREDITARY VaD.
The discovery of families with hereditary dementia related to cerebral small vessel disease (SVD) has advanced the understanding of VaD, and SIVaD in particular. Notably, studies in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a hereditary SVD caused by mutations in the NOTCH3 gene, have delineated the cognitive profile of pure SIVaD (21), the cognitive mechanisms of apathy (a clinically relevant manifestation of SIVaD) (22), the neuroimaging correlates of VCI (reviewed by Dichgans and Leys [23] and in the following text), the role of disease modifiers and resilience factors (24), and the therapeutic response to pharmacological interventions (25).
EPIDEMIOLOGY
Dementia is strongly associated with age and, in younger individuals, is often linked to genetic disorders. The incidence and prevalence of dementia rises exponentially from the age of ~75 years in developed countries (1). However, the age-specific prevalence and incidence of all-cause dementia has fallen over the past few decades in part due to better education, living conditions, and health care (26). Prevalence of mild cognitive impairment (MCI) without dementia is also strongly age-related, being more common than dementia in the younger old, with dementia being proportionally higher in the oldest patients (27).
VaD is generally stated to be the second most common cause of dementia in later life in Caucasian populations, although it may be the most common cause in East Asia, and there are few data from other ethnic groups (28,29). However, there are few recent epidemiological data on dementia subtypes partly due to classification difficulties: mixed neurodegenerative and cerebrovascular pathology is common and in fact is present in the majority of older old individuals (>75 years) dying with dementia (30) (see also Neuropathology section). Due to the lack of contemporary population-based estimates, there are few data on changes of VaD prevalence over time. However, the reduction in dementia prevalence and incidence seen in men more than women in the United Kingdom in association with falling vascular event rates has been suggested to be linked to a reduction specifically in the vascular contribution to dementia. The prevalence of VCI is uncertain, but it is a risk factor for progression to dementia and also for mortality (31).
DEMENTIA AFTER STROKE.
Overall, about 1 in 10 patients have dementia before their stroke, and 1 in 10 develop new dementia in the first year after a first-ever stroke with lower risks thereafter (32). However, rates vary considerably depending on clinical characteristics, and there are strong stepwise associations with stroke severity: pre-event dementia prevalence is ~20% in severe stroke (NIHSS >10) versus only ~5% in TIA, and new 1-year post-event dementia occurs in over one-third of severe strokes (NIHSS >10) compared with only 8% of minor strokes (NIHSS <3) and 5% of TIAs (33). Relative to the background population rate, dementia is brought forward by ~25 years for severe stroke, compared with ~4 years for minor stroke and ~2 years for TIA (33).
The risk of MCI is also increased post-stroke (34) and TIA (35), with acceleration in the rate of cognitive decline compared with pre-stroke rates and greater acute declines in global cognition in African-Americans, in males, and after cardioembolic or large artery stroke (36,37). Transient cognitive impairment (~30% of TIA/minor stroke) (38) or frank delirium (~25% of hospitalized stroke) (18) may occur with many patients improving after the event. Individual patient cognitive trajectories are heterogeneous and difficult to predict, but initial improvement may be followed by longer-term cognitive decline, especially in older patients with lower baseline cognition suggesting that such transient changes may be a marker of cognitive fragility and lack of cognitive reserve (38).
RISK FACTORS.
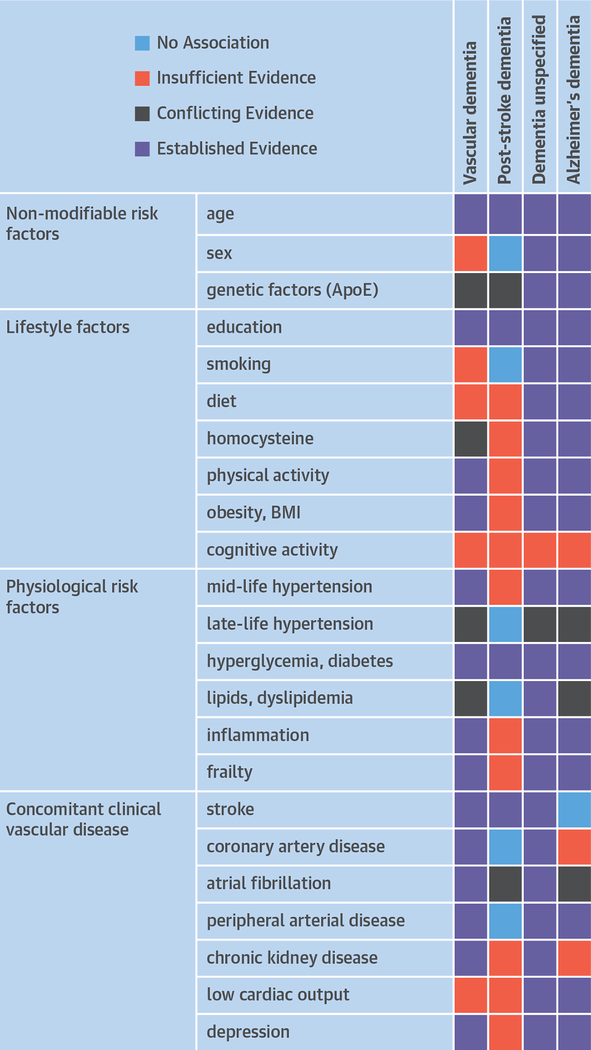
The risk factors for dementia overlap with those for stroke supporting the concept of a shared susceptibility, as also suggested by the epidemiological relationship between these 2 disorders (33) (Figure 2). The overwhelming risk factor for all-cause dementia is increasing age (26,28,29,33). Other unmodifiable risk factors include female sex, although the relationship with VaD is less clear with recent data suggesting no association at least post-stroke (33). Genetic factors may also play a role, but apart from rare disorders such as CADASIL, few specific risk genes are known (39). Apolipoprotein E4 is a strong risk factor for AD especially in women, but the relationship with VaD and PSD is less clear and requires further study (39).
FIGURE 2. Risk Factors for Dementia.
Risk factors for vascular dementia, post-stroke dementia, dementia of unspecified etiology (unspecified dementia), and Alzheimer’s dementia. Data derived from Dichgans and Leys (23) and Pendlebury et al. (134). Note that recent genetic and epidemiological evidence implicates AD as a risk factor for stroke (135).
Modifiable risk factors can be divided into protective factors versus those that increase the risk of dementia (40). Protective factors include markers of increased cognitive reserve (resilience against age- and disease-related change) including higher education/IQ, occupation, social networks, and cognitive and physical activity (41). Mediterranean diet has been suggested to reduce the risk of cognitive decline, but there is a lack of data specifically on VaD (8).
There is strong evidence linking midlife hyper-tension and diabetes to both vascular and Alzheimer’s dementia, although many studies may have included cases of mixed pathology (8,40). Indeed, diabetes seems to primarily increases the burden of cerebrovascular pathology, but not the frequency of Alzheimer’s pathology (42). The relationship between late-life hypertension and dementia is unclear, and it appears unrelated to PSD at least to 5-year follow-up in contrast to diabetes (33). There is some evidence for a link between midlife cholesterol and obesity and later-life dementia (8,40). Smoking is linked to an increased risk of cognitive decline, although relationships between smoking and specifically VaD are unclear (8,40). Late-life depression is a risk factor particularly for VaD and may have an underlying vascular pathology (43).
Cerebrovascular disease, both symptomatic and asymptomatic, is a powerful risk factor for VaD. Risk of dementia post-stroke is driven by age and stroke lesion burden/location (severity, prior/recurrent stroke, dysphasia), together with pre-morbid markers of brain susceptibility/reserve (educational level, pre-morbid dependency, severity of leukoaraiosis), baseline cognitive score, and diabetes (32,33,44). Risk of dementia after TIA is therefore low in the absence of other markers of susceptibility/reduced reserve (33). Hemorrhagic stroke may carry slightly higher dementia risks than ischemic stroke for strokes of similar severity (33), and risks are higher in superficial/lobar versus deep bleeding due to associations with cerebral amyloid angiopathy (CAA) (44). Other putative and possibly modifiable risk factors for VaD include systemic inflammation (45).
PATHOBIOLOGY
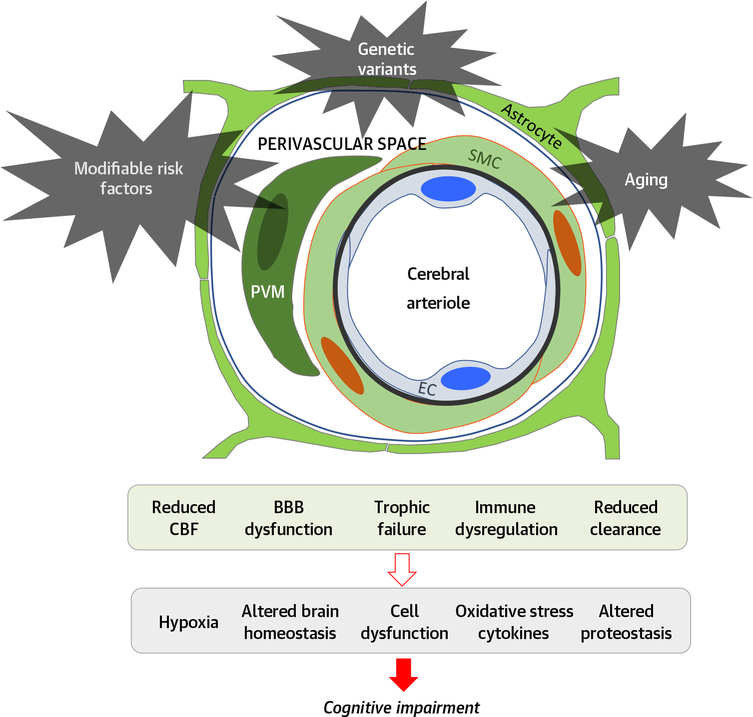
Cerebrovascular cells are closely related to brain cells, and their interaction plays a critical role in brain development, maintenance, and function, both in health and disease. The concept of the neurovascular unit emphasizes the unique relationship between brain cells and the vasculature, their functional interactions, and coordinated reaction to injury (Figure 3) (11). Here, we will briefly review key aspects of neurovascular structure, function, and pathobiology relevant to VCI.
FIGURE 3. Pathobiology of Neurovascular Dysfunction in Vascular Cognitive Impairment.
Modifiable risk factors (hypertension, lifestyle factors, and so on), genetic factors (ApoE, and so on), and aging impair key functions of the neurovascular unit leading to the alterations in brain function underlying cognitive impairment. Notice that the neurovascular unit is involved not only in flow regulation, but also in other vital functions that are essential for maintaining brain health. BBB = blood-brain barrier; CBF = cerebral blood flow; EC = endothelial cell; PVM = perivascular macrophage; SMC = smooth muscle cell.
ANATOMY OF THE CEREBROVASCULAR NETWORK.
The brain is supplied by an “outside in” vascular network. Originating from large cerebral arteries running on the brain’s surface, pial arteries form a highly anastomotic network and dive into the brain parenchyma (penetrating arteries). At the base of the brain, penetrating vessels arise directly from the circle of Willis and proximal branches, and ascend to supply the basal ganglia. Unlike pial vessels and capillaries, penetrating vessels have few collateral branches so that occlusion of a single vessel is sufficient to cause small ischemic lesions (lacunar infarcts) (46). Also, the deep subcortical white matter (WM), supplied by long penetrating arteries where perfusion pressure is predicted to be low, is thought to be especially vulnerable to hemodynamic insufficiency (11).
Penetrating vessels are surrounded by perivascular spaces (Virchow-Robin spaces) that may serve as a drainage pathway connected to meningeal lymphatics exiting the skull (47). Perivascular spaces house several cell types, including perivascular macrophages, fibroblasts, and other cells (48,49). As the arterioles morph into capillaries, the vascular and glial basement membranes merge and the perivascular space disappears.
In capillaries, one-third of the endothelial surface is covered by pericytes, and both cell types are wrapped by astrocytic end-feet covering about two-thirds of the capillary circumference (50). Capillaries are a major site of the blood-brain barrier (BBB), which is characterized by: 1) tight junctions that seal adjacent endothelial cells; 2) a low rate of endothelial vesicular transport (transcytosis); and 3) a vast repertoire of bidirectional molecular transporters regulating the molecular exchange between blood and brain (51).
CBF AND ITS REGULATION.
CBF is exquisitely controlled to meet the ever-changing demands of brain cells and to cope with daily blood pressure fluctuations. Endothelial cells are a major regulator of vasomotor tone, mainly by releasing vasoactive molecules, such as the potent vasodilator nitric oxide (NO) (52).
CBF autoregulation refers to the ability of arteries and arterioles to dilate or constrict when intravascular pressure decreases or increases, respectively, to maintain CBF relatively constant over a range of systemic blood pressures (normally from 60 to 150 mm Hg) (53). The property of smooth muscle cells to constrict in response to increased transmural pressure (myogenic tone) plays a major role in this mechanism (53).
Functional hyperemia serves to match CBF delivery with the energy needs of the brain and to clear metabolic waste, thus maintaining the brain’s metabolic homeostasis. Because the brain lacks energy reserves, the increases in energy demands induced by neural activity are met by increasing the delivery of oxygen and glucose through blood flow (11). The cellular and molecular bases of functional hyperemia have not been completely elucidated, but involve elaborate signaling mechanisms between neurons, glia and vascular cells at all levels of the cerebrovascular tree (11).
PATHOGENIC MECHANISMS UNDERLYING VCI.
Chronic hypoperfusion has long been implicated in VCI pathogenesis. In support of this hypothesis, chronic CBF reductions have been shown to produce WM injury, lacunar infarcts, hemorrhages, brain atrophy, and memory impairment in rodents, some of which are exacerbated by ApoE4 (54,55). In humans, cerebral hypoperfusion has been documented in several cross-sectional studies in both sporadic and genetic SVDs, with lower global, gray matter, and WM blood flow. However, it remains unclear whether the CBF reduction is a causative factor or a reflection of reduced metabolic demands and whether other aspects of neurovascular function are also involved (Figure 3). Indeed, longitudinal studies provided conflicting results as to whether low CBF at baseline in normal-appearing WM precedes the development of new WM hyperintensities (56). Moreover, the association between reduced CBF and WMH is weaker when accounting for age or dementia diagnosis (56).
Experimental studies have amply demonstrated that hypertension and aging, 2 leading risk factors for VaD and SVD, have profound impact on vessel wall structure and CBF regulation (57,58). Recent human studies using BOLD or arterial spin labeling-MRI provided convincing evidence that cerebrovascular reactivity is altered in patients with SVD (59,60). Moreover, a recent post-mortem study in patients with VaD showed that endothelium-dependent arteriolar dilation was significantly reduced in the WM-penetrating arterioles (61). However, a causal relationship between altered microvascular hemodynamic dysfunction and tissue damage remains to be established.
Growing evidence supports a relationship between higher pulsatility in large intracranial arteries and SVD (62). Aging and hypertension are associated with loss of elasticity in the arterial walls and vascular stiffening (63), but how these factors may contribute to brain dysfunction and damage remains to be established. Loss of autoregulation resulting in reduced CBF, microvascular damage from hydrodynamic stress, and increased amyloid-β deposition have been proposed as potential mechanisms (64).
The BBB limits entry of potentially neurotoxic plasma components, such as fibrinogen, and blood cells into the brain (65,66). Several animal models of hypertension or aging are associated with increased BBB permeability, neuronal loss, and WM degeneration (58). Chronic hypoperfusion has the potential to promote BBB leakage (55), and BBB permeability may be increased in humans with SVD and VaD (67), as well as AD (68). Widening of perivascular spaces, a hallmark of SVD, has been proposed to reflect dysfunction of fluid clearance, which may further result in the accumulation of harmful waste products potentially damaging for the brain (69) (Figure 3).
CELLULAR AND MOLECULAR MECHANISMS.
Major vascular risk factors lead to endothelial dysfunction and damage, which, in turn, may cause neurovascular dysfunction, increased BBB permeability, and micro-vascular thrombosis. A key consequence of endothelial dysfunction is reduced NO bioavailability (70). For example, reactive oxygen species (ROS) scavenge NO or suppress NO synthesis by inactivating critical cofactors (10). On the other hand, inhibitory phosphorylation of endothelial NO synthase by Rho kinase suppresses endothelial NO and leads to cognitive impairment (11,71). ROS produced by a NOX2-containing NADPH oxidase via angiotensin II receptor type 1 have been implicated in the endothelial dysfunction and cognitive impairment in a model of chronic hypertension (72). In this model, the major cellular source of the ROS has recently been localized to perivascular macrophages (72). Dysfunctional endothelial cells can also secrete toxic factors that block oligodendroglial differentiation, hence impairing myelination, essential for WM integrity (56). Furthermore, endothelial cells may undergo deleterious proteomic changes with aging. For instance, upregulation of brain endothelial cell–derived acid sphingomyelinase, a key sphingolipid metabolizing enzyme, contributes to age-related BBB disruption by increasing caveolae-mediated transcytosis (73).
In addition to smooth muscle cell degeneration, loss or dysfunction of pericytes recently emerged as likely key contributors to brain lesions. Several studies suggest that pericyte coverage decreases with age in rodents, monkeys, and humans (74). Yet, reliable information on pericyte integrity in VaD is missing. Pericyte loss is associated with early BBB breakdown, reduced functional hyperemia, cerebral hypoperfusion, and hypoxia that ultimately lead to prominent WM damage, neuronal loss, and cognitive deficits (75).
The microvascular matrisome, the ensemble of proteins constituting the extracellular matrix (ECM) as well as proteins associated with the ECM, is another emerging contributor. Genetic studies revealed that most monogenic forms of SVDs are caused by mutations in genes encoding matrisome proteins (76). Moreover, pathological alteration of the vascular ECM, namely an abnormal elevation of tissue inhibitor of metalloproteinase-3, has been linked to a channelopathy-like defect that underlies cerebrovascular dysfunction in a mouse model of CADASIL (77). Perivascular cells, although still unexplored, are attractive candidates to cause changes in the ECM.
NEUROPATHOLOGY
The neuropathology of VaD is heterogeneous and complex. The most widely recognized neuropatho-logical substrates include infarcts, hemorrhages, and global hypoxic ischemic brain injury. WM injury including demyelination with or without axonal loss is also common in persons with VaD, but is not specific and may also occur in the context of neuro-degenerative dementia, for example, AD. Similarly, cortical atrophy and hippocampal sclerosis may be related to both focal and diffuse hypoxic brain injury, but are also not specific to VaD and are also seen in neurodegenerative diseases. The pathogenesis of vascular tissue injury includes systemic, cardiac, and, most commonly, primary cerebrovascular etiologies. The latter includes both SVD and large vessel disease, both of which are very common in the aging brain and make a significant contribution to dementia, including both VaD and AD (78).
SMALL VESSEL DISEASE.
SVD is an umbrella term that includes various pathological findings, including small infarcts, microscopic infarcts, microbleeds, arteriolosclerosis, intracranial atherosclerosis, and CAA (Figure 4). Enlarged perivascular spaces and WM pallor are also common important pathological features of SVD. SVD is highly prevalent in the aging brain. Infarcts are present in about 50% of older people (age 90 years), and most of these infarcts are small gross infarcts or microinfarcts (see the following text) (79,80). Unlike other age-related pathologies (e.g., AD and Lewy bodies [81,82]), infarcts continue to increase in the oldest age groups (82). In most people, these small infarcts are not diagnosed during life (79). Arteriolosclerosis, intracranial atherosclerosis, and CAA all increase the odds of both small infarcts, for example, lacunar infarcts, microinfarcts, and hemorrhages (83,84). Interestingly, arteriolosclerosis and CAA also increase the odds of dementia in older persons, even after controlling for infarcts and hemorrhages (78). This implies that there is a subthreshold degree of tissue injury without infarction that is associated with cognitive impairment. Some of this tissue injury may be in the form of WM disruption and cortical atrophy noted in imaging studies (see Imaging section). Enlarged perivascular spaces, seen both in neuropathology and imaging studies, also provide evidence of SVD and have been associated with aging, hyper-tension, cerebrovascular disease, and cognitive impairment (69).
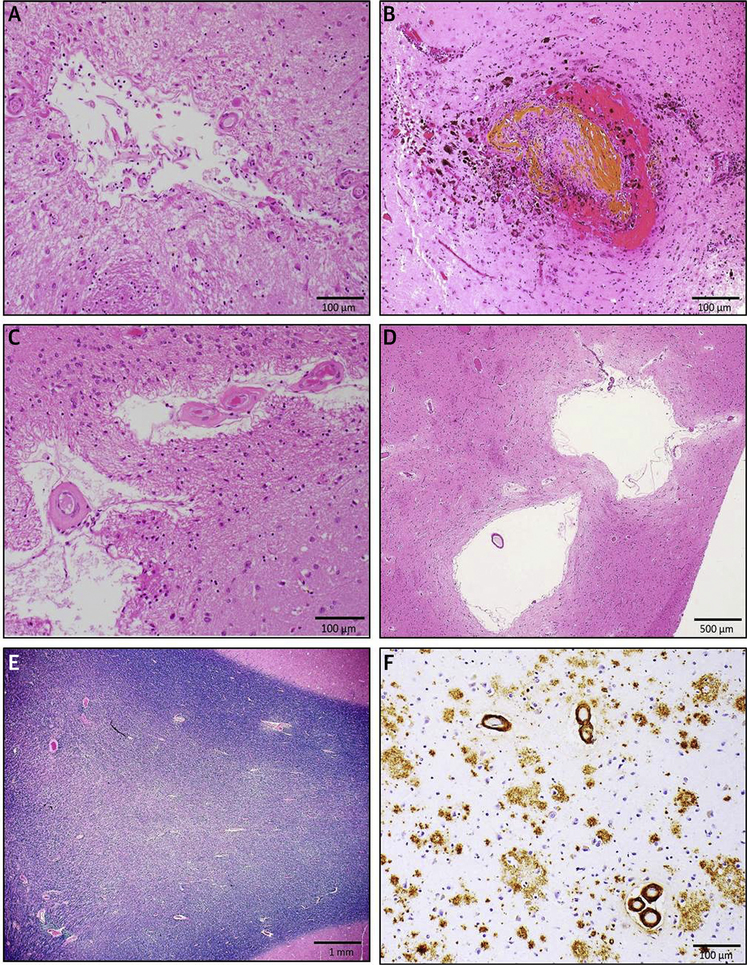
FIGURE 4. Neuropathology of VCI.
Small-vessel disease pathologies: (A) chronic microscopic infarct in the anterior caudate nucleus on hematoxylin & eosin stain; (B) microbleed surrounding damaged cortical blood vessel in the temporal lobe on hematoxylin & eosin stain; (C) arteriolosclerosis in the basal ganglia on hematoxylin & eosin; (D) enlarged perivascular spaces on hematoxylin & eosin stain; (E) white matter pallor in the posterior watershed region on Luxol fast blue and hematoxylin stain; and (F) amyloid angiopathy and Alzheimer’s disease pathology in the midfrontal cortex (immunohistochemistry with the anti-β-amyloid antibody 4G8).
SMALL AND MICROSCOPIC INFARCTS.
It has become increasingly evident that small and microscopic infarcts play a key role in VaD and other dementia syndromes, especially dementia in older persons (79,85). Microscopic infarcts are infarcts that are not seen on gross pathological examination but are seen microscopically, whereas macroscopic or gross infarcts are visible with the naked eye. This definition notably differs from imaging studies, which consider any infarct <3 mm to be microscopic. Some studies suggest a major effect of multiple cortical small and microscopic infarcts on cognitive performance (85,86), although subcortical lacunar infarcts have also been shown to be related to cognitive impairment (87). Because the number, size, and location of infarcts, other coexisting pathologies, and clinical resilience are all important factors in the expression of pathology, it is difficult from a pathological perspective to determine whether any specific constellation of SVD pathology will result in a vascular, mixed, or Alzheimer’s type dementia.
LARGE INFARCTS.
Large or cystic infarcts (typically >10 to 15 mm in greatest dimension) are often related to large-vessel intracranial or extracranial athero-sclerosis or heart disease. Both size and location of infarcts is important in the occurrence of dementia. Of course, larger infarcts are more often clinically recognized as stroke and pose a risk for PSD. PSD may present clinically as a VaD, mixed dementia, or as AD. Confirmation of the diagnosis of “pure” VaD requires autopsy confirmation given that it is quite common to have unrecognized AD or other neurodegenerative pathologies.
MICROBLEEDS AND OTHER HEMORRHAGES.
It is well known that CAA is associated with lobar hemorrhages and SVD without vascular amyloid, for example, in hypertension, with basal ganglia hemorrhages. However, these macrohemorrhages are less common than smaller hemorrhages, microbleeds, and cortical superficial siderosis, resulting from focal subarachnoid hemorrhage, often seen in CAA. Although microbleeds and superficial siderosis can be seen on pathological specimens, imaging studies are better at overall detection of these lesions. Multiple studies have shown that microbleeds are associated with other evidence of SVD, are more common in those with cognitive impairment, and may be predictive of cognitive decline (88,89). Number, location, size, and coexisting pathologies may increase the likelihood of cognitive impairment.
OVERLAP WITH NEURODEGENERATIVE PATHOLOGY.
Although VaD is present in about 10% of older persons with dementia, more commonly, cerebro-vascular disease and ischemic injury coexist with Alzheimer’s and other neurodegenerative pathologies, such as Lewy bodies, seen in Parkinson disease and dementia with Lewy bodies, and TDP-43, a DNA-RNA binding protein implicated in amyotrophic lateral sclerosis and frontotemporal dementia (80,90). Indeed, several large pathological studies have shown that mixed or multietiology dementia is the most common type of dementia in aging (80,90). In addition, these studies have shown the vascular pathology accounts for about one-third of all dementia cases (30). Studies have also shown that at any level of AD pathology, having macroscopic infarcts, microinfarcts, atherosclerosis, arteriolosclerosis, and/or CAA each increase the likelihood that a person will exhibit a dementia syndrome (78). In addition to having an additive effect with neuro-degenerative pathologies, some studies suggest that specific vessel disease pathologies promote neuro-degenerative pathologies. For instance, some but not all studies have shown that cerebral atherosclerosis is associated with increased deposition of amyloid in the aging brain (91,92). More recently, some studies have shown a link between arteriolosclerosis and age-related TDP-43 pathology (93,94). Conversely, in persons with AD pathology, there are well recognized decreases in perfusion, changes in the blood brain barrier, and alteration of the neurovascular unit including pericytes (11,51,68).
IMAGING
Neuroimaging is critically important in the diagnosis and management of VCI. Although large infarcts, extensive WM alterations, or advanced atrophy can be visualized by computer assisted tomography, MRI is well suited to visualize and quantify brain alterations related to SVD (95), and findings using this imaging modality will be discussed in detail.
WHITE AND GRAY MATTER IMAGING ABNORMALITIES IN SVD.
Direct imaging of the small penetrating vessels on MRI remains challenging, especially at routinely used field strengths. Although ultra-high field MRI at 7-T (and above) holds the potential to characterize small vessels both in structure and function (96,97), most neuroimaging manifestations captured by MRI are parenchymal alterations thought to arise from small vessel pathology. A detailed overview of the broad spectrum of SVD-related lesions together with consensus terminology has been provided by the STRIVE (STandards for ReportIng Vascular changes on nEuroimaging) initiative (95).
The most common subcortical imaging manifestations are WMH and lacunes of presumed vascular origin (Figure 5). Hemorrhagic manifestations of SVD include microbleeds, larger intracranial hemorrhages, focal subarachnoid hemorrhage, and cortical superficial siderosis. The latter is tightly linked to CAA and has prognostic relevance (98).
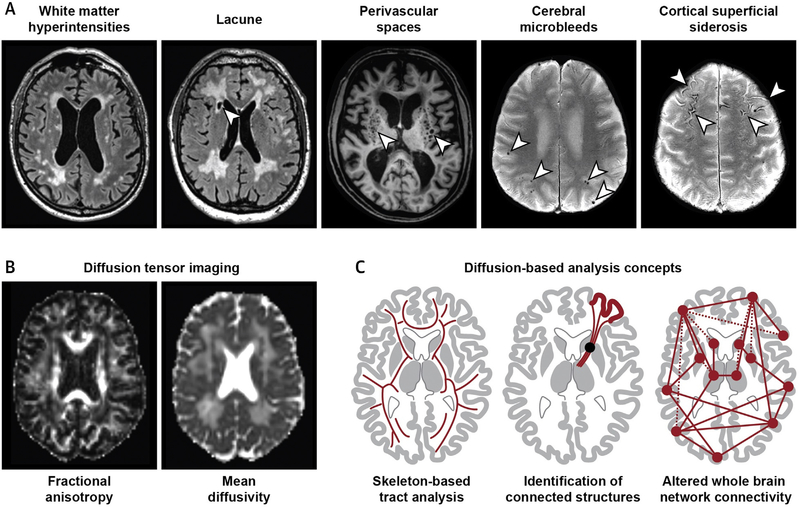
FIGURE 5. Neuroimaging of Vascular Cognitive Impairment.
(A) Signal alterations on conventional magnetic resonance imaging (visible lesions) in small vessel disease (SVD). The arrowheads indicate the pathology mentioned at the top of each brain scan. (B) Diffusion tensor imaging shows increased water diffusivity in the brain of an SVD patient. (C) Diffusion imaging can be analyzed using different strategies, including regional and global approaches.
As discussed in the Neuropathology section, enlarged perivascular spaces and brain atrophy have also been recognized as important imaging hallmarks of SVD. Volume loss can be found both for white and grey matter, again highlighting that SVD is not only a subcortical, but also cortical disease (99). Conversely, the presence of brain atrophy in SVD illustrates that SVD imaging markers are not specific, because brain volume loss is primarily a hallmark of neurodegenerative diseases. At present, there are no specific imaging modalities or molecular imaging markers (e.g., PET ligands) that can reliably distinguish vascular injury from neurodegenerative pathology.
QUANTIFYING SVD BURDEN AND PROGRESSION.
Quantitative measures of SVD imaging abnormalities have been proposed as markers of disease burden and progression in cross-sectional and longitudinal studies, as well as clinical trials. Yet, quantifying SVD is challenging. Visual rating can suffer from low inter-rater reliability, and volumetric measurement of WMH is time-consuming and shows only a weak association with clinical deficits (100).
Major progress towards the fully automated assessment of SVD burden and progression has been made with diffusion imaging, which quantifies the movement of water molecules in brain tissue. Water mobility is increased in SVD patients even in brain regions that appear normal on conventional MRI scans, and diffusion tensor imaging has increasingly been used to quantify these alterations (Figure 5). Likely contributors are increased extracellular water content and altered WM fiber structure (101). Although these alterations may not be disease specific, diffusion metrics have proven to be informative risk markers. In comparison with traditional markers, such as lesions or brain volume, they show a stronger association with clinical deficits and SVD progression (102). Advanced post-processing techniques, such as skeleton-based analysis (Figure 5) and histogram analysis, enable reliable quantification in a fully automated way (103).
SECONDARY NEURODEGENERATION AFTER ACUTE INFARCTS.
Recent studies have drawn attention to remote effects of subcortical lesions. Cross-sectional studies have demonstrated an association between the extent of subcortical lesions and cortical thickness (104). In prospective studies, incident subcortical lesions were associated with cortical thinning specifically in connected cortical regions identified by diffusion-based tractography (Figure 5) (105). These studies identify secondary neurodegeneration as a source of cortical atrophy in SVD, thus offering a potential target for future interventions (106).
CONNECTIVITY AND NETWORK DEGRADATION.
Neuroimaging has provided in vivo evidence that VCI may be a brain network disorder. Although the assessment of functional connectivity in SVD by resting-state functional MRI is hampered by low reproducibility (107), structural connectivity can be reliably determined using diffusion imaging, whole brain tractography, and network construction (Figure 5). Graph theoretical analysis allows quantifying the properties of the structural networks, and recent analyses have shown that altered network structure can account reasonably well for the association between SVD neuroimaging lesions and cognitive deficits (108). In particular, abnormal organization of rich clubs, a set of highly interconnected regions within the brain network, was found to contribute to cognitive impairment (109). As noted for secondary neurodegeneration, these observations have shifted the attention from focal lesions to more widespread changes in SVD and VCI (99).
MANAGEMENT
There is limited evidence from randomized controlled trials (RCTs) that interventions to control vascular risk factors lower the risk of incident dementia or cognitive decline (23,40,110,111). In the SYST-EUR (Systolic Hypertension in Europe) trial, a blood pressure (BP) reduction of 8.3/3.8 mm Hg was associated with a marginally significant reduction in the risk of dementia (112). Various other trials failed to demonstrate a beneficial effect of BP lowering on dementia risk or cognitive function (40,111). However, the recently published SPRINT (Systolic Blood Pressure Intervention Trial) MIND found that intensive BP lowering to <120 mm Hg compared with a target <140 mm Hg reduces the risk of both MCI and the combined endpoint of MCI or dementia in adults with increased risk for cardiovascular disease but without diabetes and without history of stroke (113). Interestingly, intensive BP lowering was further associated with a smaller increase in WMH on brain MRI (113). In the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) active treatment in patients with cerebrovascular disease lowered the risk of cognitive decline by 19% (114). However, there is no evidence from secondary stroke prevention trials that BP lowering reduces the risk of incident dementia (115). Similarly, there is no evidence that treatment of hyperglycemia and diabetes reduces the risk of dementia or cognitive decline (42). However, the benefit of glycemic control on multiple target organs is sufficiently documented to recommend risk factor control. In the Heart Protection Study and PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) trial, statin treatment had no effect on dementia incidence and cognitive decline (116,117). Importantly, however, none of the listed trials used cognitive function as the primary endpoint. More-over, many trials recruited subjects who were at low risk of dementia and had a short treatment duration, short follow-up, and a high number of dropouts with potential bias from differential dropout (40).
Observational studies suggest lifestyle factors as a target for dementia prevention. In the FABS (Fitness for the Aging Brain Study), a 6-month program of physical activity in adults with subjective memory impairment resulted in a modest improvement in cognition during follow-up (time frame: 18 months) (118). In contrast, the LIFE (Lifestyle Interventions and Independence for Elders) study found no improvements in global or domain-specific cognitive function by a 24-month moderate-intensity physical activity program compared with a health education program among sedentary older adults (119). Similarly, the DAPA (Dementia and Physical Activity) trial, which was conducted in patients with mild to moderate dementia, found no beneficial effect of a moderate to high-intensity aerobic and strength exercise training program on cognitive deterioration (120).
Observational data also suggest that smoking cessation reduces the risk of dementia including VaD (121). However, there are no interventional studies that examined the effect of smoking cessation or weight reduction, another modifiable lifestyle factor (40,111), on cognitive decline. Although a low educational level is associated with dementia risk, there is no evidence for a beneficial effect of education or structured cognitive interventions on disease progression (23,40).
The PREDIMED (Prevención con Dieta Mediterránea) trial found a beneficial effect of a Mediterranean diet supplemented with olive oil or nuts on cognitive function in cognitively normal elderly volunteers after a follow-up of 4 years (122). However, the study was later retracted and republished due to methodological issues raising questions about the validity of the results (123). Adherence to a Mediterranean diet was also part of FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability), which randomized subjects to a multicomponent intervention (vascular risk monitoring, exercise, nutritional advice, cognitive training) or general health advice. Patients in the intervention group showed modest improvements on cognitive scores (124). However, 2 other trials showed no benefit of a multidomain intervention on cognitive functioning (125,126). Also, there was no beneficial effect of 3 polyunsaturated fatty acid supplementation on cognitive decline in elderly people with memory complaints (126).
Oral anticoagulation may preserve cognitive function in patients with atrial fibrillation (AF). A retrospective analysis of registry data from 440,106 Swedish patients with a hospital diagnosis of AF found oral anticoagulation at baseline to lower the risk of incident dementia by 29%. Those on treatment over at least 80% of the observational period had 48% lower risk of dementia than those who were not on oral anticoagulants (127). This observation supports the general notion that strategies for stroke prevention should also be effective in preventing dementia.
In light of the previously mentioned data, the American Heart Association/American Stroke Association recommends checking health status with Life’s simple 7 (nonsmoking, physical activity at goal levels, healthy diet consistent with current guideline levels, body mass index <25 kg/m2, blood pressure <120/80 mm Hg, total cholesterol <200 mg/dl, and fasting blood glucose <100 mg/dl) to maintain optimal brain health (111), and provides specific recommendations on risk factor management (8,111).
SYMPTOMATIC TREATMENT.
Strategies for symptomatic treatment of VaD include the prescription of cholinesterase inhibitors (galantamine, donepezil, rivastigmine) the N-methyl D-aspartate antagonist memantine, and various Chinese medicines (25,128–130). In RCTs, both cholinesterase inhibitors and memantine showed small benefits on cognition. However, with the exception of donepezil, there were no beneficial effects on the Clinicians’ Global Impression of Change scale and behavioral and functional scales, thus questioning the use of cholinesterase inhibitors and memantine in VaD (128). Nevertheless, some expert statements and guidelines recommend considering donepezil for cognitive enhancement in VaD (8). Treatment plans in VaD should further address comorbidities, such as behavioral and psychological symptoms, support for patients and caregivers, and maximizing independence (23).
MARKERS FOR MONITORING DISEASE PROGRESSION.
Prevention and treatment trials for VaD/VCI have sparked interest in biological markers that could serve as adjunct outcome measures and help differentiate VCI from other causes of cognitive decline. Proposed markers include quantitative and semi-quantitative measures of WMH on brain MRI (95,131), composite scores for the burden of vascular brain lesions (132), and metrics derived from diffusion tensor imaging (103). In the absence of circulating or CSF markers specific for VCI, assessment of biofluids is of limited value for disease monitoring or diagnosis, but it may help exclude other causes of dementia, as is also true for PET imaging.
CONCLUSIONS AND FUTURE DIRECTIONS
The evidence reviewed in the previous sections highlights the critical role of neurovascular function in the maintenance of brain health and the significant contribution of vascular causes to age-related dementia. A renewed interest in vascular causes of cognitive dysfunction has led to several advances. New classification criteria have been developed for VCI and VaD based on both clinical and imaging criteria, and more practical tools have been introduced to assess the cognitive deficits. Advances have also been made in the pathobiology of VCI and VaD, providing a more nuanced understanding of the pathogenic factors converging on the cerebral microvasculature, especially in the WM, to induce cognitive dysfunction. Although neuropathological investigations have revealed a previously-unappreciated multiplicity of the brain lesions underling VCI and VaD and the frequent coexistence with neurodegenerative pathology, advances in brain imaging now provide the opportunity to detect these lesions in vivo and to assess and quantify their effect on brain structure and function.
Despite these advances, several knowledge gaps remain:
The epidemiology of cognitive impairment caused by vascular factors is less well understood compared with other forms of dementia. Most studies have focused on unspecified dementia, and there is incomplete information on the incidence/prevalence of VCI and VaD. It remains to be defined how the new classification systems for VCI work in practice, and whether they can be used in epidemiological studies of VCI, especially when applied at the population level. In addition, it would be important to develop risk model/scores for the prediction of dementia risk in individual patients. In this regard, the use of new imaging tools may aid risk prediction.
A deeper understanding of the pathobiology of VCI and VaD is needed to develop new diagnostic and therapeutic interventions. To this end, animal models that better recapitulate the human disease would be needed to provide new mechanistic insight, develop biomarkers, and test therapies. Most investigations have focused on the effect of reduced CBF on cognition. However, the cognitive effect of other aspects of neurovascular function, especially endothelial function and proteostasis (Figure 3), must be explored in greater depth.
There is also urgent need of reliable biomarkers for early diagnosis and monitoring disease progression. Although a global BBB dysfunction may be an early marker of disease (59), longitudinal studies, large enough to avoid potential confounders, would be needed to better understand the contribution of BBB leakage to brain damage and to define its role as a biomarker.
Limited progress has been made in the management of VCI and VaD, and disease-modifying treatments are not available. In contrast to the wealth of research in AD therapeutic development, drug discovery efforts in VCI/VaD are limited. A vigorous effort involving the basic scientific community is needed to identify “druggable” targets based on pathogenic pathways identified in animal models. These targets would then need to be translated into therapies to be tested in clinical trials. Considering the frequent overlap between VCI and AD pathologies, such efforts are certainly warranted because they would benefit both conditions.
There is insufficient evidence that risk factor control affects the clinical course of the disease. What would be needed are adequately powered prevention trials with longer treatment and follow-up that: 1) account for the methodological challenges of cognitive trials; and 2) are accompanied by surrogate endpoints, such as brain imaging. Such trials are feasible but would require considerable resources.
As highlighted in the Neuropathology section, most cognitive impairment in the elderly arises from multiple pathologies, of which the vascular component is currently the only treatable and preventable one. Moreover, dementia and stroke share the same risk factors, and stroke doubles the likelihood of developing dementia. Indeed, a decrease in stroke incidence is associated with a concomitant decrease in dementia (see Epidemiology section), justifying a concerted effort for the joint prevention of stroke and dementia endorsed by all of the major brain, stroke, and dementia organizations (133).
In the absence of disease-modifying treatments, measures to prevent cerebrovascular disease and promote brain health are the only viable options currently available to contain the rapidly expanding burden of one of the most vexing diseases affecting the aging world population.
HIGHLIGHTS.
Age-related dementia is growing alarmingly worldwide and is estimated to affect 150 million people by 2050.
Cerebrovascular alterations are a major cause of dementia, but are also a culprit in AD.
New insights into pathobiology, prevention, and diagnosis have emerged, but therapies are not yet available.
Maintaining vascular health and preserving brain function may mitigate the public health impact of dementia.
Acknowledgments
Dr. Iadecola is supported by National Institutes of Health grants R01-NS34179, R37-NS089323, R01-NS100447, R01-NS095441, and R01-NS/HL3785; and has served on the Strategic Advisory Board of Broadview Ventures. Dr. Duering is supported by the Deutsche Forschungsgemeinschaft (DU 1626-1-1) and the Alzheimer Forschung Initiative e.V. (16018CB). Dr. Joutel is supported by the European Union (Horizon 2020 Research and Innovation Programme SVDs@target under the grant agreement no. 666881) and the National Research Agency, France (ANR-16-RHUS-0004). Dr. Pendlebury is supported by the NIHR Oxford Biomedical Research Centre. Dr. Schneider is supported by National Institutes of Health grants R01AG10161, R01AG042210, UH2NS100599, R01AG017917, RF1AG015819, and R01AG034374; and has served as a consultant for AVID Radiopharmaceuticals. Dr. Dichgans is supported by the Deutsche For-schungsgemeinschaft (EXC 2145 SyNergy, CRC1123 B3, DI 722/13-1); the European Union’s Horizon 2020 research and innovation programme (grant agreements No 666881 [SVDs@target] and No 667375 [CoSTREAM]), and the German Center for Neurode-generative Diseases (DZNE; DEMDAS-2).
ABBREVIATIONS AND ACRONYMS
- AD
Alzheimer’s disease
- ADL
activities of daily living
- BBB
blood-brain barrier
- CAA
cerebral amyloid angiopathy
- CBF
cerebral blood flow
- ECM
extracellular matrix
- MCI
mild cognitive impairment
- MoCA
Montreal cognitive assessment
- NO
nitric oxide
- PSD
post-stroke dementia
- SIVaD
subcortical ischemic vascular dementia
- VaD
vascular dementia
- VCI
vascular cognitive impairment
- WMH
white matter hyperintensities
REFERENCES
- 1.Alzheimer’s Disease International. World Alzheimer’s Report 2018. Available at: https://www.alz.co.uk/research/world-report-2018. Accessed May 7, 2019.
- 2.World Health Organization. Global action plan on the public health response to dementia 2017–2025. 2017. Available at: http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/. Accessed May 7, 2019.
- 3.IADRP. International Alzheimer’s Disease Research Portfolio. 2018. Available at: https://iadrp.nia.nih.gov/. Accessed May 7, 2019.
- 4.Mast H, Tatemichi TK, Mohr JP. Chronic brain ischemia: the contributions of Otto Binswanger and Alois Alzheimer to the mechanisms of vascular dementia. J Neurol Sci 1995;132:4–10. [DOI] [PubMed] [Google Scholar]
- 5.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32: 632–7. [DOI] [PubMed] [Google Scholar]
- 6.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet 1974;2:207–10. [DOI] [PubMed] [Google Scholar]
- 7.Hachinski VC, Bowler JV. Vascular dementia. Neurology 1993;43:2159–60. author reply 2160–1. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004;5:347–60. [DOI] [PubMed] [Google Scholar]
- 11.Iadecola C The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017;96:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement 2018;14: 280–92. [DOI] [PubMed] [Google Scholar]
- 13.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–41. [DOI] [PubMed] [Google Scholar]
- 14.Looi JC, Sachdev PS. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology 1999;53:670–8. [DOI] [PubMed] [Google Scholar]
- 15.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards neuro-psychological battery after TIA and stroke. Stroke 2012;43:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zietemann V, Georgakis MK, Dondaine T, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology 2018;91:e1838–50. [DOI] [PubMed] [Google Scholar]
- 17.Pendlebury ST, Klaus SP, Thomson RJ, et al. Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (III) applicability of cognitive tests. Stroke 2015; 46:3067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldenbeuving AW, de Kort PL, Jansen BP, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology 2011;76:993–9. [DOI] [PubMed] [Google Scholar]
- 19.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994;24:145–53. [DOI] [PubMed] [Google Scholar]
- 20.Koski L, Xie H, Konsztowicz S, Tetteh R. French-English cross-linguistic comparison and diagnostic impact of the AD-8 dementia screening questionnaire in a geriatric assessment clinic. Dement Geriatr Cogn Disord 2010;29:265–74. [DOI] [PubMed] [Google Scholar]
- 21.Peters N, Opherk C, Danek A, Ballard C, Herzog J, Dichgans M. The pattern of cognitive performance in CADASIL: a monogenic condition leading to subcortical ischemic vascular dementia. Am J Psychiatry 2005;162:2078–85. [DOI] [PubMed] [Google Scholar]
- 22.Le Heron C, Manohar S, Plant O, et al. Dysfunctional effort-based decision-making underlies apathy in genetic cerebral small vessel disease. Brain 2018;141:3193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dichgans M, Leys D. Vascular Cognitive Impairment. Circ Res 2017;120:573–91. [DOI] [PubMed] [Google Scholar]
- 24.Chabriat H, Herve D, Duering M, et al. Predictors of clinical worsening in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: prospective cohort study. Stroke 2016;47:4–11. [DOI] [PubMed] [Google Scholar]
- 25.Dichgans M, Markus HS, Salloway S, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol 2008;7:310–8. [DOI] [PubMed] [Google Scholar]
- 26.Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol 2017; 13:327–39. [DOI] [PubMed] [Google Scholar]
- 27.De Ronchi D, Palmer K, Pioggiosi P, et al. The combined effect of age, education, and stroke on dementia and cognitive impairment no dementia in the elderly. Dement Geriatr Cogn Disord 2007; 24:266–73. [DOI] [PubMed] [Google Scholar]
- 28.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology 1998;51:728–33. [DOI] [PubMed] [Google Scholar]
- 29.Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Dis Elderly Research Group. Neurology 2000;54:S4–9. [PubMed] [Google Scholar]
- 30.Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol 2018;84: 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wentzel C, Rockwood K, MacKnight C, et al. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology 2001;57:714–6. [DOI] [PubMed] [Google Scholar]
- 32.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8: 1006–18. [DOI] [PubMed] [Google Scholar]
- 33.Pendlebury ST, Chen PJ, Welch SJ, et al. , for the Oxford Vascular Study. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol 2019;18:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sexton E, McLoughlin A, Williams D, et al. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur Stroke J 2019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rooij FG, Schaapsmeerders P, Maaijwee NA, et al. Persistent cognitive impairment after transient ischemic attack. Stroke 2014; 45:2270–4. [DOI] [PubMed] [Google Scholar]
- 36.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine DA, Wadley VG, Langa KM, et al. Risk factors for poststroke cognitive decline: the REGARDS Study (Reasons for Geographic and Racial Differences in Stroke). Stroke 2018;49: 987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pendlebury ST, Wadling S, Silver LE, Mehta Z, Rothwell PM. Transient cognitive impairment in TIA and minor stroke. Stroke 2011;42:3116–21. [DOI] [PubMed] [Google Scholar]
- 39.Markus HS, Schmidt R. Genetics of vascular cognitive impairment. Stroke 2019;50:765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke 2012;43: 3137–46. [DOI] [PubMed] [Google Scholar]
- 41.Stern Y, Chetelat G, Habeck C, et al. Mechanisms underlying resilience in ageing. Nat Rev Neurosci 2019;20:246. [DOI] [PubMed] [Google Scholar]
- 42.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018;14: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013;202: 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moulin S, Labreuche J, Bombois S, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol 2016;15:820–9. [DOI] [PubMed] [Google Scholar]
- 45.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010;375:773–5. [DOI] [PubMed] [Google Scholar]
- 46.Shih AY, Blinder P, Tsai PS, et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 2013;16:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 2018;100:375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faraco G, Park L, Anrather J, Iadecola C. Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med (Berl) 2017;95:1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018;554:475–80. [DOI] [PubMed] [Google Scholar]
- 50.Korogod N, Petersen CC, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 2015;4:e05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev 2019;99:21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenblum WI. Endothelium-dependent responses in the microcirculation observed in vivo. Acta Physiol (Oxf) 2018;224:e13111. [DOI] [PubMed] [Google Scholar]
- 53.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Sciences, 2009. [PubMed] [Google Scholar]
- 54.Koizumi K, Hattori Y, Ahn SJ, et al. Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun 2018;9:3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci (Lond) 2017;131:2451–68. [DOI] [PubMed] [Google Scholar]
- 56.Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci 2017; 131:635–51. [DOI] [PubMed] [Google Scholar]
- 57.Santisteban MM, Iadecola C. Hypertension, dietary salt, and cognitive impairment. J Cereb Blood Flow Metab 2018;38:2112–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 2017;312:H1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol 2018; 31:36–43. [DOI] [PubMed] [Google Scholar]
- 60.Huneau C, Houot M, Joutel A, et al. Altered dynamics of neurovascular coupling in CADASIL. Ann Clin Transl Neurol 2018;5:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagi Z, Brandner DD, Le P, et al. Vasodilator dysfunction and oligodendrocyte dysmaturation in aging white matter. Ann Neurol 2018;83:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Y, Thrippleton MJ, Blair GW, et al. Small vessel disease is associated with altered cerebro-vascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab 2018. October 8 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 2017;97:1555–617. [DOI] [PubMed] [Google Scholar]
- 64.Iulita MF, Noriega de la Colina A, Girouard H. Arterial stiffness, cognitive impairment and dementia: confounding factor or real risk? J Neurochem 2017;144:527–48. [DOI] [PubMed] [Google Scholar]
- 65.Strickland S Blood will out: vascular contributions to Alzheimer’s disease. J Clin Invest 2018;128:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci 2018; 19:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barry Erhardt E, Pesko JC, Prestopnik J, Thompson J, Caprihan A, Rosenberg GA. Bio-markers identify the Binswanger type of vascular cognitive impairment. J Cereb Blood Flow Metab 2018. March 7 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019;25:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018;114:1462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faraco G, Brea D, Garcia-Bonilla L, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci 2018;21:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Silva TM, Faraci FM. Microvascular dysfunction and cognitive impairment. Cell Mol Neurobiol 2016;36:241–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faraco G, Sugiyama Y, Lane D, et al. Perivascular macrophages mediate the neuro-vascular and cognitive dysfunction associated with hypertension. J Clin Invest 2016;126:4674–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park MH, Lee JY, Park KH, et al. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron 2018;100:167–82.e9. [DOI] [PubMed] [Google Scholar]
- 74.Erdo F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab 2017;37:4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 2018;24:326–37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Joutel A, Haddad I, Ratelade J, Nelson MT. Perturbations of the cerebrovascular matrisome: a convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab 2016;36: 143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Capone C, Dabertrand F, Baron-Menguy C, et al. Mechanistic insights into a TIMP3-sensitive pathway constitutively engaged in the regulation of cerebral hemodynamics. Elife 2016;5:e17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 2016;15:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 2003;60:1082–8. [DOI] [PubMed] [Google Scholar]
- 80.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017;134: 171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kovacs GG, Milenkovic I, Wohrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuro-pathol 2013;126:365–84. [DOI] [PubMed] [Google Scholar]
- 82.James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 2012;307:1798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 2017;27:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 2015;85:1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42: 722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 2007;62:406–13. [DOI] [PubMed] [Google Scholar]
- 87.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol 2007;62: 59–66. [DOI] [PubMed] [Google Scholar]
- 88.Vinke EJ, de Groot M, Venkatraghavan V, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging 2018;71:32–40. [DOI] [PubMed] [Google Scholar]
- 89.Yilmaz P, Ikram MK, Niessen WJ, Ikram MA, Vernooij MW. Practical small vessel disease score relates to stroke, dementia, and death. Stroke 2018;49:2857–65. [DOI] [PubMed] [Google Scholar]
- 90.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204. [DOI] [PubMed] [Google Scholar]
- 91.Sadleir KR, Bennett DA, Schneider JA, Vassar R. Elevated Abeta42 in aged, non-demented individuals with cerebral atherosclerosis. Curr Alzheimer Res 2013;10:785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yarchoan M, Xie SX, Kling MA, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 2012;135:3749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 2014;137:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nelson PT, Trojanowski JQ, Abner EL, et al. “New Old Pathologies”: AD, PART, and Cerebral Age-Related TDP-43 With Sclerosis (CARTS). J Neuropathol Exp Neurol 2016;75:482–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Invest Radiol 2014; 49:307–13. [DOI] [PubMed] [Google Scholar]
- 97.Geurts LJ, Zwanenburg JJM, Klijn CJM, Luijten PR, Biessels GJ. Higher pulsatility in cerebral perforating arteries in patients with small vessel disease related stroke, a 7T MRI Study. Stroke 2018. December 11 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wollenweber FA, Opherk C, Zedde M, et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 2019;92:e792–801. [DOI] [PubMed] [Google Scholar]
- 99.Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol 2018;14:387–98. [DOI] [PubMed] [Google Scholar]
- 100.Duering M, Gesierich B, Seiler S, et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology 2014;82:1946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duering M, Finsterwalder S, Baykara E, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement 2018;14:764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeestraten EA, Lawrence AJ, Lambert C, et al. Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology 2017;89:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol 2016;80:581–92. [DOI] [PubMed] [Google Scholar]
- 104.Seo SW, Lee JM, Im K, et al. Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol Aging 2012;33: 1156–67. [DOI] [PubMed] [Google Scholar]
- 105.Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology 2015;84:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duering M, Schmidt R. Remote changes after ischaemic infarcts: a distant target for therapy? Brain 2017;140:1818–20. [DOI] [PubMed] [Google Scholar]
- 107.Lawrence AJ, Tozer DJ, Stamatakis EA, Markus HS. A comparison of functional and tractography based networks in cerebral small vessel disease. Neuroimage Clin 2018;18:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tuladhar AM, van Uden IW, Rutten-Jacobs LC, et al. Structural network efficiency predicts conversion to dementia. Neurology 2016; 86:1112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tuladhar AM, Lawrence A, Norris DG, Barrick TR, Markus HS, de Leeuw FE. Disruption of rich club organisation in cerebral small vessel disease. Hum Brain Mapp 2017;38:1751–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larsson SC, Markus HS. Does treating vascular risk factors prevent dementia and alzheimer’s disease? a systematic review and meta-analysis. J Alzheimers Dis 2018;64:657–68. [DOI] [PubMed] [Google Scholar]
- 111.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017; 48:e284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998;352:1347–51. [DOI] [PubMed] [Google Scholar]
- 113.Williamson JD, Pajewski NM, Auchus AP, et al. , for the SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003;163:1069–75. [DOI] [PubMed] [Google Scholar]
- 115.Zonneveld TP, Richard E, Vergouwen MD, et al. Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev 2018;7:CD007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 117.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30. [DOI] [PubMed] [Google Scholar]
- 118.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027–37. [DOI] [PubMed] [Google Scholar]
- 119.Sink KM, Espeland MA, Castro CM, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE Randomized Trial. JAMA 2015;314:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 2018;361:k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi D, Choi S, Park SM. Effect of smoking cessation on the risk of dementia: a longitudinal study. Ann Clin Transl Neurol 2018;5:1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 2015;175:1094–103. [DOI] [PubMed] [Google Scholar]
- 123.Agarwal A, Ioannidis JPA. PREDIMED trial of Mediterranean diet: retracted, republished, still trusted? BMJ 2019;364:l341. [DOI] [PubMed] [Google Scholar]
- 124.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–63. [DOI] [PubMed] [Google Scholar]
- 125.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016;388:797–805. [DOI] [PubMed] [Google Scholar]
- 126.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017;16: 377–89. [DOI] [PubMed] [Google Scholar]
- 127.Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018;39:453–60. [DOI] [PubMed] [Google Scholar]
- 128.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol 2007;6:782–92. [DOI] [PubMed] [Google Scholar]
- 129.Jia J, Wei C, Chen S, et al. Efficacy and safety of the compound Chinese medicine Sai-LuoTong in vascular dementia: a randomized clinical trial. Alzheimers Dement (N Y) 2018;4: 108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Man SC, Chan KW, Lu JH, Durairajan SS, Liu LF, Li M. Systematic review on the efficacy and safety of herbal medicines for vascular dementia. Evid Based Complement Alternat Med 2012;2012: 426215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schmidt R, Scheltens P, Erkinjuntti T, et al. White matter lesion progression: a surrogate endpoint for trials in cerebral small-vessel disease. Neurology 2004;63:139–44. [DOI] [PubMed] [Google Scholar]
- 132.Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014;83:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hachinski V, Ganten D, Lackland D, Kreutz R, Tsioufis K, Hacke W. Implementing the Proclamation of Stroke and Potentially Preventable Dementias. Int J Stroke 2018;13:780–6. [DOI] [PubMed] [Google Scholar]
- 134.Pendlebury S, Rothwell P, for the Oxford Vascular Study. Incidence and prevalence of dementia associated with TIA and stroke: rates and risk factors in a population-based cohort. Lancet Neurol 2019;18:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Traylor M, Adib-Samii P, Harold D, et al. Shared genetic contribution to ischaemic stroke and Alzheimer’s disease. Ann Neurol 2016;79:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]