Abstract
Current fascia research is allowing for an interdisciplinary understanding of the body’s anatomical, biomechanical, and neurological connectivity via the fascial network. Fascial research and its application has been validated and established in various clinical areas of research. The purpose of this study was to apply the current knowledge of the fascial system to general exercise protocols. This study involved 20 women, ages 30–60 years, who were novice weight trainers, mostly sedentary, and with no injuries, excessive pain or disease. The 10-week study compared strength gain changes between a strength training regimen control group (10) and a treatment group (10) with the same strength routine along with a fascial system exercise protocol. Statistical analysis was completed using a repeated measure design to determine differences between baseline and final measures of strength between groups. The repeated measures analysis of variance revealed no significant differences between treatment and control groups between pre and post trials. The analysis did find significant differences in strength across trials for both groups in the variables of leg press (Treatment =+62 lbs., Control = +67 lbs.), leg extension (Treatment =+61 lbs., Control = +45.5 lbs.), and chest press (Treatment =+19.5 lbs., Control = +16.5 lbs.). These results may be attributed to the control group receiving sufficient stimulus to the fascial system to produce similar results to that of the treatment group or due to training time was not sufficient to elicit an effect of the fascial training.
Keywords: Fascial adaptation, fascial exercise, strength training
INTRODUCTION
The overload principle is defined as challenging a specific system or tissue with exercise beyond a point that the system or tissue is accustomed producing an adaptation to a new homeostatic level (11). While this overload principle is widely accepted, current research suggests that several aspects of muscle function, including strength gains, are connected intimately to the sensory and mechanical organ known as fascia (3, 6, 27, 34, 35). Fascia connects and surrounds every muscle and organ as it forms a continuous matrix from head to toe (3). The potential of this matrix of fascia to influence muscle tissue is great.
The fascial system is the three-dimensional matrix of the body-wide tensional network of connective tissue that includes, ligaments, tendons, joint capsules, retinacula, and other fibrous collagenous tissues with a common definition that has emerged with fascia being defined as the uninterrupted body-wide tensional network that surrounds and penetrates all structures from head to toe (19). Throughout history and even in current times, medical students are asked to strip away this tissue in order to view the origin and insertion points of the muscles as well as view other structures as separate entities such as organs, nerves, and vessels (19, 38). While this may be a useful demonstration for anatomical investigation purposes, this practice of stripping away the fascia may not be productive in explaining muscle or other tissues or systems in physiological terms.
Healthy fascia appears in a two-directional, (lattice) arrangement of this collagen fiber network. A larger degree of crimp (wavy appearance) of individual collagen fibers allows for an increase in elastic storage capacity for optimal force production (33). One unique property of fascia is its elastic capacity used to store kinetic energy. In healthy tissue, fascia can assist with workloads that are beyond the capacity of the muscle contraction because of the elastic quality. In some movements, such as jumping or leaping, the workload produced beyond the capacity of the muscle is called the catapult mechanism (18). Diminishing health in this tissue can occur because of lack of movement, scarring from injury/surgery, poor biomechanics, or poor posture. The tissue can then present itself as scattered and/or adhering to one another causing pain and reduced range of motion (ROM) (30). This fascial matrix remodeling is a result of repetitive mechanical strain that activates the function of fibroblasts, the cell that has most of the function in collagen biosynthesis and organization (9, 20).
Fascia holds the key to developing form and function of the body. It is richly innervated with mechanoreceptors that detect pressure changes, stretch, and vibration and are involved in proprioception and nociception (pain perception) (29). Fascia also has contractile properties like that of smooth muscle due to the presence of myofibroblasts as they contain alpha smooth muscle actin. Myofibroblasts develop from regular fibroblasts due to an increase in mechanical strain and specific cytokines. This can influence musculoskeletal dynamics and resting muscle tone (35). Force transmission created by this fascial system challenges the traditional myotendinous pathway model of force transfer to the bone and joint to produce movement. This force transmission can be studied from how the intramuscular connective tissue, (endomysium, perimysium, epimysium) affect structures in this environment, outside of this environment, and beyond the muscle compartment (25, 37).
One main focus in fascial research is the application in therapeutic disciplines. Manual therapists have refined hands on therapies, such as myofascial release, to address painful conditions stemming from the fascial system. However, researchers have identified specific movement categories that focus on exercises that address the unique properties of fascia. This can include the use of soft pressure, fluid movement, slow and dynamic stretching, and elastic bouncing movements (e.g., light hopping). Increases in ROM, pain reduction, and increases in proprioception can result from training the fascial system (30). The relationship between fascial health and training aspects, such as increasing strength, may be of interest since most disruption occurs in that soft tissue from injury, surgery, and overuse which may affect the integrity of muscle function in several capacities (32).
The purpose of this study was to determine how potentially increasing the health and function of the fascial system affected strength outcomes in healthy female adults ranging in age from 30 to 60 years in a traditional weight training program following American College of Sports Medicine (ACSM) guidelines.
For this study, it was hypothesized that engaging in a specified training and conditioning program to improve the health and function of the fascial system would yield significant strength gains compared to a non-fascial training regimen.
METHODS
Participants
An a priori power analysis using G-Power 3.1 software (Univeristat Kiel, Germany) determined that the minimum sample size for a 2×2 repeated measures ANOVA to determine within-between interactions was 16 total subjects. The calculations assumed an α= 0.05, power ≥0.80 and an effect size of 0.40. The actual power for the repeated measures ANOVA with 16 subjects is 0.84. This study included 20 female participants between the ages of 30 to 60 years with a mean age of 39.9 (sd 8.735). Participants were required to be sedentary with no more than one day a week of any type of exercise or not exercising at all. All participants had never performed this type of weight training before and were considered a novice participant. Additional requirements included the lack of current injuries, severe movement restrictions, or taking any medication. There were no weight, height, or body composition restrictions. All prospective candidates were asked to fill out a pre-screening questionnaire to evaluate the above requirements. The University of Texas of the Permian Basin Institutional Review Board approved the protocol, and all participants filled out an informed consent and a Physical Activity Readiness Questionnaire (PAR-Q) prior to testing. The final selection used a random sample from all eligible prospective participants. Thirty-six participants total were recruited for the study. Five of the participants were dropped at the beginning of study for not replying to adherence check ins, six participants were dropped half way through the study for the same reason, and five participants chose to drop out due to emergencies and time constraints. Twenty participants finished the study with 10 in the control group and 10 in the treatment group. This study was executed without any injury to any participant.
Protocol
Participants were matched in pairs by height and weight and then randomly assigned to either the treatment or control group. Participants in the treatment group were given the materials they needed to execute the fascial training program. This included a 5 foot long wooden dowel rod, 8cm massage ball, a 3-foot long by 4 inch in diameter foam roller, an instructional DVD, and a program guidebook. The control group only received a program guidebook. All participants were instructed on how to log their weight training workouts.
A five-minute treadmill warm-up was performed followed by the 1RM test on specified Life Fitness (Rosemont, IL) selectorized weight machines to determine a baseline strength level as well as the appropriate starting weight for each exercise (Table 1).
Table 1.
Weight machines used for strength training protocol.
| Muscle Group | Machine Type |
|---|---|
| *Chest | Seated Chest Press |
| *Quadriceps | Seated Leg Extension |
| *Legs/Glutes | Seated Leg Press |
| +Hamstrings | Seated Leg Curl |
| +Back | Seated Row |
| +Shoulders | Seated Shoulder Press |
| +Abdominals | Seated Trunk Curl |
Denotes the exercises used to determine 1RM for testing purposes
Denotes exercises that were included to provide a well-rounded routine for the sake of training
Participants in the treatment group met with the principal investigator individually after collecting their baseline strength data to review specific concepts for each category of movement on the DVD as shown in Table 2. A demonstration was also given for the foam roller, massage ball, and dowel rod. The principal investigator checked in by email every week for compliance and by phone for the first two weeks for coaching on technique. Appointments at the YMCA (study facility) to instruct and coach in person were also made available. Participants were asked about their confidence level following the DVD and anything that was not clear in the first week, was assisted to a level of full confidence.
Table 2.
Treatment group DVD exercises.
| Movement Category | Exercise | Description |
|---|---|---|
| Fascial Release | Foam Rolling | Legs, Glutes, Upper back, Lats, Spine |
| Slow Dynamic Stretch | Front/Back of Calf stretch | Calf stretch (hold), roll to top of foot (hold) 3× |
| Hip Flexors, torso stretch | Split stance, tuck hip and move to and fro 4×. | |
| Pause. Raise same arm as stretching hip and lean to opposite direction. 1× each side | ||
| Forward Flexion to extension | Slowly flex forward, pause. Roll up, arms overhead, slight hyperextension. Pause. 3× | |
| Faster Dynamic Stretch | Rotation with front arm reach (crossing mid-line) | Rotate torso as extend arm in front and cross mid-line. Switch arms. 1 minute |
| Torso rotation with posterior arm reach | Rotate torso and reach same arm as rotating back behind body. Switch arms. 1 minute | |
| Lateral Line Stretch | Raise arm up by ear as same side hip slides out parallel to ground. Switch arms. 1minute. | |
| Deep anterior stretch | Reach arm by ear, knees bend and push forward, back neutral. Switch arms. 1 minute. | |
| Elastic Recoil | Light hopping | Light bouncing with two feet leaving the ground or one foot at a time. 1 minute. |
| Floating Arms | Arms at side. Explode (spring) arms up and come to mid-line. Repeat. 1 minute. | |
| Floating Back | Flex forward. Pause. Slight countermovement and spring back up. 1 minute. | |
| Fluid Motion (dowel) | Stir the Pot | Legs wide. Dowel inside right foot. Right arm crosses mid-line to left and makes large circle. 6×. Repeat other side. |
| Squat to Reach | Dowel horizontal in both hands as squat. Reach overhead as stand straight. 6× | |
| Around the World | Flexed forward. Left foot in front. Rotate right and lift left arm with dowel up. Continue overhead and return to center. 6×. Switch. | |
| Refined Movement | Spinal Articulation | Supine bridge position. Undulating movements performed on small section of spine-10 seconds. Lower spine a bit and repeat. |
| Very Slow Leg Stretch | Side lying. Foot flexed as leg pushes out at a chosen angle very slowly. Pause 5 seconds. Point toe and bring leg in and choose another angle at hip. 6×. Switch legs. |
All participants were instructed to perform only the designated weight training protocol two times a week on non-consecutive days and no more than 30 minutes of moderate intensity cardiovascular training (i.e., able to still hold a conversation) three times a week. The treatment group was instructed to perform the exercises on the DVD only two times a week on nonconsecutive days (Table 2). This DVD was created only for this study and was not used before this study. However, some of the concepts were used in a ten month class (2× a week) the principal author taught to validate and become familiar with best practices. Some of the material on the DVD were exercises suggested by the literature and other exercises were the creation of the principal author based on the categories of movement that research papers outlined.
Participants were allowed to decide to use the DVD on the same day before the weight training program or on a separate day. This was done to consider time constraints of the participants and ensure adherence. With regards to timing of the fascial training program and results acquired, this type of intervention would not keep the fascial system dehydrated for long enough (tissue too dry) or make the tissue change it’s elasticity for long enough (tissue too lax) before executing the weight training regimen. The warm up protocol, mentioned below, was in place to have both groups starting in the same manner for the weighted portion of the study.
Both groups were instructed to warm-up on the treadmill or stationary bike for 10 minutes before doing any strength training. Participants were asked not to perform any extended cardiovascular exercise (i.e., more than the 10 minute warm-up) before the strength training program so as to preserve energy stores for heavy lifting. Both groups could not engage in any other exercise program and had to follow only the exercise instructions in the program guidebook.
The initial evaluation determined the starting point for all participants’ weight training programs as well as instructions given to operate the machines. Participants were then instructed to record all exercises, sets, and repetitions in their logbook as well as any observations as to performance, mood, energy level, and general comments about exercise. With regards to weight training progression, participants were told that once they reached three sets of 12 repetitions for an exercise, the weight must be increased in 5 pound increments. Proper weight increase could be verified by the weight training program standards set forth as follows. For each exercise set, the weight should be such that a minimum of 8 repetitions could be reached but no more than 12 could be performed. This was in place for safety and uniformity reasons. For the safety of these novice weight training participants, the first two weeks comprised of the same weight training exercises but an endurance protocol of 15–18 repetitions was set.
Participants were contacted every week to check for compliance and discuss any concerns, answer questions, and provide encouragement. The investigator also made individual appointments to meet in the gym to answer any questions related to performance on the weight training machines or to assist with DVD exercise instruction.
At the end of the 9th week, participants were contacted to make an appointment for the final evaluation that comprised of another 1RM test after completing the 10-week program. At this time, log sheets were turned into the investigator and participants were instructed on how to proceed with weight training for the next four weeks. It was suggested that returning to an endurance program (such as what was done for weeks one and two) would be beneficial.
Statistical Analysis
A 2 × 2 repeated measures analysis of variance was used to compare the results between the two groups for both pre- and post-test strength variables. Descriptive statistics were produced for body weight and the weight lifted. The dependent variables were strength gains in chest press, leg press and leg curl. The independent variable was the type of intervention (either the treatment group or the control group). An a priori probability to determine significant differences was set at p < .05. All analysis were completed using SPSS version 23 statistical package.
RESULTS
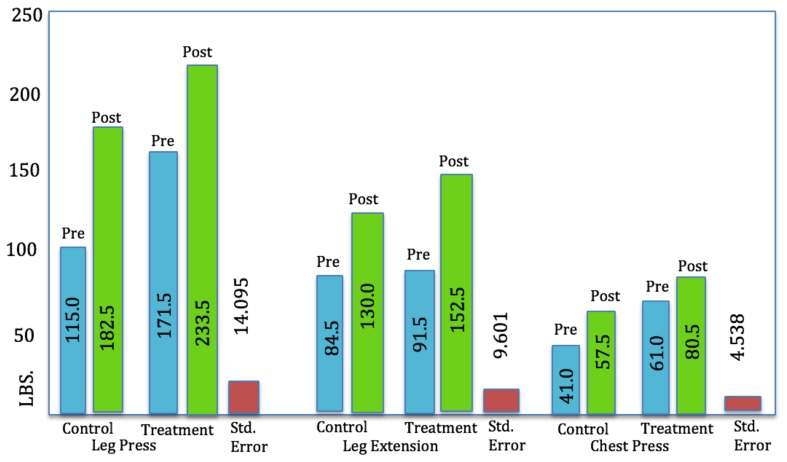
The means and standard deviations for the various analyses are presented in Table 3. The treatment group lost an average of 3 pounds more in body weight when compared to the control group. In terms of the weight training, the treatment group was able to lift an average of 15 more pounds post-test for the leg extension and an average of 3 more pounds for the chest press. The control group lifted an average of 6 more pounds for the leg press (Table 3.)
Table 3.
Descriptive statistics for body weight and weight lifted.
| Variable | Treatment | Control | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre test | Post Test | Pre Test | Post Test | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body Weight | 186.10 | 31.203 | 179.40 | 29.796 | 175.40 | 42.518 | 172.00 | 39.936 |
| Leg Press* | 171.50 | 51.804 | 233.50 | 55.580 | 115.00 | 23.094 | 182.50 | 40.500 |
| Leg Exten.* | 91.50 | 26.358 | 152.50 | 41.113 | 84.50 | 27.432 | 130.00 | 23.452 |
| Chest Press* | 61.00 | 15.951 | 80.50 | 18.174 | 41.00 | 5.164 | 57.50 | 14.577 |
Note:
p < .05 significance
The omnibus repeated measures analysis found no significant differences in strength gains over time between the treatment and control groups (F = 0.364, p > 0.05) A post hoc analysis of differences between specific dependent variables was completed (Table 4).
Table 4.
Post hoc analyses of specific dependent measures.
| Variable | Trial | Group | Trial * Group | |||
|---|---|---|---|---|---|---|
| Mean Square | F | Mean Square | F | Mean Square | F | |
| Body Weight | 255.025 | .194 | 819.025 | .622 | 27.225 | .021 |
| Leg Press | *41925.625 | 21.104 | *28890.625 | 14.543 | 75.625 | .038 |
| Leg Exten. | *28355.625 | 30.759 | 2175.625 | 2.360 | 600.625 | .652 |
| Chest Press | *3240.000 | 15.730 | *4622.500 | 22.442 | 22.500 | .109 |
Note:
p < .05 significance
Although there were significant differences between the dependent variables across time for individuals in both groups and also significant differences between the groups initially, there were no significant differences between the interactions of groups over time. When examining only the time component, the difference between trials (pre and post) regardless of group, had statistically significant differences between pre and post trials (p < .0005) for the weighted exercises (i.e., leg press, leg extension, chest press). This suggests that the participants had significant gains in strength variables regardless of the protocol. When examining the group differences, the difference between the treatment and control groups, regardless of pre and post, there were statistically significant differences for the leg press (p = .001) and the chest press (p < .0005). Body weight and leg extension changes were not significantly different. The lack of significance with regards to the leg extension may have something to do with the type of exercise it is compared to the leg press and chest press. The leg extension is a single joint exercise, and the leg press and chest press are multi-joint exercises. When discussing the fascial system, there is a global tensional network that should be considered. More global tension called upon for movement (the larger, multijoint movements), may have more of an effect on muscle function. The Trial*Group interaction (difference between groups over time periods) revealed no statistical significance at p = 0.05 (Figure 1).
Figure 1.
Pre- and post-test, means for strength gains over time.
DISCUSSION
A critical analysis of the literature suggests that specific types of movements address fascial tissue by providing the correct physiological strain that activates a response in the properties (e.g., viscoelastic, contractile, sensory) of the fascial system. While no movement or exercise regimen is absent of facial system participation, these movements are designed to provide a specific tensional strain (rather than compression) through variance of speed/direction and globally focused work needed to stimulate and renew the architecture of this fibrous collagenous soft connective tissue based on these properties (30). The amount, duration, and speed of the load allows fascia to exhibit a potential for both elastic and plastic deformation in a non-linear fashion (15, 39). Fascial viscoelasticity is dependent upon the integration of the architecture, composition, and water content of connective tissues (10). Tensional changes in connective tissue signal an immediate reorganization of the fibroblast cytoskeleton that causes a change in tissue stiffness and viscosity. It does this through cell signaling, gene expression, matrix adhesions that modify the connective tissue tension, and biochemistry (23). These studies explain the basis for the principles of fascial training and the movements chosen to represent those principles. The movement categories found in the current research protocol for fascial training address the relationship of the muscle-tendon complex by allowing muscles to contract isometrically (in most exercises on the DVD) and allow the focus to be on the tendinous tissue as the converging area of a global fascial system. This concept is based on studies (7, 8) that illustrate that in movements such as walking and jumping, muscle fiber contracts at a nearly constant length while the tendon performs a stretch-shorten cycle. This assists with efficient force generation.
The fascial system contains several mechanoreceptors (intrafascial receptors) that are sensitive to strong stretches, sustained pressure, and tangential forces (lateral stretch) as incorporated in the present study. Fascia also contains an abundant amount of interstitial myofascial tissue receptors that function as mechanoreceptors that respond to tension and/or pressure. The amount of intramuscular connective tissue (fascia within a muscle) and its morphological distribution has a high degree of variation between muscles with differing functions (27). These variations could be addressed in a focused and multimodal connective tissue training program such as the one in the current study. It must also be mentioned that since these movements are using only body weight and gravity in limited forms, muscle tissue can only be affected to a certain extent.
The present study suggested participating in a combination of movements (e.g., the treatment group’s DVD) to address the properties and function of fascia as a stand-alone program to train and condition the fascial system. The intention was not to be a specific warm-up or cool down for the strength training activities required in the study.
It was hypothesized that a 10-week fascial training program (e.g., treatment group) would make more significant increases in strength gains over that of the control group. The hypothesis was based on the premise that a better functioning (e.g., increased elasticity) fascial system would produce a significant improvement in strength in the treatment group. The main purpose of the treatment group’s fascial training program was to renew the fascial system by affecting the fibroblast and encouraging collagen turnover. A complete fascial training program, such as in the current study, contains various categories of movements in varying directions with varying degrees of intensity, duration, and speed to affect the fibroblast and create a renewed fiber architecture and increased elastic storage capacity (16, 30).
The basic premise is that connective tissue is highly adaptable and when regularly put under increasing or steady strain, the tissue architecture is better equipped to meet new demands because the fibroblasts adjust their matrix remodeling activity. It was thought that the treatment group’s program would help to increase the elasticity in the fascial components of the muscle to thus increase the amount of stored energy and result in greater strength gains. This reasoning can be substantiated by the relationship of muscle and fascia, and in this case, the fascia as intermuscular, intramuscular, and epimysial fasciae working neurologically and biomechanically together (38).
The result in the current study revealed that there were no significant differences in strength gains between the control and treatment groups. There were significant differences between trials (pre and post) across time regardless of the group. This result may be explained by research that shows that with three to five weeks of resistance training in novice participants, considerable improvements in strength occurred due to improved neural function but not necessarily increases in muscle mass (26, 13). In the early stages of training, it was found that resistance training produced a 92% improvement in maximal strength but only a 23% increase in muscle cross-sectional area. Improvements in neural drive and recruitment patterns are a large part of these strength gains. A more recent review confirms this finding by Ikai and Fukinaga (5) but also adds that strength improvements at this stage can also include changes in the architecture of muscle fibers as well as the parallel and series elastic components (1). This change in the elastic component of the muscle (fascia) at this early stage could explain the control group’s ability to have similar results as the treatment group.
There are pathways of force transmission other than the traditional myotendinous junction, as force can also be transmitted by the entire surface circumference of myofibrils onto the extracellular matrix (12, 36). The intervention for the treatment group was thought to enhance this fascial communication to produce a significant result. While the treatment group may have experienced this conditioning from the fascial exercise program, the control group may have had similar results due to the architectural changes in the parallel elastic components (mentioned above) as that is what this updated view of force transmission is based on. Furthermore, epimuscular myofascial force transmission can cause substantial effects on muscle adaptation through mechanical interaction between epimuscular fascia and myofibers (14). Since both groups participated in a weight training program (mechanical loading), all participants had the opportunity to condition this aspect of the fascial system and thus produced no significant outcome between groups.
Another concept that may explain accepting the null hypothesis is the adaptation time for fascial tissue. It is possible to work with a minimal amount of strain, consistently, and experience a training effect in fascial tissue. However, this conditioning result is accumulated slowly (e.g., six months to two years) but is long lasting to produce improvements in strength and elasticity of the global fascial net (16, 30). This may explain why the treatment group did not show any significant strength gains over the control group since the study was only for 10 weeks. Furthermore, since the participants were novice weight trainers, the stimulus to the muscle and related fascial structures may have been enough to create such an equality between groups. In other words, the stimulus to the connective tissue in the control group through resistance training was enough to create a similar adaptation without the fascial exercises of the treatment group. This may be because exercise of various types and loads have an effect on skeletal muscle connective tissue through enhanced collagen synthesis. To explain, tendons experience an increase in cross-sectional area with resistance training (17, 24). A significant elevated collagen synthesis response can be produced from a single loading bout as well as consistent long-term loading. However, the amount of collagen that actually is deposited into the load-bearing structure of the tendon to increase tendon size or a change in mechanical function is not known (16, 21, 22). This may explain the remarkable strength gains for all participants. It would have been ideal to run this study for six to eight months, but that was much too long for a program with novice weight trainers as well as maintaining consistent participation that long to yield a valid result.
When discussing any point in human physiology, it is impossible to isolate muscle performance from the influence of elastic structures. Muscle speed, force, and power are influenced by the exchange of energy between these elastic structures and the muscle tissue as well as the external environment. The cross-bridge elastic behavior with the whole muscle and associated tendon impacts muscle energetics, mechanics, and neuromotor control (28). Fascia’s impact on ROM, altered sensory input, and increased tissue stiffness makes a good case for the material included in the treatment group’s program. However, it may be that the participants in the control group did not have any significant fascial restrictions and that the weight training program was enough stimulus to the fascial system to allow similar adaptations as the treatment group in both strength and ROM. While research indicates that a healthy fascial system could improve fluid dynamics, oxygen utilization, and waste removal to assist with muscle performance, the stimulus to the treatment group was not enough to create a significant outcome. When considering aspects of muscle physiology, earlier researchers (2) made a point that the proprioceptors in the muscle and its connective tissue may limit the development of muscular force output due to the effect of their protective mechanisms. The question could be raised that the treatment group’s program was refining the proprioceptive properties in the muscle too much to make significant strength gains over the control group. Other researchers (4) explain that many experts believe that resistance training can lead to a reduction in the sensitivity of these proprioceptors. This decreased sensitivity leads to greater force production because of muscle disinhibition. This highlights conflicting results in the relationship between connective tissue and muscle tissue as resistance training commences and continues. With regards to this study, it may be better suited to have participants engage in a fascial training program months prior to starting a heavy strength training program and not participate in each simultaneously. Once fascial conditioning has been established, a fascial training program, or part of a program, could be executed in regularly spaced intervals (e.g., once every 2–3 weeks) to maintain fascial conditioning while engaging in a heavy weight training program.
One main limitation in the current study was the length of time for the intervention. A longer study (e.g., 6+ months) may be more suited to investigate this slower adapting fascial system as research suggests. However, comparing fascia’s adaptations with strength gains, as in the current study, may be difficult as participants try to engage in such a rigorous program for a long length of time. For future studies, other parameters of human performance, such as muscular power or endurance may be more suitable to examine fascial tissue. Investigating these performance parameters may be better applied to a more experienced population, such as collegiate or professional athletes. Since the fascial system is also involved in the medical and therapeutic realms, future studies might want to investigate a fascial training regimen for various orthopedic conditions, diseases, and special populations.
REFERENCES
- 1.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534(Pt 2):613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caiozzo VJ, Perrine JJ, Edgerton VR. Training-induced alterations of the in vivo force-velocity relationship of human muscle. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:750–754. doi: 10.1152/jappl.1981.51.3.750. [DOI] [PubMed] [Google Scholar]
- 3.Findley T, Shalwala M. Fascia research congress evidence from the 100 year perspective of Andrew Taylor Still. J Bodyw Mov Ther. 2013;17(3):356–364. doi: 10.1016/j.jbmt.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Fleck SJ, Kraemer WJ. Designing resistance training programs. Champaign, IL: Human Kinetics Publishers Inc; 1987. [Google Scholar]
- 5.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Medicine. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 6.Fujimaki S, Machida M, Wakabayashi T, Asashima M, Takemasa T, Kuwabara T. Functional overload enhances satellite cell properties in skeletal muscle. Stem Cells Int. 2016;4:1–11. doi: 10.1155/2016/7619418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukashiro S, Hay DC, Nagano A. Biomechanical behavior of muscle-tendon complex during dynamic human movements. J Appl Biomech. 2006;22:131–147. doi: 10.1123/jab.22.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga T, Kawakami Y, Kubo K, Kanehisa H. Muscle and tendon interaction during human movements. Exerc Sport Sci Rev. 2002;30:106–110. doi: 10.1097/00003677-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Grinnell F. Fibroblast mechanics in three-dimensional collagen matrices. J Bodyw Mov Ther. 2008;12(3):191–193. doi: 10.1016/j.jbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilak F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology. 2000;37(1–2):27–44. [PubMed] [Google Scholar]
- 11.Holloszy John O, Coyle Edward F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(4):831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 12.Huijing PA. Muscle as a collagen fiber reinforced composite material: force transmission in muscle and whole limbs. J Biomech. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 13.Ikai M, Fukunaga T. A study on training effect on strength per unit cross-sectional area of muscle by means of ultrasonic measurement. Eur J Appl Physiol. 1970;28:173–180. doi: 10.1007/BF00696025. [DOI] [PubMed] [Google Scholar]
- 14.Jaspers RT, Yucesoy CA, Huijing PA. Roles of fascia in molecular biology of adaptation of muscle size Fascia: The Tensional Network of the Human Body. London: Churchill Livingstone; 2012. [Google Scholar]
- 15.Kirilova M. Time-dependent properties of human umbilical fascia. Connect Tissue Res. 2012;53(1):21–28. doi: 10.3109/03008207.2011.604452. [DOI] [PubMed] [Google Scholar]
- 16.Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19:500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 17.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 2007;191(2):111–121. doi: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- 18.Kram R, Dawson TJ. Energetics and bio mechanics of locomotion by red kangaroos (Macropus rufus) Comp Biochem Physiol. 1998;B120:41–49. doi: 10.1016/s0305-0491(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 19.Kumka M, Bonar J. Fascia: A morphological description and classification system based on a literature review. J Can Chiropr Assoc. 2012;56(3):179–191. [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong EH, Findley TW. Fascia – Current knowledge and future directions in physiatry: narrative review. J Rehabil Res Dev. 2014;51(6):875–884. doi: 10.1682/JRRD.2013.10.0220. [DOI] [PubMed] [Google Scholar]
- 21.Langberg H, Rosendal L, Kjær M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjær M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Phyisiol. 1999;521(Pt1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol. 2005;288(3):C747–C756. doi: 10.1152/ajpcell.00420.2004. [DOI] [PubMed] [Google Scholar]
- 24.Mackey AL, Heinemeier KM, Koskinen SOA, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res. 2008;49:165–168. doi: 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]
- 25.Meijer HJM, Rijkelijkhuizen JM, Huijing P. Effects of firing frequency on length-dependent myofascial force transmission between antagonistic and synergistic muscle groups. Eur J Appl Physiol. 2008;104:501–513. doi: 10.1007/s00421-008-0788-5. [DOI] [PubMed] [Google Scholar]
- 26.Moritani T, DeVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med Rehabil. 1979;58(3):115–130. [PubMed] [Google Scholar]
- 27.Purslow P. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Physiol. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- 28.Roberts TJ. Contribution of elastic tissues to the mechanics and energetics of muscle function during movement. J Exp Biol. 2016;219:266–275. doi: 10.1242/jeb.124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleip R. Fascial plasticity – A new neurobiological explanation: Part 1. J Bodyw Mov Ther. 2003;7(1):11–19. [Google Scholar]
- 30.Schleip R, Müller D. Training principles for fascial connective tissues: scientific foundation and suggested practical applications. J Bodyw Mov Ther. 2013;17(1):103–115. doi: 10.1016/j.jbmt.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Selye H, Heuser G. Fourth annual report on stress. Montreal: Acta, Inc; 1954. [Google Scholar]
- 32.Solomonow M. Neuromuscular manifestations of viscoelastic tissue degradation following high and low risk repetitive lumbar flexion. J Electromyogr Kinesiol. 2012;22:155–175. doi: 10.1016/j.jelekin.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Staubesand J, Baumbach KUK, Li Y. La structure find de l‘aponévrose jambiére. Phlebologie. 1997;50:105–113. [Google Scholar]
- 34.Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J. 2010;81:11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 35.Tozzi P. Selected fascial aspects of osteopathic practice. J Bodyw Mov Ther. 2012;16(4):503–519. doi: 10.1016/j.jbmt.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Trotter JA, Purslow PP. Functional morphology of the endomysium in series-fibered muscles. J Morphol. 1992;212:109–122. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- 37.Turrina A, Martinez-Gonzalez MA, Stecco C. The muscular force transmission system: role of the intramuscular connective tissue. J Bodyw Mov Ther. 2013;17(1):95–102. doi: 10.1016/j.jbmt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 38.van der Wal J. The architecture of the connective tissue in the musculoskeletal system: An often overlooked functional parameter as to proprioception in the locomotor apparatus. Int J Ther Massage Bodywork. 2009;2(4):9–23. doi: 10.3822/ijtmb.v2i4.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yahia LH, Pigeon P, DesRosiers EA. Viscoelastic properties of the human lumbodorsal fascia. J Biomed Eng. 1993;15(5):425–429. doi: 10.1016/0141-5425(93)90081-9. [DOI] [PubMed] [Google Scholar]