Abstract
Functional Fitness Training (FFT) programs are characterized by utilizing a high volume of training and using a variety of high intensity exercises. While FFT are growing in the number of practitioners and popularity, the relationship between physiological biomarkers and subjective scales in the specific context of FFT has not yet been evaluated in the literature. The purpose of the present study was to monitor the time-course response of cytokines (IL-10 and 1L-1β), immune variables (C-reactive protein -CRP and immunoglobulin A-IgA), hormonal milieu (cortisol-C, total testosterone-TT, free testosterone-FT and testosterone/cortisol-T/C ratio), creatine kinase-CK, muscle performance (countermovement jump height) and perceived well-being (WB) following a functional fitness competition. Nine amateur male athletes (age 27.1 ± 4.1 years; training experience 2.2 ± 1.3 years) completed five workouts over three consecutive days of FFT-competition. All variables were measured before, 24 h, 48 h, and 72 h following the last day of competition. The FFT-competition induced a decrease in IL10/IL1β ratio approximately 5% after 24h, 21% after 48h and 31% after 72h. Delta T/C ratio remained unchanged during the post-competition period. IgA displayed a significant increase 24h and 72h post FFT-competition. The WB status score was higher 72h after the FFT-competition as compared with pre-competition. The present findings suggest that FFT-competition induces transient changes in some inflammatory and hormonal biomarkers, and perceived well-being seems to be efficient to detect changes in muscle performance.
Keywords: Extreme conditioning programs, immune response, performance, well-being, high-intensity functional training
INTRODUCTION
Functional Fitness Training (FFT) programs, for example CrossFit®, Insanity®, Gym Jones®, and others are characterized by utilizing a high volume of training and using a variety of high intensity exercises. It is common to have a fixed time to perform the greatest quantity of repetitions as can be completed, or to perform a specific task in a minimal amount of time, with or without short rest between sets (5, 19). While FFT programs are growing in the number of practitioners and popularity, a debate has been established between what is observed in the scientific literature and anecdotal reports from athletes, coaches, and physicians about safety and benefits (3). With respect to FFT-competitions, scientific reports describing competition induced-stress are even smaller.
It has been demonstrated that a FFT session resulted in increased acute oxidative stress as compared with a traditional session of high-intensity treadmill running in males with 3 or more months of FFT experience (10). Tibana et al. (17) revealed that two consecutive days of FFT induced a high metabolic response, elicited significant increases in interleukin-6 (IL-6) and an increase in pro/anti-inflammatory cytokine balance without significant impairment in muscle power in the recovery period. Thus, FFT with either reduced recovery between sessions or reduced time within the competition could lead to physical and/or mental fatigue associated with lower well-being (WB) and accompanied by decreased athletic performance. The negatively influenced hormonal milieu may result in immunosuppression and increased risk of injury/illness (2). To our knowledge, the temporal relationship of changes in biomarkers and WB responses after three consecutive days of FFT-competition has not been investigated.
There is an evident importance of using methods to control the training stress of physiological variables, such as testosterone (T), cortisol (C), T/C ratio, immune biomarkers, creatine kinase (CK), and performance analysis (2). However, these measurements are expensive and not common in daily training or competition, while stress perception scales would be very practical and inexpensive. It has been proposed that psychological variables, such as the WB scale, can detect changes in fatigue, as well as muscle soreness, and can be a useful tool in monitoring training induced-stress (12). It is important to note that the relationship between such physiological biomarkers and subjective scales in the specific context of FFT-competition has not yet been evaluated in the literature. Thus, the aim of the present study was to monitor the time-course response of physiological, psychological and performance markers following a FFT-competition. The initial hypothesis was that the FFT-competition increases inflammatory response as well as metabolic stress, impairing the hormonal milieu, and decreasing muscle performance.
METHODS
Participants
Nine participants aged 27.1 ± 4.1 years with experience in FFT were recruited through advertisements. Participant characteristics are presented in Table 1. All participants were free of injury and known illness, were not using drugs to enhance performance, and had a minimum experience of six months with FFT. Moreover, they were interviewed by the researcher and reported to have previous experience in resistance and cardiovascular training before engaging in FFT-competition. Indirect maximal aerobic capacity (VO2max) (11) and strength (1RM), described in table 1, were assessed two weeks before competition. Participants were advised to refrain from ingesting alcohol for 24 h before all tests, avoid any exercise 48 h before competition days, and to maintain their normal daily diet during the study. All participants signed an informed consent document and the study was approved by the University Research Ethics Committee for Human Use (2.698.225; 7 June 2018) and conformed to the Helsinki Declaration on the use of human participants for research.
Table 1.
Anthropometric and performance measurements of the participants [mean (95% CI)].
| N = 9 | |
|---|---|
| Anthropometric variables | |
| Age, years | 27.1 (23.5 – 28.7) |
| FFT Experience, months | 28.9 (15.8 – 41.9) |
| Height, cm | 174.0 (170.3 – 177.6) |
| Weight, kg | 79.3 (72.9 – 85.6) |
| BMI, kg/m2 | 26.2 (24.5 – 27.8) |
| Body fat, % | 9.2 (7.0 – 11.4) |
| Performance variables | |
| VO2max, mL/kg/min | 50.8 (47.8 – 53.8) |
| Back squat, kg | 161.7 (142.6 – 180.6) |
| Front squat, kg | 139.6 (121.6 – 157.8) |
| Clean, kg | 122.4 (109.4 – 135.7) |
| Snatch, kg | 88.6 (72.6 – 104.4) |
Note: FFT, Functional Fitness Training
Protocol
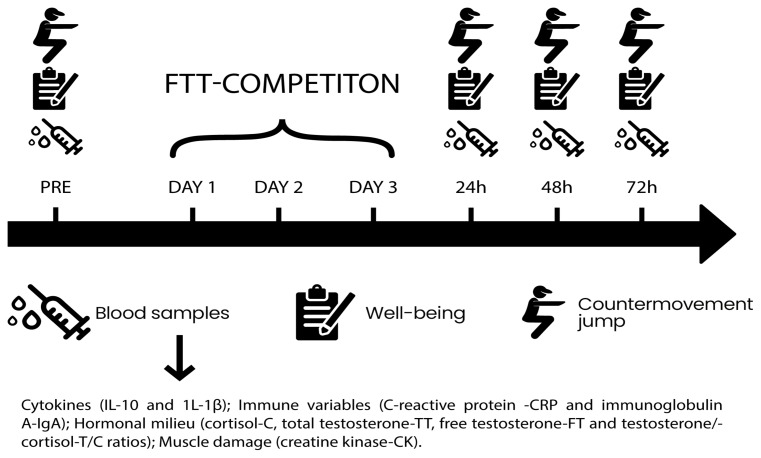
This study was designed to analyze the time-course effects of three days of FFT-competition on physiological, psychological and performance responses in trained adult men. In this study, the competition was the independent variable, while the dependent variables consisted of changes in interleukin-10 (IL-10), interleukin-1β (IL-1β), IL-10/IL-1β ratio, free and total testosterone, cortisol, T/C ratio, creatine kinase, immunoglobulin A, C-reactive protein, well-being and countermovement jump (Figure 1). The workouts of the FFT-competition are presented in Table 2.
Figure 1.
Protocol of the research and its relation to the moment of competition
Table 2.
Schematic representation of the competition.
| Workout 1 Day 1 7:00pm |
Workout 2 Day 2 02:00pm |
Workout 3 Day 2 7:00pm |
Workout Day 3 01:00 pm |
Workout 5 Day 3 5:30 pm |
|---|---|---|---|---|
|
Set individual for athlete Athlete A: Row 500 meters/30 strict handstand push-ups/15 Ring Muscle-ups. Athlete B: Row 1000 meters/24 strict handstand push-ups/12 Ring Muscle-ups. Athlete C: Row 1500 meters/18 Strict handstand push-ups/9 Ring Muscle-ups. |
A set for each athlete: 15 fat bar hang power cleans (40 kg); 20 meters overhead walking lunges (40 kg); 25 toes to bars; 10 fat bar shoulder to overhead (40 kg); 20 meters overhead walking lunges (40 kg). |
For time - All 3 Athletes 27 burpees box jump-over 21 legless rope-climb |
A set for each athlete with each weight. 15 meters handstand walk Squat snatches* 15 meters handstand walk |
For time - All 3 Athletes 30 calories of assault bike 20 Thrusters (50kg) 40 calories of assault bike 16 Thrusters (60kg) 50 calories of assault bike 12 Thrusters (70kg) 60 calories of assault bike 8 Thrusters (75kg) |
Note.
Snatch weights and reps by set: Set 1 – 6 reps (60kg); Set 2 – 4 reps (70kg); Set 3 - 2 reps (85kg)
Blood samples were collected at 24 hours before FFT-competition and 24, 48 and 72 hours after FFT-competition by venipuncture from the antecubital vein. The samples were collected in 5-milliliter evacuated tubes (Vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). After coagulation, the samples were centrifuged at 2.900 RPM, the resultant serum divided into several aliquots, and frozen at −80 °C until analysis.
Hormonal analyses were performed using commercial kits, c-reactive protein (CRP) lot 167404 [Roche], and Immunoglobulin IgA lot 196435 [Roche]; specific for humans in an automatic device (Cobas E601 - Roche) for both CRP and Immunoglobulin IgA by the electrochemiluminescence method. Hormone concentrations (total testosterone, free testosterone and cortisol) were analyzed using the E411 Roche (Roche, Inc., Chaska, MN, USA) through dioxetane-based chemiluminescent. The testosterone/cortisol ratio (TT/C) was calculated after obtaining values of total testosterone and cortisol.
Data were collected as previously described by Tibana et al. (17). Participants reported to the laboratory between 08:00 and 10:00 a.m., and blood samples (15 mL) were drawn from the antecubital vein into vacutainer tubes (Becton Dickinson, Brazil). Samples were then centrifuged at room temperature at 2500 rev*min−1 for 15 min. All participants were encouraged to avoid smoking, alcohol and caffeine consumption to avoid any influence on these parameters. Serum was removed and frozen at −80°C for further analysis. Serum was analyzed for amyloid A using a DADE Dimension RXL clinical chemistry analyzer (Dade-Behring, Inc, Newark, DE, USA). The analyzer was calibrated daily using Liquid-Assayed Multiqual (Bio-Rad, Hercules, CA, USA), and two levels of quality control with known concentrations. In addition, serum IL-10 (anti-inflammatory cytokine) and IL-1β (pro-inflammatory cytokine) were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BioLegend’s ELISA Max Deluxe, San Diego, CA, USA). Standard curves were generated using commercially available microplate reader-compatible statistical software (MicroWin 2000, Microtek Laborsysteme GmbH, Overath, Germany). All samples were determined in duplicate to guarantee precision of the results. For all measures, the mean intra-assay coefficient of variation was 3.0–9.0%, and the inter-assay coefficient of variation was <5.0%. The ratio between IL-10 and IL1β (IL-10/IL-1β ratio) was calculated in order to stablish a ratio between changes in anti-and pro-inflammatory cytokines. An increase in the IL-10/IL-1β ratio corresponds to a better anti-inflammatory status and a decrease in the ratio corresponds to a high pro-inflammatory status.
Data were collected as previously described in Twist et al. (20). Whole-blood creatine kinase activity was assessed from a single fingertip capillary sample with the subject in a seated position. After pre-warming the hand, a sample of blood (30 μL) was obtained and analyzed using a colorimetric assay procedure (Reflotron, Boehringer Mannheim, Germany). Before each test session, quality control (calibration) measurements were undertaken according to the manufacturer’s recommendations. The “normal” reference range for creatine kinase activity, as provided by the manufacturer, is 24–195 U/L.
The questionnaire assessed participants fatigue, sleep quality, general muscle soreness, stress levels and mood on a five-point scale. Overall well-being was then determined by summing the five scores as described elsewhere (12, 18).
Participants completed an assessment for vertical jump performance through a countermovement jump (CMJ). Flight time, calculated as the difference between landing and take-off time, was recorded using a wearable inertia sensor (PUSH™ band, PUSH Inc., Toronto, Canada). The CMJ was performed with hands held firmly on the hips and participants were instructed to jump as high as possible. The jump was performed at a self-selected countermovement depth and no instruction was given on what countermovement depth to use with flight time used to estimate the jump height (cm).
Statistical analysis
The data are expressed as means and 95% confidence interval (CI). Shapiro-Wilk tests were applied to check for normality distribution of the variables assessed. In cases of non-normal distribution, the variables were log transformed to base before analysis and this improved approximation to a normal distribution. Delta values were calculated using the difference between a specific time point and baseline value (ex.: delta 24h = 24h – baseline value) and were used to represent the magnitude of the change from baseline. Repeated measures ANOVA was used to compare hormonal, inflammatory, muscle damage and WB status between baseline values and after FFT-competition at different time points. Tukey’s post-hoc test was applied in the event of significance. Correlation between WB and physiological or performance variables were evaluated using Pearson product moment coefficients. Based on an alpha error of 0.05 and a power (1 – β) of 0.80, the sample effect size f was 0.82 for CK, 0.52 for CMJ, 0.46 for WB, 0.82 for Immunoglobulin IgA, 0.51 for cortisol, 0.51 for free and total testosterone, 0.54 for CRP, 0.26 for IL-1 and 0.56 for IL-10. The level of significance was p ≤ 0.05 and SPSS version 20.0 (Somers, NY, USA) software was used.
RESULTS
Hormonal responses pre- and 24h, 48h and 72h post-FFT-competition are presented in table 3. IgA had a statistically significant increase (p < 0.05) after 24h and 72h post-FFT-competition. Cortisol, free testosterone and total testosterone had a statistically significant decrease (p < 0.05) after 48h of post-FFT-competition.
Table 3.
Hormonal responses pre- and post-functional fitness competition [mean (95% CI)]
| Pre | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| IgA (mg/dL) | 251.9 (178.9 – 324.8) | 256.4* (180.4 – 332.5) | 249.4 (166.8 – 332.1) | 258.0* (181.8 – 334.1) |
| Cortisol (ug/dL) | 13.4 (9.2 – 17.6) | 9.1 (7.3 – 11.0) | 7.7* (5.8 – 9.6) | 10.2 (8.7 – 11.8) |
| Free testosterone (pg/mL) | 28.8 (14.9 – 43.5) | 26.5 (15.2 – 37.8) | 22.8* (14.3 – 31.4) | 27.7 (16.3 – 39.1) |
| Total testosterone (ng/mL) | 7.1 (3.5 – 10.7) | 6.5 (3.7 – 9.3) | 5.6* (3.5 – 7.7) | 6.8 (4.0 – 9.6) |
| T/C ratio | 0.72 (0.16 – 1.28) | 0.78 (0.30 – 1.25) | 0.79 (0.42 – 1.16) | 0.71 (0.35 – 1.07) |
Note: IgA, immunoglobulin A; T, testosterone; C, cortisol;
Significantly different from Pre (p ≤ 0.05).
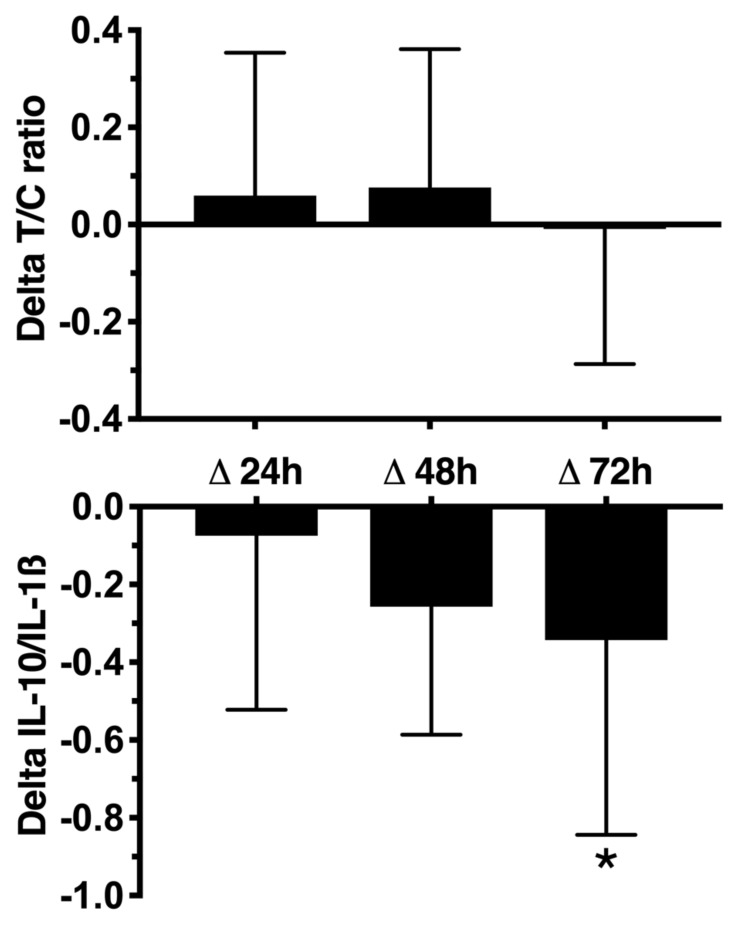
No statistically significant differences (p > 0.05) were observed in the absolute values of inflammatory responses after the FFT-competition (table 4). However, the IL10/IL1β ratio presented a statistically significant decrease (p < 0.05) after 72h. IL10/IL1β ratio decreased approximately 5% (p = 0.41) after 24h, 21%(p = 0.11) after 48h and 31% (p = 0.02) after 72h. Delta T/C ratio remained stable during the post-competition period, however, delta IL10/IL1β ratio was significantly lower 72h than 24h after competition (figure 2).
Table 4.
Inflammatory responses pre- and post-functional fitness competition [mean (95% CI)]
| Pre | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| CRP (mg/dL) | 0.08 (0.01 – 0.15) | 0.16 (−0.03 – 0.35) | 0.18 (−0.04 – 0.40) | 0.18 (0.01 – 0.36) |
| IL1β (pg/mL) | 2.91 (2.52 – 3.31) | 2.74 (2.53 – 2.95) | 2.76 (2.61 – 2.91) | 2.87 (2.68 – 3.09) |
| IL10 ( pg/mL) | 4.26 (2.74 – 5.78) | 3.89 (2.91 – 4.87) | 3.40 (2.32 – 4.48) | 3.27 (2.61 – 3.93) |
| IL10/IL1β ratio | 1.50 (0.92 – 2.08) | 1.42 (1.06 – 1.78) | 1.24 (0.84 – 1.64) | 1.16* (0.88 – 1.43) |
Note. CRP: C-reactive protein; IL1β: interleukin 1-beta; IL10: interleukin 10;
Significantly different from Pre (p ≤ 0.05).
Figure 2.
Delta Testosterone (T)/cortisol (C) ratio and delta interleukin 10 (IL10)/interleukin 1-beta (IL-1β) ratio pre- and post-functional fitness competition. *p ≤ 0.05 for Δ24h.
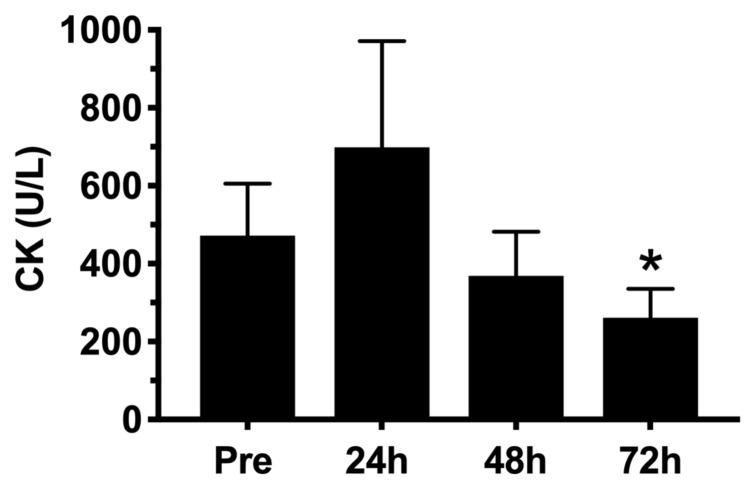
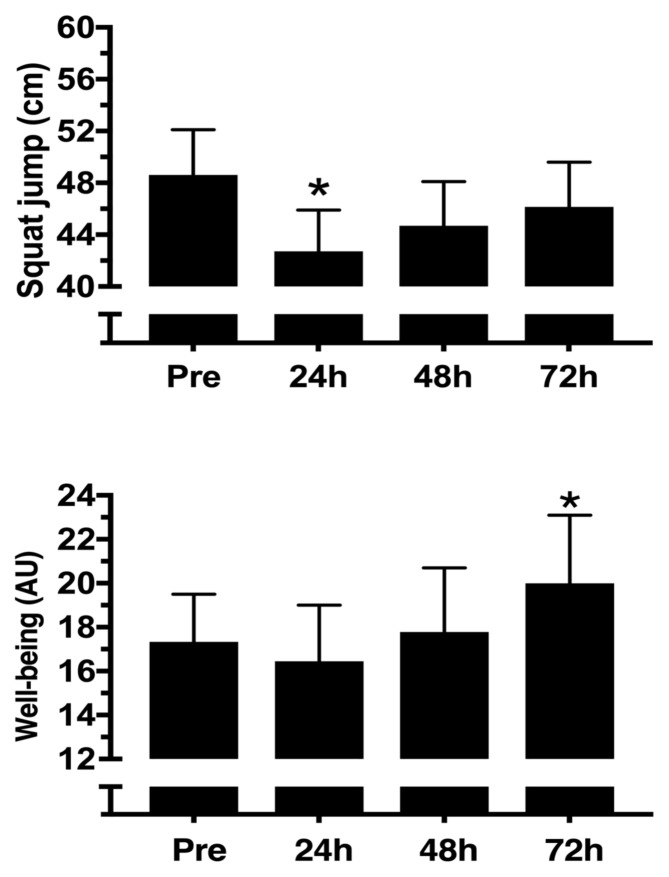
Despite jump performance presenting a statistically significant decrease (p < 0.05) 24h after the competition [42.7 (39.6 – 45.9) cm post-24h versus 48.6 (45.1 – 52.1) cm pre-competition], post-24h plasma CK had no difference from pre-competition [698.7 (495.4 – 971.9) U/L post-24h versus 472.0 (338.8 – 605.2) U/L pre-competition]. Post-exercise jump performance at 48h and 72h had no statistical difference from baseline [48.6 (45.1 – 52.1) cm]. Plasma CK had a statistically significant decrease (p < 0.05) 72h after the competition [261.2 (186.5 – 336.0) U/L] when compared to pre-competition (figures 3 and 4).
Figure 3.
Serum creatine kinase (CK) pre- and post-functional fitness competition [mean (95% CI)]. *Significantly different from Pre (p ≤ 0.05).
Figure 4.
Squat jump performance and well-being status pre- and post-functional fitness competition [mean (95% CI)]. *Significantly different from Pre (p ≤ 0.05).
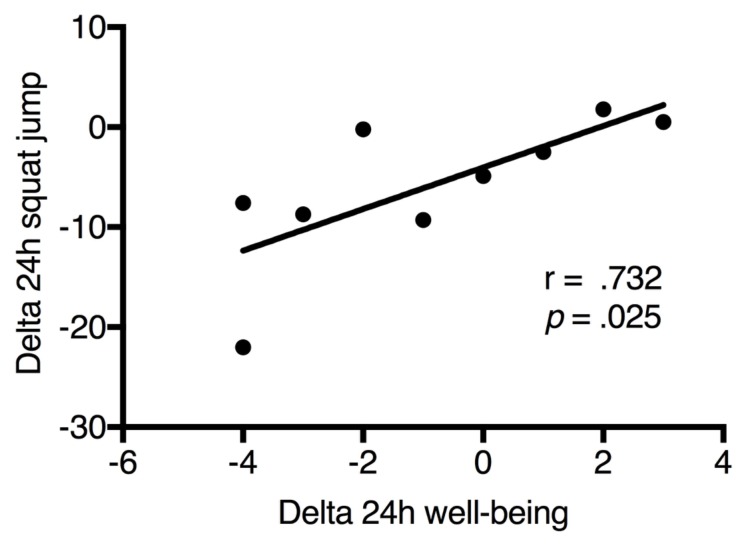
After 24h and 48h following the FFT-competition, WB status had no statistically significant differences (p > 0.05) from pre-competition. However, the WB status score was significantly higher after 72h of completion when compared to the pre-competition values (figure 4). Absolute values of WB did not present any statistically significant correlations (p > 0.05) with the absolute values of physiological or performance variables. However, delta changes in the first 24h of WB showed a statistically significant correlation (r = 0.732, p = 0.025) with delta changes in the first 24h of squat jump performance (figure 5).
Figure 5.
Correlation between the changes in 24h of well-being status and changes in 24h of squat jump performance.
DISCUSSION
The aim of this study was to monitor the time-course response of physiological, psychological and performance markers following the FFT-competition. The main findings were: (a) three days of FFT-competition induced a transient disturbance in the metabolic, hormonal milieu, and muscle performance 72 h after the competition in trained individuals, as expected; (b) IL-10/IL-1β decreased 72 h (31%) following the FTT-competition as compared with baseline; (c) findings related to psychological variables (perceived WB) showed a lack of correlation between hormone concentrations, metabolic response and immune variables.
To the best of our knowledge, this is the first study to analyze the time-course response of biomarkers and psychological variables following a functional fitness competition. The results may be useful to future exercise prescription before and after a competition and to understand the biomarkers and psychological responses for preventing nonfunctional overreaching, through the reduction of training volume in the week before and after the competition. The main difficulties in comparing our results obtained after a FFT-competition in participants who participated in teams is that previously published studies were performed only with acute sessions that analyzed one (8, 10) or two days of training (17) during workouts that were performed individually. For example, Heavens et al. (8) showed that after an adapted protocol from functional fitness (which consisted of a descending pyramid scheme of back squat, bench press and deadlift, beginning with 10 repetitions of each, then 9, then 8 and so on until 1 repetition on the final set) the biomarkers related to muscle damage were significantly increased (IL-6 immediately post-exercise for men:~3 pg/mL; women:~3.5 pg/mL; CK in both men and women, the peak concentration was at +24 hours). However, in the present study men with more than 2 years of experience were analyzed, whereas Heavens et al. (8) used men and women without experience in functional fitness. This aspect (untrained status) can explain the exacerbated hormonal and biochemical response observed by Heavens et al. (8), as the most well-known factor that influences recovery from exercise-induced muscle damage is previous muscle damage. This phenomenon is known as the ‘repeated bout effect’ (9, 15).
In the study by Tibana et al. (17) it was found that two consecutive FTT sessions elicited significant changes in metabolic responses (blood lactate and glucose), and cytokine levels (IL-6, IL-10, and osteoprotegin) 48 h after the last session in trained males (16). Interestingly, IL-10 decreased 24 h and 48 h (~40%; 0.018) after the second session as compared with baseline. Similarly, in the present study, IL-10/IL-1β ratio displayed a decrease at 24 h (~5%), 48 h (~21%) and 72 h (~31%) following the FTT-competition as compared with baseline, thus demonstrating that the disruption in the balance of anti and pro-inflammatory cytokines remained for at least 3 days after the competition.
Regarding the hormonal response following a functional fitness competition, many studies have used the T/C ratio to represent the anabolic:catabolic balance during the training season or during the process of recovery after a competition, however results are conflicting. Coutts et al. (7) and Nunes et al. (14), demonstrated that changes in plasma cortisol and testosterone measures were not correlated with changes in training load or performance in rugby and basketball athletes, respectively. Similarly, Agostinho et al. (1) demonstrated that despite the existence of a large variation in internal training load during an entire annual training periodization, there was little change in resting salivary T/C ratio concentration in judo athletes. Nonetheless, a recent case study by Bazyler et al. (4) examined the time course of physiological (total testosterone, cortisol, TNF-α, IL-6) and performance changes (squat jumps and dynamic mid-thigh pulls) in a national level female weightlifter following three competition phases over 28-weeks of training. The authors demonstrated that despite the decrease in IL-6 and TNF-α coupled with increases in the T:C ratio in response to the large decreases in training volume-load following the national championship, this change does not adequately explain competition performance. In the present study, there was no correlation between hormone concentrations, metabolic response and immune variables with changes in performance (countermovement jump) which is in agreement with the previous articles and demonstrates that hormonal changes cannot have applicability in the short term.
On the other hand, as squat jump and dynamic mid-thigh pull performance changes corresponded with weightlifting performance at each competition, these tests appear to provide a better indication of competition preparedness than serum markers of stress and inflammation. In the current investigation pre-FFT-competition CK values were above the recommend clinical values (~200 U/L), which may indicate excessive training in the days preceding the competition. Coaches should review their pre-FFT-competition training strategies to avoid fatigue and performance decrements during the competition. The application of a tapering period could be interesting in these situations (13) along with competition-specific workouts that are of a lower intensity/volume. Nevertheless, the clinical (but not statistically significant) increase in plasma CK activity 24 h after FFT-competition (~50%) can indicate muscle fiber damage or impairment in muscle architecture after a functional fitness competition. The peak CK value of 698.7 (495.4 – 971.9) U/L, measured 24 h after the FFT-competition, even with no differences from baseline, is comparable to CK activities reported by Wiechmann et al. (21) after mixed martial arts matches (829±753 U/L); Takarada (16) after competitive rugby matches (1081±159 U/L); and Heavens et al. (8) following an acute FFT session in male non-athletes (~700 U/L). Therefore, it can reasonably be assumed that muscle damage or impairment in muscle architecture following a three-day FFT-competition can be presented as in other sports. Although serum CK activity has been considered a popular measure of muscle damage due to both the simplicity of sample collection and very high variability of this measure, a poor temporal correlation with muscle recovery exists (8).
Interestingly, from a temporal perspective, although the 72-hour interval was sufficient to normalize the CK indexes, the time period was not sufficient to reestablish countermovement jump height performance after the competition. These data demonstrate that analyzing only biochemical and hormonal markers following training or competition may not demonstrate a real-world scenario for athletes.
With respect to non-invasive and non-exhaustive measures of assessing fitness and/or wellness (e.g. stress, fatigue), psychological monitoring is also purported to be effective and inexpensive in assessing individual responses to training (6, 12, 20). In support of this notion, previous studies have showed that the WB scale can detect changes in fatigue, muscle soreness and can be a useful tool in monitoring training induced-stress. McLean et al. (12) and Twist et al. (20) endorse the use of psychometric measurements that provide an effective and easy method for assessing fatigue in professional rugby league players. Buchheit et al. (6) found that training load, submaximal exercise heart rate and wellness measures, but not salivary C-reactive protein, can be used to monitor training-induced changes in recovery status and fatigue, as well as positive changes in both generic and sport specific running performance in Australian Football players. Our data showed a lack of correlation between hormone concentrations, metabolic response and immune variables with WB score. Even with no correlation, WB score was higher 72h following the competition as compared with the pre-competition, while IL-10/IL-1β ratio 72 h (−31%) following the FFT-competition was not normalized. This suggests a dissociation of hormonal changes after competition with the perception of WB among these athletes. However, WB score had a correlation with the squat jump, a performance marker. These findings are of importance in a practical setting, suggesting that coaches could use the WB scale because it is a very practical and inexpensive tool for monitoring fatigue after the FTT-competition.
Some limitations of the present study should be highlighted, such as the reduced number of participants, lack of diet control and the absence of female participants. Finally, taking into consideration that we analyzed biomarker responses before and after the FFT-competition in participants who were on teams, these responses would probably be different if the participants had competed individually. Therefore, further studies comparing individual vs team are needed to elucidate these outcomes. Nonetheless, future studies including muscle biopsies could improve the understanding of the muscle environment and not only selected systems, such as the immune or metabolic systems.
Conclusions
Three consecutive days of FFT-competition elicited a significant disturbance in metabolic, hormonal milieu and muscle performance following 72 h of the competition. This highlights the importance to expose practitioners to appropriate loads to enhance physical qualities which provide a protective effect against the disturbances elicited by the FFT-competitions, leading to greater physical outputs and resilience in competition. Furthermore, these data may be useful to coaches for monitoring fatigue and prescribing training (lower volume sessions and/or resting days) in days following a multi-day FFT-competition. Lastly, psychometric measurement tools seem to be an effective and easy method for assessing fatigue in participants after the competition.
ACKNOWLEDGEMENTS
The authors thank the athletes of the FFT-competition for their availability before, during and after competition. There were no funding sources.
REFERENCES
- 1.Agostinho MF, Moreira A, Julio UF, Marcolino GS, Antunes BM, Lira FS, Franchini E. Monitoring internal training load and salivary immune-endocrine responses during an annual judo training periodization. J Exerc Rehabil. 2017;13(1):68–75. doi: 10.12965/jer.1732850.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antualpa K, Aoki MS, Moreira A. Salivary steroids hormones, well-being, and physical performance during an intensification training period followed by a tapering period in youth rhythmic gymnasts. Physiol Behav. 2017;179:1–8. doi: 10.1016/j.physbeh.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Aune KT, Powers JM. Injuries in an extreme conditioning program. Sports Health. 2017;9(1):52–58. doi: 10.1177/1941738116674895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazyler CD, Mizuguchi S, Zourdos MC, Sato K, Kavanaugh AA, DeWeese BH, Breuel KF, Stone MH. Characteristics of a national level female weightlifter peaking for competition: A case study. J Strength Cond Res. 2017;32(11):3029–3038. doi: 10.1519/JSC.0000000000002379. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron MF, Nindl BC, Deuster PA, Baumgartner N, Kane SF, Kraemer WJ, Sexauer LR, Thompson WR, O’Connor FG. Consortium for health and military performance and american college of sports medicine consensus paper on extreme conditioning programs in military personnel. Curr Sports Med Rep. 2011;10(6):383–389. doi: 10.1249/JSR.0b013e318237bf8a. [DOI] [PubMed] [Google Scholar]
- 6.Buchheit M, Racinais S, Bilsborough JC, Bourdon PC, Voss SC, Hocking J, Cordy J, Mendez-Villanueva A, Coutts AJ. Monitoring fitness, fatigue and running performance during a pre-season training camp in elite football players. J Sci Med Sport. 2013;16(6):550–555. doi: 10.1016/j.jsams.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Coutts A, Reaburn P, Piva TJ, Murphy A. Changes in selected biochemical, muscular strength, power, and endurance measures during deliberate overreaching and tapering in rugby league players. Int J Sports Med. 2007;28(2):116–124. doi: 10.1055/s-2006-924145. [DOI] [PubMed] [Google Scholar]
- 8.Heavens KR, Szivak TK, Hooper DR, Dunn-Lewis C, Comstock BA, Flanagan SD, Looney DP, Kupchak BR, Maresh CM, Volek JS, Kraemer WJ. The effects of high intensity short rest resistance exercise on muscle damage markers in men and women. J Strength Cond Res. 2014;28(4):1041–1049. doi: 10.1097/JSC.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 9.Huang MJ, Nosaka K, Wang HS, Tseng KW, Chen HL, Chou TY, Chen TC. Damage protective effects conferred by low-intensity eccentric contractions on arm, leg and trunk muscles. Eur J Appl Physiol. 2019;119(5):1055–1064. doi: 10.1007/s00421-019-04095-9. [DOI] [PubMed] [Google Scholar]
- 10.Kliszczewicz B, Quindry CJ, Blessing LD, Oliver DG, Esco RM, Taylor JK. Acute exercise and oxidative stress: Crossfit(TM) vs. Treadmill bout. J Hum Kinet. 2015;47:81–90. doi: 10.1515/hukin-2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klusiewicz A, Borkowski L, Sitkowski D, Burkhard-Jagodzinska K, Szczepanska B, Ladyga M. Indirect methods of assessing maximal oxygen uptake in rowers: Practical implications for evaluating physical fitness in a training cycle. J Hum Kinet. 2016;50:187–194. doi: 10.1515/hukin-2015-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean BD, Coutts AJ, Kelly V, McGuigan MR, Cormack SJ. Neuromuscular, endocrine, and perceptual fatigue responses during different length between-match microcycles in professional rugby league players. Int J Sports Physiol Perform. 2010;5(3):367–383. doi: 10.1123/ijspp.5.3.367. [DOI] [PubMed] [Google Scholar]
- 13.Mujika I, Padilla S. Scientific bases for precompetition tapering strategies. Med Sci Sports Exerc. 2003;35(7):1182–1187. doi: 10.1249/01.MSS.0000074448.73931.11. [DOI] [PubMed] [Google Scholar]
- 14.Nunes JA, Moreira A, Crewther BT, Nosaka K, Viveiros L, Aoki MS. Monitoring training load, recovery-stress state, immune-endocrine responses, and physical performance in elite female basketball players during a periodized training program. J Strength Cond Res. 2014;28(10):2973–2980. doi: 10.1519/JSC.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 15.Peake JM, Neubauer O, Della Gatta PA, Nosaka K. Muscle damage and inflammation during recovery from exercise. J Appl Physiol. 1985;122(3):559–570. doi: 10.1152/japplphysiol.00971.2016. 2017. [DOI] [PubMed] [Google Scholar]
- 16.Takarada Y. Evaluation of muscle damage after a rugby match with special reference to tackle plays. Br J Sports Med. 2003;37(5):416–419. doi: 10.1136/bjsm.37.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibana RA, de Almeida LM, Frade de Sousa NM, da Nascimento DC, Neto IV, de Almeida JA, de Souza VC, de Lopes MF, de Nobrega OT, Vieira DC, Navalta JW, Prestes J. Two consecutive days of crossfit training affects pro and anti-inflammatory cytokines and osteoprotegerin without impairments in muscle power. Front Physiol. 2016;7:260. doi: 10.3389/fphys.2016.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibana RA, Sousa NMF, Prestes J, Feito Y, Ferreira CE, Voltarelli FA. Monitoring training load, well-being, heart rate variability, and competitive performance of a functional-fitness female athlete: A case study. Sports (Basel) 2019;7(2):35. doi: 10.3390/sports7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibana RA, Sousa NMFd. Are extreme conditioning programmes effective and safe? A narrative review of high-intensity functional training methods research paradigms and findings. BMJ Open Sport Exerc Med. 2018;4(1):e000435. doi: 10.1136/bmjsem-2018-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twist C, Waldron M, Highton J, Burt D, Daniels M. Neuromuscular, biochemical and perceptual post-match fatigue in professional rugby league forwards and backs. J Sports Sci. 2012;30(4):359–367. doi: 10.1080/02640414.2011.640707. [DOI] [PubMed] [Google Scholar]
- 21.Wiechmann GJ, Saygili E, Zilkens C, Krauspe R, Behringer M. Evaluation of muscle damage marker after mixed martial arts matches. Orthop Rev (Pavia) 2016;8(1):6209. doi: 10.4081/or.2016.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]