Abstract
Objective
To explore the timing of relapse following drug discontinuation and its relationship to estimated plasma levels and elimination half-life by comparing data from a randomized, placebo-controlled discontinuation study of cariprazine with those from similarly designed and conducted randomized control trials of other oral atypical antipsychotics (AAPs).
Methods
Data from a long-term, randomized, double-blind, placebo-controlled relapse prevention study in participants with schizophrenia (NCT01412060) were analyzed. Similarly designed, published studies of other AAPs were used for comparison. Time to drug-placebo relapse separation and relapse rates were estimated from Kaplan–Meier curves and evaluated descriptively. Separation was defined as a sustained difference of ≥5% incidence of relapse between the AAP and placebo curves.
Results
The Kaplan–Meier curve for cariprazine showed a time to drug-placebo relapse separation at 6–7 weeks after randomization, compared to the Kaplan–Meier curves for the other AAPs, which showed earlier separation at 1–4 weeks. The placebo relapse rates at 4 weeks after randomization were 5% for cariprazine and 8–34% for other AAPs. Geometric mean values of model-predicted plasma concentrations for total active cariprazine moieties (sum of cariprazine, desmethyl-cariprazine, and didesmethyl-cariprazine) were 20.0 and 6.1 nM at 2 and 4 weeks after discontinuation, respectively. Elimination half-lives of other AAPs and their active metabolites (<4 days) suggest that plasma concentrations would be low or negligible at 2–4 weeks after last dose.
Conclusion
Discontinuation of cariprazine treatment appeared to be associated with a delayed incidence of relapse compared with other AAPs, which may be due to the longer half-life of cariprazine and its active metabolites.
Keywords: cariprazine, schizophrenia, half-life, occupancy
Introduction
Schizophrenia is a severe, chronic, and heterogeneous disorder that has a profound impact on the health, social, and economic well-being of patients, families, caregivers, and society.1,2 Relapse prevention is critical for improving long-term outcomes in patients with schizophrenia.3 Randomized, placebo-controlled, double-blind trials have demonstrated that continued antipsychotic treatment was significantly more effective than drug discontinuation for relapse prevention (number needed to treat [NNT]=3–5).3 However, partial adherence and nonadherence are key threats to symptomatic stability and functional achievements,4 and these rates have been reported to be as high as 50%, which can limit the efficacy of pharmacotherapy.5,6 Factors contributing to partial adherence and nonadherence include patient knowledge, attitudes toward their illness and the medication, and past experiences with their illness and its treatment.7 Furthermore, the medical cost of partial adherence and nonadherence is estimated to be approximately 40% of health care spending for schizophrenia.8
Long-acting injectable antipsychotics (LAIs) have shown superiority over oral antipsychotics in preventing hospitalization in real-world mirror-image9 and cohort10 studies. Since pharmacologic characteristics of LAIs and oral antipsychotics are equivalent, it is believed that the superiority of LAIs in these studies is due to assured medication delivery, as nonadherence is apparent upon missing an injection, and LAIs have substantially longer half-lives compared with oral agents.11 Supporting the concept that the half-life of pharmacotherapy may be important in preventing relapse, a study comparing the effect of oral versus LAI formulations of paliperidone found that patients who withdrew from treatment remained relapse-free longer if they had been treated with an LAI versus oral formulation.12 Despite the potential advantages of LAIs, oral antipsychotics are considered to be the first-line treatment and are more commonly prescribed than LAIs. This may be due to negative attitudes toward LAIs (eg, side effects, costs) and clinicians primarily prescribing LAIs to patients with chronic schizophrenia and a history of partial adherence or nonadherence and/or multiple relapses.13,14 It has also been hypothesized that oral atypical antipsychotics with longer half-lives, in a manner similar to LAIs, may be able to bridge gaps in appropriate medication intake by providing prolonged drug exposure during periods of decreased adherence.15
Cariprazine, an oral dopamine D3-preferring D3/D2 receptor partial agonist, serotonin 5-HT1A receptor partial agonist, and 5-HT2A receptor antagonist,16 is approved for the treatment of adults with schizophrenia (US and Europe) and adults with manic, mixed, or depressive episodes of bipolar I disorder (US). Cariprazine forms two major metabolites, desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR), which are pharmacologically equipotent to and have relatively similar in vitro binding profiles compared with cariprazine.17,18 Half-lives based on time to reach steady state are 2–4 days for cariprazine, 1–2 days for DCAR, and 1–3 weeks for DDCAR; the effective half-life of total CAR (molar sum of cariprazine, DCAR, and DDCAR) is approximately 1 week.17 At steady state, the most prominent moiety is DDCAR, distantly followed by cariprazine and then DCAR.
In contrast to cariprazine and its active metabolites, the half-lives of all other oral antipsychotics are considerably shorter, in the range of 7–91 hrs (eg, aripiprazole, asenapine, brexpiprazole, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, ziprasidone);15 the half-lives of their active metabolites are on the same order of magnitude as the parent drug. An exception is risperidone, which has a half-life of 3 hrs, whereas its active metabolite, 9-OH risperidone (paliperidone), has an approximate half-life of 25 hrs,19 which is consistent with most other once-daily oral antipsychotics.
The overall objective of this post hoc, exploratory study was to investigate if there could be a connection between the timing of relapse following drug discontinuation and estimated plasma levels (and/or elimination half-life) by comparing data from a randomized, placebo-controlled discontinuation study of cariprazine with those from similarly designed and conducted randomized, controlled trials of other oral atypical antipsychotics.
Methods
Cariprazine relapse prevention study design
Data from a long-term, randomized, double-blind, fixed-dose, placebo-controlled relapse prevention study in participants with schizophrenia (NCT01412060) were analyzed; detailed methods for this study are published elsewhere.20 Briefly, participants stabilized with cariprazine during a 20-week open-label phase were randomized (1:1) to continue cariprazine (3, 6, or 9 mg/d) or switch to placebo for up to 72 weeks of double-blind treatment. In order to continue to double-blind treatment, participants were required to meet the following stabilization criteria: Positive and Negative Syndrome Scale (PANSS)21 total score ≤60; decrease from baseline ≥20% in PANSS total score; Clinical Global Impressions-Severity of Illness (CGI-S)22 score ≤4; score ≤4 on each of the 7 PANSS items (delusions, conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, hostility, uncooperativeness, and poor impulse control); and no significant tolerability issues (investigator judged). The primary outcome for the relapse prevention study was time to relapse during double-blind treatment. The P-value was based on the log-rank test; HR and 95% CI were based on Cox proportional hazards regression model, with treatment group as an explanatory variable.
Relapse was defined by objective rating scale criteria and subjective clinical measures. Participants were deemed as relapsed if they met any of the following rating scale criteria: increase ≥30% in PANSS total score for participants who scored ≥50 at randomization or increase ≥10 in PANSS total score for participants who scored <50 at randomization; score >4 on any of the 7 PANSS items (defined above); CGI-S score ≥2 point increase from randomization. Relapse was also judged to occur if, in the investigator’s opinion, the participant was hospitalized due to worsening of schizophrenia; exhibited deliberate self-injury or aggressive/violent behavior; or had clinically significant suicidal/homicidal ideation.
This study was conducted in compliance with the International Conference on Harmonisation Guidances on General Considerations for Clinical Trials and Good Clinical Practice and the Declaration of Helsinki. This study was also approved by institutional review boards or ethics committees and government agencies (Table S1). All participants provided written informed consent after receiving a complete description of the studies.
Table S1.
List of independent ethics committee and institutional review boards
| Country | Independent ethics committees/institutional review boards |
|---|---|
| USA | Copernicus Group |
| Sharp Healthcare IRB | |
| University of Texas - Houston Health Science Center Office of Research Support Committees |
|
| India | Ethics Committee, Shanti Nursing Home, Society for Psychiatric Update and Research |
| IMS - Independent Ethics Committee | |
| Ethical Committee Sheth VS Hospital | |
| Institutional Ethics Committee, Office of Research Cell, C.S.M. Medical University | |
| Vijayawada Institute of Mental Health and Neurosciences Hospital – Ethics Committee |
|
| Clinic Ethics Forum | |
| DMHC Ethics Committee | |
| Independent Ethics Committee, 57, Brahmin Mitra Mandal Society | |
| Kanpur Medical Ethics Committee | |
| Safe Search Independent Ethics Committee | |
| Yash Society Ethics Committee | |
| Ethics Committee, Radianz Healthcare and Research | |
| Central Ethics Committee, Nitte University | |
| Manipal University Ethics Committee, Manipal University | |
| The Ethics Committee, R.K. Yadav Memorial Mental Health and De-addiction Hospital | |
| Romania | Academia de Stiinte Medicale, Comisia Nationala de Bioteca a Medicamentului si a Dispozitivelor Medicale |
| Comisia Nationala de Etica pentru Studiul Clinic al Medicamentului | |
| Slovak Republic | Eticka komisia NsP sv.Barbory Roznava a.s, |
| Eticka komisia pri LNsP MUDr. Ivana Stodolu Liptovsky Mikulas, | |
| Eticka komisia Vseobecnej nemocnice Rimavska Sobota NaP | |
| Eticka komisia Fakultnej nemocnice Trnava | |
| Eticka komisia Univerzitnej nemocnice Ruzinov Bratislava | |
| Eticka komisia LF UK a Univerzitnej nemocnice | |
| Ukraine | Ethics Commission at Kyiv City Clinical Psychoneurological Hospital #1 |
| Ethics Commission of Kyiv City Psychoneurological Hospital #2 | |
| Ethics Commission of Communal Institution Lviv Regional Clinical Psychiatric Hospital | |
| The Ethics Commission of State Medical and Preventive Institution Central Clinical Hospital of Ukrzaliznytsia |
|
| Ethics Committee of the Kharkiv Regional Clinical Psychiatric Hospital #3 | |
| Ethics Commission at SE Institute of Neurology, Psychiatry and Narcology of NAMS of Ukraine | |
| Ethics Commission of Communal Institution Kherson Regional Psyciatric Hospital of Kherson Regional Council | |
| Ethics Commission at Communal Institution O.I. Yushchenko Vinnytsia Regional Psychoneurologic Hospital | |
| Ethics Commission of Crimea Republic Institution Psychoneurological Dispensary | |
| Ethics Commission of Communal Institution Dnipropetrovsk Clinical Psychiatric Dnipropetrovsk Regional Council | |
| Ethics Commission at Crimea Republic Institution Clinical Psychiatric Hospital #1 | |
| Ethics Commission at City Clinical Psychoneurological Hospital #1 | |
| Ethics Commission at Communal Therapeutic and Preventative Institution “Regional Clinical Psychiatric Hospital” | |
| Ethics Commission at Odessa Regional Medical Centre of Mental Health | |
| Ethics Committee of Regional Psycho-Neurological Hospital #3 |
Relapse prevention study designs for the other oral atypical antipsychotics
Data from published, similarly designed relapse prevention studies of other oral antipsychotics23–29 were used for comparison. In these studies, the primary outcome measure was time from randomization to relapse or impending relapse analyzed using standard statistical methods (eg, log-rank test); relapse and stability criteria were defined similarly to the cariprazine study (Table S2) and participants were required to meet stabilization criteria before proceeding to the next study phase (eg, stabilization to randomized). Across studies, length of stabilization phase ranged from 8 to 36 weeks, and double-blind, randomized phase was placebo-controlled and ranged from 16 to 52 weeks; however, due to the limited number of studies available, these factors could not be rigorously evaluated. Two placebo-controlled, discontinuation studies (aripiprazole and ziprasidone)30,31 were excluded from these analyses because the studies did not include a stabilization phase on the tested antipsychotic; rather, the enrolled participants had been stabilized on any antipsychotic and were then switched to either aripiprazole or ziprasidone, or to placebo.
Table S2.
Detailed stability criteria used in the relapse prevention studies
| Drug | Checkpoints | Stability criteria |
|---|---|---|
| CAR1 | Meet at week 8/20 | PANSS total score≤60; ≥20% decrease in PANSS total score from baseline; CGI-S≤4; score≤4 on each of the 7 PANSS items (delusion, conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, hostility, uncooperativeness, and poor impulse control); no significant tolerability issue (investigator judged) |
| BRX2 | 12 weeks continuous | Outpatient status; PANSS total score≤70; score of ≤4 on each of the 4 PANSS items (conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content); CGI-S≤4; no current suicidal behavior as assessed by C-SSRS; no violent or aggressive behavior resulting in injury or property damage |
| QTP XR3 | Meet at week 8/16 | On established dose; CGI-S≤4; PANSS total score≤60 at week 0 (enrollment; inclusion criteria), with no change ≥10 points in PANSS total score from week 0 to 8 and from 0 weeks to 0 (randomization baseline) |
| OLZ4 | 8 weeks continuous | Did not meet relapse criteria (an increase in any BPRS positive item to >4, and either an absolute increase of ≥2 on that specific item or an absolute increase of ≥4 on the BPRS positive subscale [conceptual disorganization, hallucinatory behavior, suspiciousness, unusual thought content]; hospitalization due to positive psychotic symptoms; a priori secondary definition of relapse was a completed suicide or a serious suicide attempt [as determined by the investigator]) |
| LUR5 | 12 weeks continuous | On a stable dose for 4 weeks prior to randomization; PANSS total score≤70, with PANSS item scores≤4 on all positive subscale items and “uncooperativeness” item, and a CGI-S<4. Note: two excursions (defined as 70<PANSS total score≤80 and/or a CGI-S=4 and/or PANSS positive subscale item=5) were permitted (post-stability attainment), except during the last 4 weeks of stabilization phase. |
| ASN6 | 26 weeks continuous | Did not experience loss of stability (PANSS total score increase of ≥20% increase in PANSS total score or 12 pts from baseline), or met any exclusion criteria (PANSS total score>75, CGI-S>3, score≥4 on items [unusual thought content, conceptual disorganization, hallucinatory behavior, hostility, or uncooperativeness]), or ISST scores of 2 on items 7, 10, or 11 |
| ILO7 | 12 weeks continuous | On stable dose for at least 4 weeks prior to randomization; CGI-S≤4, five individual PANSS positive symptoms (delusions, conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, hostility) and uncooperative item score≤4, PANSS total score ≤70, and not requiring any new acute treatment service intervention |
| PAL ER8 | Meet at week 7/8 and 9–14a | Stable dosage regimen; PANSS total score≤70; CGI-S≤4; scores≤4 for PANSS items for delusions, conceptual organization, hallucinatory behavior, suspiciousness/persecution, hostility, and uncooperativeness. |
Notes: aObtained from https://clinicaltrials.gov/ct2/show/NCT00086320.
Abbreviations: ASE, asenapine; BPRS, Brief Psychiatric Rating Scale; BRX, brexpiprazole; CAR, cariprazine; CGI-S, Clinical Global Impression Scale – Severity; C-SSRS, Columbia-Suicide Severity Rating Scale; ER or XR, extended release; ILO, iloperidone; ISST, InterSePT Scale for Suicidal Thinking; LUR, lurasidone; OLZ, olanzapine; PAL, paliperidone; PANSS, Positive and Negative Syndrome Scale; PBO, placebo; QTP, quetiapine.
Relapse analyses
Kaplan-Meier (K-M) curves from published relapse prevention studies of oral antipsychotics were used to estimate the time to curve separation between drug and placebo relapse rates. Separation was defined as a sustained difference of ≥5% incidence of relapse between drug and placebo curves; the 5% cutoff was selected because this threshold was roughly the point at which participants being treated with the drug generally had a significantly lower incidence of relapse compared with those being treated with placebo. A sensitivity analysis using a 10% cutoff, which corresponds to NNT≤10, was also performed to provide a threshold of established clinical relevance.32 In addition, a post hoc analysis of the cariprazine study was conducted to evaluate the time to curve separation in the subset of participants who were stabilized on 3–6 mg/d cariprazine (doses within the US Food and Drug Administration [FDA] and European Medicines Agency [EMA] recommended dose range).
Plasma concentrations of total CAR were obtained from participants who were randomized to placebo in the cariprazine study by using individual-level predictions derived from population pharmacokinetic models.33 Elimination half-lives of oral antipsychotics and their active metabolites, if available, were obtained from the prescribing information of each drug; these values were used to estimate plasma concentrations after drug discontinuation. The proportions of each active moiety at steady state (relative to total active moieties) were calculated using mean trough plasma or serum concentrations,34–38 but if this information was not available, then proportions were obtained from the literature or the drug’s prescribing information. Study characteristics such as stability criteria, length of open-label stabilization phase, and discontinuation protocol were evaluated descriptively for between-study differences.
Analyses compared the time to drug-placebo relapse separation of 5% and 10%; differences in time to drug-placebo relapse separation and placebo relapse rates at 4 weeks after randomization between each oral antipsychotic and their respective placebo group were compared across drugs descriptively. The time to drug-placebo relapse separation was also compared with the time at which PANSS total scores separated between drug and placebo (if information was published). The potential relationship between delayed relapse (eg, time to drug-placebo relapse separation and placebo relapse rates) and weighted elimination half-life was evaluated by visual inspection. Weighted elimination half-life is the summation of each drug and, if applicable, the active metabolite’s elimination half-life multiplied by its proportion at steady state relative to total active moieties; for ranges, the median was used. A sensitivity analysis was also performed using the longest reported elimination half-life or elimination half-life of the most prominent moiety at steady state of each drug. Statistical analysis could not be performed to evaluate between-study differences due to the limited number of available studies.
Results
This analysis included eight published placebo-controlled relapse prevention studies of oral atypical antipsychotics that had an open-label stabilization phase (8–36 weeks); a summary of these studies is presented in Table 1. All studies required participants to meet stability criteria prior to continuing to the randomized, double-blind phase (Table 1, Table S2). Five studies23,25–28 required participants to meet stability criteria for >8 continuous weeks; the other three studies20,24,29 required participants to meet stability criteria at prespecified time points. Only one study24 tapered discontinuation to placebo; all others were assumed to have used an abrupt discontinuation protocol.
Table 1.
Relapse prevention study characteristics
| Study | Stabilization | Stability criteriab | Number of DB patients |
DB length (weeks) | |
|---|---|---|---|---|---|
| Design | Length (weeks)a | ||||
| Asenapine27 | OL | 26 | 26 weeks continuous | ASE=194 PBO=192 |
26 |
| Brexpiprazole23 | SB | 12–40 | 12 weeks continuous | BRX=97 PBO=105 |
<52c |
| Cariprazine20 | OL | 20 | Meet at weeks 8 and 20 | CAR=101 PBO=99 |
26–72 |
| Iloperidone28 | OL | 15–25 | 12 weeks continuous | ILO=153 PBO=150 |
<26c |
| Lurasidone26 | OL | 12–24 | 12 weeks continuous | LUR=144 PBO=141 |
28 |
| Olanzapine25 | OL | 8–14 | 8 weeks continuous | OLZ=224 PBO=102 |
<30c |
| Paliperidone ER29 | OL | 14 | Meet at weeks 8 and 14e | PAL=105 PBO=102 |
16–40c |
| Quetiapine XR24,d | OL | 16 | Meet at weeks 8 and 16 | QTP=94 PBO=103 |
≤39c |
Notes: aLength includes cross-titration, run-in, and stabilization periods, if applicable, and ranges indicate that patients could move to randomization phase once stability criteria were met; bdetails on stability criteria are provided in Table S1; cvariable length is due to positive interim results and subsequent early study termination; dQTP XR was the only study that had a tapered discontinuation protocol (4-day cross titration); estability criteria obtained from https://clinicaltrials.gov/ct2/show/NCT00086320.
Abbreviations: ASE, asenapine; BRX, brexpiprazole; CAR, cariprazine; DB, double-blind; ER or XR, extended release; ILO, iloperidone; LUR, lurasidone; OL, open-label; OLZ, olanzapine; PAL, paliperidone; PBO, placebo; QTP, quetiapine; SB, single-blind.
Cariprazine treatment (3–9 mg/d) was associated with a significantly delayed time to relapse compared with placebo (P=0.0039, HR [95% CI]=0.52 [0.33, 0.82]).39 An analysis was also performed to evaluate the time to relapse in a subgroup of participants that received doses of cariprazine within the FDA- and EMA-recommended range (1.5–6 mg/d). Treatment with cariprazine doses within this range (ie, 3–6 mg/d) was also associated with a significantly delayed time to relapse compared with the corresponding placebo group (ie, participants that were stabilized on cariprazine 3–6 mg/d during the open-label phase; P=0.026, HR [95% CI]=0.49 [0.25, 0.93]). In all studies of other oral antipsychotics, treatment with drug compared with placebo was also associated with a significantly delayed time to relapse or impending relapse (P<0.05 for all).23–29
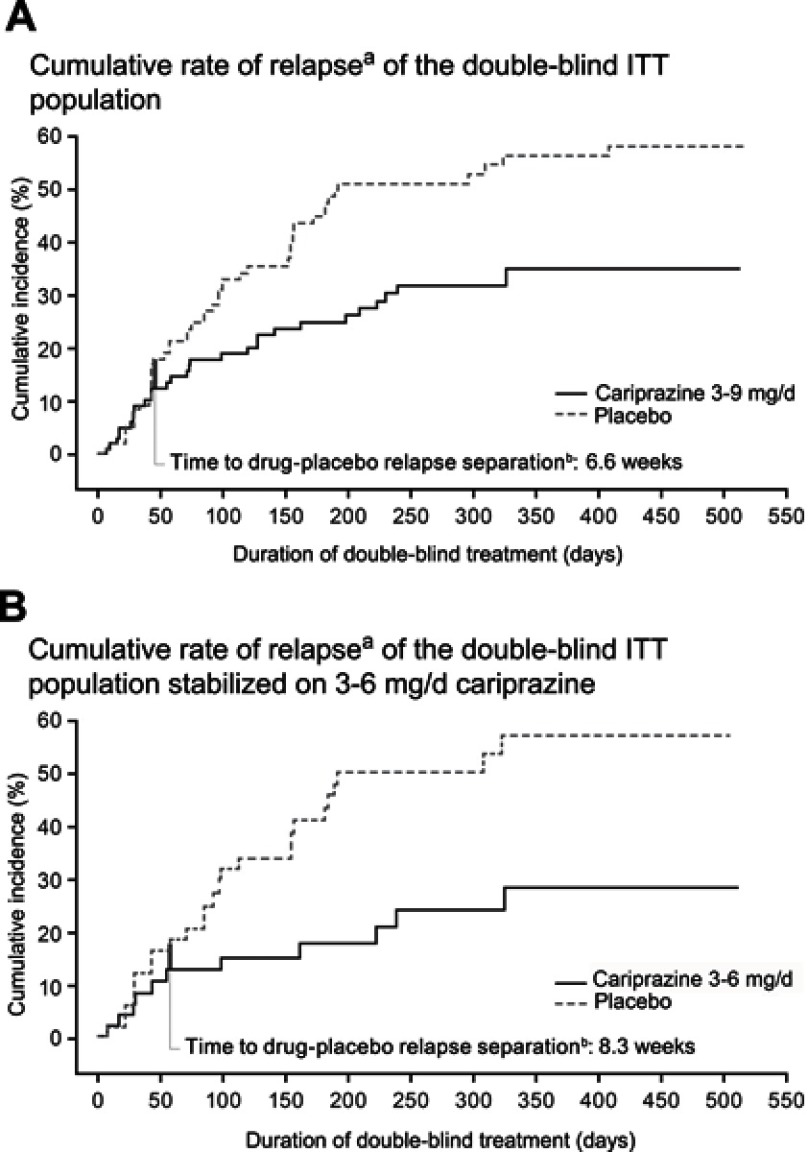
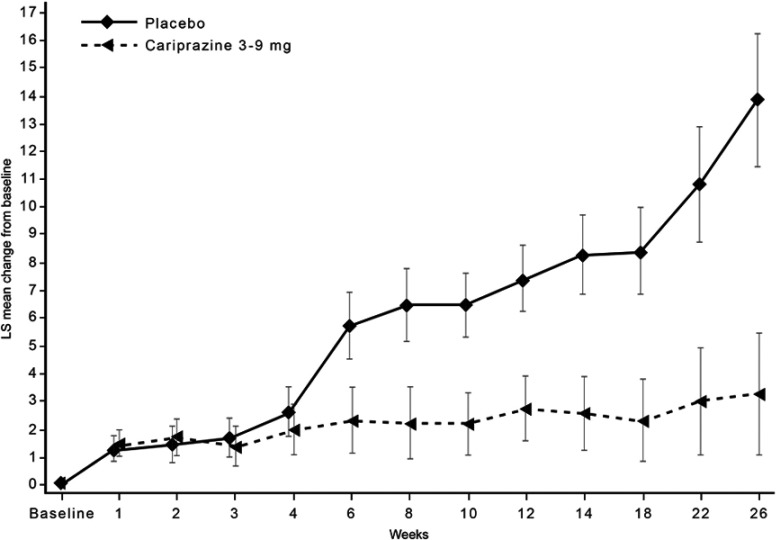
The K-M curve for cariprazine treatment (3–9 mg/d) showed a time to drug-placebo relapse separation at 6.6 weeks after randomization (Figure 1A); this correlated with time to separation of PANSS total score change between cariprazine and placebo (Figure S1). For participants stabilized in the recommended 3–6 mg/d dose range, the K-M curve showed that time to drug-placebo relapse separation occurred even later, at 8.3 weeks, when compared with its corresponding placebo group (Figure 1B).
Figure 1.
Cumulative rate of relapse.
Notes: aKaplan–Meier curves for time to relapse in the final intent-to-treat (ITT), randomized population during the double-blind period for cariprazine 3–9 mg/d versus the overall placebo group (A) and for cariprazine 3–6 mg/d versus its corresponding placebo group (B); bthick vertical bar represents 5% incidence of relapse and indicates the time to drug-placebo relapse separation. Panel A is adapted from © 2019 Allergan. Used with permission.
K-M curves from studies of other oral antipsychotics showed earlier separation between drug and placebo, ranging between 1 and 4 weeks (Table 2). Two of these studies published by-week PANSS total score data; in those studies, time to drug-placebo relapse separation also coincided with the between-group separation of PANSS total score change (lurasidone: ~3 weeks vs 4 weeks, respectively; paliperidone ER: 1.6 weeks vs 1 week, respectively).26,29
Table 2.
Placebo relapse rate, time to drug-placebo relapse separation, and elimination half-lives of drugs and major active metabolites
| Drug | PBO relapse at week 4 (%)a | Time to drug-PBO relapse separation (weeks)a | Elimination half-life of drug and major active metabolitesb | Proportionc at steady state of drugs and active metabolites | |
|---|---|---|---|---|---|
| 5% Separation | 10% Separation | ||||
| CAR | 5 | 6.6 | 12.0 | CAR: 2–4 days DCAR: 1–3 days DDCAR: 1–3 weeksd |
CAR: 23.3% DCAR: 7.2% DDCAR: 69.5%e |
| BRX | 8 | 4.0 | 6.1 | BRX: 91 hrs | No CNS active metabolites |
| QTP XRf | 13 | 2.7 | 3.4 | QTP: 7 hrs Norquetiapine:12 hrs |
QTP: 30.3–35.7% Norquetiapine: 64.3–69.7%g |
| OLZ | 15 | 2.6 | 4.1 | OLA: 30 hrs | No CNS active metabolites |
| LUR | 15 | 2.7 | 8.2 | LUR: 18 hrs ID-14283: 7.5–10 hrsh |
LUR: 70% ID-14283: 26%i |
| ASE | 18 | 1.1 | 2.4 | ASE: ~24 hrs | No CNS active metabolites |
| ILO | 24 | 1.1 | 3.3 | ILO: 18–33 hrs P95: 23–31 hrs P88: 26–37 hrs |
ILO: 32.5% P95: 48% P88: 19.5%j |
| PAL ERf | 34 | 1.6 | 3.3 | PAL ER: 23 hrs | No CNS active metabolites |
Notes: aEstimated from final intent-to-treat population Kaplan–Meier curve; separation was defined as a sustained difference of ≥5% or ≥10% incidence of relapse between drug and placebo curves; belimination half-life values were obtained from the prescribing information of each drug unless otherwise specified; cproportion of drug and active metabolites is relative to total active moieties; delimination half-lives for cariprazine, DCAR, and DDCAR are based on time to steady-state values; eproportions calculated using model-predicted Cmin values at steady state from a population pharmacokinetic modeling study (A.P. and T.C., unpublished data, 2014); fplease note that QTP XR and PAL ER are sustained-release formulations; gproportions calculated using serum concentrations from Bakken et al (2011)35 and Bakken et al (2015)34 with the assumption that steady-state pharmacokinetic parameters are similar for the IR and XR formulations as reported by Bui et al (2013);36 helimination half-life for ID-14,283 was obtained from Citrome (2011);37 iproportions are from Meyer et al (2009)38 and based on mean serum concentrations; jproportions at steady state are from extensive metabolizers in the prescribing information for ILO.
Abbreviations: ASE, asenapine; BRX, brexpiprazole; CAR, cariprazine; CNS, central nervous system; DCAR, desmethyl-cariprazine; DDCAR, didesmethyl-cariprazine; ER or XR, extended release; ILO, iloperidone; LUR, lurasidone; OLZ, olanzapine; PAL, paliperidone; PBO, placebo; QTP, quetiapine.
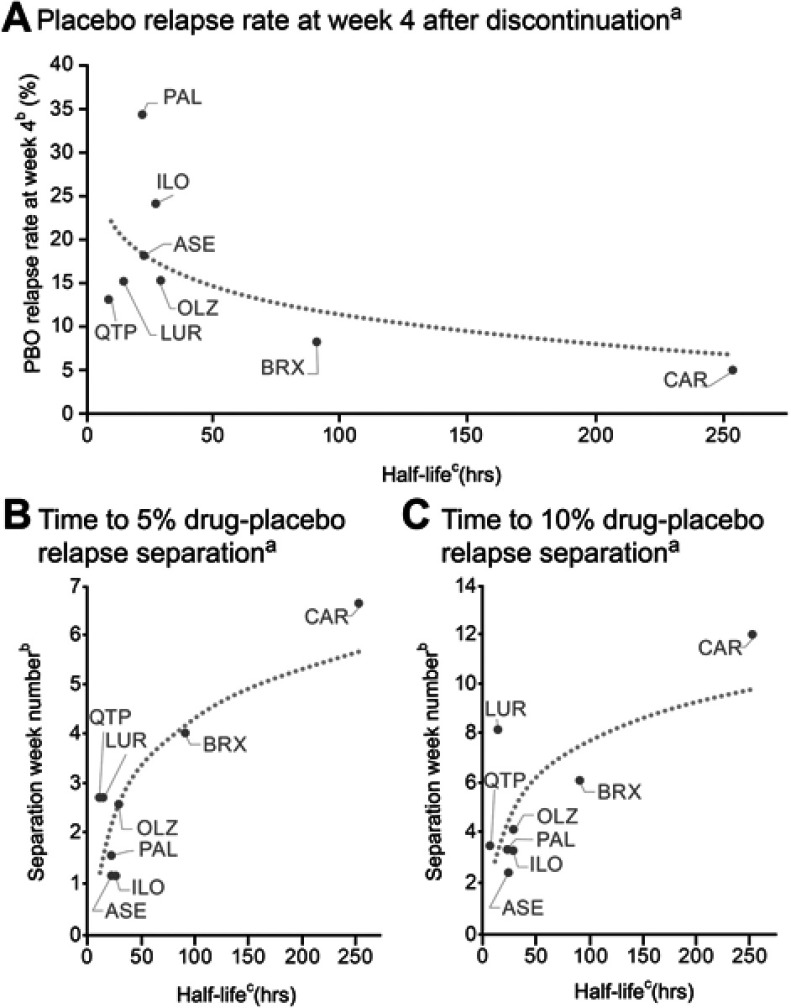
In the cariprazine study, overall placebo relapse rate at week 4 was estimated to be 5%; the estimated placebo relapse rates for the other oral antipsychotics were higher, ranging from 8% to 34% at 4 weeks after last dose (Table 2). Brexpiprazole, with the second longest half-life after cariprazine, had the second lowest placebo relapse rate and second longest time to drug-placebo relapse separation. The remainder of the oral antipsychotics all had half-lives of approximately 1 day or less; correspondingly, these compounds had the highest placebo relapse rates at week 4 and shortest time to drug-placebo relapse separation following treatment discontinuation.
Placebo relapse rate and time to drug-placebo relapse separation appeared to be correlated with elimination half-life (Table 2); a graphical representation plotting relapse outcomes versus weighted elimination half-life is shown in Figure 2. A similar trend was seen for time to drug-placebo separation with clinically relevant separation defined as ≥10% sustained difference (equivalent to an NNT=10) compared with separation defined as ≥5% sustained (Figure 2B-C; Table 2). Analyses were performed using the longest drug or metabolite elimination half-life, as well as using median elimination half-life of the most prominent moiety instead of weighted half-life; results were similar regardless of half-life calculation method (data not shown).
Figure 2.
Drug half-life versus placebo relapse rate (A) and separation from active drug (B-C).
Notes: aLogarithm regression fits were generated to provide a visual guide; bestimated from final intent-to-treat population Kaplan–Meier curve; cweighted half-life values based on elimination half-life and steady-state proportions of each drug (and its metabolites) were used (Table 2).
Abbreviations: ASE, asenapine; BRX, brexpiprazole; CAR, cariprazine; ILO, iloperidone; LUR, lurasidone; OLZ, olanzapine; PAL, paliperidone; PBO, placebo; QTP, quetiapine.
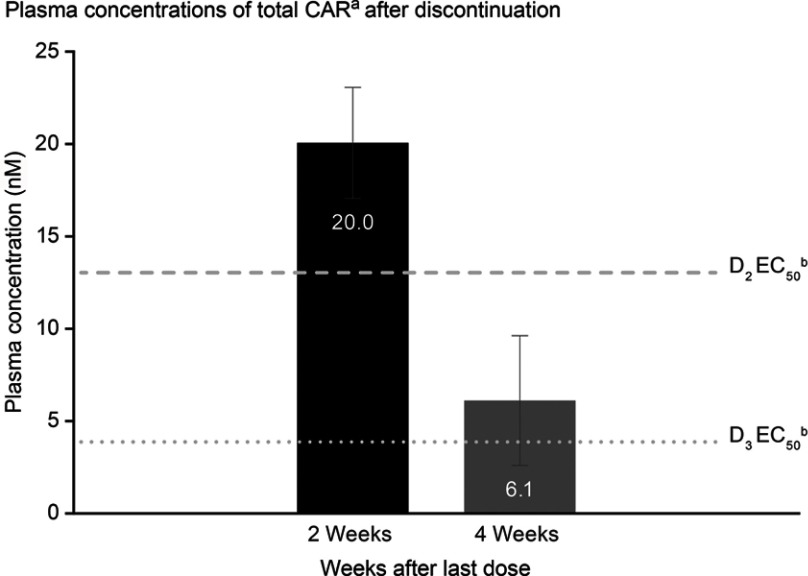
Geometric mean values of model-predicted plasma concentrations for total CAR were 20.0 and 6.1 nM at 2 and 4 weeks after last cariprazine dose, respectively (Figure 3). Based on published EC50 estimates for D2 and D3 receptor occupancy by total CAR, which are 13.0 and 3.8 nM, respectively,40 D2 and D3 receptors are predicted to remain occupied at 2 and 4 weeks after last cariprazine dose. Geometric mean values of model-predicted plasma concentrations for DDCAR, which is the most prominent moiety at steady state, were 18.5 and 5.5 nM at 2 and 4 weeks after last cariprazine dose, respectively. Elimination half-lives of other oral antipsychotics and their active metabolites (Table 2) suggest that plasma concentrations and D2 and D3 receptor occupancy for those compounds would be low or negligible at 2 and 4 weeks after last dose.
Figure 3.
Plasma concentrations of total CARa after discontinuation.
Notes: aGeometric mean values (geometric SD) of model-predicted total CAR (combined cariprazine, DCAR, and DDCAR) plasma concentrations in patients randomized to placebo during double-blind period; these predicted values are in line with observed data from a pharmacokinetic clinical study;17 bEC50 values for D2 and D3 receptor occupancy were obtained from Girgis et al (2016).40
Abbreviations: DCAR, desmethyl-cariprazine; DDCAR, didesmethyl-cariprazine; total CAR, combined cariprazine, DCAR, and DDCAR.
Discussion
This analysis examined the relationship between the trajectories of the incidence of relapse and the plasma concentrations of oral antipsychotics in participants with schizophrenia after drug discontinuation (eg, randomization to double-blind placebo). In all of the relapse prevention studies that were included in this post hoc, exploratory study, treatment with oral antipsychotics was significantly superior versus placebo at delaying schizophrenia relapse or impending relapse.20,23-29,39 This study found that there was a delayed incidence of schizophrenia relapse following discontinuation of cariprazine treatment relative to discontinuation of treatment with the other oral antipsychotics as reported in separate studies. The incidence of relapse at 4 weeks following discontinuation of cariprazine treatment (5%) was approximately 2–7 times lower than that observed following drug discontinuation in other relapse prevention studies (8–34%). In addition, time to separation between cariprazine and placebo for estimated relapse incidence was 6.6 weeks compared with 1–4 weeks in the other studies. For all oral antipsychotics, the lower placebo relapse rate at week 4 and the longer time to drug-placebo relapse separation were associated with a longer elimination half-life. Factors other than half-life, such as length of open-label stabilization phase and type of drug discontinuation protocol, were relatively consistent across studies. These factors do not appear to be differentiating factors for relapse rate trajectories in the placebo group or time to drug-placebo relapse separation following antipsychotic discontinuation; however, due to the limited number of studies available, a rigorous evaluation could not be performed. Although the primary analysis in the cariprazine study included some participants who received a cariprazine dose outside the recommended range (9 mg/d), time to drug-placebo relapse separation for the approved dose range of 3–6 mg/d was even longer compared with the 3–9 mg/d dose range, suggesting that the 9 mg/d dose was not a differentiating factor for the delayed time to drug-placebo relapse separation. Overall, relapse occurred in 49.5% of placebo- and 29.7% of cariprazine-treated participants.39 The relapse rates at the study endpoint on active medication in the cariprazine study are similar to what has been reported in a meta-analysis of relapse prevention studies in schizophrenia, which found that antipsychotics (27%) significantly reduced relapse rates at 1 year versus placebo (64%);3 thus, the overall relapse rate also does not appear to be a differentiating factor for the delayed relapse seen after the discontinuation of cariprazine.
The long half-life of cariprazine is primarily due to the presence of an active metabolite (DDCAR) with an extended half-life (1–3 weeks) that is considerably longer than that of the parent drug (2–4 days). In contrast, half-lives for the active metabolites of the compared antipsychotics are approximately the same as that of the parent drug (<4 days). At steady-state and under fixed-dosing conditions, DDCAR is the most prominent moiety (Table 2).33 Based on the geometric mean values of model-predicted plasma concentrations of DDCAR and its relative value to total CAR, the presence of DDCAR alone may be enough to occupy D2 and D3 receptors at 2 and 4 weeks after last dose.
Occupancy of dopamine receptors is believed to be the main mechanism of action for antipsychotics.41,42 It is believed that 65–80% occupancy of striatal dopamine receptors is associated with optimal therapeutic efficacy, although an analysis of the CATIE trial found that over half of the patients in remission had D2 occupancy levels below 65%.43 This observation may suggest that during maintenance treatment, lower levels of D2 occupancy may be sufficient to delay relapse and provide maintained treatment benefits for stabilized patients. In the cariprazine study, predicted plasma concentrations of total CAR suggest that patients who have discontinued from cariprazine treatment may have substantial D2 occupancy at 2 weeks after last dose and moderate D2 occupancy at 4 weeks after last dose. Additionally, plasma concentrations of cariprazine indicate that D3 receptors are substantially occupied at 2 and 4 weeks after last dose; while it is unknown what roles D3 receptors have in relapse prevention in patients with schizophrenia, it is believed that they may be involved in modulating cognition, mood, reward, and negative symptoms.42,44–46
Relapse can be decreased by uninterrupted pharmacotherapy,47 but in reality, most outpatients are only partially adherent to their medication regimen.48,49 After an initial hospital stay, schizophrenia and other psychotic disorders are the leading cause of 7-day readmission and third leading cause of 30-day readmission.50 In several naturalistic studies, nonadherence rates in patients with schizophrenia ranged from 30% to 60%; however, these numbers appear to drop to approximately 10% or lower in randomized clinical trials where selected patients agreeing to take part in a research study are monitored by health care professionals and have regular access to health care.7 This finding indicates that better measures are needed in real-world settings to deliver continued treatment to patients on a regular basis. LAIs were introduced as a possible solution; however, not all patients are willing to take an LAI, and despite having been on the market for several years, their usage rate among patients with schizophrenia still pales in comparison to oral antipsychotics.13 Thus, given the evidence seen with LAIs,9–12 oral medications that can provide the patient with sufficient coverage from when they are discharged from the hospital to the time of their first outpatient appointment may be able to reduce rates of relapse and rehospitalization. Half-lives of LAIs range from 4–6 days (eg, risperidone-LAI) to 1–2 months for other LAIs (eg, aripiprazole-LAI [30–57 days], paliperidone-LAI [24–49 days], olanzapine-LAI [approximately 30 days]).51–55 With an effective half-life of approximately 1 week and occupancy of dopamine receptors for up to 4 weeks after the last dose, cariprazine may be an example of an oral antipsychotic that can effectively bridge gaps to provide more beneficial medication coverage.
Results from this study need to be interpreted within its limitations. For example, time to drug-placebo relapse separation and placebo relapse rate were estimated from published K-M curves since primary data were not available for analysis. Another limitation is the lack of standardized definition of curve separation. For this study, 5% and 10% separation were used; however, it is possible that the results may be affected if a different percent separation value was used. It should also be noted that in the cariprazine study, participants received 20 weeks of open-label cariprazine and were at steady-state dosing by the time of randomized withdrawal; as such, it is unclear how relapse rates would change if cariprazine was taken on an inconsistent basis and then abruptly discontinued. Further, the beneficial property of delayed relapse is not well understood and has not been empirically validated. Additionally, due to differences in study designs between the cariprazine relapse prevention study and published studies of some oral antipsychotics, these analyses should be interpreted cautiously and could not include comparisons with some frequently used oral antipsychotics (eg, aripiprazole, ziprasidone) as published studies with a similar design were not available. Future head-to-head, prospective, randomized clinical trials are needed to confirm the results of this post hoc, exploratory study.
In conclusion, the delayed incidence of relapse seen after discontinuation of cariprazine compared with other oral atypical antipsychotics appears to be at least partially due to the particularly long half-life of cariprazine and its active metabolites, which is substantially longer than the half-life of the other oral atypical antipsychotics and their active metabolites. Results from this analysis suggest that the long half-life of cariprazine may result in additional protection from schizophrenia relapse in cases of partial adherence, though prospective comparative trials are needed to confirm these results.
Supplementary materials
By-week change from baseline in PANSS total score (MMRM).
Abbreviations: LS, least squares; MMRM, mixed-effects model for repeated measures; PANSS, Positive and Negative Syndrome Scale.
References
- 1.Durgam S, Earley W, Li R, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr Res. 2016;176(2–3):264–271. doi: 10.1016/j.schres.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 2.Fleischhacker WW, Hobart M, Ouyang J, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: A randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2017;20(1):11–21. doi: 10.1093/ijnp/pyw076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peuskens J, Trivedi J, Malyarov S, et al. Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: A randomized, placebo-controlled trial in clinically stable patients. Psychiatry (Edgmont). 2007;4(11):34–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Beasley CM, Jr., Sutton VK, Hamilton SH, et al. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23(6):582–594. doi: 10.1097/01.jcp.0000095348.32154.ec [DOI] [PubMed] [Google Scholar]
- 5.Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30(1):69–77. doi: 10.1177/0269881115620460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane JM, Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349–355. doi: 10.4088/JCP.10m06306 [DOI] [PubMed] [Google Scholar]
- 7.Weiden PJ, Manning R, Wolfgang CD, et al. A randomized trial of Iloperidone for prevention of relapse in schizophrenia: The REPRIEVE Study. CNS Drugs. 2016;30:735–747. doi: 10.1007/s40263-016-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2007;27(1):6–14. doi: 10.1097/JCP.0b013e31802dda4a [DOI] [PubMed] [Google Scholar]
Acknowledgments
Writing and editorial assistance was provided by Katharine Fang, PhD, of Prescott Medical Communications Group (Chicago, IL), a contractor of Allergan. The authors thank Laishun Chen, PhD, for contributing to early discussions regarding plasma concentrations and EC50. A poster of the results in this manuscript was previously presented at the 171st Annual Meeting of the American Psychiatric Association, May 5-9, 2018, New York City, NY (abstract not published) and the 2019 Congress of the Schizophrenia International Research Society, April 10-14, 2019, Orlando, FL (this poster’s abstract was published in Schizophr Bull. 2019;45 Suppl 2:S317; https://academic.oup.com/schizophreniabulletin/article/45/Supplement_2/S317/5434430). Financial support for this work was provided by Allergan and Gedeon Richter Plc.
Availability of Data and Material
Allergan will share de-identified patient-level data and/or study-level data, including protocols and clinical study reports, for this clinical trial. To request access to the data, the researcher must sign a data use agreement. All shared data are to be used for non-commercial purposes only. More information can be found on http://www.allerganclinicaltrials.com/.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. C. Correll has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Angelini, Boehringer-Ingelheim, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante-ProPhase, Medscape, Merck, Neurocrine, Noven, Otsuka, Pfizer, Recordati, ROVI, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He has served on a Data Safety Monitoring Board for Boehringer-Ingelheim, Lundbeck, ROVI, Supernus, and Teva. He has received royalties from UpToDate and grant support from Janssen and Takeda, and he is also a shareholder of LB Pharma. R. Jain has received grant funding from, or served as a consultant, on advisory boards or on speakers’ bureaus for Addrenex, Alkermes, Allergan, AstraZeneca, Avanir, Forum, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Rhodes, Supernus, Shionogi, Shire, Sunovion, Supernus, Takeda, Teva, and Tris Pharmaceuticals. J. Meyer has received speaking or advising fees from Acadia, Alkermes, Allergan, Arbor Scientia, Bristol-Myers Squibb, Merck, Neuroscience Education Institute, Neurocrine, Otsuka America, Sunovion, and Teva. A. Periclou, T. Carrothers, M. Patel, and W. Earley are employees of Allergan. Á. Barabássy is an employee of Gedeon Richter Plc. Allergan and Gedeon Richter Plc. The authors report no other conflicts of interest in this work.
References
- 1.Fleischhacker WW, Arango C, Arteel P, et al. Schizophrenia–time to commit to policy change. Schizophr Bull. 2014;40(Suppl3):S165–S194. doi: 10.1093/schbul/sbu006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67 [DOI] [PubMed] [Google Scholar]
- 3.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. doi: 10.1016/S0140-6736(12)60239-6 [DOI] [PubMed] [Google Scholar]
- 4.Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159(1):103–108. doi: 10.1176/appi.ajp.159.1.103 [DOI] [PubMed] [Google Scholar]
- 6.Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–1192. doi: 10.1176/ps.2007.58.9.1187 [DOI] [PubMed] [Google Scholar]
- 7.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. doi: 10.1002/wps.20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivares JM, Pinal B, Cinos C. Comparison of long-acting antipsychotic injection and oral antipsychotics in schizophrenia. Neuropsychiatry. 2011;1(3):275–289. doi: 10.2217/npy.11.24 [DOI] [Google Scholar]
- 9.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–965. doi: 10.4088/JCP.13r08440 [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603–619. doi: 10.1093/schbul/sbx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brissos S, Veguilla MR, Taylor D, Balanza-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198–219. doi: 10.1177/2045125314540297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiden PJ, Kim E, Bermak J, Turkoz I, Gopal S, Berwaerts J. Does half-life matter after antipsychotic discontinuation? a relapse comparison in schizophrenia with 3 different formulations of paliperidone. J Clin Psychiatry. 2017;78(7):e813-e820. doi: 10.4088/JCP.16m11308 [DOI] [PubMed] [Google Scholar]
- 13.De Risio A, Lang AP. History and therapeutic rationale of long acting antipsychotics. Curr Clin Pharmacol. 2014;9(1):39–52. [DOI] [PubMed] [Google Scholar]
- 14.Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. doi: 10.4088/JCP.15032su1 [DOI] [PubMed] [Google Scholar]
- 15.Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(Suppl 2):S12–S21. doi: 10.1016/S0924-9338(10)71701-6 [DOI] [PubMed] [Google Scholar]
- 16.Kiss B, Horvath A, Nemethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328–340. doi: 10.1124/jpet.109.160432 [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Kubota T, Iwakaji A, Imada M, Kapas M, Morio Y. Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des Devel Ther. 2016;10:327–338. doi: 10.2147/DDDT.S95100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193–206. doi: 10.1517/17425255.2013.759211 [DOI] [PubMed] [Google Scholar]
- 19.Álamo C. The pharmacological role and clinical applications of antipsychotics’ active metabolites: paliperidone versus risperidone. Clin Exp Pharmacol. 2013;03:01. doi: 10.4172/2161-1459 [DOI] [Google Scholar]
- 20.Durgam S, Earley W, Li R, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr Res. 2016;176(2–3):264–271. doi: 10.1016/j.schres.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Mental H, Psychopharmacology Research B, Early Clinical Drug Evaluation P; Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, Md.: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 23.Fleischhacker WW, Hobart M, Ouyang J, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2017;20(1):11–21. doi: 10.1093/ijnp/pyw076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peuskens J, Trivedi J, Malyarov S, et al. Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomized, placebo-controlled trial in clinically stable patients. Psychiatry (Edgmont). 2007;4(11):34–50. [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley CM Jr., Sutton VK, Hamilton SH, et al. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23(6):582–594. doi: 10.1097/01.jcp.0000095348.32154.ec [DOI] [PubMed] [Google Scholar]
- 26.Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30(1):69–77. doi: 10.1177/0269881115620460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane JM, Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349–355. doi: 10.4088/JCP.10m06306 [DOI] [PubMed] [Google Scholar]
- 28.Weiden PJ, Manning R, Wolfgang CD, et al. A randomized trial of iloperidone for prevention of relapse in schizophrenia: The REPRIEVE Study. CNS Drugs. 2016;30(8):735–747. doi: 10.1007/s40263-016-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2007;27(1):6–14. doi: 10.1097/JCP.0b013e31802dda4a [DOI] [PubMed] [Google Scholar]
- 30.Arato M, O’Connor R, Meltzer HY, Group ZS. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207–215. [DOI] [PubMed] [Google Scholar]
- 31.Pigott TA, Carson WH, Saha AR, et al. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–1056. [DOI] [PubMed] [Google Scholar]
- 32.Citrome L. Quantifying clinical relevance. Innov Clin Neurosci. 2014;11(5–6):26–30. [PMC free article] [PubMed] [Google Scholar]
- 33.Periclou A, Phillips L, Bihorel S, et al. Characterization of population pharmacokinetics of cariprazine and its major metabolites. American Psychiatric Association 169th Annual Meeting; May 14–18, 2016; Atlanta, Georgia. [Google Scholar]
- 34.Bakken GV, Molden E, Hermann M. Impact of genetic variability in CYP2D6, CYP3A5, and ABCB1 on serum concentrations of quetiapine and N-desalkylquetiapine in psychiatric patients. Ther Drug Monit. 2015;37(2):256–261. doi: 10.1097/FTD.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 35.Bakken GV, Rudberg I, Molden E, Refsum H, Hermann M. Pharmacokinetic variability of quetiapine and the active metabolite N-desalkylquetiapine in psychiatric patients. Ther Drug Monit. 2011;33(2):222–226. doi: 10.1097/FTD.0b013e31821160c4 [DOI] [PubMed] [Google Scholar]
- 36.Bui K, Earley W, Nyberg S. Pharmacokinetic profile of the extended-release formulation of quetiapine fumarate (quetiapine XR): clinical implications. Curr Med Res Opin. 2013;29(7):813–825. doi: 10.1185/03007995.2013.794774 [DOI] [PubMed] [Google Scholar]
- 37.Citrome L. Lurasidone for schizophrenia: a brief review of a new second-generation antipsychotic. Clin Schizophr Relat Psychoses. 2011;4(4):251–257. doi: 10.3371/CSRP.4.4.5 [DOI] [PubMed] [Google Scholar]
- 38.Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18(11):1715–1726. doi: 10.1517/13543780903286388 [DOI] [PubMed] [Google Scholar]
- 39.Earley W, Guo H, Luchini R. Modified cariprazine relapse prevention clinical trial results. Schizophr Res. 2018;199:452–453. doi: 10.1016/j.schres.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 40.Girgis RR, Slifstein M, D’Souza D, et al. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology (Berl). 2016;233(19–20):3503–3512. doi: 10.1007/s00213-016-4382-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–S5. doi: 10.1017/S1461145704004110 [DOI] [PubMed] [Google Scholar]
- 42.Stahl SM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 4th ed. Cambridge, New York: Cambridge University Press; 2013. [Google Scholar]
- 43.Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D(2) receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682–685. doi: 10.1097/JCP.0b013e3182979a0a [DOI] [PubMed] [Google Scholar]
- 44.Gross G, Drescher K. The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handb Exp Pharmacol. 2012;213:167–210. [DOI] [PubMed] [Google Scholar]
- 45.Leggio GM, Salomone S, Bucolo C, et al. Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. Eur J Pharmacol. 2013;719(1–3):25–33. doi: 10.1016/j.ejphar.2013.07.022 [DOI] [PubMed] [Google Scholar]
- 46.Sokoloff P, Le Foll B. The dopamine D3 receptor, a quarter century later. Eur J Neurosci. 2017;45(1):2–19. doi: 10.1111/ejn.13390 [DOI] [PubMed] [Google Scholar]
- 47.Kane JM. Treatment strategies to prevent relapse and encourage remission. J Clin Psychiatry. 2007;68(Suppl 14):27–30. [PubMed] [Google Scholar]
- 48.Masand PS, Roca M, Turner MS, Kane JM. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia: a review. Prim Care Companion J Clin Psychiatry. 2009;11(4):147–154. doi: 10.4088/PCC.08r00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remington G, Teo C, Mann S, Hahn M, Foussias G, Agid O. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261–263. doi: 10.1097/JCP.0b013e31828568bc [DOI] [PubMed] [Google Scholar]
- 50.Fingar KR, Barrett ML, Jiang HJ A Comparison of All-Cause 7-Day and 30-Day Readmissions, 2014. HCUP Statistical Brief #230. October 2017. Available from: www.hcup-us.ahrq.gov/reports/statbriefs/sb230-7-Day-Versus-30-Day-Readmissions.pdf. Accessed July 10, 2018.
- 51.Gefvert O, Eriksson B, Persson P, et al. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta) in patients with schizophrenia. Int J Neuropsychopharmacol. 2005;8(1):27–36. doi: 10.1017/S1461145704004924 [DOI] [PubMed] [Google Scholar]
- 52.Hard ML, Mills RJ, Sadler BM, Wehr AY, Weiden PJ, von Moltke L. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase i study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617–624. doi: 10.1007/s40263-017-0447-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potkin SG, Preda A. Aripiprazole once-monthly long-acting injectable for the treatment of schizophrenia. Expert Opin Pharmacother. 2016;17(3):395–407. doi: 10.1517/14656566.2015.1114100 [DOI] [PubMed] [Google Scholar]
- 54.Owen RT. Paliperidone palmitate injection: its efficacy, safety and tolerability in schizophrenia. Drugs Today (Barc). 2010;46(7):463–471. doi: 10.1358/dot.2010.46.7.1514647 [DOI] [PubMed] [Google Scholar]
- 55.Heres S, Kraemer S, Bergstrom RF, Detke HC. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299–312. doi: 10.1097/YIC.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
By-week change from baseline in PANSS total score (MMRM).
Abbreviations: LS, least squares; MMRM, mixed-effects model for repeated measures; PANSS, Positive and Negative Syndrome Scale.
Data Availability Statement
Allergan will share de-identified patient-level data and/or study-level data, including protocols and clinical study reports, for this clinical trial. To request access to the data, the researcher must sign a data use agreement. All shared data are to be used for non-commercial purposes only. More information can be found on http://www.allerganclinicaltrials.com/.