Abstract
Background
It is unclear whether low density lipoprotein receptor-related protein 11 (LRP11), a newly found lipoprotein receptor regulatory protein, has the carcinogenic effects in cervical cancer.
Methods
Bioinformatics analysis, immunohistochemical (IHC) staining and evaluation, cell proliferation assay, flow cytometry, transwell migration and invasion assays, Western blotting, growth of LRP11-silenced cells in athymic nude mice were performed in this research.
Results
We found that LRP11 expression was higher in high-grade squamous intraepithelial lesions (HSIL) and cervical cancer tissue than in normal cervix, and high expression of LRP11 was associated with differentiation degree (P=0.0266), indicating poor prognosis (P=0.0210). The silencing of LRP11 in SiHa and CaSki cell lines inhibited cell proliferation, reduced migration and invasion and suppressed cell growth in nude mice, which possibly related to cell cycle protein regulation of CDK 2/4, cyclin D1/E1, MMP-2/9, and VEGF. Furthermore, LRP11 showed substantial positive correlation with P16 in vivo and in vitro.
Conclusion
LRP11 plays important roles in proliferation, migration and invasion, with the potential to be a useful prognostic marker and therapeutic target for patients with HSIL and cervical cancer.
Keywords: HPV, cervical cancer, LRP11, P16, cell cycle
Introduction
Cervical cancer is the most common gynecological malignancy. According to the International Agency for Research on Cancer (IRAC) 2018 GLOBOCAN statistical report, the number of new cases of cervical cancer worldwide in 2018 was 569,847 and the number of deaths was 311,365. The proportion of new cases (13.1%) and the proportion of deaths (6.9%) ranked fourth among female tumors.1 Chinese cancer statistics report published in 2015 found that rates of incidence and mortality increased yearly from 2000 to 2011; furthermore, the incidence rate was the second highest among women in the 34–44 age group.2 Therefore, cervical cancer still imposes a heavy economic burden around the world and severely endangers women’s health. In addition to persistent high-risk human papillomavirus (HPV) infection, there are many risk factors involved in carcinogenesis.
Lipids play critical roles in cellular survival, interaction, proliferation and death, because they are involved in cellular signaling, cell membranes, and cell-cell interactions. These cellular processes are strongly related to oncogenic processes, particularly transformation, progression, and metastasis, suggesting that bioactive lipids are mediators of a number of carcinogenesis processes.3 The low-density lipoprotein receptor‐related protein families are transmembrane proteins, limited to the regulation of cholesterol homeostasis by receptor‐mediated endocytosis of lipoprotein particles; however, there is growing experimental evidence that the other members of the gene family have additional physiological functions, including signal transduction.4 Members of the LRP family, including LDLR related protein 1 (LRP1), LRP2/glycoprotein330/megalin, very low-density lipoprotein (VLDL) receptor (VLDLR); LR11 (also known as sorLA); apolipoprotein E (apoE) receptor type 2 (apoER2, LRP8, LR7/8B); LRP3, MEGF7/LRP4, LRP5, and LRP6, LR32 (also called LRP1B), as well as the LRP11.5,6 Many studies have reported that family members LRP1 and LRP1B serve as prognostic markers in colon cancer, breast cancer, thyroid cancer, urothelial and clear-cell renal cell carcinoma that they play important roles in carcinogenesis.7–15 LRP11, as a newly described member of the LRP family, was found highly expressed in cervical cancer patients and was associated with poor prognosis according to our bioinformation analysis. However, its function remains unclear, only Fatima’s research found that LRP11 in the form of exosomes could promote the occurrence and development of head and neck cancer by affecting lipid metabolism and membrane trafficking.16 To evaluate the role of LRP11 in cervical cancer, we sampled the tumor tissue obtained from HSIL and cervical cancer patients and examined their LRP11 expression levels. Through gene silencing in cervical cancer cell lines, we described the effects of LRP11 on proliferation, cell cycle arrest, apoptosis, migration and invasion of cancer cells. For many studies have reported that P16 plays important roles in the proliferation through regulating the expression of cell cycle associated protein, and now it is widely used as a biomarker for CIN and cervical cancer patients. Therefore, we used P16 as a reference.17–19
Methods and materials
Bioinformation databases
LRP11 bioinformation analysis were performed from the GEO dataset (https://www.ncbi.nlm.nih.gov/geoprofiles), Oncomine database (https://www.oncomine.org), GEPIA database (http://gepia.cancer-pku.cn/). In the GEO dataset, we need to download the original data of LRP11 in the form of GEO profile, which can be opened by Excel software. The mRNA expression level of LRP11 is relatively quantified. Therefore, we use GraphPad software directly for student’s t-test and mapping. In the Oncomine database and the GEPIA database, we can directly input LRP11 and other related molecular names, then we can obtain corresponding expression quantitative graphs, expression related graphs and survival prognosis graphs.
Tissue samples
Fresh tissue samples (12 normal cervix and 12 cervical cancer) were collected in our surgery department. Paraffin-embedded tissue samples came from 39 normal controls (healthy or uterus benign tumor cases), 40 HSIL patients and 50 cervical cancer patients who were admitted to Qilu Hospital of Shandong University from 2009 to 2012. The paraffin-embedded tissue blocks were then made into tissue microarray (TMA) blocks. All clinical information, including age, HPV infection status, ThinPrep cytologic test (TCT) results, colposcopy and pathological diagnosis were all examined, and patients were staged according to 2009 FIGO staging guidelines. All patients have provided written informed consent, and that this was conducted in accordance with the Declaration of Helsinki. The ethics committee of Qilu Hospital approved this study, and the approval number is KYLL-2017–560.
Immunohistochemical (IHC) staining and evaluation
The TMA blocks were cut into 4-µm sections. The slides were then incubated with antibodies against P16 (1:200, Abcam, USA, ab 189034) and LRP11 (10 μg/mL, R&D Systems, USA, AF8355). Immunohistochemical staining and evaluation procedures were described previously.20 The primary antibody was incubated overnight at 4 °C refrigerator for 12–16 h and the secondary antibody was incubated for 30–60 min at room temperature.
Cell lines and maintenance
The following human cervical cancer cell lines were used in this study: SiHa, human cervical squamous carcinoma, HPV 16-positive; CaSki, human cervical adeno-squamous carcinoma, HPV 16-positive. All these cell lines used in our study were purchased commercially from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). SiHa cells were maintained in minimum essential medium (MEM), CaSki cells were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium, and all media (HyClone Laboratories, Logan, UT, USA) were supplemented with 10% fetal bovine serum (FBS; BI, Kibbutz Beit-Haemek, Israel). They were all incubated in standard culture conditions (5% CO2, 37 °C).
Transduction of the LRP11-silencing plasmid
To further clarify the roles of LRP11 in cervical cancer, we transduced SiHa and CaSki cell lines with LRP11 small hairpin RNA (shRNA) plasmids to target the expression of LRP11. The plasmid was designed by He Yuan Co., Ltd. (Shanghai, China). The lentiviral vectors pLKD-CMV-G&PR-U6 was used to promote transduction efficiency. First, we design a shRNA interference fragment for human LRP11. The interference fragment was constructed to the downstream of the U6 promoter of the lentiviral vector (pLKD-CMV-G&PR-U6-LRP11-shRNA) by molecular biological means, and the vector can express the green fluorescent protein EGFP and puromycin resistance while interfering with the target gene. The EGFP is convenient for observing the working state of the vector, and the puromycin resistance is convenient for screening stable cell lines interfered by the target gene. After 7 days of selective culture with puromycin dihydrochloride (2 μg/mL; Amresco, Solon, OH, USA), the stably silenced cell lines were used to perform the subsequent experiments. For convenience, we designated the LRP11 knockdown cell lines as sh-LRP11, while the control cell lines were designated as NC.
Cell proliferation assay
Cell survival rate was assessed using Cell Counting Kit-8 (CCK-8) (Tongren, Shanghai, China). According to the manufacturer’s instructions, 2×103 cells were seeded in each well of a 96-well plate, and incubated for 0, 12, 24, 48, 72 h. Then, 10 µL of CCK-8 reagent was added to each well including the negative control at 4 h before measuring the optical density (OD) at 450 nm using a microplate reader (Infinite 2000; Tecan, Männedorf, Switzerland).
Flow cytometry
The distributions of NC and sh-LRP11 cells at various stages of the cell cycle and apoptosis were measured by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA), as previously described.20–22
Transwell migration and invasion assays
The abilities of migration and invasion were measured by the Transwell assays, and the procedures have been described previously.21
Western blotting
Total protein was extracted from the samples, as previously described.20–22 Primary antibodies used were anti-LRP11, anti-P16, anti-CDK 2 (1:1000, Abcam, ab 32147), anti-CDK 4 (1:1000, Cell Signaling Technology, Danvers, MA, USA, #12790), anti-Cyclin D1 (1:1000, Cell Signaling Technology, Danvers, MA, USA, #2978), anti-cyclin E1 (1:1000, Abcam, ab 3927), anti-cleaved-PARP (1:1000, Cell Signaling Technology, Danvers, MA, USA, #5652T), anti-BcL-xL (1:200, Santa Cruz Biotech, sc-8392), anti-Bax (1:200, Santa Cruz Biotech, sc-7480), anti-MMP-2 (1:1000, Abcam, ab 92536), anti-MMP-9 (1:1000, Cell Signaling Technology, Danvers, MA, USA, #13667), anti-VEGF (1 μg/mL, Abcam, ab 46154), and anti-β-actin (1:1000, Cell Signaling Technology, #4970). β-actin is currently recognized as very stable internal control. For mammalian cell expression, it refers to a protein encoded by the housekeeping gene. Secondary antibodies were anti-rabbit and anti-mouse IgG peroxidase conjugates (1:5000, Merck Millipore, MA, USA), as well as anti-sheep IgG cell and tissue staining kit (R&D Systems, USA, CTS019). The ImageQuant LAS 4000 system (GE Healthcare Life Sciences, Logan, UT, USA) was used to detect these proteins, and the results were analyzed by ImageJ software (NIH, Bethesda, MD, USA).
Growth of LRP11-silenced cells in athymic nude mice
The mice used in this study were purchased from Weitong Lihua Biotechnology Co., Ltd. (Beijing, China). Age of the mice is 5 weeks old. Ten female athymic nude mice were randomly divided into two groups (5 mice/group). When the fusion rate reaches 80%, SiHa cells transduced with either the sh-LRP11 or the NC plasmid were digested by 0.25% trypsin and collected by centrifugation in 1000rpm for 4 min. Then they were counted on a hemocytometer plate and the concentration was adjusted to 5×106 cells/mL with serum-free medium, and the mice were subcutaneously injected. The tumor volume and size were measured as described: The tumour volume was measured every there days after tumour formation (approximately 8 days), and the tumour size was calculated according to the following formula: volume = (width)2×length/2.22 Then, 41 days later, the xenografts were removed from the mice, weighed and photographed. Growth curves were then drawn. All animal experiments were performed on the basis of “The Detailed Rules and Regulations of Medical Animal Experiments Administration and Implementation” (document no. 1998–55; Ministry of Public Health, People’s Republic of China). The ethics committee of Qilu Hospital approved this study, and the approval number is KYLL-2017–560.
Statistical analysis
Statistical analyses were conducted with GraphPad Prism 5.01 software (GraphPad Software Inc., San Diego, CA, USA). All experiments were repeated at least three times, and the results were presented as mean ± the standard deviation (SD). Statistical comparisons were performed using Student’s t-test, Pearson r test, Chi-square test, Yate’s continuity corrected Chi-square test and Fisher’s exact test. Finally, the Kaplan-Meier method and the log-rank test were used to analyze the survival data. P<0.05 was considered statistically significant.
Results
Expression of LRP11 and P16 in normal, HSIL, and cervical cancer tissues and their associations
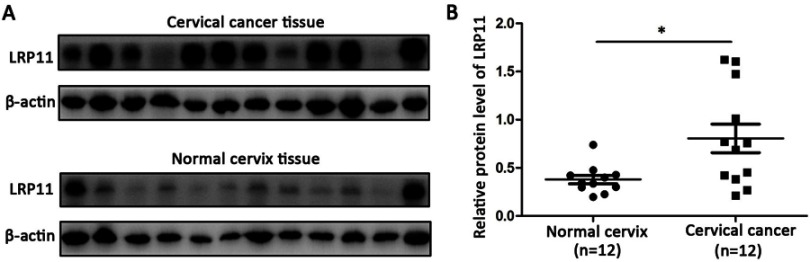
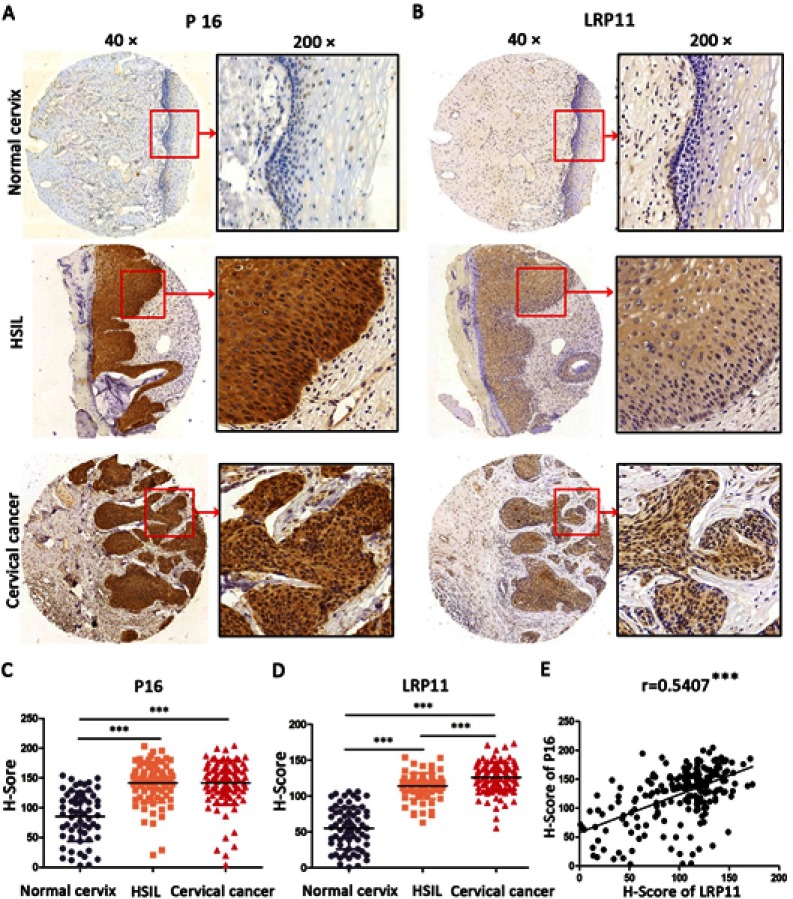
To examine whether LRP11 is expressed in cervical cancer, relative protein expression levels of LRP11 in 12 normal cervical and 12 cervical cancer tissues were assessed by Western blotting (Figure 1A). The protein levels of LRP11 in the cervical cancer samples were significantly higher than those of normal tissues (P<0.05) (Figure 1B). These results agreed with our bioinformation analysis: The expression of LRP11 mRNA increased in HPV infection tissue and HPV progression tissue in GEO DataSets (Figure S1A), and the Oncomine database also showed a same trend in cervical cancer tissue, compared with the normal cervix uteri (Figure S1B and C). Then, IHC was used to compare expression levels of P16 and LRP11 in normal, HSIL, and cervical cancer tissues (Figure 2). Expression levels of P16 were higher in HSIL and cervical cancer tissues than in the normal cervix (P<0.001) (Figure 2A and C), and LRP11 expression levels were significantly correlated with pathological level changes (P<0.0001) (Figure 2B and D). Furthermore, we performed Pearson r tests to determine the correlation between the expression of LRP11 and P16. We found that LRP11 expression was positively correlated with P16 expression (r=0.5407, P<0.001) (Figure 2E).
Figure 1.
LRP11 expression in normal and cervical cancer tissues. (A) Protein expression of LRP11 was determined by Western blotting in 12 normal cervical tissues and 12 tumor tissues. (B) Quantification of protein expression levels as shown in A. *P<0.05.
Figure 2.
Expression of LRP11 and P16 in cervical cancer tissues and their correlation. (A and B) The expression of P16 and LRP11 in normal cervix, high squamous intraepithelial lesion (HSIL) and cervical cancer tissues (×40 and ×200). (C and D) The H-Score of P16 and LRP11 in normal cervix, HSIL and cervical cancer. (E) The correlations between expression of P16 and LRP11. ***P<0.001.
Relationship between LRP11 and P16 expression and clinicopathological characteristics, and their correlation with overall survival rates in cervical cancer patients
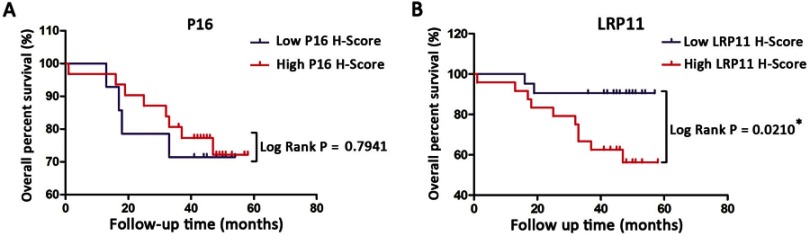
The results are summarized in Table 1. We found that LRP11 expression correlated with cervical cancer differentiation (P<0.05), but did not correlate with age, histology, clinical stage, tumor size, lymph node metastasis (LNM) or lymph vascular space involvement (LVSI). The expression levels of P16 did not correlate with any. We also gathered survival data from these cervical cancer patients, including 33 survivors, 12 deaths and 5 patients lost to follow-up. OS time in cervical cancer patients with high LRP11 expression tumors was shorter than those with low LRP11 tumors (P<0.05); however, no significant association was found between the expression P16 and OS (Figure 3A and B). The survival results agreed with our bioinformation analysis (Figure S1D and E).
Table 1.
Association between LRP11/P16 expression and clinicopathological factors
| Variables | No. | Expression of LRP11 | Expression of P16 | ||||
|---|---|---|---|---|---|---|---|
| Low | High | P-value | Low | High | P-value | ||
| Age | 0.6149 | 0.5000 | |||||
| ≤45 | 19 | 7 | 12 | 5 | 14 | ||
| >45 | 31 | 15 | 16 | 11 | 20 | ||
| Histology | 0.2457 | 0.5571 | |||||
| SCC | 47 | 22 | 25 | 15 | 32 | ||
| Adenocarcinoma | 3 | 0 | 3 | 1 | 2 | ||
| Clinical Stage | 0.1493 | 0.2035 | |||||
| Stage I | 37 | 19 | 18 | 10 | 27 | ||
| Stage II/III | 13 | 3 | 10 | 6 | 7 | ||
| Differentiation | 0.0266* | 0.3185 | |||||
| Low/Moderate | 27 | 8 | 19 | 7 | 20 | ||
| High | 23 | 14 | 9 | 9 | 14 | ||
| Tumor Size | 0.3567 | 0.2787 | |||||
| <4 cm | 39 | 19 | 20 | 11 | 28 | ||
| ≥4 cm | 11 | 3 | 8 | 5 | 6 | ||
| LNM | 0.4280 | 0.9604 | |||||
| Negative | 42 | 20 | 22 | 14 | 28 | ||
| Positive | 8 | 2 | 6 | 2 | 6 | ||
| LVSI | 0.9432 | 0.8201 | |||||
| Negative | 40 | 18 | 22 | 13 | 27 | ||
| Positive | 10 | 4 | 6 | 3 | 7 | ||
Note: *Statistically significantly value.
Abbreviations: SCC, squamous cell carcinoma; LNM, lymph node metastasis; LVSI, lymph vascular space involvement.
Figure 3.
Kaplan-Meier overall survival (OS) curve for cervical cancer patients and the correlation of OS with P16 expression (A) and LRP11 expression (B). *P<0.05.
Effects of LRP11 silencing on P16 expression
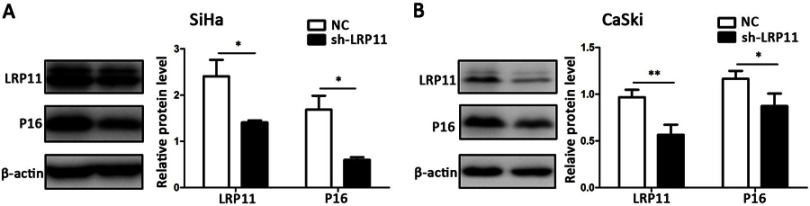
To study the influence of LRP11 on expression of P16, we performed Western blotting. The LRP11 protein knockdown efficiency is close to 50%, so we can carry out subsequent experiments to study its function. And the influence of LRP11 knockout to cycle-related molecule P16 is obvious. Compared with their respective control groups, the silencing of LRP11 in SiHa, CaSki resulted in a decline in P16 expression (P<0.05) (Figure 4).
Figure 4.
The effect of LRP11 silencing on P16. (A) The protein levels of LRP11 and P16 were determined by Western blotting in SiHa, as well as quantification of protein expression levels (B) CaSki protein levels, similar to that of SiHa. *P<0.05, **P<0.01, compared with the NC groups.
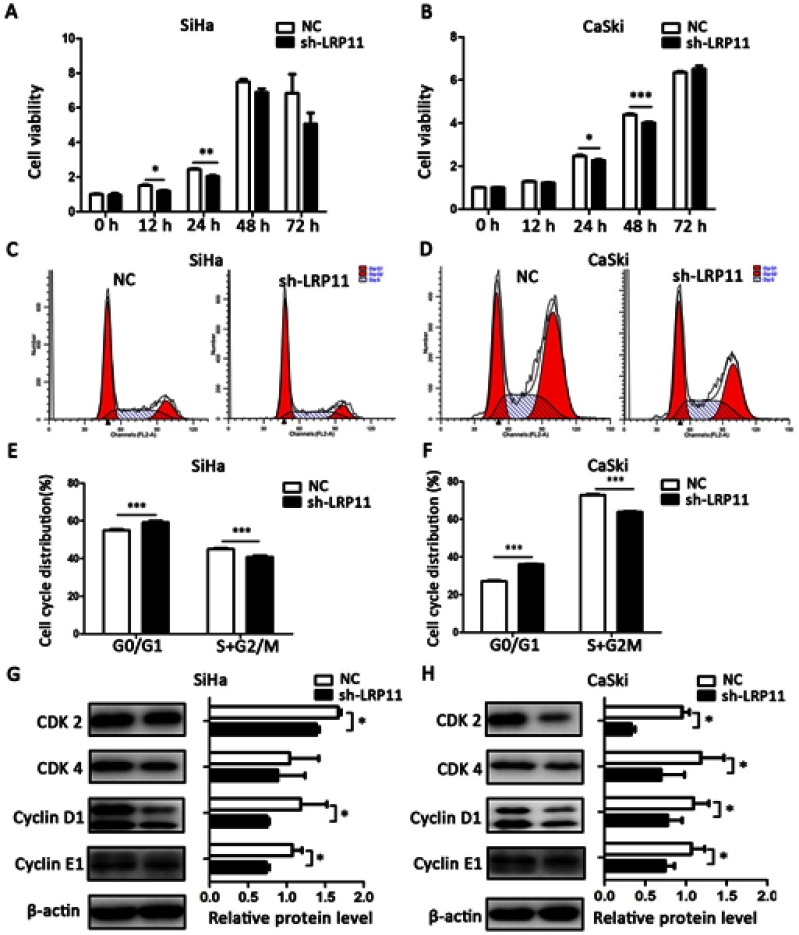
LRP11 increases cell viability and accelerates the cell cycle
To confirm whether LRP11 affects cell viability and the cell cycle, CCK-8 and flow cytometric assays were performed. The cell viability of the SiHa sh-LRP11 group was lower at 12 and 24 h (Figure 5A), and the cell viability of the CaSki sh-LRP11 group was lower at 24 and 48 h (Figure 5B), than in the NC groups. We also measured the cell cycle distribution using flow cytometry. Compared with the NC groups, the sh-LRP11 groups of SiHa and CaSki were arrested in the G0/G1 phase (Figure 5C–F). We also found that cell cycle regulatory proteins, including CDK 2/4 and cyclin D1/E1, were influenced by LRP11 expression. These proteins were expressed at lower levels in the SiHa and CaSki cells with silenced LRP11 than in the NC groups (Figure 5G and H). In addition, the expression of CDK 2/4 positively correlated with LRP11 expression in our bioinformation analysis (r=0.38/0.12, P<0.001/0.05) (Figure S1F and G).
Figure 5.
Effect of LRP11 on cell viability and cell cycle distribution. (A and B) After 0, 12, 24, 48, 72 h, viabilities of the SiHa and CaSki cell lines were assessed using the CCK-8 assay. (C and D) Cell cycle distributions of cells transfected with shRNA and their NC groups were assessed by flow cytometry. (E and F) Cell cycle phase distribution was expressed as the percentage of total cells as shown in C and D. (G and H) After shRNA transfection, the protein expression levels of CDK 2-cyclin E1 and CDK 4-cyclin D1 were determined and analyzed by Western blotting. *P<0.05, **P<0.01, ***P<0.001 compared with the NC groups.
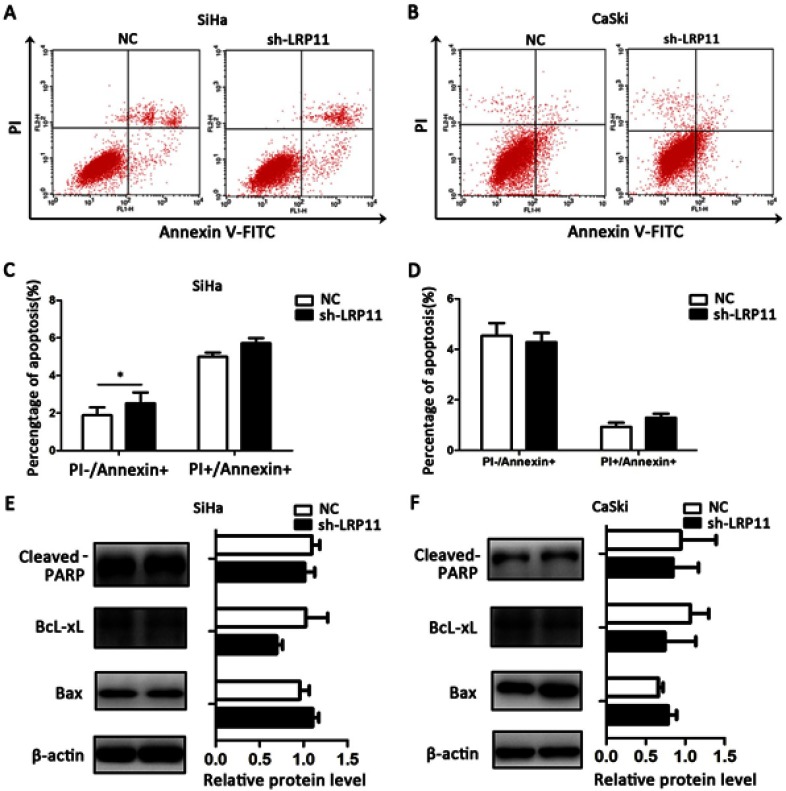
LRP11 has little effect on apoptosis
To study whether LRP11 expression affects cervical cancer cell death, we performed flow cytometry apoptosis assays. We found that silenced LRP11 in the SiHa cell line led to a small increase in early apoptosis (PI-/Annexin+), with no significant change in late apoptosis (PI+/Annexin+) (Figure 6A and C); silencing of LRP11 in the CaSki cell lines gave no significant change in either early or late apoptosis (Figure 6B and D). The Western blotting results revealed that changes in expression of apoptosis-related proteins, including cleaved PARP, BcL-xL, Bax, agreed with the change of apoptotic appearance, however without statistical significance (Figure 6E and F).
Figure 6.
Effect of LRP11 on apoptosis. (A) Knockdown of LRP11 by shRNA induced early apoptosis of sh-LRP11 SiHa cells. (B) Knockdown of LRP11 by shRNA induced no change of apoptosis in CaSki cells. (C and D) Quantification of A and B. (E and F) After shRNA transfection, protein expression levels of cleaved PARP, Bcl-xL and Bax were analyzed by Western blotting. *P<0.05 compared with the NC groups.
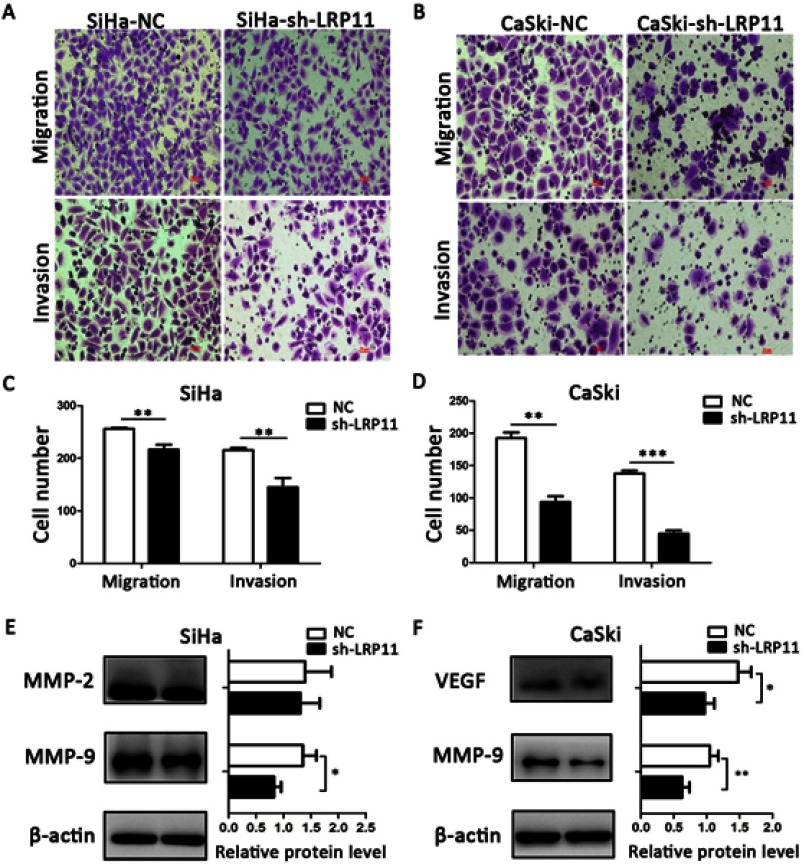
LRP11 promotes migration and invasion
Compared with the NC groups, the number of migrated and invading cells was significantly lower in the SiHa sh-LRP11 (P<0.01) and CaSki sh-LRP11 groups (P<0.001) (Figure 7A–D). To examine whether LRP11 affected Matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor (VEGF) at the protein level, we performed Western blotting. The results showed that most of the MMP-2, MMP-9 and VEGF were downregulated with the silencing of LRP11 (Figure 7E and F), and the difference was statistically significant.
Figure 7.
Effect of LRP11 on migration and invasion of SiHa and CaSki cells. (A and B) Silencing LRP11 in SiHa and CaSki cells resulted in decreased migration and invasion. (C and D) Quantification of the images of A and B. (E and F) Western blotting was performed to identify the protein levels of MMP-2, MMP-9 and VEGF after the silencing LRP11 in the SiHa and CaSki cells; *P<0.05, **P<0.01, ***P<0.001 compared with the NC groups.
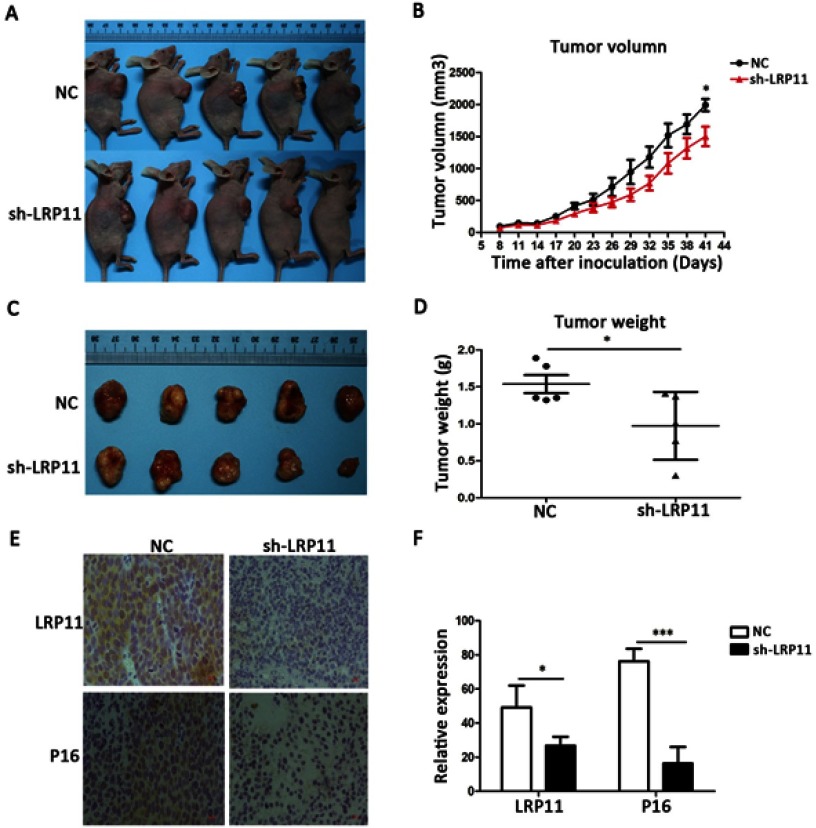
Knockdown of LRP11 suppresses growth of cervical cancer in vivo
To examine whether the silencing of LRP11 in cervical carcinoma inhibited tumor growth in vivo, SiHa cells transduced with control plasmid and sh-LRP11 were injected subcutaneously into athymic nude mice. Tumor volumes were then measured for 41 days. As shown in Figure 8A and B, sh-LRP11 tumors grew at a slower rate than did tumors in the NC group after day 41 (P<0.05). Furthermore, the average size and weight of sh-LRP11 tumors were smaller and lighter than in the control groups (P<0.05) (Figure 8C and D). IHC showed that P16 expression was lower in the sh-LRP11 group (P<0.05) (Figure 8E and F), agreeing with the in vitro results.
Figure 8.
Effect of LRP11 sh-RNA on tumor growth in vivo. SiHa cells transduced with sh-LRP11 were subcutaneously injected into nude mice. (A and B) Tumor volumes were measured for 41 days. (C and D) At day 41, nude mice were sacrificed and the tumors were weighed. (E) LRP11 and P16 expression of the tumor in vivo by IHC. (F) H-Score of LRP11 and P16 in image E. *P<0.05, ***P<0.001 compared with the NC groups.
Discussion
Although the HPV vaccine has been used to prevent cervical cancer, it is marketed in mainland of China for a short period of time (the bivalent vaccine Cervarix and the tetravalent vaccine Gardsil were approved in 2016 and 2017 respectively). As a consequent, it is estimated that it will take at least 20 years to effectively reduce the high incidence rate of cervical cancer through large-scale vaccination, during which a large number of new HPV-infected patients and cervical cancer patients will appear. Besides, the screening methods and treatment of cervical cancer have certain shortcomings, such as the low specificity of HPV testing and the low sensitivity of TCT examination, so it is especially important to find new targets for diagnosis and therapy for these cervical cancer patients.
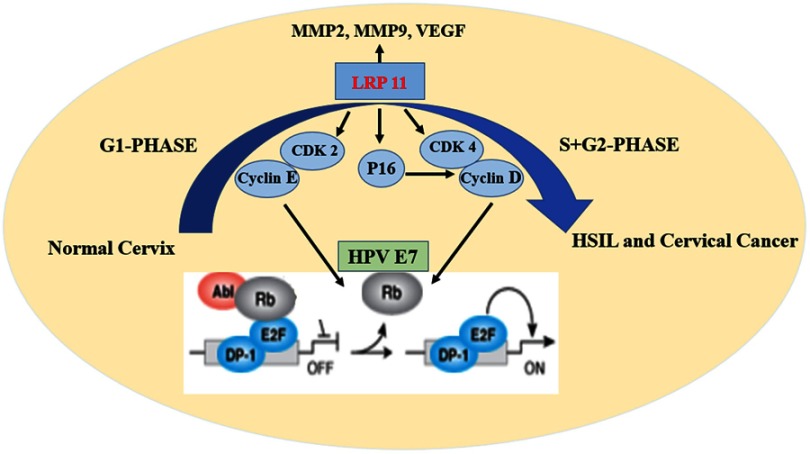
As we all know, a stable cell cycle and its regulation are critical for normal cervix cells. In the G1 phase of normal cells, hypophosphorylated retinoblastoma protein (Rb) binds to the E2F-DP1 transcription factor, and together with HDAC forms an inhibitory complex that inhibits downstream key transcriptional activities, controlling excessive cell proliferation. When normal proliferation activities are required, CDK 2-cyclin E and CDK 4/6-cyclin D work together to deactivate the dynamic transcriptional complex containing Rb and E2F.23,24 Cervical cancer is primarily caused by persistent HPV infection, after the oncogene integration of HPV, it produces substantial amounts E7 oncoprotein. E7 then combines with Rb, leading to numbers of disorders of cell proliferation and cervical cancer (Figure 9).25–29
Figure 9.
Schematic model of the effect of LRP11 on cervical cancer cells.
Through our bioinformation analysis, we found that LRP11 expression was elevated in HSIL and cervical cancer patients and that this correlated with overall survival rate. In addition, we found that LRP11 was positively correlated with CDK 2/4, suggesting that LRP11 may be related to the cell cycle. Based on previous report, we also found that in a variety of tumors, including cervical cancer, breast cancer, prostate cancer, hepatocellular carcinoma, and retinoblastoma, LRP 5/6/8 could activate the Wnt/β-catenin signaling pathway, lead to the accelerated cell cycle progression, and eventually promote the development of cervical cancer.6 Therefore, it is worth of exploring the carcinogenic effects of LRP11 in cervical cancer.
In the present study, to verify the results of the bioinformation analysis, we performed Western blots and IHC, and the results were consistent. Furthermore, we measured expression levels of P16, which has been reported to participate in cell cycle regulation and is now used for the clinical detection of HSIL in cervical cancer patients.30–34 We found that LRP11 expression correlated with cancer differentiation and could be used as a prognostic marker, an advantage over P16 measurement. Consistent with our results, Hoang’s study also found that osteosarcoma patients with positive LRP5 expression showed a trend on decreased of event-free survival.5 In vivo experiments, we only performed gene silencing and did not overexpress the LRP11 in cancer cell lines, because the expression levels of LRP11 in the cervical cell lines were all relatively high, and the effect of interference may be greater. The results of CCK-8 and flow cytometry assays showed that LRP11 indeed accelerated the cell cycle by influencing the expression of CDK 2-cyclin E and CDK 4-cyclin D, and had little effect on apoptosis. This is consistent with the effect of LRP6 in the proliferation of human triple negative breast cancer (TNBC) cell lines.35 Some scholars speculated that the high expression of LRP family members could help the cancer cells absorb more cholesterol than the normal cells and provide an extra energy to promote their uncontrolled growth by accelerating the cell cycles.36,37 Furthermore, the Transwell results showed that LRP11 promoted migration and invasion of cancer cells by regulating the expression of MMP2, MMP9 and VEGF, which have been reported to be related to migration and invasion.38–42 This was similar to the effects of LRP1 in breast carcinoma.8 Finally, the tumor formation experiment in nude mice demonstrated the tumorigenic potential of LRP11.
Our study has several limitations: one is the small number of clinical patients, the other is that we found LRP11 and P16 had a great correlation in in vivo and in vitro experimental results. We should perform coimmunoprecipitation (Co-IP) to confirm their relationship; however, this experiment needs highly specific monoclonal antibodies against P16 and LRP11. A monoclonal antibody against LRP11 has not been produced yet.
In conclusion, the present study suggested that LRP11 was involved in human HSIL and cervical cancer progression. Higher expression of LRP11 in cervical cancer tissue was significantly associated with tumor differentiation and poor overall survival. The silencing of LRP11 in cervical cancer cell lines showed that LRP11 was involved in cell proliferating, possibly occurring through its effects on the CDK 2-cyclin E and CDK 4-cyclin D cell cycle pathways (Figure 9). All these results indicated that LRP11 has great potential to be used as a prognostic marker and therapy target for cervical cancer patients.
Acknowledgment
The study was conducted at Qilu Hospital, Shandong University and was supported by the National Key R&D Program of China (2016YFC1302900 and 2016YFC0902901), the National Natural Science Foundation of China (NSFC, 81572559), the Key Research Project of Shandong Province (2017CXGC1210) and the National Science and Technology Project of China (2015BAI13B05).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Perrotti F, Rosa C, Cicalini I, et al. Advances in lipidomics for cancer biomarkers discovery. Int J Mol Sci. 2016;17(12):1992. doi: 10.3390/ijms17121992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39(3):219–228. doi: 10.1080/07853890701214881 [DOI] [PubMed] [Google Scholar]
- 5.Chung NS, Wasan KM. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev. 2004;56(9):1315–1334. doi: 10.1016/j.addr.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Roslan Z, Muhamad M, Selvaratnam L. The roles of low-density lipoprotein receptor-related proteins 5, 6, and 8 in cancer: a review. J Oncol. 2019;2019:4536302. doi: 10.1155/2019/4536302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benes P, Jurajda M, Zaloudik J, Izakovicova-Holla L, Vacha J. C766T low-density lipoprotein receptor-related protein 1 (LRP1) gene polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2003;5(3):R77–R81. doi: 10.1186/bcr591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang HS, Kim J, Lee HJ, Kwon BM, Lee DK, Hong SH. LRP1-dependent pepsin clearance induced by 2ʹ-hydroxycinnamaldehyde attenuates breast cancer cell invasion. Int J Biochem Cell Biol. 2014;53:15–23. doi: 10.1016/j.biocel.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 9.Boulagnon-Rombi C, Schneider C, Leandri C, et al. LRP1 expression in colon cancer predicts clinical outcome. Oncotarget. 2018;9(10):8849–8869. doi: 10.18632/oncotarget.24225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta N, Kumar Thakur B, Chakraborty A, Das S. Butyrate-induced in vitro colonocyte differentiation network model identifies ITGB1, SYK, CDKN2A, CHAF1A, and LRP1 as the prognostic markers for colorectal cancer recurrence. Nutr Cancer. 2019;71(2):257–271. [DOI] [PubMed] [Google Scholar]
- 11.Feng C, Ding G, Ding Q, Wen H. Overexpression of low density lipoprotein receptor-related protein 1 (LRP1) is associated with worsened prognosis and decreased cancer immunity in clear-cell renal cell carcinoma. Biochem Biophys Res Commun. 2018;503(3):1537–1543. doi: 10.1016/j.bbrc.2018.07.076 [DOI] [PubMed] [Google Scholar]
- 12.Langbein S, Szakacs O, Wilhelm M, et al. Alteration of the LRP1B gene region is associated with high grade of urothelial cancer. Lab Invest. 2002;82(5):639–643. doi: 10.1038/labinvest.3780458 [DOI] [PubMed] [Google Scholar]
- 13.Ni S, Hu J, Duan Y, et al. Down expression of LRP1B promotes cell migration via RhoA/Cdc42 pathway and actin cytoskeleton remodeling in renal cell cancer. Cancer Sci. 2013;104(7):817–825. doi: 10.1111/cas.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prazeres H, Torres J, Rodrigues F, et al. Chromosomal, epigenetic and microRNA-mediated inactivation of LRP1B, a modulator of the extracellular environment of thyroid cancer cells. Oncogene. 2017;36(1):146. doi: 10.1038/onc.2016.143 [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Sun P, Gao C, et al. Down-regulation of LRP1B in colon cancer promoted the growth and migration of cancer cells. Exp Cell Res. 2017;357(1):1–8. doi: 10.1016/j.yexcr.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 16.Qadir F, Aziz MA, Sari CP, et al. Transcriptome reprogramming by cancer exosomes: identification of novel molecular targets in matrix and immune modulation. Mol Cancer. 2018;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuschenbach M, Wentzensen N, Dijkstra MG, von Knebel Doeberitz M, Arbyn M. p16INK4a immunohistochemistry in cervical biopsy specimens: a systematic review and meta-analysis of the interobserver agreement. Am J Clin Pathol. 2014;142(6):767–772. doi: 10.1309/AJCP3TPHV4TRIZEK [DOI] [PubMed] [Google Scholar]
- 18.Kurshumliu F, Thorns C, Gashi-Luci L. p16INK4A in routine practice as a marker of cervical epithelial neoplasia. Gynecol Oncol. 2009;115(1):127–131. doi: 10.1016/j.ygyno.2009.06.020 [DOI] [PubMed] [Google Scholar]
- 19.Jia WL, Ding L, Ren ZY, et al. [Effects of both folic acid, p16 protein expression and their interaction on progression of cervical cancerization]. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(12):1647–1652. doi: 10.3760/cma.j.issn.0254-6450.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 20.Han S, Wang Y, Shi X, et al. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp Cell Res. 2018;371(1):222–230. doi: 10.1016/j.yexcr.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 21.Han S, Li Y, Zhang J, et al. Roles of immune inhibitory molecule B7-H4 in cervical cancer. Oncol Rep. 2017;37(4):2308–2316. doi: 10.3892/or.2017.5481 [DOI] [PubMed] [Google Scholar]
- 22.Han S, Shi X, Liu L, et al. Roles of B7-H3 in cervical cancer and its prognostic value. J Cancer. 2018;9(15):2612–2624. doi: 10.7150/jca.24959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28(33):2925–2939. doi: 10.1038/onc.2009.170 [DOI] [PubMed] [Google Scholar]
- 24.Kim YT, Zhao M. Aberrant cell cycle regulation in cervical carcinoma. Yonsei Med J. 2005;46(5):597–613. doi: 10.3349/ymj.2005.46.5.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science (New York, N.Y.). 1989;243(4893):934–937. doi: 10.1126/science.2537532 [DOI] [PubMed] [Google Scholar]
- 26.Lee KA, Shim JH, Kho CW, et al. Protein profiling and identification of modulators regulated by the E7 oncogene in the C33A cell line by proteomics and genomics. Proteomics. 2004;4(3):839–848. doi: 10.1002/pmic.200300626 [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Kumar P, Das BC. HPV: molecular pathways and targets. Curr Probl Cancer. 2018;42(2):161–174. doi: 10.1016/j.currproblcancer.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 28.Oyervides-Munoz MA, Perez-Maya AA, Rodriguez-Gutierrez HF, et al. Understanding the HPV integration and its progression to cervical cancer. Infect Genet Evol. 2018;61:134–144. doi: 10.1016/j.meegid.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Huang X, Zhang Y. Involvement of human papillomaviruses in cervical cancer. Front Microbiol. 2018;9:2896. doi: 10.3389/fmicb.2018.02896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuovo GJ, de Andrade CV, Wells SI, Brusadelli M, Nicol AF. New biomarkers of human papillomavirus infection in acute cervical intraepithelial neoplasia. Ann Diagn Pathol. 2018;36:21–27. doi: 10.1016/j.anndiagpath.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Elliott A, Hoffa AL, Herring N, Houser PM. Potential influence of p16 immunohistochemical staining on the diagnosis of squamous cell lesions in cervical biopsy specimens: observation from cytologic-histologic correlation. Cancer Cytopathol. 2018;126(12):1003–1010. doi: 10.1002/cncy.22063 [DOI] [PubMed] [Google Scholar]
- 32.Hu H, Zhao J, Yu W, et al. Human papillomavirus DNA, HPV L1 capsid protein and p16(INK4a) protein as markers to predict cervical lesion progression. Arch Gynecol Obstet. 2019;299(1):141–149. doi: 10.1007/s00404-018-4931-1 [DOI] [PubMed] [Google Scholar]
- 33.Miralpeix E, Genoves J, Maria Sole-Sedeno J, et al. Usefulness of p16(INK4a) staining for managing histological high-grade squamous intraepithelial cervical lesions. Mod Pathol. 2017;30(2):304–310. doi: 10.1038/modpathol.2016.168 [DOI] [PubMed] [Google Scholar]
- 34.Vasiljevic N, Carter PD, Reuter C, et al. Role of quantitative p16(INK4A) mRNA assay and digital reading of p16(INK4A) immunostained sections in diagnosis of cervical intraepithelial neoplasia. Int J Cancer. 2017;141(4):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma J, Lu W, Chen D, Xu B, Li Y. Role of Wnt co-receptor LRP6 in triple negative breast cancer cell migration and invasion. J Cell Biochem. 2017;118(9):2968–2976. doi: 10.1002/jcb.25956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou T, Zhan J, Fang W, et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC). BMC Cancer. 2017;17(1):269. doi: 10.1186/s12885-017-3239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuya Y, Sekine Y, Kato H, Miyazawa Y, Koike H, Suzuki K. Low-density lipoprotein receptors play an important role in the inhibition of prostate cancer cell proliferation by statins. Prostate Int. 2016;4(2):56–60. doi: 10.1016/j.prnil.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Wang Q, Shen S, Wei X, Li G. HIF-1alpha/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phytother Res. 2019;33(3):798–807. doi: 10.1002/ptr.6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Liu M, Song Y, Li C, Zhang S, Ma L. KLF2 inhibits the migration and invasion of prostate cancer cells by downregulating MMP2. Am J Mens Health. 2019;13(1):1557988318816907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh NT, Huang CY, Li CC, Wang IC, Lee MF. MED28 and forkhead box M1 (FOXM1) mediate matrix metalloproteinase 2 (MMP2)-dependent cellular migration in human nonsmall cell lung cancer (NSCLC) cells. J Cell Physiol. 2019;234(7):11265–11275. [DOI] [PubMed] [Google Scholar]
- 41.Xu F, Si X, Wang J, Yang A, Qin T, Yang Y. Nectin-3 is a new biomarker that mediates the upregulation of MMP2 and MMP9 in ovarian cancer cells. Biomed Pharmacother. 2019;110:139–144. doi: 10.1016/j.biopha.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 42.Lu P, Chen J, Yan L, et al. RasGRF2 promotes migration and invasion of colorectal cancer cells by modulating expression of MMP9 through Src/Akt/NF-kappaB pathway. Cancer Biol Ther. 2019;20(4):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]