ABSTRACT
Exposure to war and violence has major consequences for society at large, detrimental impact on people’s individual lives, and may also have intergenerational consequences. To gain more insight into these intergenerational consequences, research addressing the impact of the Holocaust on offspring is an important source of information. The aim of the current study was to systematically review the mechanisms of intergenerational consequences by summarizing characteristics in Holocaust survivors and their offspring suggested to impact the offspring’s mental health. We focused on: 1) parental mental health problems, 2) (perceived) parenting and attachment quality, 3) family structure, especially parental Holocaust history, 4) additional stress and life events, and 5) psychophysiological processes of transmission. We identified 23 eligible studies published between 2000 and 2018. Only Holocaust survivor studies met the inclusion criteria. Various parent and child characteristics and their interaction were found to contribute to the development of psychological symptoms and biological and epigenetic variations. Parental mental health problems, perceived parenting, attachment quality, and parental gender appeared to be influential for the mental well-being of their offspring. In addition, having two survivor parents resulted in higher mental health problems compared to having one survivor parent. Also, there was evidence suggesting that Holocaust survivor offspring show a heightened vulnerability for stress, although this was only evident in the face of actual danger. Finally, the results also indicate intergenerational effects on offspring cortisol levels. Clinical and treatment implications are discussed.
KEYWORDS: Holocaust, intergenerational, trauma, offspring
HIGHLIGHTS
• The aim was to review the mechanisms of intergenerational consequences of the holocaust.• Survivor mothers were more influential for the well-being of their offspring than fathers.• Having two survivor parents resulted in higher mental health problems compared to one.• Heightened vulnerability for stress in offspring was found in the presence of actual danger• The results indicated intergenerational effects with regard to cortisol levels.
Abstract
La exposición a la guerra y la violencia tiene consecuencias importantes para la sociedad en general, un impacto perjudicial en la vida individual de las personas, y también puede tener consecuencias intergeneracionales. Para obtener más información sobre estas consecuencias intergeneracionales, la investigación que aborda el impacto del Holocausto en la descendencia es una fuente importante de información. El objetivo del presente estudio fue revisar sistemáticamente los mecanismos de las consecuencias intergeneracionales resumiendo las características de los sobrevivientes del Holocausto y sus descendientes, que podrían impactar la salud mental de la descendencia. Nos centramos en: 1) los problemas de salud mental de los padres, 2) la calidad (percibida) de la crianza y el apego, 3) la estructura familiar, especialmente antecedentes del Holocausto de los padres, 4) el estrés y los eventos de la vida adicionales, y 5) los procesos psicofisiológicos de la transmisión. Identificamos 23 estudios elegibles publicados entre 2000 y 2018. Solo los estudios de sobrevivientes del Holocausto cumplieron con los criterios de inclusión. Se descubrió que diversas características de los padres y de los hijos y su interacción contribuyen al desarrollo de los síntomas psicológicos y las variaciones biológicas y epigenéticas. Los problemas de salud mental de los padres, la crianza percibida, la calidad del apego, y el género parental parecieron influir en el bienestar mental de sus hijos. Además, tener dos padres sobrevivientes resultó en mayores problemas de salud mental en comparación con tener uno de los padres sobrevivientes. Además, hubo evidencia que sugiere que los descendientes de los sobrevivientes del Holocausto muestran una mayor vulnerabilidad al estrés, aunque esto fue solo evidente ante el peligro real. Finalmente, los resultados también indican los efectos intergeneracionales en los niveles de cortisol de la descendencia. Se discuten las implicaciones clínicas y de tratamiento.
PALABRAS CLAVE: Holocausto, intergeneracional, trauma, descendencia
Abstract
暴露于战争和暴力会对整个社会产生重大影响,会对人们的个人生活造成不利影响,并也可能产生代际影响。为了更深入地了解这些代际影响,探讨大屠杀对后代影响的研究是一个重要的信息来源。本研究的目的是通过总结大屠杀幸存者及其后代中暗示会影响后代心理健康的特征来系统地回顾代际影响的机制。我们重点关注:1)父母的心理健康问题,2)(感知到的)父母养育和依恋质量,3)家庭结构,尤其是父母的大屠杀史,4)额外的应激和生活事件,以及5)传递的心理生理过程。我们确定出23项发表于2000年至2018年间符合条件的研究。只有大屠杀幸存者的研究符合纳入标准。多种亲子特征及其互动方式被发现会促进心理症状的发展、生物及表观遗传变异。父母的心理健康问题、感知到的养育、依恋质量和父母的性别似乎对他们后代的心理健康有影响。此外,有两名幸存者父母相较于有一名幸存者父母的后代会出现更多的心理健康问题。另外有证据表明,大屠杀幸存者后代会表现出更高的应激脆弱性,尽管只是在面对实际危险时才明显。最后,结果也表明了在后代皮质醇水平上的代际影响。文中还讨论了临床和治疗意义。
关键词: 大屠杀, 代际, 创伤, 后代
1. Introduction
War and violence have been part of human history. Nowadays more than 65 million people around the world have been forced to leave home as a result of armed conflicts; more than 21 million of them are refugees of whom more than half younger than 18 years of age (www.UNHCR.org). Exposure to war and violence not only has major consequences for society at large but also has detrimental impact on people’s individual lives. Besides trauma-related psychopathology of those exposed, violence and war may also have intergenerational consequences (Betancourt, 2015; Danieli, 1998; Havinga et al., 2017). The term ‘transmission’ of trauma has been used to describe these consequences, defined as thoughts, feelings, and behaviours generated from the survivors’ experiences and transmitted to their offspring (Fonagy, 1999; Kretchmar & Jacobovitz, 2002; Munroe et al., 1995). While some definitions describe similar symptoms for survivors and their offspring, other describe a more indirect process, through which, consciously or unconsciously, the experiences of the earlier generation influence (first and second generation) parenting attitude and behaviour (Baider et al., 2000; Van IJzendoorn & Schuengel, 1996). A better understanding of the intergenerational impact of violence and war is important not only from a theoretical perspective but also paramount for generating ideas for (more) effective interventions to help minimize these consequences in survivors of war.
While empirical research on the intergenerational consequences of violence and war focused mainly on offspring of Holocaust survivors, exceptions have also considered other violence-stricken populations such as refugees and survivors of repressive regimes and torture (Bloch, 2018; Sangalang, Jager, & Harachi, 2017; Sangalang & Vang, 2017). Methodologically, this field has evolved from clinical case studies in the 1960s to descriptive patient group studies in the seventies, and to studies including clinical and non-clinical groups in the eighties and nineties (Danieli, 1998; Solkoff, 1981, 1992). In the last two decades, integrative reviews reached the conclusion that, overall, Holocaust survivor offspring (HSO) did not present quantitatively more signs of mental health problems than non-survivor offspring. The authors of these analyses do acknowledged, however, the existence of a group of offspring characterized by psychopathological symptoms (in)directly related to their parents’ war experiences, their parents’ war-related psychopathology, and/or the impact of growing up in a Holocaust survivor family (Felsen, 1998; Kellermann, 2001; Solomon, 1998; Van IJzendoorn, Bakermans-Kranenburg, & Sagi-Schwartz, 2003). In addition, a review by Leen-Feldner et al. (2013) among parents with PTSD (i.e., including but not restricted to Holocaust survivors) suggested that parental symptoms of PTSD are associated to various offspring mental health problems, including internalizing-type problems, general behavioural problems, and altered hypothalamic-pituitary-adrenal axis functioning. The important question then arises how parental war experiences contribute to the mental health problems of the HSO.
The aim of this systematic review was to increase our understanding of intergenerational consequences of (mass) violence by examining possible mechanisms that are associated with and may contribute to the development of mental health problems in World War II and specifically Holocaust survivor offspring. More specifically, five possible mechanisms will be evaluated that have been identified on the basis of theoretical and empirical studies as factors that may play a critical role in HSO mental health (Kellermann, 2001; Leen-Feldner et al., 2013; McGuire, Palaniappan, & Larribas, 2015; Van IJzendoorn et al., 2003). The current review focused on: (a) parental mental health problems; (b) (perceived) parenting and attachment; (c) parental Holocaust history; (d) additional stress and traumatic life events in HSO; and (e) cortisol metabolism, epigenetic factors, and genetic predisposition.
1.1. Parental mental health problems
Severe mental illness may affect not only those suffering from it but also those who are in close personal contact with them (Lombardo & Motta, 2008). For example, parents with severe anxiety and/or depression may model patterns of thinking, feeling and behaving for their children (Katz, Hammen, & Brennan, 2013; Rasic, Hajek, Alda, & Uher, 2014). Low self-esteem, distrust towards fellow human beings, and a pessimistic outlook on the world in general and on the future may be the dominant message conveyed to their offspring. We hypothesized therefore that a higher incidence of current and lifetime mental health problems and psychiatric diagnoses in Holocaust survivors are related to a higher incidence of mental health problems in HSO.
1.2. (Perceived) parenting and attachment
The attachment theory prescribes parenting that is responsive and attuned to the needs of the young child to grow up, thrive and explore the world (Bowlby, 1982; Winnicott, 1971). Parents who have to deal with unresolved problems from their past, for instance loss or maltreatment, may have difficulty in attuning to the needs of their offspring, impacting the quality of the interactions of parents with their children. Parents may, for example, exhibit frightened, frightening, or unexpected behaviour when they associate stressful situations in their current life with traumatic experiences in the past. These parenting practices or dynamics in the parent–child relationship may, in turn, underlie disorganized attachment and contribute to offspring’s mental health problems (Hesse, 1999).
Furthermore, the caregiving style of Holocaust survivor parents has been characterized by a perceived inability to provide physical and emotional care and the perceived reversal of parent and child roles, as was stated by Wiseman et al. (2002) in their qualitative assessment of the characteristics of growing up in Holocaust survivor families (as perceived by offspring). Scharf and Mayseless (2011) indicated three major themes that characterized the parent–child relationship quality of HSO: Survival issues (e.g. overprotection and fear of separation), lack of emotional resources (e.g. emotional neglect and unpredictable emotional reaction), and coercion of the child to please the parents and satisfy their needs (e.g. push to achieve and role reversal). Following this, we hypothesized that Holocaust experiences of the parents are associated with an unfavourable attachment style and related to unfavourable psychological development of HSO.
1.3. Parental Holocaust history
As a result of the mere absence of family members due to the Holocaust, the offspring may have had less family support available compared to non-Holocaust offspring. Moreover, survivor parent(s) possibly were less able to provide direct and indirect care, such as acting as an adequate role model or providing emotional support and advise (Chaitin, 2002; Krell, Suedfeld, & Soriano, 2004; Wiseman et al., 2002). Children in one-survivor families (i.e., with the other parent alive and non-survivor) may be better off compared to children in two-survivor families, as the non-survivor parent can complement some of the tasks that are difficult for the survivor parent. We therefore hypothesized that growing up in a two-survivor family versus a one-survivor family is associated with more mental health problems in offspring.
Next, both parents will exert a different influence on the child’s psychological development, for example in the processes of socialization; mothers still being dominant as a caregiver in particular when children are young (Kellermann, 2008; Wiseman et al., 2002). Besides the difference in parenting style between fathers and mothers it is becoming increasingly clear that severe stress in mothers during pregnancy can affect the development of the unborn child (Glover, 2015; Reynolds et al., 2015; Taouk & Schulkin, 2016). We thus expected a higher incidence of mental health problems in offspring of mother survivors compared to father survivors.
1.4. Additional stress and traumatic life events in HSO
Several authors have suggested a diathesis-stress model that predicts heightened vulnerability in HSO for stressful life events occurring later in life (Kellermann, 2001; Van IJzendoorn et al., 2003). In other words, HSO may show increased vulnerability to develop psychological disturbances when affected by serious physical or psychological stressors additional to the familial Holocaust experiences, like breast cancer (Baider et al., 2000) or combat experiences (Solomon, Kotler, & Mikulincer, 1988). We thus hypothesized that HSO suffer from more mental health problems as a result of cumulating negative life events than non-survivor offspring.
1.5. Cortisol metabolism, epigenetic factors, genetic predisposition
Besides the impact of psychological mechanisms linking parental trauma and offspring mental distress, a growing number of studies have considered biological and (epi)genetic mechanisms linking parental trauma with changes in offspring’s cortisol metabolism compared to offspring of non-traumatized parents (e.g. Yehuda & Bierer, 2008b; Yehuda et al., 2005). It is becoming increasingly clear that parental stress, in a pre- or post-natal period, affects the stress system of offspring leading to epigenetic and cortisol level changes (Betancourt, 2015; Heim & Binder, 2012). The hypothalamic-pituitary-adrenal (HPA)-axis and the autonomic nervous system are central elements of the biological stress system. The HPA-axis functioning includes a cascade of neuroendocrine reactions with corticotrophin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), which stimulates the secretion of the glucocorticoid cortisol and a feedback loop of cortisol binding with mineralocorticoid receptors (MR) and glucocorticoid receptors (GR). During stress, cortisol levels are high. The feedback loop prevents the stress-reaction from cortisol overshooting and promotes restoration after stress. When this stress system is activated for a longer period, however, or if there is no proper negative feedback inhibition of cortisol, basal hormone levels are not properly restored and this can lead to disturbances of the stress response and, in the long run, to the development of various types of disease. People in conditions of acute or chronic stress, and without PTSD, have heightened cortisol levels. In contrast, in individuals with PTSD, basal cortisol levels have decreased and cortisol receptors appear to be more sensitive to cortisol.
In addition, (epigenetic) alterations have been associated with parental PTSD, major depression and intergenerational effects on cortisol metabolism. FKBP5 is one of the genes that have impact on the stress response and specifically on glucocorticoid receptor sensitivity (GR-sensitivity) and responsiveness by altered methylation (Naumova et al., 2016). Increased FKBP5 methylation gives rise to decreased GR-sensitivity. Cortisol levels and responsivity thus appear to be an important index of the stress system and were therefore used as an indicator for heightened stress levels within the current review (Duthie & Reynolds, 2013; Klaassens, 2010). We hypothesized that HSO show increased basal cortisol levels, show less cortisol reactivity, and increased FKBP5 methylation.
2. Methods
2.1. Search strategy, study selection, and data coding
We conducted a systematic literature search using the Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Liberati et al., 2009) to identify studies on offspring of World War Two survivors, published between 2000 and February 2018 with regard to the aforementioned factors. This timespan was chosen because studies up to 2000 were included in comprehensive former reviews (Kellermann, 2001; Van IJzendoorn et al., 2003). The search was performed using the following databases: PsycINFO, Pilots, Ovid Medline and Embase. The domains of the search and their synonyms were combined into syntaxes using Boolean operators. Only studies written in the English language were selected. The key-words have been chosen to closely align with the central concepts in our hypotheses. The list of key-terms (including synonyms) was as follows: Second World War, Holocaust, concentration camp, survivor, child, adult offspring, second generation, mental or psychological disorders, symptoms, specific disorders (e.g. PTSD, depression, anxiety disorders, personality disorders), mental illness, symptoms, comorbidities, well-being, quality of life, identity, individuation, neurobiology, neuropsychology, genetic, epigenetic, cortisol metabolism. For the full syntax (PsycINFO) see appendix 1.
Figure 1 contains a flowchart of the study selection. World War Two survivor offspring was defined as being born after the war had ended, and having had at least one parent that was exposed to World War Two cruelties. The studies were considered eligible for inclusion if they: (a) were written in the English language, (b) included World War Two survivors and offspring and (c) contained quantitative data. Excluded were (d) narrative or qualitative studies without quantitative data and (e) case reports, dissertations, book reviews, conference reports, theoretical papers and studies which had already been incorporated in earlier reviews (Kellermann, 2001; Van IJzendoorn et al., 2003).
Figure 1.
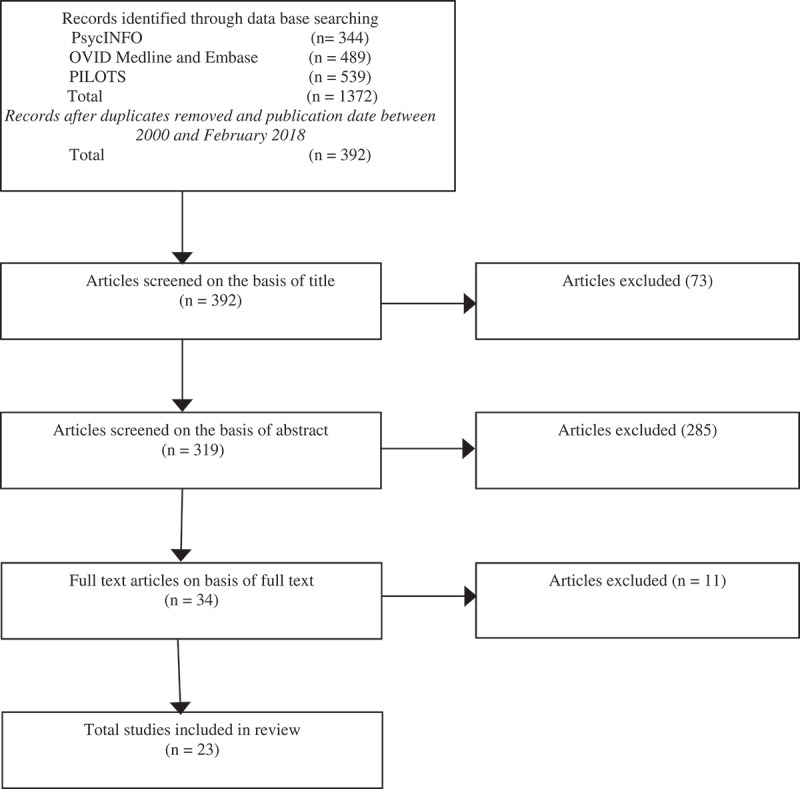
Flowchart study selection.
In the initial search, 1372 studies were retrieved. After excluding duplicates, 392 studies remained, and 319 remained after screening of titles. Next, two researchers (PD & RK) independently reviewed the abstracts. After screening the abstracts, 34 articles remained and based on the full text, the final selection consisted of 23 articles. The reviewers agreed on 98% of the selected studies. After the debate, consensus was achieved on the remaining studies. With regard to data extraction, two authors (RH & TM) independently checked the accuracy of the first reviewer (PD) who extracted the data from the 23 included studies. Disagreements were resolved by discussion.
3. Results
3.1. General study characteristics
The study characteristics of the selected studies are presented in Table 1. Several quality assessment tools were considered to evaluate the quality of the reviewed studies including the Cochrane Collaboration tool (Deeks et al., 2003; Downs & Black, 1998), the CASP-Qualitative Checklist (2018) and the quality assessment criteria forwarded by the Joanna Briggs Institute (Moola et al., 2017). Unfortunately, most criteria were not applicable to the studies included in this review and not useful to evaluate their quality. Only a few general items of the instruments were suitable. Applying only part of the criteria of those standardized checklists has the disadvantage of having an incomplete assessment score which will be hard to interpret and compare to other studies. Therefore, we focused on the relevant methodological variables (i.e., recruitment and sample details, instruments used for diagnosis, measurement of outcomes, and statistical results) mentioned in Table 1.
Table 1.
Study characteristics.
| Authors, year | HS sample characteristics: Number; gender; age; residence during the war | JC Comparison sample characteristics: Number, gender, age, residence |
HSO sample characteristics: Number; gender; age; residence during study |
JCO Comparison sample characteristics: Number, gender, age, residence |
Recruitment | HS response rate; HSO response rate | Offspring mental health complaints compared to JCO (outcome symptom measure) | Study focus |
|---|---|---|---|---|---|---|---|---|
| Bader, Bierer, Lehrner, Makotkine, Daskalakis, & Yehuda., 2014 | Convenience sample N= 69 Holocaust survivor offspring | N= 26 Jewish non-HSO | Through advertisements and participation in earlier study | NS 24-hr urinary cortisol Trend for difference in depression, life-time PTSD diagnosis, and childhood trauma |
Urinary cortisol | |||
| Baider et al., 2006 | Group 1 HSO + former breast cancer N = 193; all women; M age = 48.7, SD age = 5.0; Israel Group 3 HSO healthy: N = 176; all women; M age = 46.2, SD age = 5.8; Israel |
Group 2 former breast cancer non-traumatized parents: N= 164; all women; M age = 48.7, SD age = 6.8; Israel Group 4 healthy women non-traumatized parents: N = 143; all women; M age = 46.5, SD age = 8.1; Israel |
Former first-time breast cancer patients (i.e., no evidence of active disease at the time of study) recruited from list of all patients diagnosed with stages 1 and 2 breasts cancer in between 1994 and 2000 in two oncology centres. All HSO patients (group 1) and a random sample of non-HSO (group 2) were invited to participate. Random sample of healthy HSO (group 3) selected from the files of National Holocaust Archive Random sample from Israel Interior Ministry Census files (group 4). No current or previous psychiatric conditions. |
Group 1: 193/212 = 91% Group 2: 164/174 = 94% Group 3: 176/190 = 93% Group 4: 143/150 = 95% |
Psychological distress (intrusion, avoidance IES) sign higher in HOS with cancer than in comparisons with cancer. Of coping variables, only helplessness was different between HSO and comparisons (MAC). | Life time stressors, cancer diagnosis | ||
| Baider et al., 2008 | Mother-daughter dyads: Group 1 HS mothers N = 20; M age = 73.6, SD age = 6.6; Israel Group 3 HS mothers: N = 20; M age = 76.5, SD age = 7.6; Israel |
Mother-daughter dyads: Group 2 Non-traumatized mothers: N = 20; M age = 74.3, SD age = 10.1; Israel Group 4 Non-traumatized mothers: N= 20; M age = 72.5, SD age = 8.6; Israel |
Mother-daughter dyads: Group 1 HSO & former breast cancer N = 20; M age = 46.9, SD age = 7.1; Israel Group 3 HSO healthy: N= 20; M age = 45.4, SD age = 7.3; Israel |
Mother-daughter dyads: Group 2 former breast cancer patients: N = 20; M age = 46.3, SD age = 9.8; Israel Group 4 healthy women: N= 20; M age = 45.8, SD age = 6.8; Israel |
Random selection of 24 dyads out of each group included in Baider et al., 2006 | Group 1: 20/24 = 83% Group 2:19/24 = 79% Group 3: 22/24 = 92% Group 4: 20/24 = 83% |
Global Severity Index (BSI) differentiated between HSO with cancer and JCO with and HSO without cancer. | Life time stressors, cancer diagnosis |
| Bierer, Bader, Dasklalakis, Lehrner, Makotkine, Seckl, & Yehuda, 2014 | N= 85 HSO, 40% male; M age = 46.9, SD age = 7.6; Israel | N = 27 JCO, 48.1% male; M age = 42.6, SD age = 10.5; Israel | Through advertisements; in part among participants of earlier study. | HSO more lifetime anxiety disorder (SCID, d = 0.40), and stage-anxiety (STAI; d = 0.44)). Reduced cortisol excretion in HSO compared to JCO; 11ß-HSD-2 activity elevated in HSO compared to JCO, in particular among mothers who had been children during WWII (24-h urine sample). |
Glucocorticoid metabolism, 11ß-HSD-2 activity | |||
| Gangi et al., 2009 |
N = 40 Italian-Jew Holocaust survivor offspring, 33% had received psychotherapy. 50% Female, M age = 38, SD age = 12.4, Italy |
Comparison group of N = 37 Italian Jew offspring who were able to hide during the war. 43.2% Female, M age = 37, SD age = 13.7; Italy |
Recruited via Jewish register and after identification of those who had children. | Response rate 100% | HSO had higher anxiety levels, low self-esteem, inhibition of aggression, and relational ambivalence than JCO. | Intra-familial dynamics, e.g. organization, expression of emotions. | ||
| Halligan & Yehuda, 2002 | N= 87, 36% men, 64% women raised by parent(s) who survived the Nazi Holocaust | N = 39, 49% men, 51% women raised by parent(s) without Holocaust experience and free from current and lifetime axis I psychiatric disorders. | Participants were solicited from lists obtained from the Jewish community or responded to announcements and newspaper advertisements. In addition (N = 28) were enrolled through a specialized treatment programme. N = 7 participants new since Yehuda et al., 2001a. | Dissociative symptoms (DES) lower in JCO than sub-groups of HSO, being highest in HSO with current PTSD. | Mental health, PTSD in parents | |||
| Kellermann, 2008 | HS characteristics provided by HSO: N = 273 mothers; born between 1905–1945; 65% born in Eastern Europe, 19% born in other occupied countries, 14% non-HS N = 273 fathers; born between 1895–1946; 70% born in Eastern Europe, 19% born in other occupied countries, 9% non-HS |
N= 273 clinical offspring; 69% female; 48% born between 1945–1954, 37% born between 1955–1964, 15% born between 1965–1974; Israel | - | Consecutive admissions/referrals to a HSO specialized clinic | Information not provided | No comparison group | Identification of demographic factors | |
| Lehrner, Bierer, Passarelli, Pratchett, Flory, Bader, Makotkine & Yehuda, 2014 | N = 80, 83% women, 74% men, M age = 56.6, SD = 8.5; USA | N= 15, 60% women, 40% men, M age = 58.7, SD = 11.2; USA | Through print and online advertisements in Jewish news outlets, second generation and other Jewish electronic mailing lists, advertisements and by word-of-mouth (2010–2012). | HSO more likely than JCO to have a current anxiety disorder diagnosis (SCID; d = 0.45) and to report symptoms of depression (BDI; d= 0.78) and anxiety (STAI; d= 0.79), as well as to report more child abuse and neglect (d= 0.70; CTQ). HSO had higher 24-h urinary cortisol levels (LST). | Glucocorticoid sensitivity | |||
| Letzter-Pouw et al., 2014 | Sample one n= 172a; 48.3% women; M age 42.8, SD age = 7.3; Israel Sample two n= 161; 58.4% women; M age 55.4, SD age = 5.3; Israelis from families of European origin. At least one parent who was under Nazi or pro-Nazi occupation or domination in Europe during the Second World War. |
Sample two N= 124 parents without holocaust background; 54.4% women; M age 54.4, SD age = 5.7; |
Sample one nationally representative sample recruited by contacting everyone on a list (n = 272) provided by Ministry of Interior of persons living throughout Israel, born between 1928 and 1945 in a European country that suffered Nazi occupation and who immigrated to Israel after 1945. Sample two convenience sample community-dwelling, recruited across the country. |
Sample one HSO 63% Sample two HSO 60% |
Sample one was not compared Sample two HSO reported higher Holocaust salience (n2p = .36); transmission of burden (PPRBQ) from mother (n2p = .11) and father (n2p = .09). |
Perceived parental burden. | ||
| Letzter-Pouw & Werner, 2013 | N= 178 a dyads; (almost equally divided between both genders; M age 69.8, SD age = 5.1); 87% born in Eastern Europe, 13% born in Western Europe | N= 178 a, first-born; almost equally divided between both genders; M age = 43.8, SD age = 7.3); Israel | - | Sample recruited by contacting everyone on a representative list (N = 272) provided by national Ministry of Interior of persons living throughout Israel, born between 1928 and 1945 in a European country that suffered Nazi occupation and who immigrated to Israel after 1945. | HS 178/272 = 65%; HSO 178/272 = 65% | No comparison group | Intrusive memories in Holocaust child survivors and well-being of HSO. | |
| Sagi-Schwartz et al., 2003 | HS who immigrated as orphans from Europe to Israel during or after the war, HSO (N = 48). Born in Europe between 1926 and 1937. |
Comparison group of subjects in same age range, also born in Europe but immigrated to Israel before the war. |
N = 50 Mother offspring HSO, females, born between 1947 and 1970. |
JCO, females, born between 1947 and 1970. | Population register provided by Israeli government. Thereupon 30.000 standardized telephone calls. | HS showed more traumatic stress and less lack of resolution of trauma than JC (d = .77) HS fewer secure attachment representations than JC; HSO not different in attachment classification from JCO. |
Attachment impacted by Holocaust trauma | |
| Shrira, 2015 |
Study 1 N= 63; M age = 57.1, SD = 6.26, 61.9% women Study 2 N = 300 respondents with at least one Holocaust survivor parent. M age = 57.8, SD = 4.6, 59% women. |
N1= 43, M age= 54.7, SD= 8.56, 55.8% women N2= 150, M age = 57.12, SD= 4.64, 56.8% women. |
Study 1 Convenience sample of community-dwelling, Hebrew speaking Jewish Israelis from families of European origin living in Tel Aviv and its surroundings. Data collection in June 2012. Study 2 had similar procedures as in Study 1. Data collection 2012–2013. |
HSO reported higher Iranian nuclear threat salience (8-items) than JCO. HSO also reported more anxiety symptoms (Study 1, TMAS-SF); and psychological distress (Study 2, BSI-18). |
Coping with threat: Iranian nuclear threat salience | |||
| Shrira et al., 2011 | N= 215 born in 1945 or later, in Israel, Europe/USA or in the Former Soviet Union, with a father born in Europe/USA (except for 20 respondents who were born in Europe/USA but had a father from a non-European origin), | N = 149; neither parent had lived under Nazi or pro-Nazi occupation/domination | Probability sample drawn from the Israeli component of the Survey of Health, Ageing and Retirement in Europe. Interviewed in 2005–2006. Also, drop-off questionnaire. | 66.6% of total sample completed the questionnaire | Differences between HSO and JCO in number of major health problems, of physical symptoms and number of medications. HSO also reported higher optimism and hope, and better life satisfaction. |
Function of number of survivor parents. | ||
| Yehuda et al., 2008a | N = 211; 117 men, 167 women (M age = 43.2; SD age = 9.1; born 1938–1979) with at least one parent interned in a Nazi-concentration camp during WW II or had faced comparably severe threats in hiding. | N= 73 comparable age, with parents who were not exposed to the Holocaust or war-events. | Community sample recruited through advertisements. N = 145 new observations since Yehuda et al., 2001a. | A higher prevalence of lifetime PTSD, mood, anxiety disorders, and to a lesser extent, substance abuse disorders, was observed in HSO than in JCO (SCID, CAPS). | Maternal vs paternal PTSD and PTSD occurrence in HSO. | |||
| Yehuda & Bierer, 2008b |
N= 41 HSO N= 18 HSO with maternal PTSD; M age= 49.8, SD age =6.5; N = 6 men, N= 12 women N = 23 HSO without maternal PTSD (M age = 50.4, SD age = 7.3 N = 7 men, N= 16 women |
N= 19 JCO (M age = 44.4, SD age =9.5; N = 12 men, N= 7 women | Trauma exposure (Mississippi PTSD Scale; CTQ; PPQ) Depression symptoms (BDI) | Urinary and salivary cortisol levels PTSD risk |
||||
| Yehuda, Blair, Labinsky, Bierer, 2007a |
N= 25 HSO n = 23 HSO with PTSD n = 10 HSO without PTSD, USA |
N= 16 JCO, USA | Recruitment through advertisements. | Cortisol levels were lowest in HSO with parental PTSD (plasma levels), higher in HSO without parental PTSD and highest in JCO. | Plasma cortisol levels. | |||
| Yehuda et al., 2016 | N = 32, including both parents for 5 HSO; 37.5% male, 62.5% female, M age = 77.9, SD = 5.2. | N = 8, 25.0% male, 75.0% female, M age = 73.1, SD = 8.5. | N= 22, including multiple siblings in N = 2. 27.3% male, 72.7% female, M age = 46.0, SD = 8.0, USA | N= 9. 11.1% male, 88.9% female, M age = 47.0, SD = 8.5, USA | Dataset part of a larger sample of HSO, of which the majority was recruited 1993–1995 and longitudinally followed-up 10 years after. | Holocaust exposure had an effect on FKBP5 methylation observed in exposed parents as well as their offspring. Methylation was lower in HSO compared to controls. | Cytosine methylation within the gene encoding for FK506-binding-protein-5 (FKBP5) | |
| Yehuda, Daskalakis, Lehrner, Desarnaud, Bader, Makotikine, Flory, Bierer & Meaney, 2014 |
N = 80 HSO 75% had both parents exposed n = 53 (55.8%) maternal PTSD n = 42 (44.2%) paternal PTSD n = 15 no parental PTSD, USA |
N = 15 JCO no parental PTSD, USA | 95/120 = 79% response rate | Alterations of specific methylation were demonstrated in relation to parental PTSD and neuroendocrine outcomes. Interaction effect of paternal and maternal PTSD was found. | Influence of maternal and paternal PTSD on DNA methylation and its relationship to glucocorticoid receptor sensitivity | |||
| Yehuda et al., 2001a | N =95 (33.3% men, 66.7% women) having been born to at least one biological parent who experienced the Nazi Holocaust. | N = 40 (57% men, 43% women) Jewish individuals in the same age range who did not have a parent who was a Holocaust survivor, not necessarily without psychiatric diagnoses. | Recruitment from lists obtained from the Jewish community or through community group announcements (N = 109); or through a specialized treatment programme (N = 26). N = 42 (26 HSO; 16 JCO) subjects were new participants compared to prior publications. |
HSO differed from JCO in mean number of lifetime diagnoses, in particular PTSD, depressive and (trend:) anxiety disorders (SCID, CAPS). | Development of PTSD, depressive and anxiety disorders in HSO as a function of parental exposure and PTSD. | |||
| Yehuda et al., 2002 | N =39 (18% men, 82% women) having been born to at least one biological parent who experienced the Nazi Holocaust; USA. | N = 15 (53.3% men, 46.7% women) Jewish individuals in the same age range who did not have a parent who was a Holocaust survivor, free from psychiatric diagnoses; USA. | Described in Yehuda et al., (2000) | HSO and JCO did not differ in urinary cortisol concentration. The occurrence of (lifetime and current) psychiatric disorder was higher in HSO than in JCO. |
Cortisol levels related to severity of PTSD symptoms Number of parents affected |
|||
| Yehuda et al., 2001b | N= 51, 20 men, 31 women having been born to at least one biological parent who experienced the Nazi Holocaust. M age = 40.9, SD = 7.6; USA. | N = 41, 23 men, 18 women, with the same age range (24–60 years) who did not have a parent who was a Holocaust survivor. M age = 38.3, SD = 8.8; USA. | Participants were solicited from lists obtained from the Jewish community, through advertisements (n = 79), and via short-term group psychotherapy (n = 13). | HSO reported more emotional abuse, neglect, physical neglect and (trend:) sexual abuse than JCO (CTQ). | The impact of childhood trauma; influenced by parental trauma exposure and parental PTSD. | |||
| Yehuda et al., 2007b |
N = 33 HSO N = 23 HSO with parental PTSD N = 10 HSO no parental PTSD |
N = 16 JCO | Offspring with paternal PTSD only were not significantly different in mean cortisol level than offspring with no parental PTSD or comparison subjects (JCO). Mean cortisol levels were similar for offspring with PTSD in both parents and those with maternal PTSD only, whereas both groups differed from offspring with no parental PTSD (p= .02 and p= .045, respectively) and from comparison subjects (p= .009 and p= .02, respectively). | Cortisol levels related to parental PTSD | ||||
| Van IJzendoorn et al., 2013 | Mother-daughter dyads: HS parents: N= 32; 100% female, M age = 76.98, SD = 2.99, Israel |
JC parents: N= 33; 100% female; M age = 76.98, SD = 2.99. | Mother-daughter dyads: N= 47 HSO, M age = 47.46, SD = 4.41, daughters in Israel |
Mother-daughter dyads: N= 32 JCO M age = 47.46, SD = 4.41, daughters in Israel |
Recruitment through register of Israeli Ministry. | 82.3% first generation; 82.3% second generation | HSO showed lower cortisol levels only when surviving parents displayed more dissociation (whereas HS showed higher levels of daily cortisol versus comparisons). | Dissociation as moderating factor in the biological stress regulation system in HSO. |
a= Sample overlap, in the 2014 report, six participants were excluded as not eligible for the research purposes; HS = Holocaust Survivors; HSO = Holocaust Survivor offspring; JCO = Jewish comparisons offspring; Only outcome symptom measures relevant for the current review were included: BDI = Beck Depression Inventory (Beck et al., 1961); BSI = Brief Symptom Inventory (Derogatis & Melisaratos, 1983); CTQ = Childhood Trauma (Bernstein et al., 1997); CAPS = Clinician Administered PTSD Scale (Blake et al., 1990); DES = Dissociative Experiences Scale (Bernstein & Putnam, 1986); IES = Impact of Event Scale (Horowitz, Wilner, & Alvarez, 1979); MAC = Mental Adjustment to Cancer (Watson et al., 1988); NHSPQ/PPRBQ = New Holocaust Survivor Parenting Questionnaire/Perceived Parental Rearing behaviour Questionnaire (Kellermann, 2001); PPQ = Parental PTSD Scale (Yehuda et al., 2000; Yehuda et al., 2008a); SCID = Structured Clinical Interview for DSM-IV (Spitzer et al., 1995); STAI = Spielberger State Trait Anxiety Inventory (Spielberger, 1968); TMAS-SF = Taylor Manifest Anxiety Scale-Short Form (Bendig, 1956).
The final selection of studies all pertained to HSO; studies that focused on the intergenerational impact of any other World War Two survivors did not meet the inclusion criteria and therefore could not be included. Comparison groups in these studies mostly consisted of non-traumatized Jewish people (JCO). Most studies used convenient samples, recruited by advertisement or at conferences or meetings of Holocaust-related organizations. Sample-sizes were: Holocaust survivors N between 32 and 178, mean age range 69 to 76 year; HSO N between 20 and 300, mean age range 38 to 57; JCO N between 9 and 149, mean age range between 37 and 58. In some studies, the same, or partially overlapping, samples were used. Five studies included two generations (i.e., parents and offspring). Most studies included only offspring and data of the parents were obtained through reports of their children. Thirteen of the 23 studies included males and females, other studies consisted of only women. Participants in three studies were recruited through mental health-care providers. Usually, participants with severe mental disorders, such as psychosis or bipolar disorder, alcohol or substance dependence or major medical illness were excluded. All studies entailed a cross-sectional design. Comparing mental health problems between HSO and JCO (see Table 1), it can be concluded that HSO reported more lifetime symptoms of anxiety disorders (including PTSD according to DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association [APA], 2000)) and depression, lower self-esteem, and difficulties in interpersonal functioning, they also showed difficulties in aggression regulation, a higher vulnerability to psychological distress, and they showed biological changes such as lower cortisol levels and epigenetic changes, with lower FKBP5 methylation compared to JCO.
The results of the reviewed studies are displayed in Tables 2–6. Effect sizes are reported in the corresponding tables whenever the necessary data were provided.
Table 2.
Mental health complaints in parents and their children.
| Author | Results (assessment instruments) pertaining to parent psychopathology and functioning | Results (assessment instruments) pertaining to offspring psychopathology and functioning | Results (assessment instruments) pertaining to the association between parent and offspring psychopathology and functioning |
|---|---|---|---|
| Halligan & Yehuda, 2002 | N= 57 (66%) one or both parents with PTSD (PPQ) | Dissociative symptoms (DES) were elevated in individuals with current PTSD (CAPS), but not in those with past PTSD or with the risk factor of parental PTSD (PPQ). | 24 HSO with PTSD (27.5%) had one or both parents with PTSD (PPQ). Dissociative symptoms (DES) were only elevated in HSO with current PTSD and parental PTSD but were not elevated in other HSO with PTSD or with parental PTSD or parental Holocaust exposure without PTSD(PPQ). Of the n = 27 (31%) offspring with current or past PTSD, 24 (89%) indicated that their parent(s) had had PTSD. Of the n = 60 offspring without current or past PTSD, n = 33 (55%) indicated that their parent(s) had had PTSD (φ = .35). |
| Kellermann, 2008 | Perceived mental health father: Difficult 17% Middle 40% Good 28% Missing value 15% Perceived mental health mother: Difficult 24% Middle 37% Good 22% Missing value 17% Perceived functioning father: Fully 66% Partly 20% Impaired 1% Missing value 13% Perceived functioning mother: Fully 64% Partly 19% Impaired 3% Missing value 14% |
Not studied | Not studied |
| Letzter-Pouw & Werner, 2013 | Symptoms of psychological and physical distress M = 1.71, SD= 0.52 (BSI). 84% suffered from intrusive memories, M = 16.32, SD = 6.64 (IES) |
Symptoms of psychological and physical distress M = 1.51, SD = 0.48 (BSI). | HS psychological and physical distress (BSI) indirectly predicted offspring distress (BSI). The relation was mediated by perceived perceived mother’s “transmission” of trauma (NHSPQ/PPRBQ). |
| Letzter-Pouw et al., 2014 | PTSD symptoms M = 1.73, SD = 1.78 (CAPS) | After controlling for age, gender, education, and life events, perceived “transmission” of burden from mother (NHSPQ/ PPRBQ) (β= .31) [as well as number of survivor parents (β= .16]) predicted HSO posttraumatic symptoms (CAPS). In a separate analysis, and after controlling for age, gender, education, and life events, perceived “transmission” of burden from father (NHSPQ/ PPRBQ) predicted HSO posttraumatic symptoms (CAPS) (β= .23). |
|
| Lehrner et al., 2014 | Of the N = 80 parents with Holocaust exposure (11.6% father, 9.5% mother, 63.2% both parents), 32.6% both parents had PTSD, 23.2% maternal PTSD, and 11.6% paternal PTSD (PPQ). | 5.4% HSO had major depressive disorder, 41.1% anxiety disorder (MINI) | Not studied |
| Yehuda et al., 2001a |
N= 60 (64.5%) HS with PTSD (Parental PTSD scale) N= 33 (35,5%) HS without PTSD |
56% HSO had depressive disorder while 29% HSO had PTSD |
N = 93 HSO, of whom N= 60 (64.5%) parental PTSD; N = 33 (35.4%) HSO non-parental PTSD (PPQ); N = 42 JCO After controlling for gender, parental PTSD was associated with offspring PTSD (φ = .52) and with offspring depression (φ = .34). |
| Yehuda et al., 2001b | n = 32 (62.7%) HSO had one or both parents with PTSD, n = 17 (33%) had two parents with PTSD; n = 19 (37.3%) HSO without parental PTSD. | N= 17; 33.3% PTSD | Not studied |
| Yehuda et al., 2007a | n =13 HS with PTSD; N = 12 HS without PTSD; N = 16 JC | N = 4 (66.7%) HSO met criteria for lifetime PTSD (none for current PTSD) | Not studied |
| Yehuda et al., 2008a |
N= 211 HS N = 49 (30.4%) with paternal PTSD; N= 40 (24.8%) with maternal PTSD; N= 35 (21.7%) both parents with PTSD; N = 37 (23%) no parental PTSD (PPQ). |
69.5% HSO had any lifetime psychiatric diagnosis. More specifically, prevalence in HSO (N = 200): PTSD 19.0%; mood disorder 45.5%; anxiety disorder (32.5%); eating disorder 6.0%; substance abuse 10.5%, and adjustment disorder 10.0% (SCID DSM IV; CAPS). | Based on the prevalence rates reported for occurrence of PTSD, mood disorder or any psychiatric disorder in HSO, having a mother (OR 2.40) or both parents with PTSD (OR 3.21)increased the likelihood of having PTSD compared to having no parental PTSD. Higher association of mood disorders and parental PTSD versus non-parental PTSD (Paternal PTSD OR = 3.66, maternal PTSD OR = 3.06, both parents PTSD OR = 3.21). |
| Yehuda et al., 2014 |
N = 95 (75%) HSO both parents exposed n =31 both parents PTSD n= 53 (55.8%) maternal PTSD, n =22 only maternal PTSD; n =42 (44.2%) paternal PTSD n= 11 only paternal PTSD; n =31 no parental PTSD |
Measures of social-emotional functioning in HSO were used (BDI, CTQ, STAI, PEH, RSQ, DES. PPQ)) without reporting prevalence of psychiatric disorders in this sample. | Not studied |
| Yehuda et al., 2016 |
N= 16 (51.6%) PTSD N = 4 (13.8%) Anxiety disorder (other than PTSD) N = 9 (31.0%) Mood disorder (SCID, CAPS) As was retrospectively evaluated by HSO (PPQ): Maternal PTSD; N =11 (52.4%) Paternal PTSD; N = 11 (52.4%) Any parent with PTSD; N = 16 (76.2%) (PPQ) |
N = 8 (36.4%) Anxiety disorder (other than PTSD) N = 3 (13.6%) Mood disorder (SCID) |
Not studied |
HSO = Holocaust survivor offspring; JCO = offspring of Jewish comparisons; BDI = Beck Depression Inventory CAPS = Clinical Administered PTSD Scale (Blake et al., 1995); PDS = Posttraumatic Diagnostic scale (Foa et al., 1997); ; NHSPQ New Holocaust Survivor Questionnaire (Kellermann, 2001); PEH = Perceived Emotional Health (Flory, Bierer, & Yehuda, 2011); PPQ = Parental PTSD Scale (Yehuda et al., 2000; Yehuda et al., 2008a); PPRBQ = Perceived Parental Rearing Behaviour Questionnaire (Kellermann, 2001); RSQ = Relation Scales Questionnaire (Griffin & Bartholomew, 1994); SCID = Structured Clinical Interview for DSM-IV (Spitzer et al., 1995); STAI = Spielberger State-Trait Anxiety Inventory (Spielberger, 1968).
Table 6.
Biological parameters: cortisol, epigenetic factors and genetic predisposition.
| Author | Sample | Results on offspring biological parameters | Results pertaining to association between parent and offspring cortisol metabolism and epigenetics |
|---|---|---|---|
| Bader et al., 2014 | N = 69 | No significant difference in 24-h urinary cortisol levels between HSO and JCO. Significant difference urinary cortisol in offspring of mothers who were adults (low) and who were children (high) (r = −.35) No significant difference in each combination between HSO with mothers exposed in childhood and in adolescence or not exposed. |
Controlled for age, gender, and current depressive disorder, maternal age at Holocaust (β = −.56) and maternal PTSD (β = −.32) (d =0.43) were independent predictors of lower offspring urinary cortisol, whereas offspring childhood adversity and offspring PTSD symptoms were not. No interaction of effect of PTSD and maternal age of exposure. No relation of mother’s age at birth with cortisol levels in HSO. Significant effect of maternal PTSD (β = −.32) on HSO 24-h cortisol levels (lower levels when mother had PTSD). Significant main effect of maternal PTSD on HSO urinary cortisol d = .64. |
| Bierer et al., 2014 |
N = 85 female HSO N = 27 female JCO |
24 h cortisol level HSO lower than JCO d= 0.45 and 5α-THF d = 0.34 Total glucocorticoid d= 0.43. Significant difference in 11β-HSD-2- activity between maternal exposure in childhood and JCO p =.029. HSO showed trend significant lower levels of cortisol (d= .45), 5α-THF (d= .39) (major metabolite of cortisol) and total glucocorticoids (d= .43) compared to JCO. No sign differences in the levels of other metabolites. |
11β-HSD-2 activity significantly elevated in HSO when mothers exposed to Holocaust in childhood (β = .25). 11β-HSD-2 activity was higher between HSO mothers without PTSD than with PTSD and JCO this remained also significant after including father with PTSD as covariate. No effect of gender on 11β-HSD-2 activity. Maternal age of exposure, rather than maternal PTSD, predicted offspring 11β-HSD-2 activity (β = .34). |
| Lehrner et al., 2014 |
N = 95 HSO N = 26 JCO |
Maternal PTSD only HSO cortisol suppression on DST 69.75%, both parents PTSD 82.49% Paternal PTSD higher 24-h urinary cortisol levels in HSO than when mother had PTSD d = −.84 and when both parents had PTSD |
Maternal PTSD associated with significantly higher glucocorticoid sensitivity and lower 24-h urinary cortisol excretion in HSO (β= – .41). This was the same when both maternal and paternal PTSD was present. When only the father had PTSD, an opposite effect was observed lower glucocorticoid sensitivity and higher 24-h urinary cortisol excretion. |
| Van IJzendoorn et al., 2013 |
N= 29 survivor parents N = 45 HSO daughters N = 29 matched JC non-survivor parents N = 29 JCO daughters All female and living in Israel |
No sign difference in cortisol levels of HSO compared to non-HSO | Significantly lower levels of daily salivary cortisol in HSO with survivor parents with higher scores on dissociation (DES) d= .73 |
| Yehuda et al., 2001b |
N= 51 HSO N = 41 JCO |
N = 20 HSO + parental PTSD (24-h cortisol secretion M = 42.06 SD= 21.87) N = 8 HSO no parental PTSD (24-h cortisol secretion M = 67.90 SD= 29.82) (d = 0.895). |
24-h Urinary cortisol excretion significantly lower in offspring with parental PTSD compared to offspring without parental PTSD (d = .92) and JCO. Emotional abuse and parental PTSD appear to be associated with low cortisol and risk for PTSD. |
| Yehuda et al., 2002 |
N = 39 HSO N = 15 JCO |
Offspring cortisol levels significantly associated with sum of PTSD symptoms severity of father and mother combined (r= 0.40). | 24-h Urinary cortisol levels in HSO were associated with parental PTSD symptoms (r= −.40) as much as with their own PTSD symptoms (r = −.47). |
| Yehuda et al., 2007a |
N = 16 JCO N = 25 HSO |
N= 12 HSO no parental PTSD N = 13 HSO + parental PTSD higher cortisol DEX suppression in HSO with parental PTSD than without parental PTSD d = 0.93) or JCO (d = 0.91) but not significant between HSO without parental PTSD and JCO |
an association persisted between cortisol suppression and parental PTSD after controlling for childhood trauma and HSO own PTSD .r = −.35 |
| Yehuda et al., 2007b |
N = 33 HSO N = 16 JCO |
N = 23 HSO with parental PTSD N = 10 HSO no parental PTSD The estimated mean ± SE plasma cortisol levels were 8.92 ± .41 μg/dL for JCO; 8.84 ± 0.52 μg/dL for offspring without parental PTSD; and with one 7.39 ± 0.44 μg/dL or two parents 6.97 ± 0.56 μg/dL with PTSD. |
When the whole sample was considered, there was a significant association between mean cortisol levels and severity of parental PTSD (r42 = −0.41) that was reduced when only Holocaust offspring were considered (r26 = −0.39) and was further reduced to non-significance when examined in the smaller offspring subgroup with parental PTSD (r16 = −0.36). Offspring with maternal r = −.41 or paternal PTSD r = −.32 only however after additionally controlling for PTSD in the other parent only maternal PTSD retained significant association with HSO cortisol level (paternal r = −.21; maternal r = −.34). |
| Yehuda & Bierer, 2008b |
N = 41 HSO N = 19 JCO |
N = 6 only paternal PTSD and N = 16 no parental PTSD mean 24-h urinary cortisol level no significant difference. N = 9 both parents PTSD and N = 8 only maternal PTSD similar mean 24-h urinary cortisol and differed from no parental PTSD and JCO. HSO and maternal PTSD lower 24h urinary cortisol than without maternal PTSD (r = −.61); HSO without maternal PTSD trend lower level than JCO (r = −.17). |
Significant negative association of maternal overprotection and PTSD with offspring mean cortisol (r = −.54). |
| Yehuda et al., 2014 |
N = 120 HSO N = 95 (75%) HSO both parents exposed n = 31 both parents PTSD n= 53 (55.8%) maternal PTSD; n = 43 (44.2%) paternal PTSD n = 11 only maternal PTSD; n = 11 only paternal PTSD; n = 31 no parental PTSD |
In the absence of maternal PTSD, offspring with paternal PTSD only showed higher GR-1F promotor methylation, whereas offspring with both maternal and paternal PTSD showed lower GR-1F promotor methylation (d= .75; r = .35). GR-1F promotor methylation was negatively correlated with GR-1F expression (% methylation: r = −.35; number of methylated sites: r= −.36), indicating the validity of the GR-1F promotor methylation procedures. |
Presence or absence of maternal PTSD moderated paternal PTSD effect on GR-1F promotor methylation. Only paternal PTSD higher GR-1F promotor methylation. Both parents PTSD lower GR-1F promotor methylation. HS both parents and without PTSD no effect of exposure or interaction between maternal and paternal exposure. |
| Yehuda et al., 2016 |
N = 32 survivor parents N = 22 HSO N = 8 JC parents N = 9 JCO |
HS bin/3site 6 methylation correlated with HSO methylation at the same site (r = .44). Parental Holocaust exposure significant predictor of HSO bin/3site 6 methylation parental PTSD and FKBP5 risk-allele, childhood adversity and emotional abuse were not significant associated. FKBP5 methylation is seen in Holocaust survivors (higher than comparison) and their offspring (lower than comparison) on the same site in a functional intronic region of FKBP5 in the opposite direction (β= −.37). |
No significant associations were found of the FKBP5 risk-allele with HSO own psychopathology, trauma-exposure or other examined characteristics that might independently affect methylation of this gene. Epigenetic changes were demonstrated (by changes in methylation levels) in two generations (HS and HSO) that were correlated (r = .44). After controlling for FKBP5 risk allele the association remained (r = .56), after regression bin 3/site 6 methylation parental Holocaust exposure remained significant (β = .42). Bin/3site 6 methylation Holocaust exposed correlated with HSO methylation at the same site, the presence of FKBP5 risk-allele in both generations did not substantially alter the association of bin 3/site 6 methylation between survivor and offspring (r = .44) or within Holocaust-exposed families (r = .56). |
HSO = Holocaust survivor offspring; JCO = offspring of Jewish comparisons; 11β-HSD-2 = 11β-hydroxysteroid-dehydrogenase type 2; FKBP5 = FK506-binding-protein-5 gene; PBMCs = peripheral blood mononuclear cells; IC50-DEX = concentration at which lysozyme activity is diminished by 50%; DST = dexamethasone suppression test.
3.2. Association between parental and offspring’s mental health problems
The association between mental health problems of the Holocaust survivors and of their offspring born after the war has been the subject of five studies (see Table 2). Letzter-Pouw and Werner (2013) first of all found no direct association between survivor and offspring symptoms of psychological and physical distress in a sample of 178 Holocaust survivors and their first-born offspring. However, they did find a significant indirect relation, with survivor and offspring distress mediated by the perceived mother’s transmission of trauma by the offspring. Also, in another study, posttraumatic symptoms in offspring were significantly predicted by perceived ‘transmission’ of parental burden (medium effect size), determined by items such as ‘My parent transmitted his or her burden of the Holocaust onto me’ (Letzter-Pouw, Shrira, Ben-Ezra, & Palgi, 2014).
Other studies did find a direct association between Holocaust survivors and HSO’s mental symptoms or diagnoses, in particular Posttraumatic Stress Disorder (PTSD). In their study of 2001, first of all, Yehuda, Halligan, and Bierer (2001a) pointed to parental PTSD as a significant predictor of the occurrence of PTSD in HSO (large effect size). In a later study Yehuda, Bell, Bierer, and Schmeidler (2008a) found an association between maternal (or both parents with) PTSD and PTSD, mood disorder or any psychiatric disorders among offspring (large effect size) Next, Halligan and Yehuda (2002) investigated whether dissociative symptoms were related to the impact of parental PTSD on the mental health condition of their offspring. Parental PTSD was reported more often by HSO with PTSD than without PTSD. Their findings demonstrated that dissociative symptoms in HSO were significantly elevated in HSO with current PTSD and parental PTSD (medium effect size), whereas this elevation was absent in offspring with past PTSD, only parental PTSD or only parental Holocaust exposure (Halligan & Yehuda, 2002).
We expected a significant association between psychiatric symptoms in survivors and offspring. In line with this, evidence was found for associations between parental PTSD after Holocaust experiences and current psychiatric symptoms (including PTSD and anxiety/mood symptoms) in offspring. The correlation between parental PTSD and depression or PTSD among HSO appeared to be different among offspring samples with paternal, maternal or both parents with PTSD. A significant relation with large effect sizes has been found between maternal PTSD and PTSD in offspring, while PTSD in both parents may be related to either PTSD or depressive symptoms in the next generation (Yehuda et al., 2008a).
3.3. Perceived parenting and attachment
The perceived quality of the parent–child relationship, the (perceived) quality of parenting and the family climate, and/or attachment characteristics were assessed in 10 studies (see Table 3). Letzter-Pouw and Werner (2013) reported in particular the impact of mother’s ‘transmission’ of Holocaust: offspring experienced their mothers as significantly more affectionate (small effect size) and demonstrating more over-involvement than fathers (large effect size), and this style of mothering significantly predicted posttraumatic symptoms at HSO (large effect size) (Letzter-Pouw et al., 2014). As noted above, this perceived ‘transmission’ of trauma by mother mediated the relation between survivor and offspring distress (Letzter-Pouw & Werner, 2013). Parental PTSD was significantly associated with higher reported occurrence of child maltreatment in HSO, more specifically emotional abuse and neglect, physical neglect and sexual abuse (medium to large effect sizes) (Yehuda, Blair, Labinsky, & Bierer, 2007a; Yehuda, Halligan, & Grossman, 2001b). Mental health symptoms were highly correlated (large effect size) with emotional abuse, physical neglect and high CTQ scores (Yehuda et al., 2007a).
Table 3.
Perceived parenting, attachment and mental health complaints in HSO.
| Author | HSO results on attachment/perceived parenting (instruments) | Results pertaining to association between parent and offspring attachment and offspring mental health |
|---|---|---|
| Gangi et al., 2009 | HSO differed from JCO in terms of perceiving their family as expressing emotions poorly (d= .56); not being assertive and make their own decisions (d= .57); attribute greater importance on organizing and planning of family activities and responsibilities (d= .51) and put greater emphasis on following family rules (d= .43). They described their ideal family as being strongly oriented towards competition and accepting challenges (d= .64) (FES). HSO did not differ from JCO on scales of family cohesion, family conflict, achievement orientation, intellectual-cultural orientation, active-recreational orientation, and moral-religious basis. |
Not studied |
| Lehrner et al., 2014 | Emotional abuse (CTQ) was positively associated with both maternal and paternal PTSD (r = .30; r = .32). | Of family environment factors (cohesion, expressiveness, conflict, organization, and control; FES) only conflict was correlated to glucocorticoid sensitivity (LST) in HSO. When family conflict was included as a covariate including maternal and paternal PTSD and Holocaust exposure, the main effect of maternal PTSD was unchanged. Family conflict moreover, was correlated with paternal, but not maternal PTSD (r = .36). Emotional abuse and family conflict moderated the effects of parental PTSD on stress sensitivity in offspring. |
| Letzter-Pouw & Werner, 2013 | Perceived parenting: HSO reported more affection (d = .28), over-involvement (d = .54), and transmission (d = .31) of the Holocaust from mothers than fathers, no differences between mothers and fathers on punishing (NHSPQ). | The relation between HS psychological and physical distress (BSI) and HSO distress (BSI) was mediated by perceived parenting, more specifically perceived mother’s “transmission” of trauma (NHSPQ/PPRBQ). |
| Letzter-Pouw et al., 2014 | Sample one Mothers were perceived to transmit more burden to their offspring than fathers (NHSPQ) (d = .33). Sample two HSO perceived more transmission of burden from mother (d= .70) and father (d = .64) versus comparisons (NHSPQ). |
After controlling for age, gender, education, and life events, perceived “transmission” of burden from mother (NHSPQ/ PPRBQ) (β= .31) (as well as number of survivor parents (β= .16) predicted HSO posttraumatic symptoms (CAPS). In a separate analysis, and after controlling for age, gender, education, and life events, perceived “transmission” of burden from father (NHSPQ/ PPRBQ) predicted HSO posttraumatic symptoms (CAPS) (β= .23). |
| Sagi-Schwartz et al., 2003 | No differences in proportion HSO (54%) and JCO (42%) with insecure attachment (AAI). No differences in proportion HSO (17%) and JCO (8%) with unresolved loss (AAI) No difference between HSO and JCO on mother-infant interactions (disorganizing maternal behaviours; MIDBS). No differences between HSO and JCO in satisfaction with relationship with mothers (CS). |
Interaction between attachment type (secure vs insecure) x generation (first, second) indicated less insecure attachment in the second generation, both for the Holocaust and comparison group.φ = .07 In 60% of the cases, survivors and offspring show the same (secure or insecure) attachment representation. Insecure attachment in 77% of HS and HSO dyads vs. 54% in the comparison sample. Insecure attachment representation of mothers among HSO, higher but the same as intergenerational attachment representation in general (kappa = .21). No evidence of intergenerational transmission of unresolved attachment Interaction of unresolved loss and trauma between HS and HSO (φ= .53). |
| Yehuda et al., 2001b | Adult Holocaust survivor offspring reported significant more childhood trauma, particularly emotional abuse (d = 1.02), emotional neglect (d = .96) and physical neglect (d = .94), compared to non-Holocaust survivor offspring. | Parental PTSD was associated with a higher incidence of emotional abuse (66% with parental PTSD vs. 37% without parental PTSD), and physical neglect (56% vs 21%). CTQ scores were associated with PTSD in HSO for emotional abuse r = .24; emotional neglect r = .34; physical neglect r = .36 and sexual abuse r = .27. |
| Yehuda & Bierer, 2008b | HSO with maternal PTSD (n= 23) HSO without maternal PTSD (n= 18) | Based on the PBS HSO with maternal PTSD had significantly lower scores on perceived maternal care and higher scores on maternal overprotection than HSO without maternal PTSD paternal values were not significant. |
| Yehuda et al., 2007a | Parental PTSD symptoms were significantly correlated with childhood emotional abuse (r = .33) and physical neglect (r = .38) (CTQ), and total CTQ score (r = .41). | HSO mental health symptoms (CMS) were correlated with childhood emotional abuse (r = .55), physical neglect (r = .49) (CTQ) and total CTQ score (r = .52). |
| Yehuda et al., 2007b | HSO with parental PTSD reported significantly more negative consequences of being raised by Holocaust survivor parents than those without parental PTSD (d= 1.28) (CTQ). | Not studied |
| Yehuda et al., 2016 | No significant differences in childhood trauma (CTQ) between HSO and JCO. | Parental trauma, more than offspring’s own childhood trauma (CTQ), impacted on changes of epigenetic markers (methylation at different gene-sites) (β= −.36). |
HSO = Holocaust survivor offspring; JCO = offspring of Jewish comparisons; AAI = Adult Attachment Inventory (Hesse, 1999); CAPS = Clinician Administered PTSD Scale (Blake et al., 1990); CS = Caregiving Scale, Scale especially designed for this study; CTQ = Childhood Trauma Questionnaire (Bernstein et al., 1997); FES = Family Environment Scale (Moos & Moos, 1994); LST = Lysozyme suppression test; NSPHQ = New Holocaust Survivor Parenting Questionnaire (Kellermann, 2001); PPRBQ = Perceived Parental Rearing Behaviour Questionnaire (Kellermann, 2001); CMS = Civilian Mississippi Scale (Keane et al., 1988); MIDBS = Maternal Inappropriate and Disorganizing Behaviour Scale (Lyons-Ruth et al., 1999).
In order to examine the social climate in the families, the Family Environment Scale (FES) was used in two studies (Gangi, Talamo, & Ferracuti, 2009; Lehrner et al., 2014). Results indicated that offspring of Holocaust survivors could be distinguished significantly (medium effect sizes) from Jewish comparisons by a number of characteristics: They perceived their family as expressing emotions more poorly than offspring of comparison families. Next, HSO parents were likely to attribute greater importance to organizing and planning of family activities and responsibilities, and to put greater emphasis on following family rules. Family goals were considered strongly oriented towards competition and accepting challenges. Moreover, offspring reported to be less assertive and less able to make their own decisions. Holocaust offspring in this study did not differ significantly from comparisons on their reports of family cohesion, family conflict, achievement orientation, intellectual-cultural orientation, active-recreational orientation, and moral-religious emphasis (Gangi et al., 2009).
Lehrner et al. (2014) studied the moderating effects of parenting style and family functioning on the relation between survivor PTSD and offspring mental health symptoms. They found that both maternal and paternal PTSD were significantly related to emotional abuse in the family rearing style, and family conflict was significantly associated with paternal, rather than maternal PTSD (all medium effect sizes). In HSO with maternal PTSD, perceived maternal care was significantly lower while maternal overprotection was experienced as higher. No significant association with paternal PTSD was found (Yehuda & Bierer, 2008b).
The Adult Attachment Interview (AAI; Hesse, 1999) was used to assesses the attachment style between parents (caregivers) and children and adult mental representations of childhood attachment experiences, including loss and trauma experiences (Sagi-Schwartz et al., 2003). It was demonstrated that a non-clinical sample of Holocaust survivor mothers showed significantly more insecure attachment than a comparison group of non-survivor mothers. More specifically, survivor mothers scored high on unresolved attachment (i.e., either a disoriented attachment style because of lack of resolution of loss and trauma or a mixture of diverging insecure attachment styles). In contrast, Holocaust offspring showed no evidence of higher insecure attachment classification compared to the comparison group.
Finally, the data were consistent with the hypothesis that Holocaust survivor parents were not always able to be responsive and attuned to the child because of their traumatic experiences and mental symptoms. Overall, the reviewed studies demonstrated with medium to large effect sizes, a significant association between (perceived) parental PTSD symptoms and childhood trauma experiences of HSO. In particular a significant association with experiences of emotional abuse, neglect, and physical neglect (e.g. Yehuda et al., 2007a, 2001b), was found in parents, who were diagnosed with PTSD, emotional abuse and family conflict moderated the relationship between PTSD and offspring’s glucocorticoid sensitivity (Lehrner et al., 2014). Yehuda et al. (2016) reported significantly higher prediction of epigenetic consequences from parental trauma than offspring’s own childhood trauma (medium effect size).
3.4. Parental Holocaust history
Five studies specifically addressed the association of having either one or two Holocaust survivor parents, and/or survivor gender with the offspring’s mental health problems (see Table 4). Having two parents who were survivors of the Holocaust instead of one parent was significantly associated with more intrusive memories and other posttraumatic symptoms in the offspring group (Letzter-Pouw et al., 2014; Letzter-Pouw & Werner, 2013). Although being raised by at least one Holocaust survivor parent was associated with a risk of less optimal or adequate parenting in the study by Lehrner et al. (2014), no further data were provided on mental health outcomes related to which parent was a survivor in this study. Research of Letzter-Pouw and Werner (2013, 2014) indicated that offspring’s mental health problems as well as their experience of parental trauma transmission, with transmission defined by the authors as the extent to which they received the inner pains of their parents, in turn causing them to feel responsible for their parents, was significantly more pronounced in cases when their mother was a Holocaust survivor compared to when their father was (small to medium effect sizes). The offspring reported more affection, more overinvolvement, and more ‘transmission of the Holocaust’ from their mothers than from their fathers (Letzter-Pouw et al., 2014).
Table 4.
Parental Holocaust history and mental health complaints in HSO.
| Author | Results (assessment instruments) pertaining to parental Holocaust history and mental health outcomes in offspring | Results (assessment instruments) pertaining to parental gender and mental health outcomes in offspring |
|---|---|---|
| Letzter-Pouw & Werner, 2013 | 57.3% had two HS parents. Having two HS parents was associated with more intrusive memories (IES) (r= .38). |
Perceived parenthood: HSO reported more affection (d = .28), over-involvement (d = .54), and transmission (d= .31) of the Holocaust from mothers than fathers, no differences between mothers and fathers on punishing (NHSPQ). Mother’s transmission of trauma (NHSPQ) was related to psychological distress (BSI) and with offspring’s psychological coping resources Mother’s transmission of the Holocaust (NHSPQ) related to intrusive memories (IES; r = .24). No association with perceived affection, punishment or over-involvement and no association with father’s perceived parenthood (NHSPQ). |
| Letzter-Pouw et al., 2014 | Sample one 57.0% had two HS parents. HSO who had two HS parents showed more posttraumatic symptoms (CAPS) than those with one HS parent (d = .34). |
Sample one Mothers were perceived to transmit more burden to their offspring than fathers (NHSPQ) (d = .33). After controlling for age, gender, education and life events, perceived transmission from mother (β= .31) and father (NHSPQ) (β= .23) were positively related with posttraumatic symptoms (CAPS). Sample two HSO perceived more transmission of burden from mother (d = .70) and father (d = .64) versus comparisons (NHSPQ). |
| Shrira et al., 2011 | 10.2% (n = 37) had one HS parent; 49% (n = 178) had two HS parents with a higher proportion of immigrants from Europe and the USA (φ= .46). There were no differences between both groups in health reports, life satisfaction or optimism and hope. | Not studied. |
| Yehuda et al., 2001b | No significant differences between offspring with one versus two parents with PTSD on CTQ scales. | There was a similar relationship between childhood trauma (abuse and neglect; CTQ total scores) and maternal (r = .45) and paternal (r = .39) PTSD symptoms. |
| Yehuda et al., 2008a | 70.5% (n= 200) had two HS parents; of these 49 fathers had PTSD; 40 mothers had PTSD, while 35 HSO had two parents with PTSD. The relationship of HS exposure and mental health in HSO was not studied. | Prevalence of PTSD among offspring impacted by maternal (φ= .27), not paternal PTSD (φ= .12) (PPQ, CAPS). Depressive disorder in offspring was significantly associated with paternal PTSD (48.1% compared to 17.6% in the comparison group) OR = .73, maternal PTSD (46.3%) OR 2.40; the effect cumulated when both parents had PTSD (56.9%) OR 3.21. Females with a father who had PTSD were more likely to develop PTSD, while males with a father who had PTSD were slightly less likely to develop PTSD φ = .21. |
Note. HSO = Holocaust survivor offspring; JCO = offspring of Jewish comparisons; Correlation was only included when zero-order correlations were provided. SCID = Structured Clinical Interview for DSM IV (Spitzer et al., 1995); STAI = Spielberger State-Trait Anxiety Inventory (Spielberger, 1968); CTQ = Childhood Trauma Questionnaire (Bernstein et al., 1997); FES = Family Environment Scale (Horowitz, 1979); PPQ = Parental PTSD Questionnaire (Yehuda et al., 2000).
Yehuda et al. (2008a) compared offspring with either a mother, a father or both parents with a lifetime diagnosis of PTSD. Maternal PTSD was associated with a significantly higher prevalence of lifetime PTSD among offspring and the effect was stronger in the co-presence of paternal PTSD (medium effect size). Depressive disorder in offspring was significantly associated with maternal and/or paternal PTSD, while the effect was strongest when both parents had PTSD. In this study, Yehuda et al. (2008a) found a differential effect of parental PTSD on sons and daughters. Daughters of a father who had PTSD were more likely to develop PTSD, while sons with a father with PTSD were slightly less likely to develop PTSD (small effect size) (Yehuda et al., 2008a).
Finally in a study by Shrira, Palgi, Ben-Ezra, and Shmotkin (2011), no significant differences were found between offspring with one or with both parents being Holocaust survivors, they did not differ with respect to self-reported health, life satisfaction and optimism and hope (Shrira et al., 2011). The absence of significant differences between HSO having either one or two parents with Holocaust exposure was also reported by Yehuda et al. (2001b). The studies agree, although with small to medium effect sizes, with our hypothesis that parental gender is associated with the impact of mental health problems in HSO. More specifically, the presence of Holocaust survivor mothers was related to a higher prevalence of HSO distress, anxiety disorders, mood disorders, and substance abuse than in JCO. Having two survivor parents instead of one increased the risk for (biological) vulnerability, stress sensitivity, and mental health problems in adulthood. This relationship was especially pronounced in the presence of maternal PTSD. Lifetime PTSD and depression in HSO were higher in the presence of maternal PTSD and increased when paternal PTSD was also present. Daughters of a father who had PTSD were more likely to develop PTSD than sons of a father with PTSD.
3.5. Additional stress and traumatic life events in HSO
We found two studies with regard to the development of mental health symptoms resulting from additional stress and traumatic experiences (see Table 5). First of all, a study by Baider, Goldzweig, Ever-Hadani, and Peretz (2006, 2008) distinguished four groups: An HSO and a non-HSO group either with or without a diagnosis of breast cancer. The results indicated that coping with breast cancer was significantly more strongly characterized by helplessness and hopelessness in the HSO than the non-HSO group (medium effect size) (Baider et al., 2006; Baider, Goldzweig, Ever-Hadani, & Peretz, 2008). They also scored significantly higher on measures of posttraumatic symptoms (i.e., intrusions and avoidance) as well as general psychological distress when faced with a diagnosis of breast cancer compared to non-HSO cancer patients (large effect sizes). The association between symptoms and being HSO was even stronger compared to the association between having symptoms and having a diagnosis of cancer. In addition, the cumulative effect of having Holocaust parents and a diagnosis of breast cancer was significantly higher than the impact of each single factor on symptoms of depression and psychoticism but not on other BSI subscales of distress or general level of distress. Thus, the psychological burden of cancer was larger for these women than for women with non-traumatized mothers, supporting the hypothesis of heightened vulnerability in HSO (i.e., but only for depression and psychoticism) (Baider et al., 2006, 2008).
Table 5.
Heightened vulnerability to the development of mental complaints after additional stress and traumatic life events in HSO.
| Author | Additional stress/traumatic life event HSO | Results (and instruments) pertaining to association between additional HSO exposure to stress/traumatic events, coping, and mental distress |
|---|---|---|
| Baider et al., 2006 | Breast cancer | Psychological distress levels (BSI), intrusions (d = −.68) and avoidance (d = .84) (IES), and helplessness/hopelessness coping (d = .29) (MAC) higher in HSO patients with breast cancer compared to non-HSO cancer patients. No difference on other coping styles (fighting spirit, anxious preoccupation, fatalistic acceptance (MAC). Effects of being HSO on most subscales of the BSI larger compared to effect of diagnosis of cancer (e.g. effect of being HSO generation (d = .76) compared to diagnosis of cancer (d = .49) on distress levels GSI scale BSI). Interaction between having breast cancer and being HSO on depression and psychoticism (BSI). The impact of cancer on the levels of depression and psychoticism in HSO was significantly stronger than the impact of cancer in the controls. |
| Baider et al., 2008 | Breast cancer | GSI score (BSI) highest in HSO with breast cancer compared to other three groups. Controlling for the significant effects of mothers’ distress and being a second-generation daughter, among others, the impact of cancer diagnosis on daughter level of distress was not significant (GSI index BSI). |
| Shrira et al., 2011 | Various, cumulative life stressors | Cumulative life event distress (TEI) did not have more of an effect on middle-aged HSO relative to the comparison group. HSO seem to cope with stress as well as others. |
| Shrira, 2015 | Iranian nuclear threat; and the perception of a hostile world | Iranian nuclear threat salience (constructed for this study) studied in two HSO samples (n1 = 106’ n2 = 450) and related to anxiety symptoms (TMAS-S): among HSO, Iranian nuclear threat salience showed a strong relationship to anxiety symptoms (r= .33). Probing the interaction showed that, although among comparisons there was no relationship between Iranian nuclear threat salience and anxiety symptoms (β= 0.07), among HSO, Iranian nuclear threat salience showed a strong relationship to anxiety symptoms (β= 3.24) This relation was not mediated by a perception of the world as hostile by HSO. |
aPartly same sample. HSO = Holocaust survivor offspring; IES = Impact of Event Scale (Horowitz et al., 1979); GSI = Global Inventory Index, based on BSI (Derogatis et al., 1982); MAC = Mental Adjustment to Cancer (Watson, 1988); HWS = Hostile World Scenario (Shrira et al., 2011); TEI = Traumatic Events Inventory (Shmotkin et al., 2009)); TMAS-S = Taylor Manifest Anxiety Scale – Short Form (Bendig, 1956).
Shrira (2015) and Shrira et al. (2011) demonstrated that the mere threat of a disaster in itself is not related to heightened stress levels in Holocaust survivor offspring. They conducted a study among Israeli civilian HSO and JCO on the perception of threat of an Iranian nuclear attack. HSO reported no significantly higher scores of anxiety than JCO and also revealed no more other mental health difficulties (medium effect size). Our hypothesis of higher vulnerability to additional stress in HSO is partly supported by the results of this limited number of studies that indicate that having a serious illness is accompanied with relatively high levels of distress in HSO, whereas the mere threat of a disaster is not differentially related to heightened distress in HSO (Shrira, 2015; Shrira et al., 2011).
3.6. Cortisol metabolism, epigenetic factors, genetic predisposition
Eleven studies reported about intergenerational effects on cortisol levels in offspring (see Table 6). Although no significant differences were found between 24-hr urinary cortisol levels between HSO and JCO, the results indicated that parental, and specifically maternal, PTSD (i.e., status and level of symptoms) was associated with lower 24-hr urinary cortisol levels in offspring, even after accounting for offspring’s own traumatization and PTSD (Bader et al., 2014; Bierer et al., 2014; Lehrner et al., 2014; Yehuda et al., 2008a; Yehuda & Bierer, 2008b; Yehuda, Bierer, Andrew, Schmeidler, & Seckl, 2009; Yehuda et al., 2007a, 2001a, 2002; Yehuda et al., 2001b, 2007b). The general level of the 24-h urinary cortisol level is lower in HSO of parents with PTSD than in HSO of parents without PTSD and lower than in JCO. Maternal PTSD had a significantly stronger association (medium effect sizes) with the lowering of this curve than paternal PTSD (Yehuda et al., 2007b). Most of these studies were conducted with maternal survivors so evidence for paternal impact is limited. The few studies that compared the impact of paternal and maternal PTSD in HSO revealed significantly lower 24-h urinary cortisol levels when either both parents or only mothers with PTSD were concerned than when only fathers had PTSD (medium effect sizes) (Bader et al., 2014; Lehrner et al., 2014; Yehuda & Bierer, 2008b; Yehuda et al., 2007a, 2007b). Lehrner et al. (2014) observed significantly higher 24-hr urinary cortisol levels and lower glucocorticoid sensitivity in HSO with only paternal PTSD (large effect size). Van IJzendoorn, Fridman, Bakermans-Kranenburg, and Sagi-Schwartz (2013) described significantly lower levels of daily salivary cortisol in HSO in the presence of higher parental dissociation scores (large effect size). These results were confirmed by studies using dexamethasone concentrations (IC50-DEX value) to determine glucocorticoid sensitivity (Lehrner et al., 2014; Yehuda et al., 2007b). Bader et al. (2014) reported a significant association of 24-h cortisol levels in HSO with the age of the mother during Holocaust exposure (large effect size). HSO with mothers who were adult during Holocaust exposure had significantly lower 24-hr urinary cortisol levels then HSO with mothers who were adolescents, children or JCO and this was independent of the presence of parental PTSD.
Heightened baseline cortisol levels in Holocaust survivors as a result of enduring stress might have exposed HSO to heightened intrauterine cortisol levels. To protect the unborn child from exposure to heightened cortisol levels of mother, placental 11β – hydroxysteroid dehydrogenase type 2 (11β-HSD-2) is being produced which neutralizes almost 90% of maternal cortisol (Duthie & Reynolds, 2013). 11β-HSD-2 activity was significantly higher in HSO with mothers exposed to the Holocaust during childhood compared to JCO. Also, HSO without maternal PTSD showed significantly higher 11β-HSD-2 activity compared to HSO with maternal PTSD and JCO (Bierer et al., 2014). This remained significant when paternal PTSD was included. In this study, it appeared that maternal age at exposure, exposure during childhood, rather than maternal PTSD, predicted offspring’s 11β-HSD-2 activity (medium effect sizes).
Recent studies have focused on epigenetic mechanisms and PTSD. Alterations were found in relation to parental PTSD on DNA methylation and its relationship to glucocorticoid receptor sensitivity (Yehuda et al., 2016). The study by Yehuda et al. (2016) demonstrated methylation changes (large effect sizes) at the FKBP-5 gene, in the opposite direction, survivors had significantly higher and HSO significantly lower methylation. This was not significantly associated with the FKBP5-risk-allele and could not be attributed to offspring’s own trauma exposure, offspring’s psychopathology, cortisol levels or demographic characteristics that might independently affect methylation of this gene. Maternal or paternal influences on in utero effect could not be differentiated because both parents were Holocaust survivors in this sample. According to the results described above, it can be concluded that parental Holocaust trauma may affect offspring’s cortisol metabolism. Maternal symptoms as PTSD and dissociation give rise to decrease of cortisol levels, but maternal atrocities during childhood give rise to increase of cortisol levels, increased 11β-HSD-2 activity in HSO and increased FKBP-5 gene methylation. The findings of these psychobiological markers showed a significant contribution to intergenerational consequences on HSO. The epigenetic consequences (FKBP5 methylation) with large effect sizes seem to contribute to a larger part than the other psychobiological markers with medium effect sizes.
4. Discussion
The aim of this review was to increase our understanding of the impact of being raised in a family with Holocaust survivor parents on the mental health of their offspring. We conducted a systematic literature search and included 23 studies published between January 2000 and February 2018. Because of the large heterogeneity in the type of samples across the reviewed studies (e.g. only offspring or both parents and offspring, heterogeneity in comparison groups) and the large heterogeneity in the mechanisms of interest and associated measurement instruments (i.e., varying from attachment interviews to biological parameters), this systematic review was restricted to a qualitative approach (Aromataris & Munn, 2017).
This systematic review focused on the following factors that may contribute to this multi-causality: (a) parental mental health problems; (b) (perceived) parenting and attachment; (c) parental Holocaust history; (d) the occurrence of life-time HSO stressors; and (e) cortisol metabolism, epigenetic factors, and genetic predisposition. Overall, we found that intergenerational consequences may be best understood by the impact of (and interaction between) multiple factors, and not by one single factor determining mental health outcomes in offspring.
Association between parental and offspring’s mental health problems. The hypothesis of increased prevalence of psychiatric symptoms in offspring because of parental symptoms is confirmed by the findings. Parental mental health problems were clearly found to be associated with offspring’s mental health problems, in particular with regard to the occurrence of HSO mood disorders, anxiety disorders, and substance abuse. Especially parental PTSD was associated with PTSD and depressive symptoms in HSO. This last result is in line with the conclusion of the review by Leen-Feldner et al. (2013) that parental PTSD is associated with offspring PTSD.
Perceived parenting and attachment. As we expected, several factors related to (perceived) parental parenting and attachment were also found to be related to psychological functioning and mental health problems in offspring. HSO families were characterized by relatively many and/or intense conflicts within families and by less cohesion. Survivor mothers were perceived as being more over involved than fathers and in the presence of parental PTSD, there was an increased risk of emotional and physical abuse or neglect.
Parental Holocaust history. The hypothesis that growing up in a two-survivor family versus a one-survivor family and the gender of the survivor will affect the incidence of mental health problems in offspring was confirmed. The results of the studies indicated that overall Holocaust survivor mothers appeared to be more influential for the mental well-being of their offspring than fathers. In addition, having two survivor parents resulted in higher mental health problems compared to having one survivor parent. This pattern appeared evident even if parents did not show mental health problems. As we expected, findings are consistent with the view that the parental Holocaust history is associated with the development of symptoms, and especially strong when maternal PTSD is present.
Additional stress and traumatic life events in HSO. Empirical support for the hypothesis of heightened vulnerability to stress in HSO after serious life events is consistent but limited. The results of one study among HSO with cancer indicated that having a serious illness (e.g. in case of cancer) was accompanied but with higher levels of distress. In contrast to what was expected, HSO and JCO showed the same heightened vulnerability for stress in case of mere threat (e.g. nuclear threat).
Cortisol metabolism, epigenetic factors, and genetic predisposition. As a last factor of intergenerational consequences of trauma, we reviewed studies on cortisol metabolism, epigenetic factors, and genetic predisposition. The studies demonstrated intergenerational effects with regard to cortisol levels (with lower urinary cortisol levels in offspring whose mothers were adults during the Holocaust compared to offspring of younger mothers), increased methylation in specific gene-segments (FKBP5), and increased 11β–HSD-2 activity with maternal PTSD. These findings are in line with studies focusing on epigenetic and physiological consequences of other forms of mass violence and stress such as the 9/11 terrorist attack (Yehuda & Bierer, 2008b). Overall, we found indications that special attention should be paid to maternal age at exposure and parental PTSD because each may affect different components of the cortisol metabolism and bring about various changes in cortisol metabolism.
4.1. An integrative perspective
To understand intergenerational consequences of massive trauma an integrative perspective is needed. This perspective needs to include psychological, family system, and biological and sociological characteristics. It should also be noted that intergenerational consequences do not necessarily lead to psychological symptoms (Denham, 2008; Kirmayer, Gone, & Moses, 2014). However, despite the caveats in the studies included in this review, the findings provided considerable evidence in support of the hypothesis that HSO is indirectly affected by the Holocaust experiences of their survivor parents. There was also evidence that the psychobiological systems changed in response to severe stress related to Holocaust experiences: Children with mothers who have experienced the Holocaust developed an altered cortisol metabolism due to epigenetics increased FKBP-5 methylation and increased 11β–HSD-2 activity. This may provoke altered reactions to stress in HSO compared to offspring with unchanged cortisol metabolism. Depending on the type of cortisol metabolism modification, this may give rise to heightened vulnerability to stress, distress and mental symptoms but it may also bring about resilience and even increased resistance to stressful events (Bonanno & Mancini, 2012; Harel, Kahana, & Kahana, 1988).
Furthermore, in the presence of parental mental disorders, especially maternal PTSD, offspring is vulnerable to developmental problems. And less attuned parenting, family conflicts, and emotional abuse have been found to be occurring relatively often in Holocaust survivor families. Only little evidence was found for HSO to be vulnerable to traumatic or stressful life events that really happened and not to mere threat.
4.2. Methodological issues
The strength of this study is that we have systematically analysed all empirical studies that have been published on intergenerational consequences of the Holocaust in the past two decades. Further, we evaluated characteristics, including parental mental health problems, perceived parenting, gender, additional life-time stress, cortisol and epigenetics, that were only partly addressed in previous reviews within this domain.
A limitation of the current findings is that only a restricted number of researchers have addressed intergenerational consequences. Thus, the studies included in this review were designed and carried out by only a small group of scientists. Further empirical studies and replications among offspring of war survivors by a larger variety of research groups are of great importance. Another limitation is that we narrowed our focus to a selection of possible influential intergenerational factors. This selection was based on both theoretical hypotheses and factors that were proposed in earlier research (e.g. Felsen, 1998; Kellermann, 2001; Solomon, 1998; Van IJzendoorn et al., 2003). Nevertheless, there may be other relevant factors that have not been covered by the current study. For example, the birth order and the presence or absence of siblings may have an impact on the development of the HSO mental health (Kellermann, 2008; Letzter-Pouw & Werner, 2013). Examples of other factors that we did not consider and that might be of influence are: the migration history and the circumstances in the host countries; the current living conditions of feeling safe and welcome or living in a condition of continuing threat as in Israel or the influence of living among a high percentage of Holocaust survivors (Kirmayer et al., 2014).
The recruitment methods that were used in the reviewed studies varied from convenience samples (e.g. gathered through advertisement, survivor associations or networks, meetings or conferences, mental health clinics) and snowball methods to a more representative selection of participants (e.g. random selection from national case register such as the Ministry of Interior; Sagi-Schwartz et al., 2003). Both convenience sampling and representative sampling methods have advantages and disadvantages. In our review, we focused on the clinical group of HSO, which is obviously not representative of the total population of HSO. The choice was made because the sample of clinical HSO was expected to show sufficient variation in mental health problems allowing to investigate a possible association between symptoms and parental as well as offspring characteristics. Thus, studying this group could contribute to a better understanding of underlying mechanisms for presumed transmission of parental-experienced massive trauma. Furthermore, samples by recruitment with the use of a national case register suffer from low response rates (e.g. 60% in a study by Letzter-Pouw et al., 2014) and therefore reduce representativity. Participants may refuse to participate because, for example, they do not want to be reminded of the war, they feel that the Holocaust did not play a significant role in their lives, or they are afraid to become too emotional. Consequently, selective non-response may have introduced bias in the results gathered and reported (Letzter-Pouw et al., 2014; Letzter-Pouw & Werner, 2013).
Additionally, the Jewish comparison samples in the reviewed studies were from the same catchment area but with parents who were non-Holocaust survivors. These samples varied widely, depending on the method of recruitment and in- and exclusion criteria possibly introducing selection bias. The comparison samples included further differed in health status (e.g. from completely symptom-free to general population samples with selected exclusion criteria like florid psychotic disorders). Moreover, despite careful screening procedures, comparison survivor and offspring groups were not always comparable on important demographic variables like education level (e.g. non-Holocaust survivors received more education during the holocaust years), religion, or background of partner (Holocaust survivor or not).
Most studies reviewed were one-generation studies (i.e., with an absence of direct assessments in the first-generation survivors) and mental health functioning and other survivor characteristics (e.g. perceived parenthood) were estimated by the HSO, introducing retrospective bias. Symptoms of PTSD may change over time and the current symptoms or the symptoms during the last period of the parents’ lives may be more explicit in memory and therefore no proper representation of the symptoms during the earlier or entire upbringing period. Moreover, in the few two-generations studies the associations between survivor and offspring functioning were not always computed.
Finally, it is important to mention that all studies that met our inclusion criteria pertained only to Holocaust survivors. Offspring of parents who survived World War Two by hiding or in the resistance, as well as parents who survived the Japanese occupation and internment camps in Asia were not represented. This reduces the external validity, that is, the generalizability of the findings of the current review to other survivors of World War Two and survivors of other atrocities or war as well as other groups with severe parental trauma. We also have to keep in mind that Holocaust survivors might have lived in more hospitable or stable countries than the circumstances of refugees now living in temporary housing in refugee camps or unhospitable and unstable host countries.
4.3. Clinical implications and implications for future research
First of all, this research has implications with regard to diagnostic assessment. The findings of this review indicate that intergenerational effects may not be directly observed in the occurrence of particular disorders in offspring, but appear to be reflected by a diversity of mental health problems that are influenced by both parental and offspring characteristics. Diagnostic procedures should thus take this into consideration and incorporate instruments capturing this variety of symptoms and contributing factors. In addition, to minimize intergenerational consequences for children with severely traumatized parents (e.g. refugees), treatment should be provided to both survivor and offspring for their mental health problems. Also, the findings emphasize the importance of providing support for traumatized parents in raising their children (see also Ee, Sleijpen, Kleber, & Jongmans, 2013).
For future research, it is important to examine if specific characteristics of Holocaust survivors are also evident in survivors of genocide or survivors of war in general (Kirmayer et al., 2014). More insight into the presence of intergenerational consequences of war does not have to be limited to HSO but can also be developed in prospective research among survivors of current wars such as in Syria or in refugee camps. Studies entailing a longitudinal design incorporating both psychosocial and psychobiological parameters will be of great value to closely examine interference of parental characteristics with the development of offspring. Because most studies reviewed by us relied on cross-sectional designs, longitudinal research is of great importance because the consequences of parental trauma may only become visible after a long time. Our review also made clear that it is relevant not to limit future studies to offspring that experienced warfare or was born during the war but also focus on children born after the war, as (intergenerational) consequences sometimes may only become evident many years after traumatic war events. This will contribute to increasing insight on child, parental and parenting factors as well as on their mutual influence. The focus on malleable factors can be used as a starting point for (preventive) interventions.
5. Conclusion
To conclude, the available evidence suggests that both parent and child characteristics and their interaction contribute to the vulnerability and to the development of symptoms in the HSO group. Holocaust survivor mothers have been observed to be more influential for the mental well-being of their offspring than fathers, and having two survivor parents resulted in even higher mental health problems. This is confirmed by studies of intergenerational effects with regard to parental PTSD and maternal age during the Holocaust. Those studies showed strong evidence for the effect on cortisol metabolism modification and epigenetics in HSO with survivor mothers. Also, intergenerational effects have been found with regard to cortisol levels. There is some empirical support for a heightened vulnerability for stress in HSO. These results indicate that diagnostic procedures and treatment, but also future theorizing and empirical studies should be multifactorial in trying to delineate the causal factors involved in mental health functioning in intergenerational consequences of war. In this context, it is important to note that, although the current review has predominantly focused on HSO suffering from mental health problems, it is relevant to examine those parents and children who managed to cope, adjust and/or build a healthy life, as this might help unveil factors contributing to resilience. Attention should be paid to these specific psychological and biological factors safeguarding offspring against distress.
Acknowledgments
We would like to thank the staff of the Arq Psychotrauma Expert Group library for their assistance in the database search for this review.
Appendix 1.
The search syntax was: (Second World War or World War II or World War 2 or WWII or WW2 or Worldwar-2 or Worldwar-II or World War Two or 2WAR2 or Holocaust) and (Child* of Holocaust Survivor* or child* of concentration camp survivor* or second generation or adult offspring) and (adult offspring/ or daughters/ or sons/) and Acute Stress or C*PTSD or chronic trauma* or Combat disorder* or combat fatigue or Combat Neuros#s or combat stress or Complicated Trauma* or comorbid* or Complex trauma* or DES*NOS or “Disorders of Extreme Stress“ or Dual Diagnos#s or emotional trauma* or Enduring Personality Change after Catastrophic Experience* or EPCACE or Multiple Trauma* or posttraumatic neuros#s or post-traumatic neuros#s or posttraumatic psychic syndrome* or post-traumatic psychic syndrome* or posttraumatic psychos#s or post-traumatic psychos#s or posttraumatic stress or post-traumatic stress or posttraumatic syndrome* or post-traumatic syndrome* or Psychotrauma* or PTSD or shell shock or traumati#ed or traumatic stress or Type II trauma* or Type I trauma* or war neuros#s.ti,ab. and (Posttraumatic Stress Disorder/ or Emotional Trauma/ or acute stress disorder/ or combat experience/ or trauma/ or traumatic neurosis/) and (depress* or melanchol* or low mood) And (Anxiety Disorder* or panic disorder* or anxiety symptom* or panic symptom* or Anxiety attack* or panic attack* or Agoraphobia) and (exp Anxiety Disorders) and (neurotic depression* or dysthymic disorder* or chronic depression* or mood disorder*) and (exp Dysthymic Disorder) and (eating disorder* or anorexia nervosa or bulimia nervosa or Compulsive overeating or Diabulimia or Drunkorexia or Gourmand syndrome) and (exp Eating Disorders) and (Personality disorder* or obsessive-compulsive disorder or borderline or Paranoid or Schizoid or Narcissistic or Histrionic or Schizotypal or antisocial or avoidant or masochistis or sadistic or negativistic) and (exp Personality Disorders) and (exp Drug Abuse/ or exp addiction) and (addict* or alcohol* or Amphetamine or Angel Dust or Binge Drinking or Cannabis or Cocaine or Delirium Tremens or dependen* or Drug Abus* or Drug Addicti* or Drug Dependen* or Drug Habit* or Drug Overdose* or Drug psychos* or “Drug Use disorder*“ or Drug Withdrawal or Drunkenness* or Ethanol or FAE* or FASD* or Glue or Hashish or Heroin or Inhalant abus* or Intravenous Drug* or Intravenous Substance* or Marihuana or Morphine or Narcotic* or Neonatal Abstinence or Nicotine or Opiate* or Opioid* or PCP abus* or Phencyclidine or smoker* or Smoking* or Substance abus* or Substance addict* or Substance dependen* or Substance Induced or ”Substance Use” or Substance-Induced or Substance-Related or Tobacco or Withdrawal) and (somatization/ or exp somatoform disorders) and (somatization* or somatoform or Body Dysmorphic Disorder or Conversion Disorder or Hypochondriasis or Neurasthenia or Neurodermatitis or Somatization Disorder or Somatoform Pain Disorder) and (mental disorder* or mental illness or psychological disorder* or psychiatric disorder*) and (exp Mental Disorders) and (exp Symptoms) and (Arousal or Hyperarousal or Avoidance or Reexperienc* or Intrusion or Reliv* or nightmare* or sleep* or flashback* or belief* or feeling*) and (Comorbidity) and (comorbid* or Multiple Disorders or Dual Diagnosis) and (exp Mental Health/ or Well Being/ or exp ”Quality of Life”) and (mental health or well-being or Quality of Life) and (exp social identity/ or identity formation/ or Identity Crisis/ or exp Separation Anxiety/ or exp Separation Reactions/ or exp Separation Individuation) and (identit* or individuation or separation) and (Heredity or genetic* or epigenetic* or Epigenomic* or cortisol or Hydrocortisone or Epicortisol or 11?Epicortisol or Cortifair or Cortril or neurobiolog* or neuropsycholog* or DNA or Deoxyribonucleic Acid or biomarker* or ((Biologic* or Biochemical or Clinical or Laboratory or Serum or Viral or Immunologic or Immune or Surrogate) adj (end?point* or Marker*)) or phenotype*) ti,ab.and (exp Genetics/ or Hydrocortisone/ or exp Neurobiology/ or Neuropsychology/ or DNA/ or Biological Markers/ or Phenotypes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- American Psychiatric Association (APA) (2000). Diagnostic and statistical manual of mental disorders 4th ed. text revision: DSM-IV-TR. Washington, DC: APA. [Google Scholar]
- Aromataris E., & Munn Z. (2017). Chapter 1: JBI systematic reviews In Joanna Briggs Institute reviewer’s manual, 299. [Google Scholar]
- Bader H. N., Bierer L. M., Lehrner A., Makotkine I., Daskalakis N. P., & Yehuda R. (2014). Maternal age at Holocaust exposure and maternal PTSD independently influence urinary cortisol levels in adult offspring. Frontiers in Endocrinology, 5, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baider L., Goldzweig G., Ever-Hadani P., & Peretz T. (2006). Psychological distress and coping in breast cancer patients and healthy women whose parents survived the Holocaust. Psycho-Oncology, 15(7), 635–29. [DOI] [PubMed] [Google Scholar]
- Baider L., Goldzweig G., Ever-Hadani P., & Peretz T. (2008). Breast cancer and psychological distress: mothers’ and daughters’ traumatic experiences. Supportive Care in Cancer, 16(4), 407–414. [DOI] [PubMed] [Google Scholar]
- Baider L., Peretz T., Hadani P. E., Perry S., Avramov R., & De-Nour A. K. (2000). Transmission of response to trauma? Second-generation Holocaust survivors’ reaction to cancer. American Journal of Psychiatry, 157(6), 904–910. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Ward C., Mendelson M., Mock J., & Erbaugh J. (1961). Bewck depression inventory (bdi). Archives of General Psychiatry, 4(6), 561-571. [DOI] [PubMed] [Google Scholar]
- Bendig A. W. (1956). The development of a short form of the manifest anxiety scale. Journal of Consulting Psychology, 20(5), 384. doi: 10.1037/h0045580 [DOI] [PubMed] [Google Scholar]
- Bernstein D. P., Ahluvalia T., Pogge D., & Handelsman L (1997). Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of The American Academy of Child & Adolescent Psychiatry, 36(3), 340––348.. [DOI] [PubMed] [Google Scholar]
- Bernstein E. M, & Putnam F. W (1986). Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease. [DOI] [PubMed] [Google Scholar]
- Betancourt T. S. (2015). The intergenerational effect of war. JAMA Psychiatry, 72(3), 199–200. [DOI] [PubMed] [Google Scholar]
- Bierer L. M., Bader H. N., Daskalakis N. P., Lehrner A. L., Makotkine I., Seckl J. R., & Yehuda R. (2014). Elevation of 11β-hydroxysteroid dehydrogenase type 2 activity in Holocaust survivor offspring: Evidence for an intergenerational effect of maternal trauma exposure. Psychoneuroendocrinology, 48, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. D., Weathers F. W., Nagy L. M., Kaloupek D. G., Gusman F. D., Chamey D. S., & Keane T. M (1995). The development of a clinician-administerd PTSD scale. Journal of Traumatic Stress, 8(1), 75-90. [DOI] [PubMed] [Google Scholar]
- Bloch A. (2018). Talking about the past, locating it in the present: The second generation from refugee backgrounds making sense of their parents’ narratives, narrative gaps and silences. Journal of Refugee Studies, 31(4), 647–663. [Google Scholar]
- Bonanno G. A., & Mancini A. D. (2012). Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychological Trauma: Theory, Research, Practice, and Policy, 4(1), 74–78. [Google Scholar]
- Bowlby J. (1982). Attachment and loss: Retrospect and prospect. American Journal of Orthopsychiatry, 52, 664–678. [DOI] [PubMed] [Google Scholar]
- Chaitin J. (2002). Issues and interpersonal values among three generations in families of Holocaust survivors. Journal of Social and Personal Relationships, 19(3), 379–402. [Google Scholar]
- Critical Appraisal Skills Program (2018). CASP checklist. [online] Retrieved from http://www.casp-uk.net/checklists
- Danieli Y. (Ed.). (1998). International handbook of multigenerational legacies of trauma. New York, NY: Plenum Press. [Google Scholar]
- Deeks J. J., Dinnes J., D’Amico R., Sowden A. J., Sakarovitch C., Song F., … Altman D. G. (2003). Evaluating non-randomised intervention studies. Health Technology Assessment (Winchester, England), 7(27), iii–x. Retrieved from http://researchonline.lshtm.ac.uk/id/eprint/8742 [DOI] [PubMed] [Google Scholar]
- Denham A. R. (2008). Rethinking historical trauma: Narratives of resilience. Transcultural Psychiatry, 45(3), 391–414. [DOI] [PubMed] [Google Scholar]
- Derogatis L. R., & Meilisaratos N (1983). The brief symptom inventory: an introductory report. Psychological Medicine, 13(3), 595––605.. [PubMed] [Google Scholar]
- Downs S. H., & Black N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health, 52(6), 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie L., & Reynolds R. M. (2013). Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuro-endocrinology, 98, 106–115. [DOI] [PubMed] [Google Scholar]
- Ee E. V., Sleijpen M., Kleber R. J., & Jongmans M. J. (2013). Father-involvement in a refugee sample: Relations between post-traumatic stress and care-giving. Family Process, 52, 723–735. [DOI] [PubMed] [Google Scholar]
- Felsen I. (1998). transgenerational transmission of effects ot the Holocaust In Danieli Y. (Ed.), International handbook of multigenerational legacies of trauma (pp. 43–68). New York, NY: Plenum Press. [Google Scholar]
- Flory J. D., Bierer L. M., & Yehuda R. (2011). Maternal exposure to the holocaust and health complaints in offspring. Disease Markers, 30(2–3), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E. B, Cashman L, Jaycox L, & Perry K (1997). The validation of a self-report measure of posttraumatic stress disorder: the posttraumatic diagnostic scale. Psychological Assesment, 9(4), 445. [Google Scholar]
- Fonagy P. (1999). The transgenerational transmission of holocaust trauma. Attachment & Human Development, 1(1), 92–114. [DOI] [PubMed] [Google Scholar]
- Gangi S., Talamo A., & Ferracuti S. (2009). The long-term effects of extreme war-related trauma on the second generation of Holocaust survivors. Violence and Victims, 24(5), 687–700. [DOI] [PubMed] [Google Scholar]
- Glover V. (2015). Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms In Perinatal programming of neurodevelopment (pp. 269–283). New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Griffin D. W., & Bartholomew K. (1994). Models of the self and other: Fundamental dimensions underlying measures of adult attachment. Journal of Peronality and Social Psychology, 67, 340. [Google Scholar]
- Halligan S. L., & Yehuda R. (2002). Assessing dissociation as a risk factor for posttraumatic stress disorder: A study of adult offspring of holocaust survivors. The Journal of Nervous and Mental Disease, 190(7), 429–436. [DOI] [PubMed] [Google Scholar]
- Harel Z., Kahana B., & Kahana E. (1988). Psychological well-being among Holocaust survivors and immigrants in Israel. Journal of Traumatic Stress Studies, 1, 413–428. [Google Scholar]
- Havinga P. J., Boschloo L., Bloemen A. J. P., Nauta M. H., de Vries S. O., Penninx B. W. J. H., & Hartman C. A. (2017). Doomed for disorder? High incidence of mood and anxiety disorders in offspring of depressed and anxious patients: a prospective cohort study. The Journal of Clinical Psychiatry, 78, 8–17. [DOI] [PubMed] [Google Scholar]
- Heim C., & Binder E. B. (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–111. [DOI] [PubMed] [Google Scholar]
- Hesse E. (Ed.). (1999). The adult attachment interview: Historical and current perspectives. New York, NY: Guilford. [Google Scholar]
- Horowitz M., Wilner N., & Alvarez W (1979). Impact of event scale: A measure of subjective stress. Psychosomatic Medicine, 41(3), 209––218.. [DOI] [PubMed] [Google Scholar]
- Katz S. J., Hammen C. L., & Brennan P. A. (2013). Maternal depression and the intergenerational transmission of relational impairment. Journal of Family Psychology, 27, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T. M., Caddell J. M., & Taylor K. L (1988). Mississippi scale for combat-related posttraumatic stress disorder: Three studies in rehability an validity. Journal of Consulting and Clinical Psychology, 56, 85––90.. [DOI] [PubMed] [Google Scholar]
- Kellermann N. P. (2001). Transmission of Holocaust trauma–an integrative view. Psychiatry, 64(3), 256–267. [DOI] [PubMed] [Google Scholar]
- Kellermann N. P. (2008). Transmitted Holocaust trauma: curse or legacy? The aggravating and mitigating factors of Holocaust transmission. Israel Journal of Psychiatry & Related Sciences, 45(4), 263–270. [PubMed] [Google Scholar]
- Kirmayer L. J., Gone J. P., & Moses J. (2014). Rethinking historical trauma. Transcultural Psychiatry, 51(3), 299–319. [DOI] [PubMed] [Google Scholar]
- Klaassens E. R. (2010). Bouncing back. GVO drukkers & vormgevers B. V. Ponsen & Looijen. [Google Scholar]
- Krell R., Suedfeld P., & Soriano E. (2004, October). Child Holocaust survivors as parents: A transgenerational perspective. American Journal of Orthopsychiatry, 74, 502–508. [DOI] [PubMed] [Google Scholar]
- Kretchmar M. D., & Jacobovitz D. B. (2002). Observing mother-child relationships across generations: Boundary patterns, attachment, and the transmission of caregiving. Family Process, 41(3), 351–374. [DOI] [PubMed] [Google Scholar]
- Leen-Feldner E. W., Feldner M. T., Knapp A., Bunaciu L., Bumenthal H., & Armstadter A. B. (2013). Offspring psychological and biological correlates of parental posttraumatic stress: Review of the literature and research agenda. Clinical Psychology Review, 33(8), 1006–1133. [DOI] [PubMed] [Google Scholar]
- Lehrner A., Bierer L. M., Passarelli V., Pratchett L. C., Flory J. D., Bader H. N., … Yehuda R. (2014). Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology, 40, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzter-Pouw S. E., Shrira A., Ben-Ezra M., & Palgi Y. (2014). Trauma transmission through perceived parental burden among Holocaust survivors’ offspring and grandchildren. Psychological Trauma: Theory, Research, Practice, and Policy, 6(4), 420–429. [Google Scholar]
- Letzter-Pouw S. E., & Werner P. (2013). The relationship between females Holocaust child survivors’ unresolved losses and their offspring’s emotional well-being. Journal of Loss and Trauma, 18(5), 396–408. [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A., … Moher D. (2009). The PRISMA Statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6(7), e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo K. L., & Motta R. W. (2008). Secondary trauma in children of parents with mental illness. Traumatology, 8;(15), 57–67. [Google Scholar]
- Lyons-Ruth K., Bronfman E., & Atwood G J.. A relational diathesis model of hostile-helpless states of mind: Expression in mother-infant interaction In Solomon J. & George C. (Eds.), Attachment disorganization (pp. 33–70). New York, NY: Guilford. [Google Scholar]
- McGuire S., Palaniappan M., & Larribas T. (2015). The sibling relationship as a source of shared environment In Horwitz B. & Neiderhiser J. (Eds.), Gene-environment interplay in interpersonal relationships across the lifespan. advances in behavior genetics (Vol. 3). New York, NY: Springer. doi: 10.1007/978-1-4939-2923-8_4 [DOI] [Google Scholar]
- Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., … Mu P. F. (2017). Chapter 7: Systematic reviews of etiology and risk In Aromataris E. & Munn Z. (Eds.), Joanna Briggs Institute reviewer’s manual. The Joanna Briggs; . Retrieved from https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- Moos R. H., & Moos B. S (1994). Family environment scale manual. Consulting Psychologists Press. [Google Scholar]
- Munroe J. F., Shay J., Fisher L., Makary C., Rapperport K., & Zimering R. (1995). Preventing compassion fatigue: A team treatment model In C. R. Figley (Ed.), Compassion fatigue: Coping with secondary traumatic stress disorder in those who treat the traumatized (pp. 209–231). Routledge [Google Scholar]
- Naumova O. Y., Hein S., Suderman M., Barbot B., Lee M., Raefski A., … Grigorenko E. L. (2016). Epigenetic patterns modulate the connection between developmental dynamics of parenting and offspring psychosocial adjustment. Child Development, 87, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasic D., Hajek T., Alda M., & Uher R. (2014). Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: A meta-analysis of family high-risk studies. Schizophrenia Bulletin, 40, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. M., Pesonen A.-K., O’Reilly J. R., Tuovinen S., Lahti M., Kajantie E., … Räikkönen K. (2015). Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychological Medicine, 45(10), 2023–2030. [DOI] [PubMed] [Google Scholar]
- Sagi-Schwartz A., Van IJzendoorn M. H., Grossmann K. E., Joels T., Grossmann K., Scharf M., … Alkalay S. (2003). Attachment and traumatic stress in female holocaust child survivors and their daughters. American Journal of Psychiatry, 160(6), 1086–1092. [DOI] [PubMed] [Google Scholar]
- Sangalang C., Jager J., & Harachi T. W. (2017). Effects of maternal traumatic distress on family functioning and child mental health: An examination of Southeast Asian refugee families in the U.S. Social Science & Medicine, 184, 178–186. [DOI] [PubMed] [Google Scholar]
- Sangalang C. C., & Vang C. (2017). Intergenerational trauma in refugee families: A systematic review. Journal of Immigrant and Minority Health, 19(3), 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf M., & Mayseless O. (2011). Disorganizing experiences in second- and third-generation holocaust survivors. Qualitative Health Research, 21(11), 1539–1553. [DOI] [PubMed] [Google Scholar]
- Shmotkin D., & Litwin H (2009). Cumulative adversity and depressive symptoms among older adults in Israel: The diffrential role of self-oriented versus other-oriented events of potential trauma. Social Psychiatry and Psychiatric Epidemiology, 44, 579––585.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrira A. (2015). Transmitting the sum of all fears: Iranian nuclear threat salience among offspring of Holocaust survivors. Psychological Trauma: Theory, Research, Practice, and Policy, 7(4), 364–371. [DOI] [PubMed] [Google Scholar]
- Shrira A., Palgi Y., Ben-Ezra M., & Shmotkin D. (2011). Transgenerational effects of trauma in midlife: Evidence for resilience and vulnerability in offspring of Holocaust survivors. Psychological Trauma: Theory, Research, Practice, and Policy, 3(4), 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solkoff N. (1981). Children of survivors of the Nazi Holocaust: A critical review of the literature. American Journal of Orthopsychiatry, 51(1), 29–42. [DOI] [PubMed] [Google Scholar]
- Solkoff N. (1992). Children of survivors of the Nazi Holocaust: A critical review of the literature. American Journal of Orthopsychiatry, 62(3), 342–358. [DOI] [PubMed] [Google Scholar]
- Solomon Z. (1998). Transgenerational effects of the Holocaust: The Israeli perspective In Danieli Y. (Ed.), International handbook of multigenerational legacies of trauma (pp. 69–83). New York, NY: Plenum Press. [Google Scholar]
- Solomon Z., Kotler M., & Mikulincer M. (1988). Combat-related posttraumatic stress disorder among second-generation Holocaust survivors: Preliminary findings. American Journal of Psychiatry, 145, 865–868. [DOI] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L., & Lushene R. E (1968). State-Trait Anxiety Inventory (STAI): Test Manual for Form X. Consulting Psychologists Press. [Google Scholar]
- Spitzer R. L, Gibbon M., & Williams J. B (1995). Structured clinical interview for axis I DSM-IV disorders (SCID). Washington, DC: American Psychiatric Association. [Google Scholar]
- Taouk L., & Schulkin J. (2016). Transgenerational transmission of pregestational and prenatal experience: maternal adversity, enrichment, and underlying epigenetic and environmental mechanisms. Journal of Developmental Origins of Health and Disease, 7(6), 588–601. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn M. H., Bakermans-Kranenburg M. J., & Sagi-Schwartz A. (2003). Are children of Holocaust survivors less well-adapted? A meta-analytic investigation of secondary traumatization. Journal of Traumatic Stress, 16(5), 459–469. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn M. H., Fridman A., Bakermans-Kranenburg M. J., & Sagi-Schwartz A. (2013). Aftermath of genocide: Holocaust survivors’ dissociation moderates offspring level of cortisol. Journal of Loss and Trauma, 18(1), 64–80. [Google Scholar]
- Van IJzendoorn M. H., & Schuengel C. (1996). The measurement of dissociation in normal and clinical populations: Meta analytic validation of the dissociative experiences scale (DES). Clinical Psychology Review, 16, 365–382. [Google Scholar]
- Watson M., Greer S., Young J., Inayat Q., Burgess C., & Robertson B (1988). Development of a questionnaire measure of adjustment to cancer: the MAC scale. Psychological Medicine, 18(1), 203––209.. [DOI] [PubMed] [Google Scholar]
- Winnicott D. W. (1971). Playing and reality. Hove and New York: Brunner-Routledge. [Google Scholar]
- Wiseman H., Barber J. P., Raz A., Yam I., Foltz C., & Livne-Snir S. (2002). Parental communication of Holocaust experiences and interpersonal patterns in offspring of Holocaust survivors. International Journal of Behavioral Development, 26(4), 371–381. [Google Scholar]
- Yehuda R., Bell A., Bierer L. M., & Schmeidler J. (2008a). Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of Psychiatric Research, 42(13), 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., & Bierer L. M. (2008b). Transgenerational transmission of cortisol and PTSD risk. Progress in Brain Research, 167, 121–135. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Bierer L. M., Andrew R., Schmeidler J., & Seckl J. R. (2009). Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. Journal of Psychiatric Research, 43(9), 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Bierer L. M., Schmeidler J., Aferiat D. H., Breslau I., & Dolan S (2000). Low cortisol and risk for PTSD in adult offspring of holocaust survivors. American Journal of Psychiatrym, 157, 1252––1259.. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Blair W., Labinsky E., & Bierer L. M. (2007a). Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. American Journal of Psychiatry, 164(1), 163–166. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Daskalakis N. P., Bierer L. M., Bader H. N., Klengel T., Holsboer F., & Binder E. B. (2016). Holocaust exposure induced intergenerationaleffects on FKBP5 methylation. Journal of Biological Psychiatry, 80(5), 372–380. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Daskalakis N. P., Lehrner A., Desarnaud F., Bader H. N., Makotkine I., … Meaney M. J. (2014). Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. American Journal of Psychiatry, 171(8), 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Engel S. M., Brand S. R., Seckl J., Marcus S. M., & Berkowitz G. S. (2005). Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. The Journal of Clinical Endocrinology & Metabolism, 90(7), 4115–4118. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Halligan S. L., & Bierer L. M. (2001a). Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. Journal of Psychiatric Research, 35(5), 261–270. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Halligan S. L., & Bierer L. M. (2002). Cortisol levels in adult offspring of Holocaust survivors: relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology, 27(1–2), 171–180. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Halligan S. L., & Grossman R. (2001b). Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development & Psychopathology, 13(3), 733–753. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Teicher M. H., Seckl J. R., Grossman R. A., Morris A., & Bierer L. M. (2007b). Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Archives of General Psychiatry, 64(9), 1040–1048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Critical Appraisal Skills Program (2018). CASP checklist. [online] Retrieved from http://www.casp-uk.net/checklists


