Abstract
Objective
This study aimed to examine the effect of pelvic floor muscle training on the irisin (Ir) concentration in overweight or obese elderly women with stress urinary incontinence.
Methods
The number of participants included in analysis was 49: 28 women in the experimental group and 21 women in the control group. The experimental group (EG) underwent pelvic floor muscle training, whereas no therapeutic intervention was applied to the control group (CG). Irisin concentration, severity of urinary incontinence (RUIS), and body mass index (BMI) were measured in all women at the initial and final assessments.
Results
By comparing the initial and final assessment results we have been able to demonstrate statistically significant differences in the measured variables in the experimental group. No statistically significant differences in the measured variables were reported for the control group at the initial and final assessments. Moderate negative correlation was observed between the BMI results with the irisin concentration results in the EG at the initial assessment and no correlation at the final assessment. Weak positive correlation was observed between the BMI results with the irisin concentration in the CG at the initial and final assessment.
Conclusion
Further studies are necessary to observe the regulation of irisin concentration and explain mechanisms underlying these effects.
1. Introduction
Stress urinary incontinence (SUI) is the most common type of urinary incontinence (UI). Atrophy and impairment of type II fibers in the levator ani muscles play an essential role in the development of SUI in women. Type II to type I muscle fiber switching is a beneficial physiological mechanism induced by skeletal muscle adaptation to exercise. However, if the process occurs locally, close to the urethral sphincter, then it has a negative effect in the form of SUI. The dysfunction of suspensory ligaments of the urethra and/or reduced contractility of the sphincter urethrae due to myofascial dysfunction of the pelvic floor are also associated with stress urinary incontinence in women [1, 2].
We distinguish diverse types of risk factors for urinary incontinence: predisposing, decompensating, and promoting. Among these there are certain risk factors for both UI and obesity, such as a woman's age, environmental diseases, level of physical activity, diet, and occurrence of menopause. Obesity is conducive to the occurrence of urinary incontinence; however, it can also increase the severity of this condition [3]. Targeted physical activity plays a critical role in the treatment of UI and obesity. The muscle is no longer seen as a simple contractile motor, but as crossroads of more complex networks, involving a reduction of protein (synthesis and regeneration), with a parallel increase of apoptosis and protein-lysis. [4–6]. Proteins secreted by muscle fibres (myocytes) are known as myokines. The following myokines are secreted by muscle cells in response to muscle contraction: angiopoietin-like protein 4 (ANGPTL4), fibroblast growth factor 21 (FGF21), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 15 (IL15), myonectin (CTRP15), myostatin (MSTN), vascular endothelial growth factor (VEGF), follistatin (FST), and irisin (Ir) [7–11].
Irisin was described for the first time in 2012 by Boström et al. [12] as a new myokine which results from the cleavage of the type I membrane protein, fibronectin type III containing five domains (FNDC5). This cleavage is induced by the peroxisome proliferator-activated receptor γ (PPARy) transcriptional coactivator PGC-1α. Irisin is secreted from FNDC5, and the process itself is regulated by physical activity and stimulation of PGC-1α (one of the most important energy metabolism regulators). Increase of PGC-1α expression is accompanied by mitochondrial biogenesis, increase of insulin dependent glucose uptake, increase of the neuromuscular connections, and angiogenesis stimulation. Thus, the PGC-1α expression plays a critical role in maintaining carbohydrate, lipid, and energy homeostasis in the body [13–15].
2. Aim of the Study
This study aimed to examine the effect of pelvic floor muscle training on the irisin concentration in overweight or obese elderly women with stress urinary incontinence.
3. Methods
3.1. Study Design
Between January 2017 and May 2017, 62 women suffering from UI were enrolled into a randomized, double-blind, controlled study. The following was conducted in accordance with the principles of the Declaration of Helsinki. The authors of this study obtained an approval from the Local Bioethics Committee in Poland. Moreover, a statement confirming written informed consent was obtained from all the participants All deidentified data included in this are contained within this report. In order to ensure stratified randomization, the researchers allocated the subjects using a rather simple method. Specifically, envelopes with group allocation numbers were picked from a computer generated random number table [16]. The main investigator was blinded throughout the group allocation. The initial number of patients assessed for eligibility was 98. Of 36 women who were excluded in the first stage of the study: 34 participants did not meet the inclusion criteria, whereas 2 participants refused to participate. Subsequently, 62 women were randomly assigned to the experimental group (EG) who underwent pelvic floor muscle training (PFMT) or the control group (CG).
All women in EG group underwent 12 therapy sessions (3 times a week, 45-minute sessions, lasting 4 weeks) according to the proprietary program. The exercises were completed in five/six-person groups supervised by a physiotherapist. Before joining the training session, all patients were examined for posture and body correction and then prepared for a proper training by acquiring the skills of mobilising sacroiliac joints and doing exercises to improve the range of movement in the lumbar-sacral spine and the hip and knee joints. Training in breathing through abdominal and thoracic duct was also conducted. The Pelvic Floor Muscle Training (PMFT) program was based on straining fast and slow twitch muscle fibers of the pelvic floor with relaxed gluteal muscles using the transversus abdominis muscle tension, without changing the position and with changing the position as well as synchronising the muscles with breathing. The PFMT exercise was performed in lying, sitting, and standing positions. The numbers of exercises and repetitions were determined individually depending on the functional abilities in patients. However, no therapeutic intervention was applied to the control group (CG).
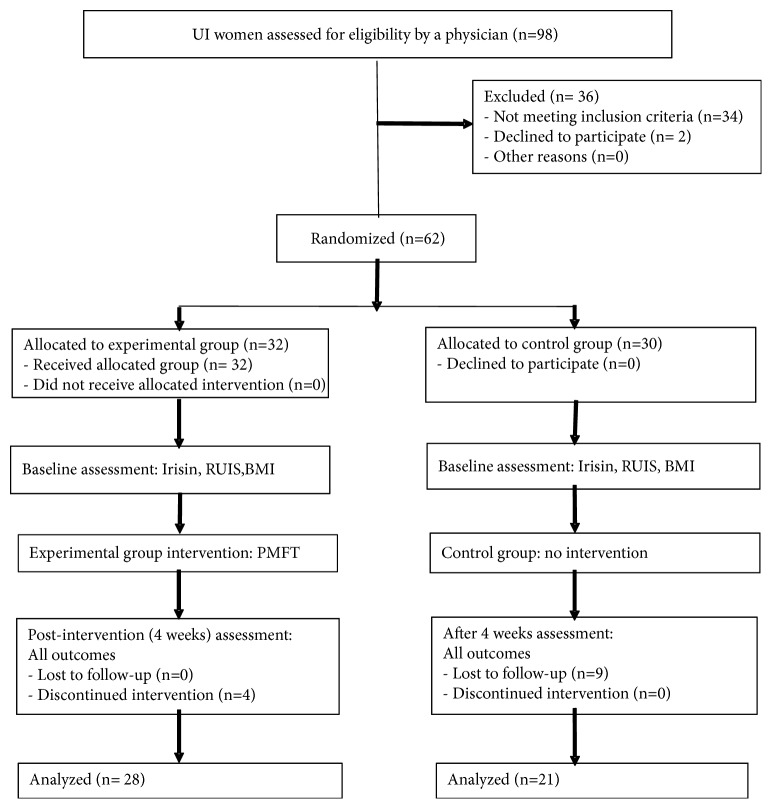
Of 13 participants who did not complete the study 4 participants withdrew from the EG during the 4-week intervention program, whereas 9 participants from the CG missed the final study visit. Overall, 49 participants have successfully completed the study (EG n = 28; CG n = 21). The randomized control trials (RCT) reporting quality have been improved using the CONSORT statement (Consolidated Standards of Reporting Trials) (Figure 1) [17].
Figure 1.
The study flow diagram.
Before the treatment, all the women were asked about the circumstances of urine loss, and the presence of comorbid conditions and contraindications to the treatment. Additionally, the Questionnaire for Urinary Incontinence Diagnosis (QUID) was used to diagnose the UI type. The QUID is a 6-item UI symptom questionnaire developed and validated to distinguish stress and urge urinary incontinence. Since the QUID includes acceptable psychometric characteristics, it can be used as a UI outcome measure in clinical trials [18].
Study inclusion criteria were as follows: age 60 years or older, a Body Mass Index (BMI) of 25 or more, diagnosed SUI, and no contradictions to the treatment (uterine tumors and myomas, urinary or genital tract infections, acute inflammations, acute infections, grade 3 or 4 hemorrhoids, stage 3 uterine prolapse, recent pelvic fractures, recent surgeries, severe urethral sphincter weakness and/or defect, suspected urethral and/or vesical fistula).
Study exclusion criteria were as follows: age < 60, a BMI under 25, diagnosed urge and mixed urinary incontinence, lack of regular physical activity, no therapeutic interventions in UI in the last three months (PFMT, Extracorporeal Magnetic Innervation (ExMI), electrostimulation, biofeedback), and the presence of contraindications to the treatment.
3.2. Measurements
To objectify the results, irisin concentrations were obtained for the EG and the CG at the initial and final assessments. Moreover, urinary incontinence severity assessment results (RUIS) and body mass index (BMI) were recorded for each study participant.
3.3. Irisin Concentration
Vacuette tubes with EDTA anticoagulant were used to draw 6 ml of fasting blood from each participant. The authors analyzed the samples using a competitive enzyme-linked immunosorbent assay (ELISA) (BIOVENDOR IRISIN ELISA kit, cat. no.: RAG018R, Brno, Czech Republic). The purified antigen competes with the antigen in the test sample for binding to an antibody that has been immobilized in a microtiter plate. This method is used for quantitative determination of irisin in human plasma. A polyclonal antibody recognizing native irisin reacts with a series of predetermined recombinant irisin standard proteins or samples under competition in the irisin-coated plate. Absorbance was measured at 450 nm in an ELISA reader. The standard curve is generated by plotting the average absorbance obtained for each standard concentration vs the corresponding irisin concentration (μg/ml), whereas irisin concentration in samples was calculated using the interpolation of the regression curve formula of a 4-parameter logistic equation.
3.4. The Revised Urinary Incontinence Scale (RUIS)
The RUIS is a valid 5-item scale that may be used to assess UI and to monitor patient outcomes after the treatment. A score of 0–3 is considered non-urinary incontinence; 4–8 mild urinary incontinence; 9–12 moderate urinary incontinence, and 13 or above severe urinary incontinence [19].
Body Mass Index (BMI) is determined as body mass in kilograms divided by height in meters squared. For adults, the BMI categories are as follows: < 18.5 underweight; 18.5–24.99 normal weight; ≥ 25.0 overweight.
3.5. Intervention
The experimental group underwent 12 therapy sessions of PFMT (45 minutes each, 3 times a week during 4 weeks).
3.6. Statistical Analyses
The collected data was analyzed statistically using the Statistica 13.1 software. Lower quartile (Q1), upper quartile (Q3), and the median were measured. Differences between the two groups were estimated using the Mann–Whitney U-test. Differences within one group were estimated using the Wilcoxon test. The correlation between measured variables was checked using the Spearman correlation coefficient. The authors defined statistical significance level as p < 0.05.
4. Results
Table 1 presents Student's t-test results and descriptive statistics for all measured variables for the EG and CG at the initial assessment. After comparing the Mann–Whitney U-test p value with the significance level of α = 0.05, the authors found no statistically significant differences between the experimental group and the control group results at the initial assessment. This confirms the homogeneity of the study groups.
Table 1.
Comparative analysis of all measured variables for the EG and CG – the initial assessment.
| Parameter | Statistics | EG (n=28) |
CG (n=21) |
p value |
|---|---|---|---|---|
| Age | IQR | 2.00 | 6.00 | 0.630 |
| Med | 62.50 | 67.00 | ||
|
| ||||
|
BMI (kg/m2) |
IQR | 2.40 | 2.85 | 1.000 |
| Med | 26.67 | 27.22 | ||
|
| ||||
|
Irisin concentration (ng/ml) |
IQR | 2.67 | 1.77 | 1.000 |
| Med | 9.02 | 5.91 | ||
|
| ||||
|
RUIS (points) |
IQR | 6.00 | 3.00 | 1.000 |
| Med | 10.00 | 7.00 | ||
EG – experimental group; CG – control group; Med – median; IQR – interquartile range;
p – significance level; RUIS – Revised Urinary Incontinence Scale; BMI-Body Mass Index
Table 2 presents Wilcoxon test results and descriptive statistics for all measured variables for the EG and CG at the initial and final assessments.
Table 2.
Comparative analysis of all measured variables for the EG and CG – at the initial and final assessments.
| Parameter | Statistics | EG (n=28) | p value | CG (n=21) | p value | ||
|---|---|---|---|---|---|---|---|
| Initial assessment | Final assessment | Initial assessment | Final assessment | ||||
|
Irisin concentration (ng/ml) |
IQR | 2.67 | 3.49 | < 0.001 ∗ | 1.77 | 3.01 | 0.079 |
| Med | 9.02 | 11.19 | 5.91 | 6.55 | |||
|
| |||||||
|
RUIS (points) |
IQR | 6.00 | 5.50 | < 0.001 ∗ | 3.00 | 3.00 | 0.124 |
| Med | 10.00 | 7.00 | 7.00 | 6.00 | |||
|
| |||||||
|
BMI (kg/m2) |
IQR | 2.40 | 2.64 | < 0.001 ∗ | 2.85 | 3.47 | 0.754 |
| Med | 26.67 | 26.28 | 27.22 | 27.34 | |||
EG – experimental group; CG – control group; Med – median; IQR – interquartile range; p – significance level; RUIS – Revised Urinary Incontinence Scale; BMI – Body Mass Index, ∗ statistical significance
After comparing the Wilcoxon test p value with the significance level of α = 0.05, the authors found a statistically significant difference in all measured variables for the experimental group at the initial and final assessments. A statistically significant increase in irisin concentration, decreases in BMI result, and an improvement in severity of UI (RUIS) were recorded for the PFMT group at the final assessment. After comparing the Wilcoxon test p value with the significance level of α = 0.05, the authors found no statistically significant differences between all measured variables for the control group at the initial and final assessments.
Table 3 presents the relationship between the irisin concentration assessment for the EG at the initial and final assessments and the UI severity (RUIS).
Table 3.
The relationship between the assessment of irisin concentration for the EG at the initial and final assessments and RUIS.
| RUIS (points) |
N | Statistics | Irisin concentration (ng/ml) | p value | |
|---|---|---|---|---|---|
| Initial assessment | Final assessment | ||||
| Mild | 9 | IQR | 2.86 | 3.42 | 0.007∗ |
| Med | 9.01 | 10.94 | |||
|
| |||||
| Moderate | 11 | IQR | 2.54 | 5.13 | 0.003∗ |
| Med | 8.20 | 10.27 | |||
|
| |||||
| Severe | 8 | IQR | 3.74 | 4.73 | 0.011∗ |
| Med | 10.23 | 12.24 | |||
EG – experimental group; Med – median; IQR – interquartile range; N – number of participants;
p – significance level; RUIS – Revised Urinary Incontinence Scale; ∗statistical significance
After comparing the Wilcoxon test p value with the significance level of α = 0.05, the authors found a statistically significant difference in irisin concentration at the final assessment in patients with severe, moderate, and mild urinary incontinence.
Table 4 presents Mann–Whitney U-test results and descriptive statistics for all measured variables for the EG and CG at the final assessment.
Table 4.
Comparative analysis of all measured variables for the EG and CG – the final assessment.
| Parameter | Statistics | EG (n=28) |
CG (n=21) |
p value |
|---|---|---|---|---|
|
Irisin concentration (ng/ml) |
IQR | 3.49 | 3.01 | 1.000 |
| Med | 11.19 | 6.55 | ||
|
| ||||
|
RUIS (points) |
IQR | 5.50 | 3.00 | 0.215 |
| Med | 7.00 | 6.00 | ||
|
| ||||
| BMI | IQR | 2.64 | 3.47 | 1.000 |
| Med | 26.28 | 27.34 | ||
EG – experimental group; CG – control group; Med – median; IQR – interquartile range; p – significance level;
RUIS – Revised Urinary Incontinence Scale; BMI – Body Mass Index
After comparing the Mann–Whitney U-test p value with the significance level of α = 0.05, the authors found no statistically significant difference in the measured variables between the experimental group and the control group at the final assessment.
In the last stage of the study, we correlated the BMI results with the irisin concentration results recorded for the experimental and control groups at the initial and final assessment. Moderate negative correlation was observed between the measured variables in the experimental group at the initial assessment (r = - 0. 435) and no correlation at the final assessment (r = - 0.187), whereas weak positive correlation was observed between the measured variables in the control group at the initial assessment (r = 0. 219) and at the final assessment (r = 0.207).
The scatter diagrams (Figures 2–5) show the association between BMI and the irisin concentration at the initial assessment and at the final assessment for the experimental and control groups.
Figure 2.
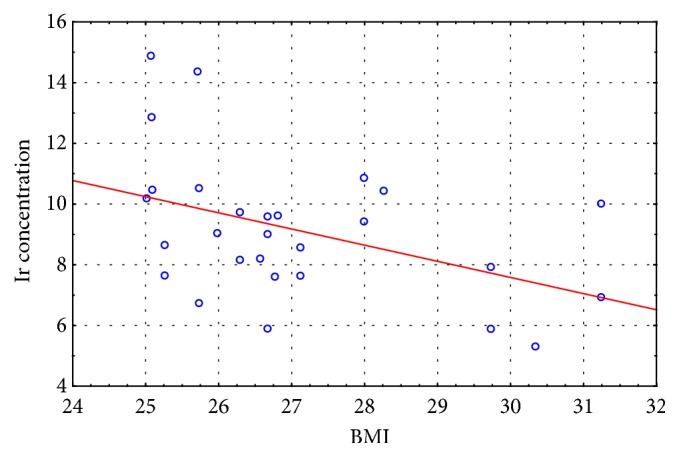
The scatter diagram: BMI to Ir concentration at the initial assessment for the experimental group.
Figure 3.
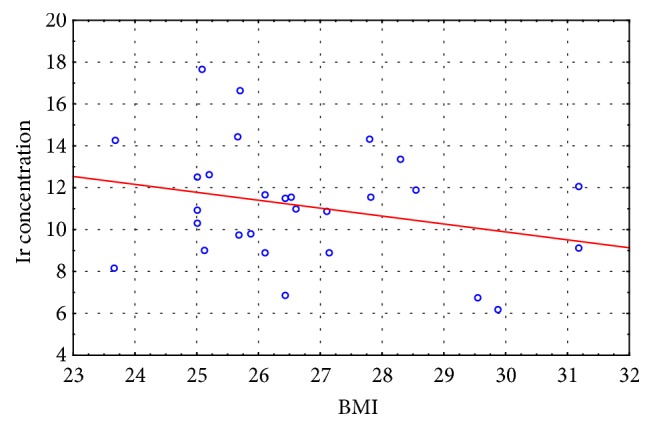
The scatter diagram: BMI to Ir concentration at the final assessment for the experimental group.
Figure 4.
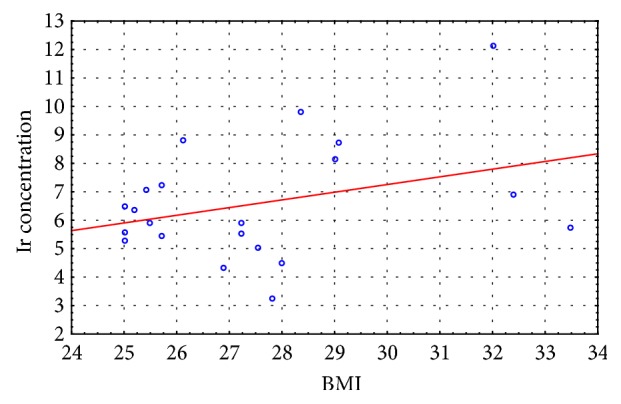
The scatter diagram: BMI to Ir concentration at the initial assessment for the control group.
Figure 5.
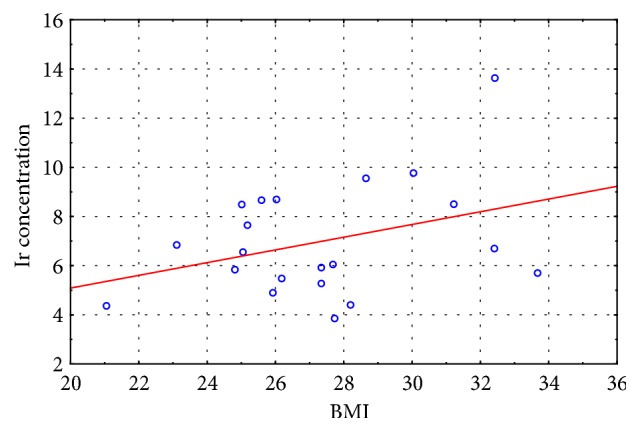
The scatter diagram: BMI to Ir concentration at the final assessment for the control group.
5. Discussion
To our knowledge, this is the first study which assesses irisin concentration after PFMTin overweight or obese elderly women with stress urinary incontinence. Valid question remains how the physical activity intensity (chronic or acute) affects irisin concentration. The authors attempted to answer this question by evaluating this myokine concentration after a chronic physical activity, i.e., a 4-week pelvic floor muscle training. Furthermore, in this study the authors assessed the Body Mass Index and the severity of urinary incontinence using the Revised Urinary Incontinence Scale (RUIS). By comparing the results at the initial and final assessments, we found a statistically significant increase in the irisin concentration for the EG (p < 0.001) and no statistically significant differences in the irisin concentration in the CG (p=0.079). Moreover, by comparing the results at the initial and final assessments, we found a statistically significant improvement in severity of urinary incontinence for the EG (p < 0.001) and no statistically significant differences in severity of UI in the CG (p=0.124). Even more interestingly, we recorded a statistically significant increase in irisin concentration in experimental group patients with mild, moderate, and severe urinary incontinence at the final assessment.
The Boström et al. study, published in 2012 [12], showed an increase in plasma irisin in eight healthy men following a 10-week aerobic training and afterwards it sparked a discussion about factors that affect myokine expression. Among such factors are age [20, 21], physical activity type [22–25], training level [23, 26, 27], anthropometric parameters [26, 28], comorbid diseases [29–31], and study design for measuring Ir concentration [32–34].
Since the study participants were overweight or obese women (BMI > 25), we also evaluated the BMI results for the EG and CG at the initial and final assessments. By comparing the results at the initial and final assessments, the authors found a statistically significant decrease in BMI results in EG (p < 0.001) and no statistically significant differences in the BMI results in the CG (p=0.754).
Because irisin concentration impacts body's metabolic profile, the authors also carried out studies to evaluate correlations between BMI results and the Ir concentration both in the EG and CG at the initial and final assessments. Moderate negative correlation was observed between the measured variables in the experimental group at the initial assessment (r = - 0. 435) and no correlation at the final assessment (r = - 0.187), whereas weak positive correlation was observed between the measured variables in the control group at the initial assessment (r = 0. 219) and at the final assessment (r = 0.207). The correlation between the irisin concentration and the BMI score is not clearly understood given that published studies offer conflicting results regarding this matter (positive correlation, negative correlation, and no correlation) [15, 29, 35–37]. Roca-Rivada et al. [37] demonstrated for the first time that white adipose tissue (WAT) also secretes FNDC5, and it can behave as an adipokine, constituting about 28% of the circulating irisin level in the blood. Research conducted using rat adipose tissue explants showed that visceral adipose tissue (VAT), and specifically subcutaneous adipose tissue (SAT), expresses and secretes FNDC5. During physical exertion the muscle tissue affects the circulating protein level, whereas in the case of obesity it is the fat tissue that actively increases the Ir concentration. Furthermore, greater skeletal muscle mass, which is a strong predictor of Ir, can also explain the positive correlation between BMI result and irisin concentration [26], while the hypotheses regarding the negative correlation of BMI and irisin concentration are associated with brown adipose tissue (BAT). Adipose tissue is heterogeneous. White adipose tissue (WAT) stores energy and its distribution greatly affects metabolic risk, whereas brown adipose tissue burns energy for thermogenesis. BAT is present and can be activated in most adult humans and total BAT activity is inversely associated with adiposity and indexes of the metabolic syndrome [38]. In overweight and obesity have been described significant low amounts of BAT, which confirms the irisin-resistance hypothesis [39, 40].
6. Conclusions
The therapeutic program induced a statistically significant increase in irisin concentration in the study participants.
The therapeutic program improved the severity of urinary incontinence in the study participants.
There was moderate negative correlation between BMI results and irisin concentration in the experimental group.
There was weak positive correlation between BMI results and irisin concentration in the control group.
Further studies are necessary to observe the regulation of irisin concentration and explain mechanisms underlying these effects.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Additional Points
Study Limitations. The authors acknowledge certain limitations of their analysis. These include a relatively small study group and the lack of assessment of long-term treatment outcomes. We therefore consider this research as a pilot study and we plan to further study this topic.
Disclosure
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
References
- 1.Petros P. E., Woodman P. J. The integral theory of continence. International Urogynecology Journal. 2008;19(1):35–40. doi: 10.1007/s00192-007-0475-9. [DOI] [PubMed] [Google Scholar]
- 2.Jóźwik M., Adamkiewicz M., et al. Budowa i czynności dna miednicy u kobiet – uaktualniony przegląd z podkreśleniem wpływu porodu drogami natury. Developmental Period Medicine. 2013;17(1):18–30. [PubMed] [Google Scholar]
- 3.Lamerton T. J., Torquati L., Brown W. J. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obesity Reviews. 2018;19(12):1735–1745. doi: 10.1111/obr.12756. [DOI] [PubMed] [Google Scholar]
- 4.Curcio F., Ferro G., Basile C., et al. Biomarkers in sarcopenia: A multifactorial approach. Experimental Gerontology. 2016;85:1–8. doi: 10.1016/j.exger.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Beyer I., Mets T., Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Current Opinion in Clinical Nutrition & Metabolic Care. 2012;15(1):12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 6.Ilich J. Z., Kelly O. J., Inglis J. E., Panton L. B., Duque G., Ormsbee M. J. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Research Reviews. 2014;15(1):51–60. doi: 10.1016/j.arr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Kersten S., Lichtenstein L., Steenbergen E., et al. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(6):969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 8.Yang S. J., Hong H. C., Choi H. Y., et al. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clinical Endocrinology. 2011;75(4):464–469. doi: 10.1111/j.1365-2265.2011.04078.x. [DOI] [PubMed] [Google Scholar]
- 9.Seldin M. M., Peterson J. M., Byerly M. S., Wei Z., Wong G. W. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. The Journal of Biological Chemistry. 2012;287(15):11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hittel D. S., Axelson M., Sarna N., Shearer J., Huffman K. M., Kraus W. E. Myostatin decreases with aerobic exercise and associates with insulin resistance. Medicine & Science in Sports & Exercise. 2010;42(11):2023–2029. doi: 10.1249/MSS.0b013e3181e0b9a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Høier B., Olsen K., Nyberg M., Bangsbo J., Hellsten Y. Contraction-induced secretion of VEGF from skeletal muscle cells is mediated by adenosine. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299(3):H857–H862. doi: 10.1152/ajpheart.00082.2010. [DOI] [PubMed] [Google Scholar]
- 12.Boström P., Wu J., Jedrychowski M. P., et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo-Quan J. I. From white to brown fat through the PGC-1α-dependent myokine irisin: implications for diabetes and obesity. Disease Models & Mechanisms. 2012;5(3):293–295. doi: 10.1242/dmm.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pukajło K., Kolackov K., Łaczmański Ł., Daroszewski J. Irisin – a new mediator of energy homeostasis. Postepy Higieny i Medycyny Doswiadczalnej. 2015;69:233–242. doi: 10.5604/17322693.1141097. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Navarrete J. M., Ortega F., Serrano M., et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. The Journal of Clinical Endocrinology & Metabolism. 2013;98(4):E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 16.Radzimińska A., Weber-Rajek M., Strączyńska A., et al. The impact of pelvic floor muscle training on the myostatin concentration and severity of urinary incontinence in elderly women with stress urinary incontinence – A pilot study. Clinical Interventions in Aging. 2018;13:1893–1898. doi: 10.2147/CIA.S177730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diallo S., Cour F., Josephson A., et al. Evaluating single-incision slings in female stress urinary incontinence: the usefulness of the consort statement criteria. Urology. 2012;80(3):535–541. doi: 10.1016/j.urology.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Bradley C. S., Rahn D. D., Nygaard I. E., et al. The questionnaire for urinary incontinence diagnosis (QUID): Validity and responsiveness to change in women undergoing non-surgical therapies for treatment of stress predominant urinary incontinence. Neurourology and Urodynamics. 2010;29(5):727–734. doi: 10.1002/nau.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansoni J., Hawthorne G., Marosszeky N., et al. The Technical Manual for the Revised Incontinence and Patient Satisfaction Tools. Centre for Health Service Development, University of Wollongong; 2011. [Google Scholar]
- 20.Daskalopoulou S. S., Cooke A. B., Gomez Y.-H., et al. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. European Journal of Endocrinology. 2014;171(3):343–352. doi: 10.1530/eje-14-0204. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto-Mikami E., Sato K., Kurihara T., et al. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0120354.e0120354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh J. Y., Mougios V., Skraparlis A., Kabasakalis A., Mantzoros C. S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism. 2014;63(7):918–921. doi: 10.1016/j.metabol.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer R. R., Shockett P., Webb N. D., Shah U., Castracane V. D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Hormone and Metabolic Research. 2014;46(2):150–154. doi: 10.1055/s-0033-1355381. [DOI] [PubMed] [Google Scholar]
- 24.Löffler D., Müller U., Scheuermann K., et al. Serum irisin levels are regulated by acute strenuous exercise. The Journal of Clinical Endocrinology & Metabolism. 2015;100(4):1289–1299. doi: 10.1210/jc.2014-2932. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya Y., Ando D., Takamatsu K., Goto K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. 2015;64(9):1042–1050. doi: 10.1016/j.metabol.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Huh J. Y., Panagiotou G., Mougios V., et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism - Clinical and Experimental. 2012;61(12):1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh J. Y., Mougios V., Kabasakalis A., et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. The Journal of Clinical Endocrinology & Metabolism. 2014;99(11):E2154–E2161. doi: 10.1210/jc.2014-1437. [DOI] [PubMed] [Google Scholar]
- 28.Elizondo-Montemayor L., Mendoza-Lara G., Gutierrez-DelBosque G., Peschard-Franco M., Nieblas B., Garcia-Rivas G. Relationship of circulating irisin with body composition, physical activity, and cardiovascular and metabolic disorders in the pediatric population. International Journal of Molecular Sciences. 2018;19(12):p. 3727. doi: 10.3390/ijms19123727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana K. S., Pararasa C., Afzal I., et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovascular Diabetology. 2017;16(1, article 147) doi: 10.1186/s12933-017-0627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pukajło K., Łaczmański Ł., Kolackov K., et al. Irisin plasma concentration in PCOS and healthy subjects is related to body fat content and android fat distribution. Gynecological Endocrinology. 2015;31(11):907–911. doi: 10.3109/09513590.2015.1065482. [DOI] [PubMed] [Google Scholar]
- 31.Wikiera B., Zawadzka K., Łaczmański Ł., et al. Growth hormone treatment increases plasma irisin concentration in patients with turner syndrome. Hormone and Metabolic Research. 2017;49(02):122–128. doi: 10.1055/s-0042-119788. [DOI] [PubMed] [Google Scholar]
- 32.Hecksteden A., Wegmann M., Steffen A., et al. Irisin and exercise training in humans—results from a randomized controlled training trial. BMC Medicine. 2013;11(1, article 235):1–8. doi: 10.1186/1741-7015-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anastasilakis A. D., Polyzos S. A., Saridakis Z. G., et al. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. The Journal of Clinical Endocrinology & Metabolism. 2014;99(9):3247–3255. doi: 10.1210/jc.2014-1367. [DOI] [PubMed] [Google Scholar]
- 34.Jedrychowski M. P., Wrann C. D., Paulo J. A., et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metabolism. 2015;22(4):734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park K. H., Zaichenko L., Brinkoetter M., et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism. 2013;98(12):4899–4907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stengel A., Hofmann T., Goebel-Stengel M., Elbelt U., Kobelt P., Klapp B. F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity—correlation with body mass index. Peptides. 2013;39(1):125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Roca-Rivada A., Castelao C., Senin L. L., et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060563.e60563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cypess A. M., Kahn C. R. Brown fat as a therapy for obesity and diabetes. Current Opinion in Endocrinology, Diabetes and Obesity. 2010;17(2):143–149. doi: 10.1097/MED.0b013e328337a81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardo M., Crujeiras A. B., Amil M., et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. International Journal of Endocrinology. 2014;2014:9. doi: 10.1155/2014/857270.857270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virtanen K. A., Lidell M. E., Orava J., et al. Functional brown adipose tissue in healthy adults. The New England Journal of Medicine. 2009;360(15):1518–1525. doi: 10.1056/nejmoa0808949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.