Abstract
Sclerosing and spindle cell rhabdomyosarcoma is a rare histologic subtype, designated in the latest WHO classification as a stand-alone pathologic entity. Three genomic groups have been defined: an infantile subset of spindle cell rhabdomyosarcoma harboring VGLL2-related gene fusions, a MYOD1-mutant subset commonly associated with sclerosing morphology, and a subset lacking recurrent genetic abnormalities. In this study, we focus on MYOD1-mutant rhabdomyosarcoma to further define their clinicopathologic and behavior characteristics in a larger patient cohort. We investigated 30 cases of MYOD1-mutant rhabdomyosarcoma (12 previously reported and 18 newly diagnosed) with an age range of 2–94 years, including 15 children. All cases showed morphology within the spectrum of spindle cell / sclerosing rhabdomyosarcoma (8 cases showing pure sclerosing morphology, 8 showing pure spindle cell morphology and 14 cases showing a hybrid phenotype of spindle, sclerosing and primitive undifferentiated areas). All tumors harbored either homozygous or heterozygous MYOD1 (p.L122R) exon 1 mutations. In 10 (33%) cases, a co-existent PIK3CA mutation was identified. Hot-spot mutations in NRAS and HRAS were each found in a single case, respectively. Follow-up was available on 22 (73%) patients with a median duration of 28 months. Local recurrence was seen in 12 (55%) and distant recurrence in 12 (55%) of cases, despite multimodality chemoradiation therapy. At last follow-up, 15 (68%) patients died of the disease, 1 patient was alive with disease and 5 had no evidence of disease. The prognosis was equally poor in pediatric and adult patients. In conclusion, MYOD1 mutation defines an aggressive rhabdomyosarcoma subset, with poor outcome and response to therapy, irrespective of age. Given that this distinct molecular subtype is characterized by an aggressive biologic behavior compared to other genetic subtypes of spindle and sclerosing rhabdomyosarcoma, the MYOD1 genotype should be used as a molecular marker in both subclassification and prognostication of rhabdomyosarcoma.
Keywords: MYOD1, sclerosing rhabdomyosarcoma, spindle cell rhabdomyosarcoma
INTRODUCTION
Rhabdomyosarcoma is the most common soft tissue sarcoma in children, accounting for 40% of all pediatric soft tissue sarcoma,1 while rhabdomyosarcoma in adults is far less common. The latest WHO classification of soft tissue tumors,2 classifies rhabdomyosarcoma into four subtypes – embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, spindle cell/sclerosing rhabdomyosarcoma and pleomorphic rhabdomyosarcoma. While alveolar rhabdomyosarcoma follow a highly aggressive course, the prognosis of embryonal rhabdomyosarcoma has significantly improved in recent years with an overall survival of 70% at 5 years for patients presenting with localized disease.3 Spindle cell/sclerosing subtype was only recently separated from the embryonal rhabdomyosarcoma group and defined as a stand alone pathologic entity based on its distinctive morphologic features. Spindle cell variant of rhabdomyosarcoma was initially described in children in the paratesticular and head and neck location, being associated with a more favorable prognosis.4, 5 A subset of spindle cell rhabdomyosarcoma were shown to have prominent hyaline sclerosis and pseudo-vascular growth pattern, suggesting morphologic overlap with the even less common sclerosing type rhabdomyosarcoma.6 As both spindle cell and sclerosing rhabdomyosarcoma have similar clinical presentations, Mentzel and colleagues suggested a histologic spectrum of a single pathologic entity.7–9 More recently, a recurrent MYOD1 L122R mutation was identified as a frequent genetic event in adult spindle cell rhabdomyosarcoma, by two independent groups10, 11 and found to be associated with altered function of MYOD1 and an aggressive clinical course.11 Subsequent studies from our group and others have identified similar hot-spot MYOD1 mutations in pediatric rhabdomyosarcoma with spindle cell/sclerosing morphology and confirmed the association with poor clinical outcomes.12–16 Here, we describe the largest cohort of MYOD1 mutant rhabdomyosarcoma to date, spanning various age groups and clinical presentations. As rhabdomyosarcoma with MYOD1 mutations is associated with a highly lethal outcome despite multimodality chemoradiation therapy, it raises an argument for a molecular subclassification of the spindle cell/sclerosing rhabdomyosarcoma based on their specific genetic signatures, and to include this variant as a separate subtype, with an unfavorable behavior comparable to alveolar rhabdomyosarcoma.
MATERIALS AND METHODS
Patient Selection and Histologic Diagnosis
Archival and personal consultation material (CRA) from adult and pediatric patients with diagnosis of spindle and sclerosing rhabdomyosarcoma was retrieved from the pathology files at Memorial Sloan Kettering Cancer Center, in which additional material was available for MYOD1 mutation testing. Thirty cases were identified in which the diagnosis of spindle and sclerosing rhabdomyosarcoma was confirmed by re-review of histologic slides and the presence of a MYOD1 mutation was identified. Formalin fixed paraffin embedded tissue was available on all the cases selected for the study. Twelve cases were previously included in the following studies by our group: 9 cases in the study by Agaram et al.13 which included 2 cases reported in Kohsaka et al.11, 10 cases in the study by Alaggio et al.14, and 3 cases in Owosho et al.15 This information also detailed in Table1. The remaining 18 cases are being genetically examined for the first time. The study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center. The morphologic features were characteristic of those described in spindle cell / sclerosing rhabdomyosarcoma,4–6, 8, 17 and showed a relatively monomorphic spindle cell proliferation arranged in long, intersecting fascicles, in a background of variably fibrotic stromal component. The tumors typically had an infiltrative growth within the adjacent skeletal muscle and adipose tissue. The nuclear pleomorphism was mild, while lacking evidence of rhabdomyoblastic differentiation. The immunohistochemical profile was based on desmin reactivity, typically diffuse and strong, at least focal reactivity for myogenin and diffuse positivity for MYOD1.
Table 1.
Clinicopathologic and Molecular features and Followup of MYOD1-mutant Rhabdomyosarcoma
| RMS# | Age | Location | Morphology | MYOD1 Ex1 p.L122R | PIK3CA | Therapy | LR | DR | FU duration (months) | FU status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 2/F | buttock | Sp, Pri | Heterozygous | WT | Chemo + RT | DR | 12 | DOD | |
| 2 | 4/F | Calf | Scl, Pri | Heterozygous | WT | na | na | |||
| 3* | 7/F | I-abd | Scl | Homozygous | WT | Chemo + RT | LR | DR | 28 | DOD |
| 4*# | 8/M | thigh | Scl, Pri (pre-Rx); Sp (post-Rx) | Homozygous | WT | Chemo | 44 | NED | ||
| 5# | 9/F | H&N | Sp | Homozygous | WT | LR | 12 | DOD | ||
| 6# | 9/M | H&N | Sp | Homozygous | WT | LR | 42 | DOD | ||
| 7 | 9/F | H&N | Scl | Homozygous | E545A | na | na | |||
| 8# | 10/F | paraspinal | Sp | Homozygous | WT | Chemo + RT | LR | DR | 35 | DOD |
| 9¥# | 10/F | buttock | Scl, Pri | Homozygous | G1049R | DR | 6 | DOD | ||
| 10 | 10/F | H&N | Scl | Heterozygous | WT | Chemo + RT | LR | 48 | NED | |
| 11# | 11/F | H&N | Scl | Homozygous | Q546R | na | na | |||
| 12# | 13/F | chest wall | Scl (pre-Rx); Sp (post-Rx) | Homozygous | E545K | Chemo + RT | LR | DR | 21 | DOD |
| 13# | 15/F | H&N | Scl | Homozygous | E542K | Chemo | LR | DR | 26 | DOD |
| 14# | 17/M | paravertebral | Sp, Scl | Homozygous | WT | DR | 24 | DOD | ||
| 15* | 17/F | thorax | Sp | Homozygous | WT | Chemo + RT | LR | DR | 68 | DOD |
| 16* | 21/M | pelvis | Sp | Homozygous | WT | Chemo | LR | DR | 42 | DOD |
| 17* | 21/F | H&N | Sp, Scl, Pri | Homozygous | M1043V | Chemo + RT | 30 | NED | ||
| 18 | 21/F | H&N | Sp, Scl | Heterozygous | - | na | na | |||
| 19* | 26/M | lower leg | Sp, Scl | Homozygous | H1047R | Chemo | 4 | NED | ||
| 20* | 31/F | H&N | Scl | Homozygous | K111E | Chemo + RT | LR | 12 | AWD | |
| 21# | 32/M | H&N | Sp, Scl | Heterozygous | WT | Chemo + RT | LR | 65 | DOD | |
| 22 | 33/M | Thigh | Sp | Homozygous | WT | na | na | |||
| 23# | 34/F | H&N | Sp, Scl | Heterozygous | E542V | Chemo | DR | 42 | DOD | |
| 24* | 36/M | forearm | Sp | Homozygous | K111 del | Chemo + RT | LR | DR | 16 | DOD |
| 25 | 38/F | Leg | Sp | Homozygous | WT | na | na | |||
| 26 | 39/M | lower leg | Sp, Scl | Heterozygous | WT | Chemo | 60 | NED | ||
| 27* | 44/F | paraspinal | Sp | Homozygous | WT | Chemo + RT | 13 | NED | ||
| 28* | 45/M | Liver | Pri, Sp | Heterozygous | WT | na | na | |||
| 29 | 77/M | lower leg | Sp, Scl | Homozygous | WT | Chemo | DR | 32 | DOD | |
| 30 | 94/M | lower leg | Scl | Homozygous | WT | na | na |
cases tested by MSK-IMPACT;
cases tested by RNAseq;
cases previously included in references 11–15; M, male; F, female; FU, follow-up; H&N, head and neck; intra-abd, intra-abdominal; Sp, spindle; Scl, sclerosing; Pri, primitive; LR, local recurrence; DR, distant recurrence; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease; LR, local recurrence; RT, external beam radiation; na, not available.
PCR and Sanger Sequencing
Genomic DNA was isolated either from fresh-frozen or archival paraffin tissue, as described previously18 in 19 samples. Targeted PCR was performed for the known MYOD1 exon 1 hot spot mutation and PIK3CA exons 9 and 20 mutations, using the following primer sequences: MYOD1 Ex1 fwd: 5’-CCTACTGTGGGCCTGCAAG-3’ and Ex1 rev: 5’-GGATCTCCACCTTGGGCAAC-3’; PIK3CA-Ex9 fwd: 5’-CCAGAGGGGAAAAATATGACAAAG-3’, PIK3CA-Ex9 rev 5’-CCATTTTAGCACTTACCTGTGACTCC-3’, PIK3CA-Ex20 fwd: 5’-CTCAATGATGCTTGGCTCTGG-3’ and PIK3CA-Ex20 rev: 5’-GTGGAATCCAGAGTGAGCTTTC-3’, using Clontech advantage 2 PCR KIT, at 64.5°C annealing temperature for 35 cycles. Direct sequencing of PCR products was performed and compared to the NCBI human MYOD1 and PIK3CA gene sequences.
MSK-IMPACT Assay
Details of the MSK-IMPACT assay have been previously published.19 Briefly, MSK-IMPACT is a comprehensive molecular profiling assay that involves hybridization capture and deep sequencing of all exons and selected introns of up to 468 oncogenes and tumor-suppressor genes, allowing the detection of point mutations, small and large insertions or deletions, and rearrangements. In addition to capturing all coding regions of the genes, the assay also captures >1000 intergenic and intronic single-nucleotide polymorphisms (tiling probes), interspersed homogenously across the genome, aiding the accurate assessment of genome-wide copy number. In total, the probes target approximately 1.5 megabases of the human genome.
RESULTS
Clinicopathologic Features
The clinicopathologic features are summarized in Table 1. There were 30 patients, including 18 females and 12 males, ranging in age from 2 to 94 years (median age – 19 yrs). The cohort included 15 (50%) children (12 females, 3 males), ranging in age from 2–17 years, and 15 (50%) adults (9 males, 6 females) with an age range of 21–94 years. The anatomic location of the tumors varied, including: head and neck (11), trunk/abdominal (paraspinal-3, chest wall-1, thorax-1, pelvic/intra-abdominal-2), extremities (lower leg-6, buttock-2, thigh-2, forearm-1) and visceral (liver-1).
Histologically, all cases showed a typical spindle cell / sclerosing morphology as previously described.13 Eight cases showed pure sclerosing rhabdomyosarcoma morphology, 8 showed pure spindle cell rhabdomyosarcoma morphology and 14 cases showed mixed features of spindle, sclerosing and primitive undifferentiated areas. (Table 1) Morphologically, the spindle cell rhabdomyosarcoma were composed of monomorphic, undifferentiated spindle cells arranged in long intersecting fascicles. (Figure 1) The growth patterns were reminiscent of myofibroblastic tumors in two cases (cases# 5 and 6) with fusiform nuclei with open chromatin and small nucleoli. In one case (case 8), the growth pattern was similar to leiomyosarcoma with intersecting fascicles of tumor with plump spindle cells and eosinophilic cytoplasm. In three cases (cases# 16, 25 and 27) the tumor morphology resembled a fibrosarcoma, with streaming cells organized in long cellular fascicles with a distinctive “herring-bone” appearance. One case (case 1) showed geographic areas of necrosis with perivascular preservation of tumor cells, resembling malignant peripheral nerve sheath tumor morphology. Tumors typically showed minimal, if any, sign of rhabdomyoblastic differentiation. Histologically, the sclerosing rhabdomyosarcoma showed spindle cells embedded in a densely hyalinized collagenous background. (Figure 1) A pseudo-vascular pattern was noted focally in most cases.
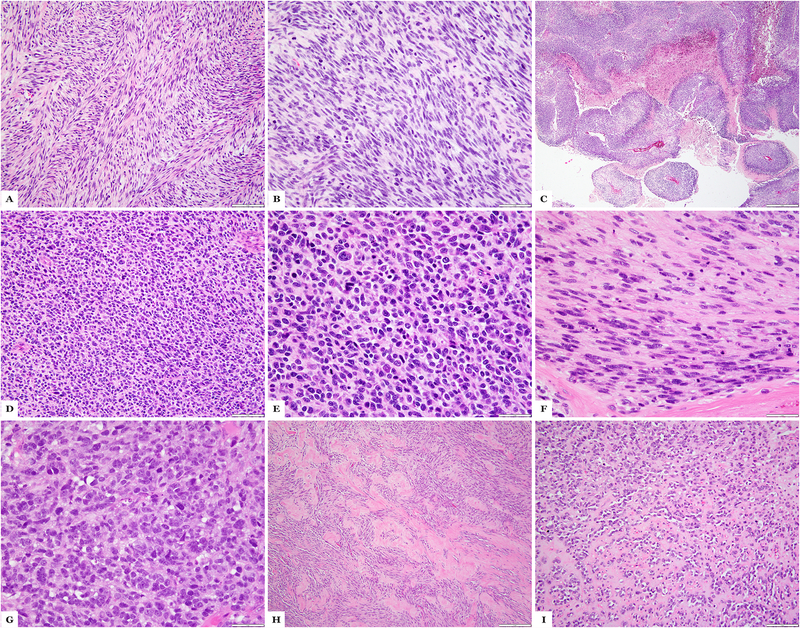
Figure 1:
MYOD1-mutant rhabdomyosarcoma with sclerosing and spindle cell morphology. (A-C) Case 4 showing areas with sclerosing morphology with small clusters of spindle cells in a sclerotic background (A), immunohistochemical stains for desmin showing cytoplasmic positivity (B) and MYOD1 showing strong diffuse nuclear positivity (C). (D-F) Case 21 showing spindle cell morphology with intersecting fascicles of monomorphic fusiform cells (D), immunohistochemical stains for desmin with strong cytoplasmic positivity (E) and MYOD1 diffuse nuclear positivity (F).
Primitive undifferentiated areas composed of sheets of round cells with scant cytoplasm and hyperchromatic nuclei (Figure 2) were seen in 5 cases. In 2 of these cases (cases# 1 and 28), there was associated spindle cell component, 2 cases (cases# 2 and 4) showed associated sclerosing component and 1 case (case# 17) had both sclerosing and spindle cell components.
Figure 2:
MYOD1-mutant rhabdomyosarcoma with primitive round to spindle cell component. (A-C) Case 1 showing areas of classic spindle cell morphology with fascicular growth (A), solid zones of primitive appearing spindle cell component (B) and geographic necrosis (MPNST-like); (D-F) Case 28 showing tumor in the liver with undifferentiated primitive round cell component (D, E) and areas of spindling (F); (G-I) Case 23, a post-therapy resection, showing primitive round cell areas (G), spindle cell morphology (H) and areas with sclerosing morphology (I). (H&E stains)
Mitotic activity was high in most tumors ranging from 10–25 per 10 high power fields. Numerous apoptotic bodies were identified in most cases. Necrosis was an uncommon feature being present in 4 cases (cases# 1, 9, 14 and 28), as focal and spotty in 3 and as geographic areas of necrosis in one (case 1, Figure 2).
Immunohistochemical staining profile was available for review in 27 of the 30 cases. Desmin was diffusely positive in all cases, while myogenin was focally positive in all but three of the tumors (cases# 4, 7 and 17). MYOD1 immunostaining was performed in 16 cases and was diffusely positive in all of the tumors tested. (Figure 1)
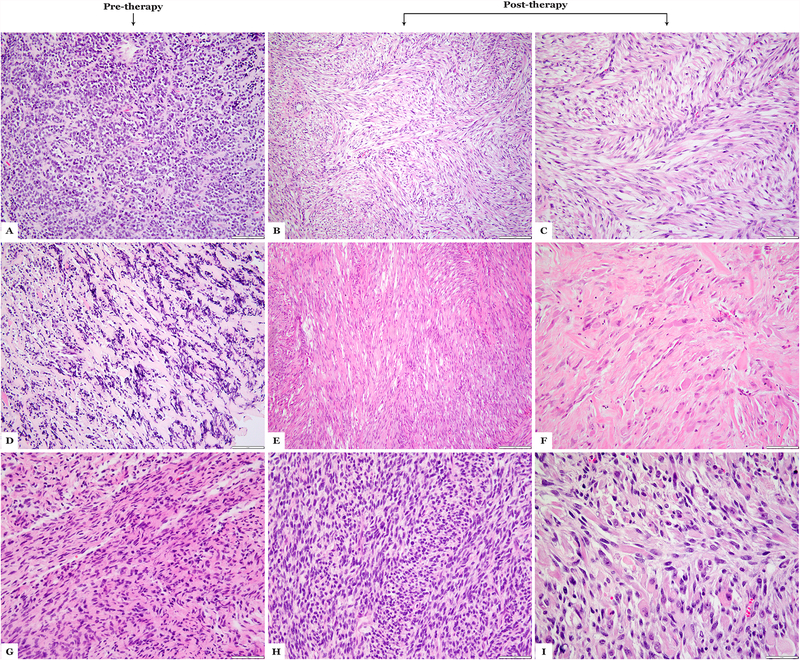
A subset of cases showed morphologic changes in the post-therapy resection when compared to the pre-therapy biopsy (Figure 3). Cases# 4 and 12 showed primitive and sclerosing morphology on the pre-treatment biopsy, and on post-therapy resections, showed predominantly spindle cell morphology with areas of maturation with rhabdomyoblastic differentiation. Cases# 23 and 26 both showed spindle, sclerosing and rhabdomyoblastic differentiation in the post-treatment samples.
Figure 3:
Morphologic spectrum of MYOD1-mutant rhabdomyosarcoma following therapy. (A-C) Case 4 showing sclerosing areas on the pre-therapy biopsy (A), while in the post-therapy resection (B,C) showing pure spindle cell morphology; (D-F) Case 12 showing a sclerosing morphology in the pre-therapy biopsy (D), while the post-therapy resection reveals a spindle cell morphology (E) and areas of maturation with rhabdomyoblastic morphology (F); (G-I) Case 24 showing spindle cell morphology on pre-therapy biopsy (G), while post-therapy resection shows cellular spindle cell areas (H) and areas of maturation (I). (H&E stains)
p.L122R MYOD1 Mutations Present in Both Sclerosing and Spindle Cell rhabdomyosarcoma
Nineteen cases were analyzed by PCR for mutations in MYOD1 exon 1. All cases showed a p.L122R (c. T365G) mutation. Targeted exome sequencing by MSK-IMPACT showed MYOD1 L122R mutations in 10 cases. In one case (case 9), previously reported by Alaggio et al.,[14] RNA sequencing data analyzed by mutation detection algorithms showed MYOD1 L122R mutations. Overall, 22 cases (73%) showed homozygosity for MYOD1 L122R presumably via loss of the other allele, while the remaining 8 (27%) were heterozygous for the MYOD1 L122R mutation.
Concurrent PIK3CA, NRAS, HRAS mutations are present in MYOD1-mutant rhabdomyosarcoma
A subset of MYOD1-mutant rhabdomyosarcoma harbor co-existing PIK3CA mutations, either in the helical domain (E542K, E545K) or in the kinase domain (H1047R).11,13 We investigated mutations at these PIK3CA hot-spots in all except one case. Ten (33%) cases showed coexistent PIK3CA mutations, 2 in exons 2 (K111del., K111E), 5 in exon 9 (E542K, E542V, E545K, E545A, Q546R) and 3 cases in exon 20 (H1047R, M1043V), respectively. (Table 1) Interestingly, 6 of the 10 cases with co-existent PIK3CA mutations showed pure sclerosing morphology, 3 cases showed hybrid spindle cell / sclerosing morphology and only 1 case showing pure spindle cell morphology.
Additionally, mutually exclusive to PIK3CA mutated cases, 1 case each showed coexistent NRAS (Q61L) (case 3) and HRAS (G13R) (case 21) hotspot mutations, respectively.
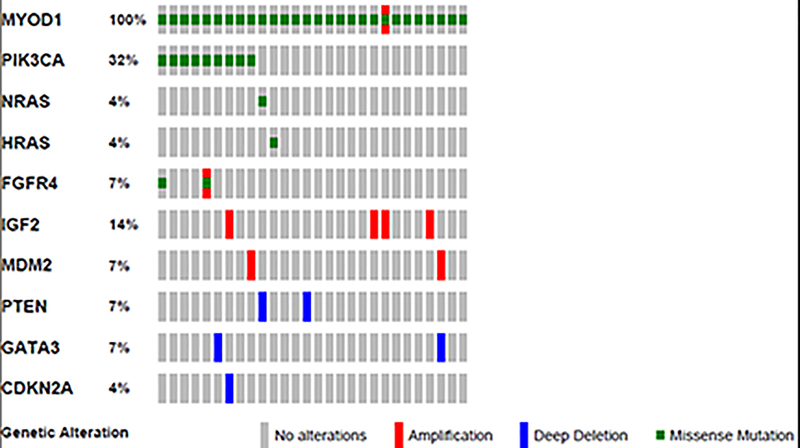
Targeted Exome Sequencing identifies additional genetic abnormalities in MYOD1-mutant rhabdomyosarcoma
Apart from the MYOD1 mutations in exon 1, targeted exome sequencing (MSK-IMPACT) showed additional genetic alterations in the 10 cases analyzed, mainly gene copy number alterations and mutations. (Figure 4)(Supplementary Table 1) Case 16 showed low level amplification of the MYOD1 gene, in addition to the exon 1 L122R MYOD1 gene mutation. Interestingly, we identified another spindle cell rhabdomyosarcoma which showed amplification of the MYOD1 gene, although it lacked MYOD1 mutations. This case (not included in the study group) occurred in a 74 year-old patient with a hard palate lesion who recurred and succumbed of disease despite chemotherapy and radiation 12 months since diagnosis.
Figure 4:
Oncoprint image showing the variant genetic alterations identified in the study cases.
IGF2 copy number gain /amplification was a frequent genetic alteration, being identified in 4 (40%) cases (cases# 15, 16, 20 and 27). However, FISH studies confirmed amplification in cases 16 and 27 (results not shown), while no IGF2 amplification could be confirmed in the remaining two cases, suggesting low level gains or spatial heterogeneity.
MDM2 gene amplification was seen in two cases (cases# 24 and 28). Case 9 also showed co-existent FGFR4 (V548M) mutation. Case 17 showed the presence of both amplification and a V550L mutation in the FGFR4 gene. Losses (deletions) of tumor suppressor genes, such as PTEN (cases# 3 and 4), GATA3 (cases# 19 and 28) and CDKN2A / CDKN2B (case# 20) were also noted. No TP53 gene alterations were identified.
MYOD1-Mutated rhabdomyosarcoma Follow a Highly Aggressive Clinical Course in both Children and Adults.
Follow-up information (Table 1) was available in 22 of the 30 cases (73%), with duration of 4–68 months (mean - 28.8 months, median - 28 months). Eleven of the patients were treated with combination of chemotherapy and radiotherapy and 7 patients were treated with chemotherapy alone. Twelve (55%) patients developed local recurrence and 12 (55%) developed distant recurrence, including 7 (32%) patients who developed local recurrence and distant recurrence. The sites of distant recurrences included lung, mediastinum, breast, abdominal soft tissue, spleen and chest wall. At last follow-up, 6 patients (27%) had no evidence of disease, 1 patient (5%) was alive with disease, and 15 patients (68%) died of the disease at 12–68 months following diagnosis. Follow-up was not available on 7 cases and one was a recent case. Based on the available follow-up, the 3-year and 4-year overall survival was 36% and 18%, respectively.
Ten of the 12 (83%) pediatric cases with follow-up data available died of the disease, and 2 were no evidence of disease. Of the 9 adult cases with follow-up data available, 5 (55%) died of the disease, 1 is alive with disease and 3 were no evidence of disease. Survival analysis showed no statistical difference of survival outcomes for pediatric and adult subgroups (Supplementary Figure 1) and for cases with and without PIK3CA mutations (Supplementary Figure 2).
DISCUSSION
MYOD1 mutations in rhabdomyosarcoma were initially reported in 2014 by two independent groups.10, 11 In order to investigate the possibility of a shared pathogenesis among the spindle cell and sclerosing rhabdomyosarcoma, spanning both age groups, our group investigated an initial cohort of 16 pediatric and adult spindle cell and sclerosing rhabdomyosarcoma13, of which 56% (9/16 cases) showed MYOD1 mutations. All 5 rhabdomyosarcomas with sclerosing histology, presenting either in pediatric or adult age-group, were positive for MYOD1 gene mutations, while only 4 (36%) of 11 spindle cell rhabdomyosarcomas showed MYOD1 mutations, 2 each in children and adults. In contrast to the study by Szuhai et al.10 where only homozygous MYOD1 mutations were identified, 4/9 mutant tumors in our study showed MYOD1 p.L122R heterozygous mutations, while the remaining were homozygous. Thus our initial findings confirmed that spindle cell and sclerosing rhabdomyosarcomas share genetic abnormalities thereby providing a strong molecular basis in supporting the classification of these two groups as a single pathologic entity. Furthermore, MYOD1 mutations were consistently identified in rhabdomyosarcoma displaying sclerosing morphology, regardless of age at presentation, in keeping with a homogeneous genetic entity.
Subsequently, focusing only on the pediatric-age group, our group identified three distinct molecular subsets of the spindle cell/sclerosing rhabdomyosarcoma.14 The first, so-called ‘congenital or infantile spindle cell rhabdomyosarcoma’, encompassing tumors presenting at birth or within one year of age, with predilection for the trunk, and harboring recurrent gene fusions, involving critical transcriptional activators of muscle-specific genes, such as VGLL2, TEAD1 and SRF.14, 20 These patients followed a favorable clinical outcome, lacking metastatic potential, all being alive and well at long term follow-up. These fusion-positive infantile spindle cell rhabdomyosarcoma group appeared to closely resemble the behavior of ETV6-NTRK3-positive infantile fibrosarcomas and we argued that the findings militated against their classification as a ‘high grade neoplasm’ and suggested downgrading of their current therapy and possibly excluding the need for chemotherapy if completely excised. The second group was that of ‘MYOD1-mutant spindle cell/sclerosing rhabdomyosarcoma’, with or without accompanying PIK3CA mutations, occurring in older children, and following a highly aggressive course with high mortality despite multimodality therapy. In that study, MYOD1 mutations was the most common genetic abnormality in pediatric spindle cell/sclerosing rhabdomyosarcoma, occurring in 64% of children beyond one year of age, and suggesting that the MYOD1 mutation can be used as a molecular biomarker to stratify these high risk patients. The third group was the remaining ‘genetically negative’ group, lacking gene fusions or MYOD1 mutations, often presenting intra-abdominally or in the genito-urinary area. This molecular-negative group followed a favorable clinical course and might represent embryonal rhabdomyosarcoma with spindle cell areas.
As head and neck is the most common anatomic site for spindle and sclerosing rhabdomyosarcoma, our group reported 13 cases of spindle cell / sclerosing rhabdomyosarcoma arising in this location, including 3 tumors with MYOD1 mutations, all showing sclerosing histology.15 When comparing the outcome of all histologic subtypes in a cohort of 99 rhabdomyosarcomas from the head and neck region, alveolar rhabdomyosarcoma and MYOD1-mutant spindle cell / sclerosing rhabdomyosarcomas had similar poor prognosis.12 In fact, the 5-year overall survival rate for embryonal rhabdomyosarcoma patients was significantly higher (82%) compared to alveolar rhabdomyosarcoma (53%) and spindle/sclerosing rhabdomyosarcoma (50%). More specifically the MYOD1-mutant positive sclerosing rhabdomyosarcoma had a 30% 5-year overall survival compared to 75% of the spindle cell rhabdomyosarcoma.12
The aim of the current study was to better define the demographics, pathologic features, and behavior in a large cohort of MYOD1 mutant positive rhabdomyosarcoma. With 30 cases (12 previously reported and 18 newly diagnosed cases), this is the largest patient cohort of MYOD1 mutant rhabdomyosarcoma to date. Our results establish MYOD1 mutation as a surrogate biomarker of poor outcome regardless of age at presentation and phenotype (spindle, sclerosing, mixed). The study included 15 children and 15 adults, with a wide age range at diagnosis, the youngest being 2 years and the oldest being 94 years of age. In children, there was a female predilection, with 12 of the 15 cases occurring in girls. In adults, there was a fairly equal distribution for males and females. In keeping with prior literature, the head and neck location was the most common site, occurring in one-third (33%) of patients, with equal predilection in both children and adults. The other common sites included the extremities and the trunk. At the molecular level, most (22/30, 73%) tumors harbored a MYOD1 homozygous mutation in exon 1 (p.L122R), while the remaining 37% of cases had a heterozygous genotype. This hot spot mutation was equally detected by either Sanger sequencing or by the MSK-IMPACT targeted cancer gene panel, a next-generation molecular analysis using paired-end sequencing. An advantage of the latter approach is the identification of co-existing mutations, such as NRAS, HRAS, FGFR4, etc.
Shukla et al.21 initially reported the presence of PIK3CA mutations in a small subset (5%, 3/60) of rhabdomyosarcoma patients. Subsequently, Kohsaka et al.11 noted an apparent association between the MYOD1 L122R mutation and concurrent mutations in PIK3CA hot spots (E542K, E545K, H1047R), suggesting that PIK3CA-mutated rhabdomyosarcoma should be screened for the MYOD1 mutation. In our initial study13, 3 of the 9 MYOD1-mutant spindle cell/sclerosing rhabdomyosarcoma showed helical domain PIK3CA mutations (E542K, E542V and E545K), all showing a sclerosing phenotype (2 pediatric and one adult). None of tumors lacking MYOD1 mutations showed PIK3CA mutations. Interestingly, in the study by Rekhi et al.16, none of the 10 MYOD1 mutant cases had co-existent PIK3CA mutations. In the current series, 33% of cases showed co-existing PIK3CA mutations with a roughly even distribution between pediatric and adult cases. In one case, reported previously by Alaggio et al., whole transcriptome sequencing identified coexistent mutations in PIK3CA and FGFR4 genes. In addition to the previously reported hot spot mutations in exons 9 and 20, one case showed a previously unreported PIK3CA exon 9 mutation (E545A) and two cases showed genetic abnormalities in exon 2 (K111E and K111del). Nine of the 10 (90%) tumors with coexistent PIK3CA mutations showed sclerosing morphology, with 6 showing pure sclerosing pattern.
Another interesting finding is the identification of co-existing mutations in the RAS pathway, with one case each showing an NRAS (Q61L) and HRAS (G13R) hotspot mutation, respectively. The clinical significance of these co-existent mutations including PIK3CA, NRAS and HRAS remains uncertain since tumors without these mutations behaved equally aggressively with poor prognosis. Finally, two cases had concurrent FGFR4 mutations (V548M and V550L).
The MYOD1 gene encodes a nuclear protein that belongs to the basic helix-loop-helix (bHLH) family of transcription factors and the myogenic factors subfamily.22 MYOD1 regulates muscle cell differentiation by inducing cell cycle arrest, a prerequisite for myogenic initiation. Mutation of Leu 122 to Arg in MYOD1 has been shown to confer reduced transcriptional activation at MYOD1 sites, together with enhanced binding to MYC sites.22
In this study, more than half of the patients developed local recurrence and distant recurrences. Previous studies have suggested that pediatric MYOD1-mutant rhabdomyosarcoma follow an aggressive course and poor prognosis.12–15 In the study by Rekhi et al.,16 7 of the 10 cases showed recurrence / metastasis and follow-up data showed 5 of the 10 cases were alive with disease. The current study confirms these prior smaller series, showing that 83% of pediatric patients died of the disease, with only 5% being alive with disease. Our findings further elucidate that MYOD1 mutant rhabdomyosarcoma has a poor prognosis in adults as well, with 55% of these patients succumbing of disease and 7% being alive with disease.
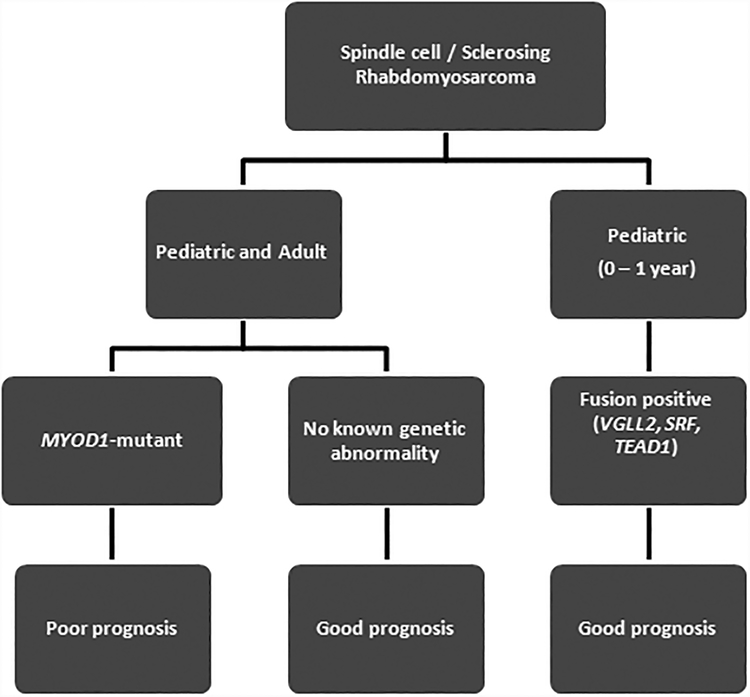
In conclusion, this study highlights the clinicopathologic features of MYOD1-mutant rhabdomyosarcoma, with morphologic spectrum ranging from spindle cell, sclerosing and mixed histology (Figure 5). Our findings also emphasize that MYOD1-mutant rhabdomyosarcoma follows an aggressive clinical course, quite similar to alveolar rhabdomyosarcoma, with poor response to multimodality therapy and unfavorable outcome in both children and adults. Given that the MYOD1 mutant rhabdomyosarcoma is associated with aggressive biologic behavior, an argument can be made that MYOD1 mutation can be used for risk stratification as well as for subclassification of this distinct molecular group within the spindle/sclerosing rhabdomyosarcoma group. (Table 2)
Figure 5:
Schematic diagram of Spindle cell / sclerosing rhabdomyosarcoma genetic subclassification.
Table 2:
Schematic showing the prognostic classification of Rhabdomyosarcoma based on the current prognostic information. (Sp / Scl RMS, Spindle / Sclerosing Rhabdomyosarcoma)
| RHABDOMYOSARCOMA | ||
|---|---|---|
| Poor Prognosis | Good Prognosis | Very Good Prognosis |
|
|
|
Supplementary Material
Supplementary Figure 1: Kaplan-Meier plot of the survival analysis for the pediatric and adult MYOD1 mutant rhabdomyosarcoma subgroups showed no statistical difference with the p-value of 0.16.
Supplementary Figure 2: Kaplan-Meier plot of the survival analysis for the MYOD1 mutant rhabdomyosarcoma with and without co-existent PIK3CA mutations showed no statistical difference with the p-value of 0.08.
ACKNOWLEDGEMENTS
The authors would like to thank Alyne Manzo for preparation of composite figures. They also thank the following pathologists and oncologists who kindly contributed case material and / or clinical follow-up information when available: Dr. Ana Burga, Englewood, NJ; Dr. Angelica Putnam, Salt Lake City, UT; Dr. Kaisa Vepsäläinen, Finland; Dr. Turpin, Cincinnati, OH; Dr. Jianying Zeng, Brooklyn, NY; Dr. Gabriel Chamyan, Miami, FL; Dr. Douglas Fair, Salt Lake City, UT.
Supported by: PO1 CA047179–15A2 (CRA), P50 CA 140146–01 (CRA), Cycle for Survival (LW, CRA).
REFERENCES
- 1.Ognjanovic S, Linabery AM, Charbonneau B, et al. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975–2005. Cancer. 2009;115:4218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher CD, Bridge JA, Hogendoorn PC, et al. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC press, 2013: Ch 7; p.127–35. [Google Scholar]
- 3.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavazzana AO, Schmidt D, Ninfo V, et al. Spindle cell rhabdomyosarcoma. A prognostically favorable variant of rhabdomyosarcoma. Am J Surg Pathol. 1992;16:229–35. [DOI] [PubMed] [Google Scholar]
- 5.Leuschner I, Newton WA Jr., Schmidt D, et al. Spindle cell variants of embryonal rhabdomyosarcoma in the paratesticular region. A report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol. 1993;17:221–30. [DOI] [PubMed] [Google Scholar]
- 6.Folpe AL, McKenney JK, Bridge JA, et al. Sclerosing rhabdomyosarcoma in adults: report of four cases of a hyalinizing, matrix-rich variant of rhabdomyosarcoma that may be confused with osteosarcoma, chondrosarcoma, or angiosarcoma. Am J Surg Pathol. 2002;26:1175–83. [DOI] [PubMed] [Google Scholar]
- 7.Mentzel T [Spindle cell rhabdomyosarcoma in adults: a new entity in the spectrum of malignant mesenchymal tumors of soft tissues]. Der Pathologe. 2010;31:91–6. [DOI] [PubMed] [Google Scholar]
- 8.Mentzel T, Katenkamp D. Sclerosing, pseudovascular rhabdomyosarcoma in adults. Clinicopathological and immunohistochemical analysis of three cases. Virchows Arch. 2000;436:305–11. [DOI] [PubMed] [Google Scholar]
- 9.Mentzel T, Kuhnen C. Spindle cell rhabdomyosarcoma in adults: clinicopathological and immunohistochemical analysis of seven new cases. Virchows Arch. 2006;449:554–60. [DOI] [PubMed] [Google Scholar]
- 10.Szuhai K, de Jong D, Leung WY, et al. Transactivating mutation of the MYOD1 gene is a frequent event in adult spindle cell rhabdomyosarcoma. J Pathol. 2014;232:300–7. [DOI] [PubMed] [Google Scholar]
- 11.Kohsaka S, Shukla N, Ameur N, et al. A Recurrent Point Mutation in MYOD1 Defines a Clinically Aggressive Subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nat Genet. 2014;46: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owosho AA, Huang SC, Chen S, et al. A clinicopathologic study of head and neck rhabdomyosarcomas showing FOXO1 fusion-positive alveolar and MYOD1-mutant sclerosing are associated with unfavorable outcome. Oral Oncol. 2016;61:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agaram NP, Chen CL, Zhang L, et al. Recurrent MYOD1 mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas: evidence for a common pathogenesis. Genes Chromosomes Cancer. 2014;53:779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alaggio R, Zhang L, Sung YS, et al. A Molecular Study of Pediatric Spindle and Sclerosing Rhabdomyosarcoma: Identification of Novel and Recurrent VGLL2-related Fusions in Infantile Cases. Am J Surg Pathol. 2016;40:224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owosho AA, Chen S, Kashikar S, et al. Clinical and molecular heterogeneity of head and neck spindle cell and sclerosing rhabdomyosarcoma. Oral Oncol. 2016;58:e6–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rekhi B, Upadhyay P, Ramteke MP, et al. MYOD1 (L122R) mutations are associated with spindle cell and sclerosing rhabdomyosarcomas with aggressive clinical outcomes. Mod Pathol. 2016;29:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiles MC, Parham DM, Qualman SJ, et al. Sclerosing rhabdomyosarcomas in children and adolescents: a clinicopathologic review of 13 cases from the Intergroup Rhabdomyosarcoma Study Group and Children’s Oncology Group. Pediatr Dev Pathol. 2004;7:583–94. [DOI] [PubMed] [Google Scholar]
- 18.Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9:3329–37. [PubMed] [Google Scholar]
- 19.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosquera JM, Sboner A, Zhang L, et al. Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosomes Cancer. 2013;52:538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla N, Ameur N, Yilmaz I, et al. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin Cancer Res. 2012;18:748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Antwerp ME, Chen DG, Chang C, et al. A point mutation in the MyoD basic domain imparts c-Myc-like properties. Proc Natl Acad Sci U S A. 1992;89:9010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Kaplan-Meier plot of the survival analysis for the pediatric and adult MYOD1 mutant rhabdomyosarcoma subgroups showed no statistical difference with the p-value of 0.16.
Supplementary Figure 2: Kaplan-Meier plot of the survival analysis for the MYOD1 mutant rhabdomyosarcoma with and without co-existent PIK3CA mutations showed no statistical difference with the p-value of 0.08.