Abstract
Vascular endothelial growth factor (VEGF) has emerged as a therapeutic target in several malignancies, including cervical cancer. Chemotherapy doublets combined with the fully humanized monoclonal antibody, bevacizumab, now constitute first-line therapy for women struggling with recurrent/metastatic cervical carcinoma. Regulatory approval for this indication was based on the phase III randomized trial, GOG 240, which demonstrated a statistically significant and clinically meaningful improvement in overall survival when bevacizumab was added to chemotherapy: 17.0 vs 13.3 months; HR 0.71; 98% CI, 0.54–0.95; p = .004. Incorporation of bevacizumab resulted in significant improvements in progression-free survival and response. These benefits were not accompanied by deterioration in quality of life. GOG 240 identified vaginal fistula as a new adverse event associated with bevacizumab use. All fistulas occurred in women who had received prior pelvic radiotherapy, and none resulted in emergency surgery, sepsis, or death. Final protocol-specified analysis demonstrated continued separation of the survival curves favoring VEGF inhibition: 16.8 vs 13.3 months; HR 0.77; 95% CI, 0.62–9.95; p = .007. Post-progression survival was not significantly different between the arms in GOG 240.
Moving forward, immunotherapy has now entered the clinical trial arena to address the high unmet clinical need for effective and tolerable second line therapies in this patient population. Targeting the programmed cell death 1/programmed death ligand 1 (PD-1/PD-L1) pathway using checkpoint inhibitors to break immunologic tolerance is promising. The immunologic landscape involving human papillomavirus-positive head and neck carcinoma and cutaneous squamous cell carcinoma can be informative when considering feasibility of checkpoint blockade in advanced cervical cancer. Phase II studies using anti-PD-1 molecules, nivolumab and pembrolizumab are ongoing, and GOG 3016, the first phase III randomized trial of a checkpoint inhibitor (cemiplimab) in cervical cancer, recently activated. Important considerations in attempts to inhibit the inhibitors include pseudoprogression and post-progression survival, abscopal effects, and immune-related adverse events, including endocrinopathies.
1. Introduction
During 2018, the American Cancer Society estimates that there will have been 13,240 new cases of cervical cancer and 4,170 deaths in the United States [1]. This is unacceptable given the availability of prophylactic human papillomavirus (HPV) vaccination and early detection of preinvasive disease via cytologic screening and/or high-risk HPV DNA testing. Worldwide, there is a disproportionate distribution of cases in resource poor settings without appropriate infrastructure to support screening programs. In 2012, cervical cancer rated as the fourth most common malignancy globally with 527,600 new cases [2,3].
In industrialized nations, invasive disease is often diagnosed during the prime years of a woman’s life (median age 49), in the midst of their careers and/or with small children at home. This patient population is more likely to be immunodeficient, abuse tobacco, and be marginalized by society due to lower socio-economic status [4–7].
Early stage cancers (FIGO IB1) may be treated by robotic radical hysterectomy with sentinel lymph node mapping and tailored adjuvant therapy. When future child-bearing is desired, fertility-preserving radical trachelectomy with lymphadenectomy may be appropriate in select cases (FIGO IB1 ≤ 2 cm). Locally advanced disease (FIGO IB2-IVA) can be cured with chemoradiation plus high-dose-rate (HDR) brachytherapy. Women who experience post-radiotherapy isolated central recurrences may be rescued via pelvic exenteration, however, this indication is becoming less frequent following widespread adoption of chemoradiation protocols with more local failures being accompanied by distant metastases. The management of women with recurrent disease who are not candidates for pelvic exenteration and those who present with metastatic (FIGO stage IVB) disease has represented an unmet clinical need for decades.
2. Part one: what has gone before
From the 1980’s to 2009 the National Cancer Institute’s (NCI) Gynecologic Oncology Group (GOG) conducted eight phase III randomized trials evaluating cytotoxic chemotherapy for metastatic and recurrent cervical cancer [8–13]. Clinically meaningful improvements in survival remained elusive and with GOG protocol 204 the regimen of cisplatin (50 mg/m2 ) plus paclitaxel (135 mg/m2 ) emerged as standard of care [14]. Response rates were short-lived and patients experienced rapid deterioration of performance status (PS) and quality of life, with early death 7–12 months from diagnosis. Furthermore, with widespread adoption of cisplatin-based chemoradiation for locally advanced disease, there was concern for platinum resistance at recurrence, thus prompting a search for an active and tolerable non-platinum doublet [15]. Topotecan plus paclitaxel was selected based on preclinical studies suggesting synergy between topotecan and microtubule-interfering agents, and phase II data which demonstrated tolerability and activity in heavily pretreated women [16,17].
2.1. A rationale to target tumor angiogenesis
The NCI’s Cancer Therapy and Evaluation Program (CTEP) permitted anti-angiogenesis therapy to also be studied based on clinical, pathologic, therapeutic, and molecular rationale. Clinically, aberrant vascular markings seen during colposcopic examination (punctuation, mosaicism, atypical vessels) in women with abnormal cervical cytology represent harbors of angiogenesis, suggesting that neovascularization is important early in pathogenesis. Molecularly, viral integration of oncogenic HPVs and expression of viral proteins E6 and E7 inhibit key cellular regulatory pathways governed by tumor suppressor gene products. Specifically, E6 increases p53 ubiquitination, and E7 inactivates retinoblastoma protein and induces hypoxia-inducible factor 1-alpha, ultimately leading to up-regulated VEGF expression which drives tumor angiogenesis [18–20].
Pathologically, angiogenesis has prognostic implications. Obermair et al. studied intratumoral microvessel density (MVD) from FIGO stage IB samples. Five-year-survival was significantly lower among patients with high MVD (63% vs 89.7%, p b .0001) [21]. Randall et al. reported that high levels of the early endothelial cell antigen, CD31, was an independent prognostic factor for progression-free survival (PFS) and overall survival (OS) [22]. Therapeutically, targeting angiogenesis in cervical cancer was first reported in a phase I study using TNP-470, an angio-inhibitory peptide derived from Aspergillus fumigatus fresenius that promotes endothelial cell cycle arrest through inhibition of methionine aminopeptidase-2. One subject with pulmonary metastases experienced a complete durable response [23,24]. In the phase II GOG 227C, Monk et al. reported that single agent bevacizumab (Fig. 1) performed well compared to historic controls, providing a median PFS and OS of 3.4 months and 7.29 months, respectively, in heavily pretreated women [25]. In the randomized phase II trial of pazopanib (an oral tyrosine kinase inhibitor (TKI) of the VEGF receptor (VEGFR)) vs lapatinib (anti-epidermal growth factor receptor), Monk et al. provided further evidence for anti-angiogenesis activity in this disease with the median OS of 50.7 vs 39.1 weeks and response rate (RR) of 9% vs 5%, favoring pazopanib [26].
Figure 1. Proposed mechanism of action of bevacizumab.
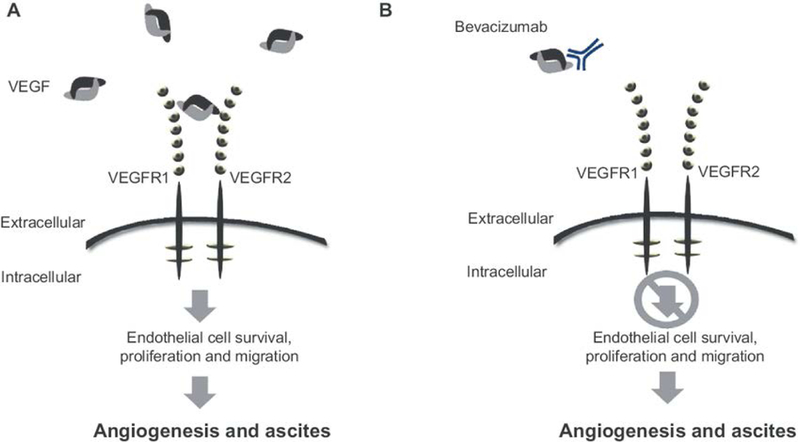
Panel A: The VEGF ligand binds VEGF receptor to initiate the molecular cascade which results in tumor angiogenesis. Panel B: Bevacizumab blocks angiogenesis through ligand-binding and sequestration. Adapted pending permission from Eskander RN, Tewari KS. Development of bevacizumab in advanced cervical cancer: pharmacodynamics modeling, survival impact and toxicology. Future Oncol. 2015;11(6); 909–22.
2.2. Gynecologic Oncology Group protocol 240
GOG 240 is a randomized, open-label, phase III trial that utilized a two-by-two factorial design to study the efficacy and toxicology of two independent factors: 1) non-platinum chemotherapy doublet, and 2) incorporation of anti-angiogenesis therapy (Fig. 2A). Each chemotherapy backbone (cisplatin-paclitaxel and topotecan-paclitaxel) was studied with and without bevacizumab, and cycles were repeated on a 21-day schedule until progression, complete response, unacceptable toxicity, and/or voluntary patient withdrawal from the trial. The primary endpoints were OS and frequency and severity of adverse events; secondary endpoints were PFS and RR. All patients had to have measureable disease by RECIST v.1. In a departure from prior studies, the study population was sanitized to maximize potential efficacy of the investigational arms. Accordingly, patients were required to have normal renal function, a urine protein: creatinine ratio (UPCR) b 1.0, GOG PS 0–1, optimization of medical co-morbidities, correction of malnutrition, and adequate control of tumor-related and neuropathic pain.
Figure 2: Gynecologic Oncology Group protocol 240.

Panel A. Trial schema. Panel B. At the second interim analysis, the addition of bevacizumab to chemotherapy was associated with increased overall survival: 17.0 vs 13.3 months [hazard ratio of death, 0.71; 98% CI, 0.54–0.95; p=0.004]. (Tewari KS, et al. New Engl J Med 2014;370:734–43. © 2014 Massachusetts Medical Society. Used with permission). Panel C. At the protocol-specified final analysis the chemotherapy plus bevacizumab arms continued to show significant improvement in OS compared with the chemotherapy-alone groups: 16.8 vs 13.3 months [hazard ratio of death 0.77; 95% CI, 0.62–0.95; p=0.007]. (Tewari KS, et al Lancet 2017;390:1654–63. © 2017 Elsevier Ltd. Used with permission).
A study population of 450 patients was expected to provide 90% power to detect a 30% reduction in the risk of death, with a one-sided alpha of 0.25%. An interim analysis was scheduled at 173 events to allow for early reporting of regimen activity according to the spending function, to drop a factor, or to close the trial for futility. The final OS analysis was scheduled at 348 deaths.
From April 2009 to January 2012, 452 women were randomized. At interim analysis in February 2012 it was determined that topotecan paclitaxel was not superior (nor inferior) to cisplatin-paclitaxel (control). These data were reported at the Society of Gynecologic Oncology’s 2013 Annual Meeting on Women’s Cancer. A nebulous signal suggesting activity due to VEGF-blockade prompted the NCI’s Data Safety and Monitoring Board to schedule a second interim analysis in December 2012 at a median follow-up of 20.8 months when 271 deaths had occurred. On February 7, 2013 the NCI issued a Press Release indicating that the trial had met one of its primary endpoints with the arms administering bevacizumab being associated with a statistically significant improvement in OS [27]. Dear Investigator and Dear Patient Letters were prepared and CTEP and Genentech, Inc. made arrangements to provide bevacizumab to patients on the chemotherapy-alone arms.
The 2013 Program Committee for the American Society of Clinical Oncology (ASCO) made a rare exception to their embargo policy and released the abstract into the public domain ahead of the meeting. It was featured in the ASCO 2013 Press Briefing and presented in the General Plenary Session where it was reported that compared with chemotherapy alone, the addition of bevacizumab was associated with increased OS (17.0 vs 13.3 months; HR for death 0.71; 98% CI, 0.54–0.95; p = .004, one-sided test) (Fig. 2B), increased PFS (8.2 vs 5.9 months; HR for progression, 0.67; 95% CI, 0.54–0.82), and increased RR (48% vs 36%; relative probability of response, 1.35; 95% CI, 1.08–1.68; p = .008, two-sided test) [28]. Health-related quality of life (HRQoL) was measured using patient reported outcomes (PROs) based on three previously validated QoL instruments. These data demonstrated that the benefits conferred by bevacizumab were not accompanied by deterioration in QoL [29].
On March 7, 2014, the protocol-specified 348 deaths required that the database be frozen for final OS analysis. The survival curves remained separated indicating that the OS benefit due to bevacizumab had been sustained in an intention-to-treat analysis: 16.8 vs 13.3 months; HR 0.77 (95% CI, 0.62–0.95; p = .007) (Fig. 2C) [30]. Post-progression survival was not significantly different between the chemotherapy plus bevacizumab and chemotherapy-alone cohorts (8.4 vs 7.1 months; HR 0.83; 95% CI, 0.66–1.05; p = .06), indicating that there was no negative rebound effect with discontinuation of anti-VEGF therapy [30].
In addition to previously recognized toxicities induced by bevacizumab (e.g., hypertension, proteinuria, and venous thromboembolism), GOG 240 identified a new adverse event: vaginal fistula. Rectovaginal and/or vesicovaginal fistula only occurred among women who had received prior pelvic irradiation, with clinically significant (i.e., grade 3+) fistula developing in 13 women (6%) treated with bevacizumab and in 1 patient (b1%) receiving chemotherapy alone [30]. No fistula resulted in surgical emergencies, sepsis, or death.
The tertiary objective of GOG 240 was to study the Moore clinical prognostic scoring system. Previously, a logistic regression model of pooled baseline characteristics from prior phase III trials had identified five independent prognostic factors for poor response [31]. Applying these factors (African-American ethnicity (a possible surrogate for poor access to healthcare), PS N0, pelvic disease, prior radiosensitizing chemotherapy, and time interval from diagnosis to first recurrence bone year) patients were separated into low- (0–1 factor), mid- (2–3 factors) and high-risk (4–5 factors) groups. During development of GOG 240 some recommended excluding the high-risk group from participation, but the need to first prospectively validate the scoring system was acknowledged. This was accomplished in GOG 240, and in subset analyses it was determined that the high-risk (and mid-risk) patients derived the greatest benefit from bevacizumab. There appeared to be no survival advantage among low-risk patients suggesting that bevacizumab may be withheld in this group, particularly in pre-irradiated women at risk for fistula [32].
Translational objectives of GOG 240 included studying the impact of tobacco use on survival [33] and whether circulating tumor cells (CTCs) could be identified. CTCs were detected, and for those women with high levels of CTCs treated with bevacizumab the PFS curves shifted to the right (HR 0.59; 95% CI, 0.36–0.96), suggesting that minimally invasive “liquid biopsies” containing high CTCs could represent a predictive biomarker for anti-angiogenesis therapy [34].
Four post-trial ad-hoc secondary analyses have served to further clarify the anti-angiogenesis landscape. Willmott et al. reported that prior pelvic radiation, pelvic tumor, concurrent tobacco use, and preexisting hypertension increase the risk of vaginal fistula. Non-irradiated patients had 0% fistula risk and a non-significant trend for improved OS compared to irradiated patients (24.5 vs 16.8 months) [35]. Seamon et al. pooled glandular cancers from GOG 240, 204, and 179 and performed binary exchange analyses which suggested that following systemic therapy, survival of patients with adenocarcinoma/adenosquamous was not significantly different from SCCA [36]. Eskander et al. confirmed that bevacizumab was active in the previously irradiated pelvis as evidenced by a doubling of the CR (s) (n = 28 vs 14) [37]. Finally, Chase et al. noted that PRO scores at discontinuation of protocol-directed therapy were lower for those who came off study for toxicity compared with disease progression (mean −10.3 vs −2.7 points). These data are instructional for early implementation of aggressive supportive care among patients experiencing significant early toxicity [38].
2.3. Regulatory approval of bevacizumab for cervical cancer and aftermath
On March 5, 2014, the United Kingdom’s Cancer Drug Fund approved bevacizumab for women in England with advanced cervical cancer. On August 14, 2014 the US Food and Drug Administration (FDA) approved bevacizumab in combination with chemotherapy for metastatic/recurrent/persistent cervical cancer. The National Comprehensive Cancer Network listed CDDP-paclitaxel-bevacizumab and topotecan paclitaxel-bevacizumab as category 1 in the September 2014 Cervical Cancer Guidelines [39]. December 22, 2014 marked SwissMedic approval, and following a Positive Opinion by the Committee on Medicinal Products for Human Use on February 27, 2015, the European Medicines Agency approved bevacizumab for women in the European Union on April 8, 2015.
Due to concerns for potential biological differences between the GOG 240 predominantly Caucasian population and Japanese women, a single-arm phase II trial was conducted in Japan. Seven Japanese patients treated with cisplatin (CDDP)-paclitaxel-bevacizumab per GOG 240 dosages/schedule, experienced an 86% RR (95% CI, 42–100%) and no significant safety signals [40]. Regulatory approval in Japan occurred on April 26, 2016. As of 2017, bevacizumab has been approved in 60 countries on six continents, including all of North and South America, Europe, Australia, at least 15 countries in the Middle East and Asia, and two African nations (Morocco and South Africa) [30]. GOG 240 represents a proof of concept of the efficacy and tolerability of anti-angiogenesis therapy in advanced virus-induced carcinoma.
Three important studies were reported in the wake of GOG 240. Using the trial database, Minion et al., studied cost-effectiveness with a Markov Model and determined that bevacizumab added $73,791 per 3.5 months of life gained (i.e., an incremental cost-effectiveness ratio (ICER) of $21,083 per month of added life). With a possible 75% reduction in the cost of bevacizumab, the ICER reduces to $6737, making antiangiogenesis therapy cost-effective at $23,580 for the 3.5 months gain in OS [41]. These observations are relevant given the recent US FDA approval of the biosimilar, Mvasi (bevacizumab-awwb).
Japanese Gynecologic Oncology Group (JGOG) 0505 reported significant non-inferiority (hazard of death, 0.994; 90% CI, 0.79–1.25; non-inferiority p = .032) in a phase III randomized trial designed to study the substitution of carboplatin (AUC 5) for cisplatin (50 mg/m2 ) in patients treated with a platinum-taxane doublet only [42]. For platinum-naïve patients (e.g., those who did not receive prior chemoradiation) cisplatin was associated with a survival advantage not observed with carboplatin.
CIRCCa was the United Kingdom’s randomized placebo-controlled phase II trial which randomized patients to six cycles of carboplatin (AUC 5) plus paclitaxel (175 mg/m2 ) with and without VEGFR1–3 oral TKI, cediranib (20 mg daily). The study closed prematurely due to withdrawal of cediranib supply, at which point 69 of a projected 80 patients had been randomized. Although PFS was significant longer in the investigational arm (8.1 vs 6.7 months; hazard of progression 0.58; 80% CI, 0.4–0.85; one-sided p = .032), grade 3+ diarrhea, grade 2–3 hypertension, and grade 3+ febrile neutropenia tracked with cediranib [43]. CIRCCa provided further testimony to the efficacy of anti-angiogenesis therapy and the feasibility of using carboplatin in this disease.
Many of the trials discussed in the first half of this review appear in Table 1.
Table 1:
Clinical trials of interest in women with recurrent and/or persistent cervix cancer.
| Study | Reference | Study Population (n) | Phase | Treatment Arm(s) | Principal Results | Toxicity | Trial’s Significance |
|---|---|---|---|---|---|---|---|
| Phase I TNP-470 | Kudelka et al [23–4] | Recurrent, or metastatic (21) | I | Single arm - TNP-470 dose escalation; starting dose 9.3 mg/m2 q 2 days for 28 with 14 day rest | 60 mg/m2 recommended dose; 1 complete response, 3 stable disease | Neurotoxicity was primary dose limiting toxicity | First report of anti-angiogenesis in cervical cancer |
| GOG-227C | Monk et al [25] | Recurrent (46) | II | Single arm - bevacizumab (15 mg/kg) q 21 days | 23.9% (11 patients) were progression free at 6 months (two-sided 90% CI, 14% to 37%) | Grade 3–4 AE included: GI, anemia, hypertension, thrombo-embolism, fistula. | Demonstrated activity and tolerability of bevacizumab in heavily pretreated patients and supported further investigation of this agent |
| Pazopanib/ Lapatinib monotherapy vs pazopanib plus lapatinib | Monk et al [26] | Stage IVB, recurrent, or persistent (230) | II | 1. Pazopanib (800 mg PO daily) 2. Lapatinib (1,500 mg PO daily) 3. Pazopanib (400 or 800 mg PO daily) plus lapatinib (1,000 or 1,500 mg PO daily) |
Pazopanib improved PFS (hazard ratio [HR], 0.66; 90% CI, 0.48 to 0.91; P = .013) and OS (HR, 0.67; 90% CI, 0.46 to 0.99; P = .045) q 21 days | Grade 3 AE included: eiarrhea. Grade 4 AE rate 9% (lapatinib) and 12% (pazopanib) | Confirms anti-angiogenesis strategy in advanced cervical cancer |
| GOG-204 | Monk et al [14] | Stage IVB, recurrent, or persistent (513) | III | 1. Cisplatin (50 mg/m2; on day 2) and paclitaxel (135 mg/m2; over 24 hours) q 21 days 2. Vinorelbine (30 mg/m2; on days 1, 8) and cisplatin (50 mg/m2; on day 1 q 21 days 3. Gemcitabine (1,000 mg/m2; on day 1, 8) and cisplatin (50 mg/m2; on day 1 q 21 days 4. Topotecan (0·75 mg/m2; on days 1–3) and cisplatin (50 mg/m2; on day 1 q 21 days |
Compared to arm 1, hazard ratios of death were 1.15 (95% CI, 0.79 to 1.67) for vinorelbine arm, 1.32 (95% CI, 0.91 to 1.92) for gemcitabine arm, and 1.26 (95% CI, 0.86 to 1.82) for topotecan arm. | AE comparable among the arms except topotecan arm experienced more leucopenia and neutropenia; paclitaxel arm experienced infection | Lead to incorporation of this doublet in GOG-240 as results demonstrated tolerability and activity in advanced cervical cancer. |
| Phase II study of topotecan and paclitaxel | Tiersten et al [17] | Recurrent, persistent or metastatic (15) | II | Paclitaxel (175 mg/m2;) and topotecan (1 mg/m2; on days 1–5) q 21 days | ORR 54% (95% CI, 29.2–76.8%) | Most common grade 3–4 AE included: anemia, leukopenia, thrombocytopenia, neurotoxicity and diarrhea | Lead to incorporation of this doublet in GOG-240 as results demonstrated tolerability and activity in advanced cervical cancer. |
| GOG-240 | Tewari et al [28, 30] | Recurrent, persistent or metastatic (452) | III | 1. Cisplatin (50 mg/m2; on day 1 or 2) and paclitaxel (135 mg/m2; or 175 mg/m2; on day 1) q 21 days with or without bevacizumab (15 mg/kg on day 1) q 21 days 2. Topotecan (0·75 mg/m2; on days 1–3) and paclitaxel (175 mg/m2; on day 1) q 21 days with or without bevacizumab (15 mg/kg on day 1) q 21 days |
Overall survival 16·8 months in the chemotherapy plus bevacizumab groups versus 13·3 months in the chemotherapy-alone groups (hazard ratio 0·77 [95% CI 0·62–0·95]; p=0·007). No difference in post-progression survival between groups. | More hypertension (grade ≥2), proteinuria (grade >3), thrombosis (grade >3) and fistula (grade >3) in bevacizumab arms | Established chemotherapy plus bevacizumab as current standard of care for recurrent, metastatic or persistent cervical cancer. |
| Randomized phase II cediranib with carboplatin and paclitaxel | Symonds et al [43] | Recurrent, or metastatic (69) previously treated with chemotherapy | II | Carboplatin (AUC of 5) plus paclitaxel (175 mg/m2;) IV q 21 day with and without cediranib 20 mg PO | ORR in cediranib group of 64%. Median PFS 8.1 months in cediranib group (80% CI 7·4–8·8), 6.7 months in placebo group [6·2–7·2); HR of 0·58 (80% CI 0·40–0·85) | The cediranib group experience more grade ≥3 or AE including diarrhea, fatigue, leucopenia, and neutropenia | Trial was closed early due to study drug supply, however results further support anti-angiogenesis in cervical cancer |
| JGOG-0505 | Kitagawa et al [42] | Japanese women with metastatic or recurrent cervical cancer previously treated with platinum, but no prior taxane (253). | III | 1. Cisplatin (50 mg/m2;) and paclitaxel (135 mg/m2;) IV q 21 days 2. Carboplatin (AUC of 5) and paclitaxel (175 mg/m2;) IV q 21 day |
Hazard of death, 0.994; 90% CI, 0.79–1.25; non-inferior p=0.032 | Thrombocytopenia, neuropathy and anemia were more common with carboplatin group. Grade 4 neutropenia, febrile neutropenia, nausea and vomiting were more common in cisplatin group | Supports the use of a carboplatin-paclitaxel-bevacizumab triplet |
| JO29569 | Sugiyama et al [40] | Japanese women with stage IVB, recurrent, or persistent (7) | II | Single arm - Cisplatin (50 mg/m2; on day 1 or 2) and paclitaxel (135 mg/m2; or 175 mg/m2; on day 1) plus bevacizumab (15 mg/kg on day 1) q 21 days | ORR 86% (95% CI, 42–100%) | Most common grade 3–4 AE included: hypertension, and neutropenia | Demonstrated tolerability of GOG-240 regimen in Japanese patients |
3. Case presentations
3.1. Case 1 background 48 year old Caucasian saleswoman.
Sporadic screening: newly diagnosed FIGO stage IVB SCCA cervix (2.5 cm primary lesion, aortocaval adenopathy, multiple, subcentimeter, FDG-avid, bilateral pulmonary nodules).
PMH: hypertension (labetalol); chronic tobacco use Laboratory: WNL
ROS: no significant vaginal bleeding, pain, hemoptysis
GOG PS = 0 3.1.1.
3.1.1. Treatment considerations
3.1.2. Therapy and follow-up Completes 3 cycles CDDP-paclitaxel-bevacizumab.
Post-C3 PET/CT: 50% reduction target lesions Pre-C4: BP 150/100 mmHg; UPCR 2+
3.1.3. Adverse event learning Hypertension (grade 2+): 25% GOG 240 bevacizumab arms (no treatment discontinuation). Timing variable.
Home BP monitoring. Titrate beta-blocker to achieve target BP. Grade 3 hypertension requires polydrug therapy: ACE inhibitors, calcium channel blockers, beta-blockers; avoid diuretics which exacerbate dehydration from chemotherapy-induced emesis. Hypertensive crisis/encephalopathy: discontinue bevacizumab. Proteinuria (grade 3+): 2% GOG 240 bevacizumab arms. UPCR prior to each cycle: ≥2+ hold bevacizumab; urinalysis and culture to detect UTI (proteinuria clears with antibiotics). Negative culture: confirm UPCR with 24-hour urine collection: do not reintroduce bevacizumab until urinary protein b2 g. Dose-limiting proteinuria reversible after bevacizumab discontinuation Nephrotic syndrome rare.
3.2. Case 2 background 52 year old Asian school teacher PET/CT: FDG-avid periaortic/mediastinal/supraclavicular lymphadenopathy CT-guided biopsy 3.5 cm left scalene node confirms recurrent cervical cancer
PMH: FIGO stage IIB cervical adenocarcinoma w/pelvic lymphadenopathy s/p CDDP-based chemoradiation plus HDR brachytherapy 3 years prior; hypertension, NIDDM (Hgb A1C 9.3), mild renal insufficiency, mild peripheral neuropathy; arthritis (left knee) GOG PS = 1
3.2.1. Treatment considerations
Moore score 2 (prior platinum, PS N 0): mid-risk (anti-angiogenesis therapy improves median OS by nearly 6 months (17.9 vs 12.1 months; HR 0.673; 95% CI, 0.51–0.91; p = .0094) [31,32].
GOG 240 not powered to study histologic subtype but bevacizumab expected to be active in adenocarcinoma: a) no difference in survival between squamous and glandular lesions in recurrent/metastatic population [36]; b) HPV 16 (SCCA) and HPV 18 (adenoCA) both encode E6 and E7 which drives VEGF expression; c) bevacizumab active in ovarian, colorectal, breast non-small cell, non-squamous lung adenocarcinomas (bevacizumab not approved in SCCA due to pulmonary hemorrhage risk)
Prior platinum with renal insufficiency/neuropathy: carboplatin appropriate
Supportive care: optimize NIDDM
3.2.2. Therapy and follow-up C4D10 carboplatin (AUC 5)-paclitaxel (175 mg/m2 )-bevacizumab (15 mg/kg).
PET/CT: 70% resolution Hgb A1C 8.1. GOG PS = 2 (arthritis) Considering knee replacement
3.2.3. Adverse event learning Wound-healing impairment by bevacizumab (t½ = 20 days).
Withhold drug 28 days prior to elective surgery; re-introduce 28 days post-surgery to avoid dehiscence.
3.3. Case 3 background 35 year old Caucasian home-maker; sporadic insurance.
PMH: FIGO stage NIB SCCA cervix s/p CDDP-based chemoradiation plus HDR brachytherapy 10 months previously (multiple treatment delays); achieved CR Re-presentation: left flank pain, gross hematuria.
PET/CT: 5 cm left pelvic sidewall mass, moderate left hydronephrosis. CT-guided biopsy: SCCA Laboratory: rising serum creatinine GOG PS = 1
3.3.1. Treatment considerations
Supportive care: left nephrostomy
Moore score 4: high-risk group (anti-angiogenesis therapy nearly doubles OS: 12.1 vs 6.3 months: HR 0.536; 95% CI, 0.32–0.905; p = .01996) [31,32].
Clinically significant vaginal fistula: 6% GOG 240 (bevacizumab arms; prior radiotherapy); increased risk with disease in irradiated field [30,35].
3.3.2. Therapy and follow-up Receives seven cycles CDDP-paclitaxel-bevacizumab.
PET/CT: 80% resolution. Pre-C8: flatus/stool per vagina Rectovaginal fistula confirmed 7 cm from anal verge. 28 days post-treatment discontinuation: transverse loop colostomy CDDP-paclitaxel-bevacizumab re-instituted 4 weeks post-op. PET/CD s/p C10: CR Status: NED 3 years, QoL satisfactory; raising 3 children
3.3.3. Adverse event learning Vaginal fistula communicating with the rectum and/or bladder may develop in previously irradiated patients receiving bevacizumab (6% incidence of grade 3+ fistula in GOG 240).
Previously irradiated patients with Moore score 0–1 may be counseled against bevacizumab use due to lack of survival advantage. Hypertension, tobacco, and/or pelvic tumor may also increase risk.
3.4. Case 4 background 42 year old African American attorney.
PMH: bulky, FIGO stage IIIB SCCA cervix (Treatment delays during chemoradiation due to CDDP-induced emesis/dehydration) s/p HDR interstitial brachytherapy (complex tumor geometry): persistent disease ROS: significant pelvic discomfort GOG PS = 2
3.4.1. Treatment considerations
Palliative care: pain control, anti-constipating regimen
GOG 240: benefit of bevacizumab in persistent disease [30].
CDDP intolerance: topotecan (0.75 mg/m2 days 1–3)-paclitaxel (175 mg/m2 day 1)-bevacizumab 15 mg/kg (day 1)
Initiate bevacizumab 42 days post-chemoradiation
3.4.2. Therapy and follow-up ER (prior to C7): acute SOB, left calf tenderness Speaks full sentences; 95% O2 saturations (RA) Helical CT: 2.5 cm left pulmonary embolus Doppler: left femoral DVT
3.4.3. Adverse event learning Venous thromboembolism (grade 3+): 8% GOG 240 with bevacizumab.
G1–3: Continue bevacizumab if: a) stable pulmonary status; b) maintain therapeutic anticoagulation (enoxaparin); c) deriving benefit from anti-angiogenesis therapy. G4 venous thromboembolism and any grade arterial thromboembolic event: discontinue bevacizumab.
4. Part two: where do we go now?
4.1. The clinical problem
The survival benefit conferred through the integration of anti-angiogenesis therapy with systemic chemotherapy for women with newly diagnosed, advanced (i.e., recurrent, persistent, and metastatic) cervical cancer represents a proof of concept of VEGF-inhibition in this disease, a proof of principle of the value of systemic therapy, and a triumph of coordinated supportive care in a marginalized patient population. Although the survival gains were accompanied by significant improvements in both PFS and response (without a concomitant deterioration in HRQoL), from the vantage point of sobriety one must acknowledge that these results represent only the proverbial foot in the door. Progress definitely, but much more is required. With few exceptions, bevacizumab is not curing anyone, and while GOG 240 addressed an unmet clinical need in a high-risk population, the trial created another population: those who progress on first-line therapy. Whereas just a few years ago, very little work was being done in this space, today several noteworthy trials are being done in this patient population.
4.2. Introduction to immuno-oncology
Cellular-based immunotherapies under investigation include adoptive transfer of autologous tumor-infiltrating T lymphocytes (TILs) and adoptive cell therapy using chimeric antigen receptor (CAR)- engineered T-cells. Progress of TILs has been impeded by the requirement for viable tumor to identify TILs to undergo expansion, and CART cell therapy involves significant safety challenges (cytokine release syndrome, “on-target, off-tumor” toxicity). Among the non-cellular based immunotherapies are therapeutic vaccines, antibody-drug conjugates, and immune checkpoint inhibitors. This review will focus on the latter group.
The nature of the antigens which allow T-cells to distinguish malignant cells from non-malignant cells has remained elusive until relatively recently. Tumor-specific mutations produce patient-specific neoantigens. The neoantigen load may serve as a predictive biomarker for immunotherapies [44]. Virus-induced cancers represent an active platform upon which novel molecules can be studied by virtue of viral antigen production during malignant transformation. Diverse antigenicity renders cancer cells vulnerable to immunologic surveillance through initiation of antigen recognition by antigen-presenting cells (APC) which activate naïve T-cell receptors (TCR). T-cell proliferation invokes an HPV-specific immune response via differentiation into effector T-cells which clear virus-infected cells. This immune response is initiated through the antigen-peptide major histocompatibility complex and regulated by immunologic checkpoints, which preserve a balance between costimulatory and inhibitory signals. The costimulatory antigen-dependent signal between CD28 on T-cells and B7–1 and B7–2 on APCs leads to fully activated T-cells. Through inhibition of immune checkpoints, cancer cells may escape T-cell immune responses [45,46]. To follow is a review of important immunotherapy trials, many of which are listed in Table 2.
Table 2:
Immunotherapy trials of interest in women with recurrent and/or persistent cervix cancer.
| Study | Reference | Study Population (n) | Phase | Treatment Arm(s) | Principal Results | Toxicity | Trial’s Significance |
|---|---|---|---|---|---|---|---|
| First-in-human REGN2810 | Papadopoulos et al. [67] | Advanced solid tumors | I | Cemiplimab (dose escalation) 1, 3, 10 mg/kg IV q 2 weeks vs cemiplimab 1, or 3 mg/kg q 2 weeks with radiotherapy | 51.7% patients had disease control, OR 18.3% (11/60) | No dose-limiting toxicities | Higher response rate when combined with radiation suggesting abscopal responses |
| Proof-of-principle abscopal trial | Golden et al. [72] | Advanced solid tumors | I | Granulocyte macrophage colony-stimulating factor and radiotherapy | 11 of 41 patient demonstrated abscopal responses | Grade 3–4 AE included: fatigue, syncope, bronchospasm, nausea and vomiting, and pulmonary embolism | Proof-of-principle trial demonstrating induced abscopal responses |
| Oak Study | Gandara et al. [70] | Recurrent non-small cell lung cancer with 2 or more prior lines of chemotherapy (850) | III | Atezolizumab (1,200 mg IV q 3) vs taxotere (75 mg/m2 IV) q 3 weeks | OS measured at progression-favored atezolizumab (8.6 vs. 6.4 months; HR 0.73; 95% CI 0.62, 0.87). Median post-progression OS was 12.7 months (95% CI=9.3–14.9). | Pre-progression 10.7% grade 3–4 AE; post-progression 6% grade 3–4 AE | Allowed for treatment beyond progression; demonstrating an OS advanced |
| CheckMate-358 | Hollebecque et al. [63] | Recurrent or metastatic HPV-related cancers (19) | I-II | Nivolumab 240 mg IV q 2 week | Preliminary results - ORR 26% (95% CI: 9.1–51.2%) in cervical cancer patients | Grade 3–4 AE: hyponatremia, syncope, diarrhea and hepatocellular injury | Durable responses demonstrated in cervical cancer patients, with at least 6 months duration |
| Phase II study of nivolumab plus ISA 101 vaccine | Glisson et al. [56] | Incurable HPV 16-positive solid tumors (24) | II | Nivolumab (3 mg/kg IV q 2 weeks) and ISA 101 (100 mcgs/peptide days 1, 22, 50) | ORR 33% (8/24). Median PFS 2.7 (95% CI, 2.3–8 months) | Grade 3–4 AE included: transaminase and lipase elevation | Improved ORR compared to CheckMate-141 trial of 16%; suggests combination with vaccine augments response |
| Keynote-12 | Seiwert et al. [52] | Recurrent or metastatic platinum-refractory HNSCC (60) | I | Pembrolizumab 10 m/kg q 3 weeks | ORR 18% (95% CI, 8–32) | Overall AE rate of 63%; most common AE was grade 1–2 fatigue | Results demonstrate activity; warranted further investigation |
| Keynote-55 | Baumi et al. [53] | Recurrent or metastatic platinum-refractory HNSCC (171) | II | Pembrolizumab (200 mg q 3 weeks) | ORR 16% (95% CI, 11–23%) | Overall AE rate of 64%; most common AE - fatigue, hypothyroidism nausea, AST increase and diarrhea | Results demonstrate activity; warranted further investigation |
| Condor | Gilbert et al. [58] | Recurrent or metastatic platinum-refractory HNSCC with low (<25%) PD-L1 expression (240) | II | Durvalumab (10 mg/kg) or tremelimumab (10 mg/kg) or durvalumab (20 mg/kg) or tremelimumab (1 mg/kg) | Ongoing | Ongoing | Ongoing |
| Hawk | Zandberg et al. [57] | Recurrent or metastatic HNSCC with high PD-L expression with prior platinum treatment (112) | II | Durvalumab 10 mg/kg | ORR of 26.54% in HPV-positive vs 7.9% in HPV-negative patients | Overall grade 3–4 AE 9.8% | Higher response rate in HPV positive tumors |
| EAGLE | Ferris et al. [59] | Recurrent or metastatic HNSCC | III | Durvalumab (10 mg/kg) or tremelimumab (1 mg/kg) or durvalumab (20 mg/kg) or tremelimumab (1 mg/kg) | Ongoing | Ongoing | Ongoing |
| CheckMate-141 | Haddad et al. [55] | Recurrent or metastatic HNSCC (362) | III | Nivolumab (3 mg/kg q 2 weeks) vs cetuximab (400 mg/m2) methotrexate (40 or 60 mg/m2) or docetaxel (30 or 40 mg/m2) | Median OS 7.5 versus 5.1 months (HR 0.71; 95% CI, 0.55–0.9). When treated beyond progression, median OS 12.7 months (95% CI, 9.7–14.6) | Grade 3–4 AE rate 13.1% in nivolumab vs. 35.1% in chemotherapy group | OS benefit when treated beyond progression; improved toxicity prolife from chemotherapy |
| Keynote-40 | Cohen et al. [54] | Recurrent or metastatic platinum-refractory HNSCC (495) | III | Pembrolizumab (200 mg q 3 weeks) vs cetuximab (400 mg/m2) methotrexate (40 or 60 mg/m2) or docetaxel (30 or 40 mg/m2) | Median OS 8.4 versus 7.1 months (HR 0.81; 95% CI, 0.66–0.99). Median OS 11.6 versus 7.9 months in patients with >50% PD-L1 expression (HR 0.54; 95% CI 0.35–0.82) | Grade 3–4 AE rate of 13.4% pembrolizumab arm, 36.3% chemotherapy arms | Improved toxicity profile compared to standard therapies. Improved survival benefit in patients with high-rate PD-L1 expression |
| REGN cutaneous | Papadopoulos et al. [62] | Cutaneous squamous cell carcinoma cohorts (26) of the advanced solid tumor (60) | I | 3 mg/kg cemiplumab q 2 weeks for up to 48 weeks | Preliminary results - ORR 46.2% (12/26 patients; 95% CI, 26.6–66.6%) | Grade 3–4 AE (19% overall) included arthralgia, rash, asthenia, AST/ALT elevation | Demonstrates activity in high mutational burden malignancy |
| NRG-9929 | NCT01711515 [48] | Primary treatment for patients with stage IB2/IIA with positive para-aortic lymph nodes only, and stage IIB/IIIB/IVA with positive lymph nodes | I | Ipilimumab (dose-escalation) after chemoradiation, IV q 3 weeks for 12 weeks | Ongoing | Ongoing | Ongoing |
| Princess Margaret & Chicago N01 Consortia | Lheureux et al. [47] | Metastatic, recurrent cervical cancer (32) | I-II | Ipilumumab 10 mg/kg IV q 3 weeks | Did not meet primary endpoint of 4 responders | Most common grade 3 AE included: anemia, diarrhea and colitis | Demonstrated safety of ipilimumab |
| Keynote-28 | Frenel et al. [65] | Recurrent cervical cancer with PD-L1 positive tumors (24) | Ib | Pembrolizumab 10 mg/kg IV q 2 weeks | ORR 17% (95% CI, 5–37%) | Grade =3 AE 21% including rash and proteinuria | Well tolerated and active in cervical cancer |
| Keynote-158 | Schellens et al. [66] | Recurrent cervical cancer with progression or intolerance to standard therapy (82) | II | Pembrolizumab 200 mg/kg IV q 2 weeks | Preliminary results - overall ORR 17% (95% CI: 8–31%); patients with >27 weeks of follow up ORR 27% (95% CI: 8–55%) | Grade ≥3 AE 12% including AST/ALT elevation and pyrexia | Demonstrates activity in cervical cancer and increasing response with longer duration of follow up |
| NRG-GYO-02 | NCT02257528 [64] | Recurrent or metastatic cervical cancer | II | Nivolumab | Ongoing | Ongoing | Ongoing |
| GOG 3016/ENGOT-cx9 (EMPOWER-Cervical 1) | NCT03257267 [68] | Recurrent or metastatic platinum- refractory cervical cancer | III | Cemiplimab vs. physician choice chemotherapy | Ongoing | Ongoing | First phase III in cervical cancer |
4.3. Breaking immunologic tolerance
To prevent overstimulation of the immune system, the cytotoxic T lymphoctye-associated antigen 4 (CTLA-4) evolved to regulate the amplitude of T-cell activation and when needed to dampen T-cell response, CTLA-4 is translocated from intracellular vesicles to the cell surface (Fig. 3). CTLA-4 was the first immune checkpoint inhibitor to be clinically targeted using ipilumumab, a fully humanized monoclonal IgG1K antibody approved by the US FDA for treatment of melanoma. A phase I–II trial evaluated the safety of 3 mg/kg dosing on a 21-day schedule in 6 women with advanced cervical cancer. Upon expansion to phase II, 37 evaluable participants received 10 mg/kg every 21 days but significant single-agent activity was absent [47]. With the NCI’s mandate to merge the cooperative groups, NRG-Oncology activated the phase I trial of ipilimumab following concurrent chemoradiation for primary treatment of locally advanced cervical cancer (GOG 9929; NCT01711515).Included for eligibility are patients with positive para-aortic nodes(i.e., essentially metastatic disease) [48]. Tremelimumab also targetsCTLA-4 but to date no trials in cervical cancer have been developed.
Figure 3. CTL-4 and the PD-L/PD-L1 pathways regulate activation of the immune system at different time points and different effector cells.

Panel A. CTL-4 functions to scale the T-cell response based on antigen binding affinity. CTL-4 resides in intracellular vesicles, and when need to dampen response it is translocated to the cellular surface. Panel B. In contrast, the PD-L/PD-L1 is utilized in peripheral tissues to damp immune responses in the setting of inflammation, limiting damage to healthy tissue. (Reproduced with from permission from Pardoll DM. Nature Reviews Cancer. 2012;12:252–64).
Unlike CTLA4, the programmed cell death 1/programmed death ligand 1 (PD-1/PD-L1) pathway is utilized in peripheral tissues to damp-en the immune responses during inflammation to limit damage to healthy tissue (Fig. 3). This pathway can also be targeted by check point inhibitors, including molecules specific for PD-1 (nivolumab, pembrolizumab, cemiplimab) or PD-L1 (durvalumab, avelumab, atezolizumab). Several trials in high risk HPV-positive tumors have al-ready reported. Impaired local cellular immunity leads to persistent infection, viral integration and concomitant expression of the viraloncoproteins, E6 and E7. Their net effect on cellular tumor suppressorgene products, p53 and pRb (retinoblastoma protein), respectively, is to create a tumor microenvironment that favors the development ofmicroinvasive carcinoma and its precursor, cervical intraepithelial neo-plasia 3 (CIN-3). The PD-1/PD-L1 pathway attenuates T-cell responses and promotes T-cell tolerance during chronic viral infections [49].Yang et al. have demonstrated that PD-1 and PD-L1 expression on cervical T-cells and dendritic cells (DCs), respectively, is associated with high risk HPV positivity and increases in parallel with increasing CIN grade. Binding of PD-L1 or PD-L2 ligands to the PD-L receptor on T-cells reduces proliferation and can induce apoptosis [50].
4.4. Immunotherapeutic insights gained from the study of shared tumor biology
Head and neck SCCAmay be carcinogen-induced or virally-induced. The former is similar to vulvar SCCAs with down-regulation of p16 and p53 mutants, while the latter recalls cervical and anal SCCAs wherein the HPV oncoproteins E6 and E7 have transformative roles with p16 upregulation. Pembrolizumab targets the PD-1 receptor and has been approved for certain populations with advanced melanoma, non-small cell lung cancer, Hodgkin’s lymphoma, and head and neck SCCA. On May 23, 2017, the US FDA issued its first tissue/site-agnostic approval, permitting patients with microsatellite instability-high or mismatch repair deficient refractory solid tumors to receive pembrolizumab [51].
The activity and tolerability of pembrolizumab in PD-L1+recurrent/metastatic head and neck SCCA was initially reported in the phase IB KEYNOTE-012 [52] and single arm phase II KEYNOTE-055 [53] studies. In the phase III randomized KEYNOTE-040 trial, Cohen et al. reported that median OS was only marginally higher with pembrolizumab (n = 247) compared to standard treatment (n=248): 8.4 vs 7.1 months; HR 0.81; 95% CI, 0.66–0.99; p=.0204. For a subset of patients with high PD-L1-expressing tumors (N50% of cancer cells), pembrolizumab was associated with dramatic improvements in median OS (11.6 vs 7.9 months; HR 0.54; 95% CI, 0.35–0.82; p=.0017) [54]. Nivolumab is a human IgG4 anti-PD-1 monoclonal antibody that has been approved for specific populations of inoperable or metastatic melanoma, and as a second-line therapy for both squamous non-small cell lung cancer and renal cell carcinoma. CHECKMATE-141 is an open label, phase III head and neck trial in which subjects were randomized 2:1 to nivolumab (3 mg/kg every 2 weeks) or standard therapy. Median OS was higher in the experimental arm (7.5 vs 5.1 months; HR for death 0.70; 97.73% CI, 0.51–0.96; p=.001). In an exploratory analysis, there was no impact of PD-L1 expression (N1% vs b1%) on survival [55]. Glisson et al. reported a 33% ORR (higher than the 30% target) with their phase II study of nivolumab plus the synthetic long-peptide HPV 16 vaccine, ISA 101, in 34 patients with incurable oropharyngeal carcinoma [56].
Durvalumab, a human IgG1K monoclonal antibody approved by the US FDA for locally advanced or metastatic urothelial carcinoma, blocks not only binding of PD-L1 (tumor) to PD-1 (T-cell), but also inhibits the interaction of PD-L1 (T-cell) with CD80 (APC). Zandberg et al. reported a 16.2% ORR in the phase II Hawk trial of high PD-L1-expressing head and neck tumors. Among those with HPV-positive cancers, an even higher response was attributed to durvalumab therapy when compared with those that were HPV-negative (29.4% ORR vs 10.8% ORR) [57]. The phase II Condor trial of PD-L1 negative tumors, (NCT02319044) and the phase III Eagle trial testing durvalumab or tremelimumab as monotherapy or in combination (NCT02369874) are ongoing [58,59].
Unfortunately, even though it has been estimated that there are over 700,000 new cases of cutaneous squamous cell carcinoma (cSCC) diagnosed annually in the United States, this disease has been excluded from national cancer registries and therefore its precise incidence is unknown. cSCC typically affects Caucasian males at a median age of 60 years. Aggressive cases are often disfiguring and lethal, causing approximately 8000 deaths in the U.S. annually. cSCC has a higher mutation burden than any other tumor type in The Cancer Genome Atlas (TCGA) and can provide insight into the feasibility of studying checkpoint inhibition in cervical cancer [60,61].
Cemiplimab (formerly REGN2810) is a human monoclonal IgG4 antibody that targets PD-1. A phase I study demonstrated an 18% ORR which included one patient with cSCC who achieved a durable CR. Papadopoulos et al. reported that in the cSCC expansion cohorts (n=26 total), cemiplimab (3 mg/kg every twoweeks for up to 48weeks) resulted in a 46.2%ORR and a disease-control rate of 69.2%.Often observed during the first assessment, responses were rapid and durable [62]. On September 8, 2017, the US FDA granted Breakthrough Therapy Designation status for cemiplimab for metastatic and locally advanced unresected cSCC.
4.5. Recurrent & metastatic cervical cancer: inhibiting the inhibitors
Analyses of TCGA RNA sequencing data involving PD-1, PD-L1 and CD8A as an immunologic marker indicates that cervical cancer clusters with tumors in which anti-PD-1 molecules have been shown to improve survival in phase III randomized trials (see NCT03257267). This includes head and neck SCCA, lung SCCA and adenocarcinoma, renal clear cell cancer, and cutaneous melanoma. CHECKMATE-358 is a phase I/II trial of nivolumab (240 mg every 14 days) in recurrent/metastatic HPV-associated malignancies. Among the 24 treated patients, 19 had cervical cancer and five had vulvar or vaginal cancer. All responses occurred in women with cervical cancer (26% ORR including one CR and four PRs lasting at least six months) [63]. NRGGY002 is an ongoing phase II study of nivolumab in recurrent cervical cancer (NCT02257528) [64].
KEYNOTE-028 is a phase IB study of pembrolizumab (10 mg/kg every 14 days for up to 24 months) in 20 cohorts with PD-L1 positive advanced solid tumors. The cervical cancer cohort was comprised of 24 women (10 had received bevacizumab) and a 17% ORR was reported (all PRs) with a median duration of response of 5.4 months. Anti-PD-1therapy was tolerable with only five patients experiencing grade 3treatment-related adverse events (AEs) and no grade 4 AEs [65]. TheKEYNOTE-158 single-arm phase II trial of pembrolizumab is ongoing (NCT02628067) but preliminary results suggest a 17% ORR among the first 47 subjects, with the ORR being independent of PD-L1 status and increasing with longer follow-up (ORR 27% with≥27 weeks follow-up) [ 66].In a phase I study of cemiplimab, partial/unconfirmed PRs were ob-served in 9.5% (n = 2) who received cemiplimab monotherapy and in40.9% (n = 9) who received cemiplimab plus hypofractioaned radio-therapy (hfRT). One CR was observed in a patient with SCCA cervixwho had received hfRT suggesting possible abscopal effect [67].
4.6. GOG 3016/ENGOT-cx9 (EMPOWER-Cervical 1)
GOG 3016 (NCT03257267) is the first phase III randomized trial of a checkpoint inhibitor in cervical cancer (Fig. 4). Eligibility includes women with recurrent and/or metastatic (SCCA or adenocarcinoma) who have progressed following frontline therapy with a platinumbased chemotherapy doublet or bevacizumab-containing triplet. Following 1:1 randomization, subjects receive either cemiplimab (350 mg every 3 weeks) or physician’s choice single agent chemotherapy. The chemotherapy menu of pemetrexed, gemcitabine, topotecan, irinotecan, or vinorelbine was assembled based on existence of phase II data in the second line setting and drug availability in regions of the world where GOG 3016 is being conducted. The primary endpoint is OS, with secondary endpoints including PFS, response, the frequency and severity of AEs, and HRQoL [68]. Similar towhat may have occurred in KEYNOTE-040 (pembrolizumab in head and neck SCCA), availability of checkpoint inhibitors through compassionate use corridors for women who progress on the control arm of GOG 3016 may dampen a survival benefit conferred by cemiplimab. Other important considerations include treatment beyond progression, abscopal effect, and immune-specific AEs.
Figure 4. Schema of the Gynecologic Oncology Group Protocol 3016 (EMPOWER-Cervical 1).

This phase III randomized clinical trial is designed to study the activity and toxicology of the anti-PD-1 molecule, cemiplimab vs physician’s choice chemotherapy as a second-line therapy for patients with platinum-resistant recurrent and/or metastatic cervical cancer. Courtesy of Matthew Fury, MD, PhD, used with permission.
4.7. Pseudo-progression and treatment beyond progression Standard RECIST endpoints such as response and PFS may underestimate the efficacy of cancer immunotherapy.
Perceived increases in tumor volume (i.e., pseudoprogression) may result from an inflammatory response to checkpoint inhibition. Immunotherapy may also alter tumor biology so that a survival advantage may manifest beyond evidence of radiographic progression [69]. This hypothesis was studied in the OAK study, a phase IIII randomized trial of atezolizumab vs docetaxel as a second line therapy in advanced non-small cell lung cancer. For the entire study population the primary analysis of OS favored atezolizumab (median 8 vs 6 months). Provided patients were either deriving benefit from atezolizumab or were clinically stable, the proto-col allowed patients to continue atezolizumab even after demonstrable progression by RECIST. Fully 50% (n = 168) of those randomized to checkpoint elected to receive post-progression treatment and in this basket it was reported at the 2017 ASCO that the median OS increased to 12.7 months, over double that of the control [70].4.8. Abscopal effect Although radiotherapy is not administered in GOG 3016, if used for palliation prior to initiating protocol- directed therapy, patients will need to be monitored for abscopal effect or responses outside of the radiation field. This rare phenomenon of “radiation as systemic therapy” may manifest following radiation-induced tumor lysis resulting in neoantigen release and cross-priming of an activated immune system stimulated by immunotherapy. When directed at tumor-specific anti-gens, the immune system can clear systemic disease. Additionally, Parikh et al.[71]obtained serial blood samples in patients with HPV-related oropharyngeal cancer and demonstrated a 2.5-fold upregulation of PD-1 expression on CD4+ T cells during chemoradiation. In a proof-of-concept trial[72], abscopal responses were induced in 11 of41 patients with oligometastases of non-small cell lung, breast and thy-mic cancer who received 35 Gy.4.9. Immune-related adverse events (irAEs)Checkpoint inhibitors are associated with unique drug-class specific adverse events that mirror autoimmune disorders[73] . Patients should be monitored for development of these inflammatory toxicities, allowing for symptom management, treatment discontinuation, or ad-ministration of immunosuppressive agents as dictated by the severity of the irAE. Complete blood counts, liver function, metabolic panels and thyroid function studies should be scheduled with each cycle. Fol-lowing treatment these studies can be obtained every 6 to 12 week for the first 6 months. Early manifestations of pneumonitis, colitis, hepatitis, nephritis, myocarditis, and encephalitis must be readily recognized and management algorithms activated expeditiously. Interrogation of fatigue should include adrenocorticotropic hormone and cortisol to assess for endocrinopathies [74].
Footnotes
Conflict of interest statement
Dr. Lindsey Minion has nothing to disclose in relation to this manuscript. Dr. Tewari reports that his institution has received research grants from Genentech, and that he is on the Speaker’s Bureau for Merck and Roche. Dr. Tewari has also participated on an Advisory Board for Regeneron in 2017 and participated on two Advisory Board.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin 68 (2018)7–30. [DOI] [PubMed] [Google Scholar]
- [2].Adegoke O, Kulasingam S, Virnig B, Cervical cancer trends in the United States: a35-year population-based analysis, J. Women’s Health (Larchmt) 21 (10) (October2012) 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Torre LA, Bray F, Siegel RL, et al. , Global cancer statistics, 2012, CA Cancer J. Clin. 65 (2) (March 2015) 87–108. [DOI] [PubMed] [Google Scholar]
- [4].Center for Disease Control and Prevention, HPV-associated cancers, http://www.cdc.gov/cancer/hpv/statistics/age.htm, Accessed date: 15 May 2017.
- [5].Waggnor SE, Darcy KM, Tian C, Lanciano R, Smoking behavior in women with locally advanced cervical carcinoma: a Gynecologic Oncology Group study, Am. J.Obstet. Gynecol. 202 (3) (March 2010) (283.e1–7). [DOI] [PubMed] [Google Scholar]
- [6].Shiels MS, Pfeiffer RM, Gail MH, et al. , Cancer burden in the HIV-infected population in the United States, J. Natl. Cancer Inst. 103 (9) (May 4 2011) 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parikh S, Brennan P, Boffetta P, Meta-analysis of social inequality and the risk of cervical cancer, Int. J. Cancer 105 (5) (July 10 2003) 687–691. [DOI] [PubMed] [Google Scholar]
- [8].Bonomi P, Blessing JA, Stehman FB, et al. , Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology study, J. Clin. Oncol. 3 (8) (August 1985) 1079–1085. [DOI] [PubMed] [Google Scholar]
- [9].Thigpen JT, Blessing JA, DiSaia PJ, Fowler WC Jr., Hatch KD, A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology study, Gynecol.Oncol. 32 (2) (February 1989) 198–202. [DOI] [PubMed] [Google Scholar]
- [10].McGuire WP 3rd,Areneau J,Blessing JA,et al. ,Arandomizedcomparativetrial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study, J. Clin. Oncol. 7 (10) (October1989) 1462–1468. [DOI] [PubMed] [Google Scholar]
- [11].Omaura GA, Blessing JA, Vaccarello L, et al. , Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Groups Study, J. Clin. Oncol. 15 (1)(January 1997) 165–171. [DOI] [PubMed] [Google Scholar]
- [12].Moore DH, Blessing JA, McQuellon RP, et al. , Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study, J. Clin. Oncol. 22 (15) (August 12004) 3113–3119. [DOI] [PubMed] [Google Scholar]
- [13].Long HJ 3rd, Bundy BN, Grendys EC Jr., et al. , Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study, J. Clin. Oncol. 23 (21) (July 20 2005) 4626–4633. [DOI] [PubMed] [Google Scholar]
- [14].Monk BJ, Sill MW, McMeekin DS, et al. , Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study, J. Clin. Oncol. 27 (28) (October 1 2009) 4649–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tewari KS, Monk BJ, The rationale for the use of non-platinum chemotherapy doublets for metastatic and recurrent cervical carcinoma, Clin. Adv. Hematol. Oncol. 8(2) (February 2010) 108–115. [PubMed] [Google Scholar]
- [16].Bahadori HR, Green MR, Catapano CV, Synergistic interaction between topotecan and microtubule-interfering agents, Cancer Chemother. Pharmacol. 48 (2001)188–196. [DOI] [PubMed] [Google Scholar]
- [17].Tieresten AD, Selleck MJ, Hershman DL, et al. , Phase II study of topotecan and paclitaxel for recurrent, persistent, or metastatic cervical carcinoma, Gynecol. Oncol. 92 (2004)635–638. [DOI] [PubMed] [Google Scholar]
- [18].Rodriguez-Freixinos V, Mackay HJ, Breaking down the evidence for bevacizumab in advanced cervical cancer: past, present and future, Gynecol. Oncol. Res. Pract. 2 (September 21 2015) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bosch FX, Lorincz A, Munoz A, et al. , The causal relation between human papillo-mav virus and cervical cancer, J. Clin. Pathol. 55 (4) (April 2002) 244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, et al. , Oncogenes and tumor angiogenesis: the HPV-16 oncoprotein activates the vascular endothelial growth factor(VEGF) gene promoter in a p53 independent manner, Oncogene 19 (40) (September 212000)4611–4620. [DOI] [PubMed] [Google Scholar]
- [21].Obermair A, Wanner C, Bilgi S, et al. , Tumor angiogenesis in stage IB cervical cancer: correlation of microvessel density with survival, Am. J. Obstet. Gynecol. 178 (2) (February 1998)314–319. [DOI] [PubMed] [Google Scholar]
- [22].Randall LM, Monk BJ, Darcy KM, et al. , Markers of angiogenesis in high-risk, early stage cervical cancer: a gynecologic group study, Gynecol. Oncol. 112 (3) (March2009) 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kudelka AP, Verschraegen CF, Loyer E, Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470, N. Engl. J. Med. 338 (14) (April 21998) 991–992. [DOI] [PubMed] [Google Scholar]
- [24].Kudelka AP, Levy T, Verschraegen CF, et al. , A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix, Clin. Cancer Rex.3 (1997) 1501–1505. [PubMed] [Google Scholar]
- [25].Monk BJ, Still MW, Burger RA, et al. , Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study, J. Clin. Oncol. 27 (7) (March 1 2009) 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Monk BJ, Lopez LM, Zarba JJ, et al. , Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer, J. Clin. Oncol. 28 (22) (August 1 2010) 3562–3569. [DOI] [PubMed] [Google Scholar]
- [27].National Cancer Institute, Bevacizumab significant improves survival for patients with recurrent and metastatic cervical cancer,https://www.cancer.gov/news-events/press-releases/2013/GOG240, Accessed date: 11 November 2017.
- [28].Tewari KS, Sill MW, Long HJ 3rd, et al. , Improved survival with bevacizumab in advanced cervical cancer, N. Engl. J. Med. 370 (8) (February 2014) 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Penson RT, Huang HQ, Wenzel LB, et al. , Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomized, phase 3 trial (NRG Oncology–Gynecologic Oncology Group protocol 240), Lancet Oncol. 16 (3) (March 2015) 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tewari KS, Sill MW, Penson RT, et al. , Bevacizumab for advanced cervical cancer, final overall survival and adverse event analysis of a randomised, controlled, open-label, phase III trial (Gynecologic Oncology Group 240), Lancet 27 (July 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moore DH, Chunqiao T, Monk BJ, et al. , Prognostic factors for response to cisplat-in-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology group study, Gynecol. Oncol. 116 (1) (January 2010) 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tewari KS, Sill MW, Monk BJ, et al. , Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG Oncology/GOG study, Clin. Cancer Res. 21 (24) (December15 2015)5480–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Waggoner SE, Java J, Monk BJ, et al. , Impact of smoking on survival among women treated with and without bevacizumab for advanced cervical cancer (CxCA): an NRG Oncology/Gynecologic Oncology Group study, Gynecol. Oncol. 137 (Suppl. 1)(April 2015) 143–144.25579119 [Google Scholar]
- [34].Tewari KS, Sill M, Monk BJ, et al. , Impact of circulating tumor cells (CTCs) on overall survival among patients treated with chemotherapy plus bevacizumab for advanced cervical cancer. An NRG Oncology/Gynecologic Oncology Group study, Gynecol. Oncol. 137 (Suppl. 1) (April 2015) 12. [Google Scholar]
- [35].Willmott L,Java J,Monk BJ,et al. ,Fistulae in women treated with chemotherapy +/−bevacizumab for persistent, recurrent or metastatic cervical cancer in GOG-240: an NRG Oncology/Gynecologic Oncology Group study, IGCS 24 (s4) (2014). [Google Scholar]
- [36].Seamon LG, Java J, Monk BJ, et al. , Prognostic impact of histology in recurrent and metastatic cervical carcinoma: an NRG Oncology/Gynecologic Oncology Group study, Br. J. Cancer (November 28 2017) . [Google Scholar]
- [37].Eskander RN, Java J, Monk BJ, et al. , Complete responses in the irradiated field following treatment with chemotherapy with and without bevacizumab in advanced cervical cancer: an NRG Oncology/Gynecologic Oncology Group study, Gynecol.Oncol. 137 (Suppl. 1) (April 2015) 28.25666606 [Google Scholar]
- [38].Chase D, Huang J, Ramondetta L, et al. , Patient-reported outcomes and toxicity at discontinuation of the therapy in advanced or persistent cervical cancer: an analysis of GOG protocol 240, IGCS 26 (s3) (2016). [Google Scholar]
- [39].Koh WJ, Greer BE, Abu-Rusturn NR, et al. , Cervical cancer, version 2.2015, J. Natl. Compr. Cancer Netw. 13 (2015) 395–404. [DOI] [PubMed] [Google Scholar]
- [40].Sugiyama T, Mizuno M, Aoki Y, et al. , A single-arm study evaluating bevacizumab, cisplatin, and paclitaxel followed by single-agent bevacizumab in Japanese patients with advanced cervical cancer, J. Clin. Oncol. 47 (1) (January 2017) 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Minion LE, Bai J, Monk BJ, et al. , A markov model to evaluate cost-effectiveness of antiangiogenesis therapy using bevacizumab in advance cervical cancer, Gynecol.Oncol. 137 (3) (June 2015) 490–496. [DOI] [PubMed] [Google Scholar]
- [42].Kitagawa R,Katsumata N,Shibata T,et al. ,Paclitaxelpluscarboplatinversuspaclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-labelrandomizedphaseIIITrialJGOG0505,J.Clin.Oncol.33 (19) (Jul12015)2129–2135. [DOI] [PubMed] [Google Scholar]
- [43].Symonds RP, Gourley C, Davidson S, et al. , Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CiRCCa): a randomized, double-blind, placebo-controlled phase 2 trial, Lancet Oncol. 16 (15) (November 2015) 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ojesina AI, Lichtenstein L, Freeman SS, et al. , Landscape of genomic alterations in cervical carcinomas, Nature 506 (7488) (February 20 2014) 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Qin Y, Ekmekcioglu S, Forget MA, et al. , Cervical cancer neoantigen landscape and immune activity is associated with human papillomavirus master regulators, Front. Immunol. 8 (June 16 2017) 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy, Science 348 (2015) 69–74. [DOI] [PubMed] [Google Scholar]
- [47].Lheureux S, Butler MO, Clarke B, Cristea MC, Martin LP, Tonkin K, et al. , Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma, JAMA Oncol. (November 162017) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].U.S. National Library of Medicine,https://clinicaltrials.gov/ct2/show/NCT01711515?term=01711515&rank=1, Accessed date: 4 November 2017.
- [49].Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC, Human papillomavirus and cervical cancer, Lancet 382 (9895) (September 7 2013) 889–899. [DOI] [PubMed] [Google Scholar]
- [50].Yang W, Song Y, Lu YL, Sun JZ, Wang HW, Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia, Immunol. 139 (2013)513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].U.S. Food and Drug Administration,https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm, Accessed date: 20 November 2017.
- [52].Seiwert TY, Burtness B, Mehra R, et al. , Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYN0TE-012): an open-label, multicenter, phase 1b trial, Lancet Oncol. 17 (7) (July 2016) 956–965. [DOI] [PubMed] [Google Scholar]
- [53].Baumi J, Seiwet TY, Pfister DG, et al. , Pembrolizumab for platinum and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study, J. Clin.Oncol. 35 (14) (May 10 2017) 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cohen EE, Harrington KJ, La Tourneu C, et al. , Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous carcinoma (R/M HNSCC): phase 3 KEYNOTE-040, Ann. Oncol. 28 (September 2017) S5. [Google Scholar]
- [55].Haddad R, Blumenschein G Jr, Fayette J, et al. , Treatment Beyond Progression With Nivolumab in Patients With Recurrent or Metastatic (R/M) Squamous Cell Carcinoma of the Head and Neck (SCCHN) in the Phase 3 Checkmate 141 Study: A Biomarker Analysis and Updated Clinical Outcomes, Annals. Oncol. 28 (Suppl 5) [Google Scholar]
- [56].Glisson B, Massarelli E, William WN, et al. , Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer, Annals. Oncol. 28 (Suppl 5) (2017). [Google Scholar]
- [57].Zandberg D, Algazi A, Jimeno A, et al. , Durvalumab for recurrent/metastatic (R/M)head and neck squamous cell carcinoma (HNSCC): preliminary results from a single-arm, phase 2 study, Ann. Oncol. 28 (suppl_5) (2017). [Google Scholar]
- [58].Gilbert J, Le Tourneau C, Mehanna H, et al. , Phase II, randomized, open-label studyof durvalumab (MEDI4736) or tremelimumab monotherapy, or durvalumab +tremelimumab, in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): CONDOR, J. Immunother. Cancer 3 (Suppl. 2) (2015) P152, 10.1186/2051-1426-3-S2-P152 (Published online 2015Nov 4). [DOI] [Google Scholar]
- [59].Ferris RL, Even C, Haddad R, et al. , Phase III, randomized, open-label study of durvalumab (MEDI4736) monotherapy, or durvalumab + tremelimumab, versus standard of care (SoC), in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): eagle, J. Immunother. Cancer 3 (Suppl. 2) (2015)P150, 10.1186/2051-1426-3-S2-P150 (Published online Nov 4). [DOI] [Google Scholar]
- [60].Karia PS, Han J, Schmults CD, Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States,2012, J. Am. Acad. Dermatol. 68 (2013) 957–966. [DOI] [PubMed] [Google Scholar]
- [61].Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, et al. , Mutational landscape of aggressive cutaneous squamous cell carcinoma, Clin. CancerRes. 15 (2014)6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Papadopoulos KP, Owonikoko TK, Johnson ML, et al. , REGN2810: a fully humananti-PD-1 monoclonal antibody for patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) - initially safety and efficacy from expansion cohorts (ECs) of phase I study, J. Clin. Oncol. 35 (15 suppl) (2017)9503. [Google Scholar]
- [63].Hollebecque A, Meyer T, Moore KN, et al. , An Open-label, Multicohort, Phase I/II Study of Nivolumab in Patients With Virus-associated Tumors (Check Mate 358): Efficacy and Safety in Recurrent or Metastatic (R/M) Cervical, Vaginal, and Vulvar Cancers, J. Clin. Oncol. 35 (15 suppl) (2017) 5504. [Google Scholar]
- [64].U.S. National Library of Medicine,https://clinicaltrials.gov/ct2/show/NCT02257528?term=NRG-GY002&rank=1, Accessed date: 4 November 2017.
- [65].Frenel JS, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, et al. ,Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial, J. Clin.Oncol. 35 (2017)4035–4041. [DOI] [PubMed] [Google Scholar]
- [66].Schellens JHM, Marabelle A, Zeigenfuss S, et al. , Pembrolizumab for Previously Treated Advanced Cervical Squamous Cell Cancer: Preliminary Results From the Phase 2 KEYNOTE-158 Study, J. Clin. Oncol. (35 suppl) (2017) 5514. [Google Scholar]
- [67].Papadopoulos KP, Crittenden MR, Johnson ML, Lockhart AC, Moore KN, Falchook GS, Afirst-in-human study of REGN2810, a monoclonal, fully human antibody to programmed death-1 (PD-1), in combination with immunomodulators including hypofractionaed radiotherapy (hfRT), J. Clin. Oncol. 34 (2016) (abstract 3024). [Google Scholar]
- [68].U.S. National Library of Medicine,https://clinicaltrials.gov/ct2/show/NCT03257267?term=REGN2810&draw=1&rank=7, Accessed date: 4 November 2017.
- [69].Wolchok JD, Hoos A, O’Day S, et al. , Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria, Clin. Cancer Res. 15(23) (December 1 2009) 7412–7420. [DOI] [PubMed] [Google Scholar]
- [70].Gandara DR, Von Pawel J, Sullivan RN, et al. , Impact of atezolizumab (atezo) treatment beyond progression (TBP) in advanced NSCLC: results from the randomizedphase III OAK study, J. Clin. Oncol. 35 (2017) (suppl:abstr 9001). [Google Scholar]
- [71].Parikh F, Duluc D, Imai N, et al. , Chemotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer, Cancer Res. 74 (2014)7205–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Golden EB, Chhabra A, Chachoua A, et al. , Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial, Lancet Oncol. 16 (2015)795–803. [DOI] [PubMed] [Google Scholar]
- [73].Kumar V, Chaudhary N, Garg M, et al. , Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy, Front. Pharmacol. 8 (2017. May 31) 311, 10.3389/fphar.2017.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Weber JS, Yang JC, Atkins MB, Disis ML, Toxicities of immunotherapy for the practitioner, J. Clin. Oncol. 33 (2015) 2092–2099.621L.E. Minion, K.S. Tewari /Gynecologic Oncology 148 (2018) 609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]


