Abstract
Rationale
Evidence from animal and human studies suggests that opiate drugs decrease emotional responses to negative stimuli and increase responses to positive stimuli. Such emotional effects may motivate misuse of oxycodone (OXY), a widely abused opiate. Yet, we know little about how OXY affects neural circuits underlying emotional processing in humans.
Objective
We examined effects of OXY on brain activity during presentation of positive and negative visual emotional stimuli. We predicted that OXY would decrease amygdala activity to negative stimuli and increase ventral striatum (VS) activity to positive stimuli. Secondarily, we examined the effects of OXY on other emotional network regions on an exploratory basis.
Methods
In a three-session study, healthy adults (N=17) received placebo, 10 and 20 mg OXY under counterbalanced, double-blind conditions. At each session, participants completed subjective and cardiovascular measures and underwent functional MRI (fMRI) scanning while completing two emotional response tasks.
Results
Our emotional tasks reliably activated emotional network areas. OXY produced subjective effects but did not alter either behavioral responses to emotional stimuli or activity in our primary areas of interest. OXY did decrease right medial orbitofrontal cortex (MOFC) responses to happy faces.
Conclusions
Contrary to our expectations, OXY did not affect behavioral or neural responses to emotional stimuli in our primary areas of interest. Further, the effects of OXY in the MOFC would be more consistent with a decrease in value for happy faces. This may indicate that healthy adults do not receive emotional benefits from opiates, or the pharmacological actions of OXY differ from other opiates.
Keywords: OxycodonE, fMRI, Opiate drugs, Emotional responses
Introduction
Non-medical abuse of prescription opioids is a significant public health problem, with total societal costs estimated at $55.7 billion per year in the USA (Birnbaum et al. 2011). Reported motives for misuse include pain relief, feelings of well-being or pleasure (i.e., a drug “high”), and reducing negative affect or anxiety (McCabe et al. 2013). People who report misusing opiates for non-pain-relief reasons (e.g., to get high and to relieve anxiety) are at greater risk for developing opiate use problems (McCabe et al. 2007). Further, individuals with preexisting mood disorders are at higher risk for opiate abuse and dependence, suggesting that relief of negative affect may contribute to opioid drug misuse (Martins et al. 2012). Yet, we know comparatively little about how opioid drugs alter emotional responses in humans or how they affect neural circuits underlying emotional processing. Here, we examined the effects of oxycodone (OXY), a widely abused prescription opioid, on brain activity in response to emotional stimuli in healthy adults.
The brain’s “emotional network” is rich in opioidergic receptors, and opiate drugs affect activity in various network structures. The emotional network includes the amygdala, which reacts more strongly to negative stimuli; the ventral striatum (VS), which reacts mainly to positive stimuli; and the insula, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and medial prefrontal cortex (MPFC), which are active when processing emotional stimuli regardless of valence (Phan et al. 2002). Of the four types of opiate receptors identified, the mu type is most extensively studied, and mu receptors are heavily represented in amygdala, VS, and ACC (Mansour et al. 1988; Pilapil et al. 1986). Consistent with this anatomical localization, mu agonist opiates alter resting brain activity in amygdala, VS, insula, ACC, OFC, and MPFC (Becerra et al. 2006; Leppä et al. 2006; Wagner et al. 2001). Imaging of the analgesic effects of mu opiates during pain shows decreased amygdala and insula activity and increased ACC activity (Adler et al. 1997; Petrovic et al. 2002; Upadhyay et al. 2012; Wagner et al. 2007; Wise et al. 2002). Indeed, some researchers have suggested that opiates are particularly effective in modulating areas implicated in “emotional” aspects of pain, while having less impact on primary somatasensory areas (Oertel et al. 2008). This evidence that opiate drugs alter neural activity in the emotional network supports the idea that some individuals may misuse opioids to modulate emotional experiences.
In addition to the imaging evidence, several lines of behavioral evidence support the idea that opiates alter processing of emotional stimuli. In inexperienced users, opiates do not consistently produce direct positive mood effects (i.e., euphoria; Walker and Zacny 1998; Walker and Zacny 1999; Zacny and Gutierrez 2003; Zacny et al. 1994; Zacny et al. 1992). However, opiates may nevertheless alter responses to “incoming” emotional stimuli in two key ways: by reducing responses to negative emotional stimuli or by increasing responses to positive emotional stimuli. In several species of animals, opiates reduce signs of separation distress from social isolation (Kalin et al. 1988; Panksepp et al. 1981). Although there is less literature on the effects of opiates on emotional responses in humans, one study found that the opioid agonist buprenorphine reduced recognition of fear expressions, suggesting blunted sensitivity to negative stimuli (Ipser et al. 2013). Conversely, blocking opioid function with naltrexone increases both neural and behavioral responses to a negative event in humans (Petrovic et al. 2008). On the other hand, opiates may increase pleasurable responses to positive stimuli. In laboratory animals, there is an extensive literature linking opioidergic functioning to expressions of pleasure when receiving a reward (Berridge et al. 2009). In humans, the opioid agonist reminfentanil increases ratings of pleasantness for neutral emotional pictures (Gospic et al. 2008), while blocking opioidergic function with naltrexone reduces behavioral and neural responsiveness to monetary and social rewards (Petrovic et al. 2008; Schweiger et al. 2013). In summary, opioid analgesics may alter emotional processing by decreasing responses to negative stimuli and increasing responses to positive stimuli.
Despite this suggestive imaging and behavioral data, there has been no previous examination of the effect of opioid analgesics on neural responses to positive and negative emotional stimuli. Here, we examined the effects of oxycodone (OXY) on behavioral and neural responses to emotional stimuli in healthy adults. We chose OXY because it is widely abused and an urgent public health concern (Compton and Volkow 2006) and because, to our knowledge, there have been no previous studies investigating the effect of OXY on brain activity. Similar to morphine and other opiates, OXY is thought to function primarily as a mu opioid agonist (Beardsley et al. 2004), although some researchers have suggested that its effects may also be mediated by kappa opioid mechanisms (Ross and Smith 1997).
We had primary and secondary a priori hypotheses that certain brain areas would be affected by OXY during emotional processing. Our primary focus was on the amygdala and VS. We predicted that OXY would decrease amygdala activity in response to negative pictures and angry/fearful faces, relative to neutral or positive stimuli, and that this effect would be accompanied by a decrease in negative ratings of negative pictures and decreased recognition of negative faces. We further predicted that OXY would increase activation of the VS in response to positive pictures and happy faces relative to neutral and negative stimuli and that this effect would be accompanied by an increase in positive ratings of positive pictures and better recognition of happy faces. Secondarily we examined on an exploratory basis the effects of OXY on the insula, ACC, OFC, and MPFC, areas of the brain associated with more general emotional arousal, rather than specific emotional valences.
Methods
Study design
The study was a three-session, within-subjects design in which healthy adults received oral placebo, 10 and 20 mg oxycodone (OXY) in counterbalanced order under double-blind conditions. At each session, participants received a capsule (containing either placebo, 10 mg OXY, or 20 mg OXY), then completed measures of self-reported and cardiovascular drug effects, and underwent functional MRI (fMRI) scanning. During scanning, participants completed two standard tasks designed to probe emotional responses: (1) The Emotional Pictures Task, in which they viewed and rated emotional pictures, and (2) The Emotional Faces Matching Task, in which they matched emotional faces. Participants also completed a control task that examined simple visual-motor activity. Sessions were separated by a minimum of 7 days (M=8.56, SD=2.93).
Participants
Healthy right-handed adults (N=17, 7 females, 10 males) were recruited using flyers and referrals from previous participants. At an initial screening interview, prospective participants completed a Structured Clinical Interview for DSM-IV (First et al. 1996), medical screening, and self-report questionnaires of health and drug use history. Inclusion criteria were the following: no lifetime Axis I or II psychiatric disorder, no history of substance abuse/dependence (including nicotine dependence), no current use of psychoactive prescription medications, no history of major medical or neurological illness, and no medical contraindications for MRI, and for women, not pregnant or planning a pregnancy. We did not exclude on smoking per se, but no participants reported smoking in the past 30 days, and none reported being “ex-smokers.” Participants were instructed to fast for at least 12 h before each session, to consume only clear liquids for at least 2 h before each session, and not to ingest any drugs other than usual amounts of caffeine for 24 h before the study. Compliance was verified using urine drug screens prior to each session. To minimize expectancies, participants were told that they might receive a stimulant, a sedative, an opioid, a cannabinoid, or a placebo. All participants provided written informed consent, and all procedures were approved by the University of Michigan Institutional Review Board.
Subjective measures
We used several standardized questionnaires to measure the subjective effects ofOXY. These consisted of (1) the Profile of Mood States (POMS; Johanson and Uhlenhuth 1980), a 72-adjective list rated on 5-point Likert scales from 0 (not at all) to 4 (extremely) that contains eight subscales (Friendliness, Anxiety, Elation, Anger, Fatigue, Depression, Confusion, and Vigor) and is sensitive to the mood effects of psychoactive drugs; (2) the state scale of the State/Trait Anxiety Inventory (STAI; Spielberger et al. 1983), a validated measure of subjective anxiety; (3) the Addiction Research Center Inventory (ARCI; Chait et al. 1985; Martin et al. 1971), a 49-item true/false scale with six subscales that measures prototypical subjective effects of several drug classes; (4) the Drug Effects Questionnaire (DEQ; Fischman and Foltin 1991), on which participants mark how much they like/dislike the drug’s effects, feel the drug’s effects, feel high, and want more of the drug, on Likert scales ranging from 0 to 4, except like/dislike, which ranged from +4 to −4; (5) a supplementary Visual Analog Scale (VAS) consisting of 20 adjectives rated on a line and scaled to a number from 0 to 100, which was designed to measure prototypical effects of OXY not captured in the other scales, such as “Dreamy” and “Nauseated.”
Cardiovascular measures
We measured blood pressure and heart rate periodically throughout the study using a standard portable blood pressure cuff. We measured oxygen saturation at the same points with a Nonin 7500F0 MRI compatible pulse oximeter (Nonin Medical, Plymouth, MN, USA).
FMRI tasks
We used two tasks to probe emotional brain functioning, the Emotional Pictures Task (EPT) and the Emotional Faces Matching Task (EFMT), both of which have been used extensively in pharmaco-fMRI studies (e.g., Bedi et al. 2009; Labuschagne et al. 2010; Phan et al. 2008; Rabinak et al. 2012; Sripada et al. 2011). Additionally, we administered a simple Visual-Motor Task (VMT) designed to examine effects of OXY during non-emotional processing (e.g., Phan et al. 2008).
EPT stimuli consisted of 270 unique images drawn from the International Affective Picture System (IAPS), a standardized and validated picture set used to evoke brief emotional responses (Lang et al. 1999). Based on standardized ratings, pictures were classified into three valence categories: pleasant (n=90), neutral (n=90), and unpleasant (n=90). Valence categories were matched on color composition and image complexity. Stimuli were divided equally into three sets, with no stimulus repeated. Participants viewed one set at each session, and order of set presentation was randomized across participants. (See Fig. 1a for a diagram of the task.) At each session, participants viewed the set over three runs in which they viewed two 20-s blocks of each picture type per run. Each block consisted of five pictures of the same type (pleasant, neutral, unpleasant, or blank) presented for 4 s each. Blocks and pictures within blocks were presented in a fixed randomized order. In between blocks of IAPS pictures, participants viewed fixation crosses positioned on a variably shaded gray background, giving a total of 12 blocks per run. These blank pictures served as a baseline condition. To ensure alertness, participants classified each IAPS picture during the 4-s viewing period using a button press (1=unpleasant, 2=neutral, 3=pleasant). During the blank blocks, participants classified the shade of the gray background by button press (1=light, 2=medium, 3=dark). Following scanning, during the 120–180-min interval postdrug, participants reviewed the emotional stimuli seen in the scanner and subjectively rated the valence (1, most unpleasant–9, most pleasant) and arousal (1, least arousing–9, extremely arousing) of each image.
Fig. 1.
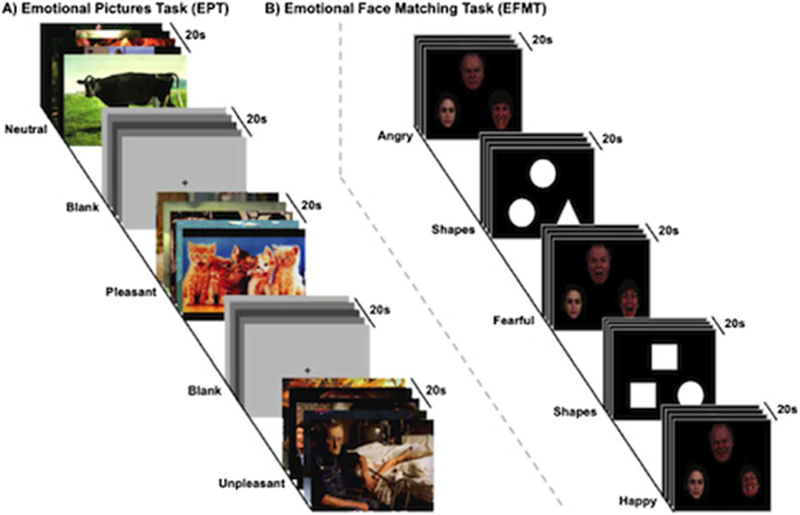
Experimental paradigms: a Emotional Pictures Task (EPT) affective images from a validated affective pictures set (Lang et al. 1999) are presented for three conditions [neutral, pleasant, unpleasant] interspersed with a variable grey fixation screen. Trials are presented for 4 s each, five times per block, two blocks per run; three runs total. b Emotional Face Matching Task (EFMT) images from a validated affective faces set (Gur et al. 2002) are presented for three conditions [two matching emotional faces (angry, fearful or happy) paired with a neutral face] interspersed with matching shapes (circle, triangle, square) paired with another shape]. Trials are presented for 5 s each, four times per block and three blocks per run per run; two runs total
EFMT stimuli consisted of photographs from a validated set of face stimuli (Gur et al. 2002) previously used in a number of pharmaco-fMRI studies (Bedi et al. 2009; Labuschagne et al. 2010; Phan et al. 2008; Sripada et al. 2011). (See Fig. 1b for a diagram of the task.) On each trial, participants viewed a triangular arrangement of faces and selected the one of two faces on the bottom that expressed the same emotion as the target face on the top. The identity of all three faces was always different, and male and female faces were equally represented. Target faces displayed one of three expressions (fearful, angry, or happy), while the probe faces on the bottom always consisted of one matching expression and one neutral expression. Twenty-second blocks in which participants matched emotional expressions were interspersed with 20-s blocks in which they matched shapes, which served as a control condition. At each session, participants completed two runs, each containing 3 blocks of matching for each face type (angry, fearful, happy), interspersed with shape matching blocks, giving a total of 18 blocks per run.
The VMT task consisted of alternating 20-s blocks in which participants saw a flashing checkerboard while pressing a single button every second or saw a white fixation cross on a black screen while remaining still. At each session, participants completed two runs, and in each run, they saw three visual-motor blocks interspersed with four fixation blocks for a total of seven blocks.
Brain image acquisition and preprocessing
Scanning was performed with blood-oxygenation-level-dependent (BOLD)-sensitive whole-brain fMRI on a 3.0 Tesla GE Signa System (General Electric) using a standard radiofrequency coil and associated software. Whole-brain functional scans were acquired using a T2*-weighted reverse spiral sequence (30-ms echo time (TE), 2-s repetition time (TR), 90° flip, 22-cm field of view (FOV), 64 × 64 matrix, 43 contiguous 3-mm axial slices aligned with the anterior commissureposterior commissure line). A high-resolution T1 scan (3D-SPGR; 1.8-ms TE, 9-ms TR, 15° flip, 25.6-cm FOV, 256× 256 matrix, 124 1.2-mm axial slices) was also acquired for anatomical localization.
Two participants failed to complete all three scans due to scanner issues, and data from one participant did not meet criteria for quality and scan stability (<2.5-mm displacement, <1.5° of rotation), resulting in a final N=14 in all fMRI analyses. Preprocessing was completed in Statistical Parametric Mapping 8 software (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK). The first four volumes in each run were discarded to allow for T1 equilibration effects. Preprocessing consisted of slice time correction, spatial realignment, normalization to the Montreal Neurologic Institute template through use of non-linear warping algorithm, spatial smoothing with a Gaussian 8-mm full-width-half-maximum kernel and high-pass temporal filtering with a cutoff of 128 s.
Procedure
Sessions were conducted from 9 a.m. to 5 p.m. After pregnancy and recent drug use tests, subjects first completed baseline measures of subjective mood and drug effects (POMS, STAI, ARCI, DEQ, and VAS) and cardiovascular measures (blood pressure, heart rate, and oxygen saturation). Participants then ingested an opaque gel capsule containing 10 or 20 mg oxycodone HCL (Purdue Pharma, Stamford, CT) with dextrose filler or placebo capsule containing only dextrose. All capsules were administered to subjects under double-blind conditions, with administration order randomized.
Participants remained in a waiting room, completing subjective and cardiovascular measures at 30 and 60 min. After the 60-min assessment, participants entered the scanner. We expected subjective drug ratings to peak at 90 min, so this placed the peak effects during scanning (Zacny and Gutierrez 2003). During the scan, participants completed the emotional tasks in counterbalanced order, followed by the visual-motor task and a 5-min resting scan during which subjects were instructed to lie still with their eyes open, focus on a fixation cross, and not think of anything in particular. After exiting the scanner at 120 min, participants again completed subjective and cardiovascular measures and the detailed ratings of the IAPS pictures, as described above. Subjective and cardiovascular measures were repeated at 180 and 240 min, after which time participants were cleared to leave the laboratory. After completing all three sessions, participants were debriefed and informed of the contents of the capsules.
Statistical analyses
Subjective, cardiovascular, and behavioral data were analyzed using within-subjects ANOVA in SPSS. fMRI data were analyzed using the general linear model as implemented in SPM8. In first-level analyses, regressors representing each condition type (EPT=pleasant, neutral, unpleasant, and blank; EFMT=angry fearful, happy, and shapes; VMT=checker-board and fixation) were convolved with the canonical hemo-dynamic response function. We then constructed individual contrast maps for each participant within each drug session contrasting conditions of interest with their respective control conditions (EPT=pleasant vs blank, neutral vs blank, and unpleasant vs blank; EFMT=angry vs shapes, fearful vs shapes, and happy vs shapes; VMT=checkerboard vs fixation).
In the second-level analysis, participants were treated as a random effect in a flexible factorial ANOVA with Emotion and Drug as within-subject factors, for the emotion tasks, or a one-way ANOVA with Drug as a within-subject factor for the VMT task (the checkerboard vs fixation contrast is contained in the contrast maps for this analysis, so there was no second independent variable). We chose flexible factorial ANOVA, which provides omnibus tests of effects, with follow-up only on significant omnibus effects, to control for the increase in type I error that might result from comparing multiple levels of our variables separately (e.g., comparing 10 mg to placebo (PL) and 20 mg to PL in separate analyses).
We first determined whether our emotion manipulations were effective in inducing activity in the emotional network by examining both an exploratory whole-brain analysis with p<0.001, uncorrected, and a 20-voxel extent—to determine whether the primary differences in activation across emotional conditions fell within emotional network areas—and conducting targeted ANOVA of activity extracted from anatomically determined regions of interest (ROIs) defining the amygdala and nucleus accumbens (NAcc, a key region within the VS, as the entire VS is difficult to anatomically define. Our accumbens ROI was based on the Harvard–Oxford structural atlas included in the FMRIB Software Library; www.fmrib.ox.ac.uk). These manipulation checks are presented in detail in Supplementary Material.
We then conducted an exploratory whole-brain analysis with p<0.001, uncorrected, and a 20-voxel extent on the Drug and Drug×Emotion interactions, to determine whether the primary observed effects of OXY fell within our a priori areas of interest and to provide unbiased information for generation of future hypotheses. If we observed effects in our primary a priori areas of interest (amygdala or NAcc) in this liberal initial analysis, we then conducted a region of interest (ROI) analysis using anatomical masks from the AAL database and Harvard–Oxford structural atlas and subjected these areas to a family-wise error (FWE) correction for multiple comparisons within the small volume correction (SVC). If we observed effects in our secondary exploratory areas of interest, parameter estimates (β weights, a.u.) were extracted from functional 5-mm spheres surrounding peak activations in regions of interest and subjected to follow-up analyses to explore the direction of the effect.
There is also the possibility of individual differences in responses to OXY, with some individuals receiving emotional “benefits” from OXY while others do not. To investigate this, we examined the correlations between OXY-induced “beneficial” changes in behavioral responses and the effects of OXY in our key a priori areas. Specifically, we examined the extent to which reductions in negative ratings for negative pictures and decreased recognition of negative faces correlated with effects of OXY on amygdala activity, and the extent to which increases in positivity ratings for positive pictures and improved recognition of positive faces correlated with NAcc activity.
Results
Subjective effects
Oxycodone produced significant subjective effects in our healthy adult volunteers. On the POMS, OXY increased Fatigue (time×drug interaction F[2, 24] =6.18, p=0.007, n2p=0.34), and on the STAI, it increased anxiety (timexdrug interaction F[10, 140]=2.59, p=0.006, n2p=0.16). It also increased Pentobarbital-Chlorpromazine-Alcohol Group (PCAG) ratings on the ARCI, a measure of sedation, and the lysergic (LSD) ratings, which tap dysphoric/somatic symptoms (PCAG time×drug interaction F[10, 130] = 6.18, p<0.001, n2p=0.32; LSD time×drug interaction F[10, 130]=4.31, p<0.001, n2p=0.25). On the DEQ, participants reported feeling a drug effect, feeling high, and disliking the drug (Feel drug×time interaction F[10, 140]=7.39, p<0.001, n2p=0.35; High drug×time interaction F[10, 140]=2.98, p<0.002, n2p=0.18; Dislike drug×time interaction F[10, 140]=4.34, p<0.001, ifp=0.24). On the VAS, OXY decreased ratings of Clearheaded, Stimulated, Energetic, Focused, and Alert and increased Tired, Drowsy, Dreamy, Worn Out, and Nauseated (Clearheaded drug×time interaction F[10, 140]=4.33, p<0.001, n2p=0.24; Stimulated drug×time interaction F[10, 140] =2.09, p=0.03, n2p=0.13; Energetic drug×time interaction F[10, 140]=2.76, p<0.001, n2p=0.16; Focused drug×time interaction F[10, 140]=2.40, p=0.01, n2p=0.15; Alert drug×time interaction F[10, 140] = 2.60, p=0.006, n2p=0.16; Tired drug×time interaction F[10, 140]=3.27, p=0.001, n2p=0.19; Drowsy drug×time interaction F[10, 140]=4.30, p<0.001, n2p=0.24; Dreamy drug×time interaction F[10, 140]=2.45, p=0.01, n2p= 0.15; Worn Out drug×time interaction F[10, 140]=4.74, p<0.001, n2p=0.25; Nauseated drug×time interaction F[10, 140]=7.04, p<0.001, n2p=0.34). Figure 2 depicts results for the STAI, PCAG, High, Dreamy, and Nausea across the course of the session to demonstrate the typical time course of various subjective drug effects relative to the scanning session.
Fig.2.
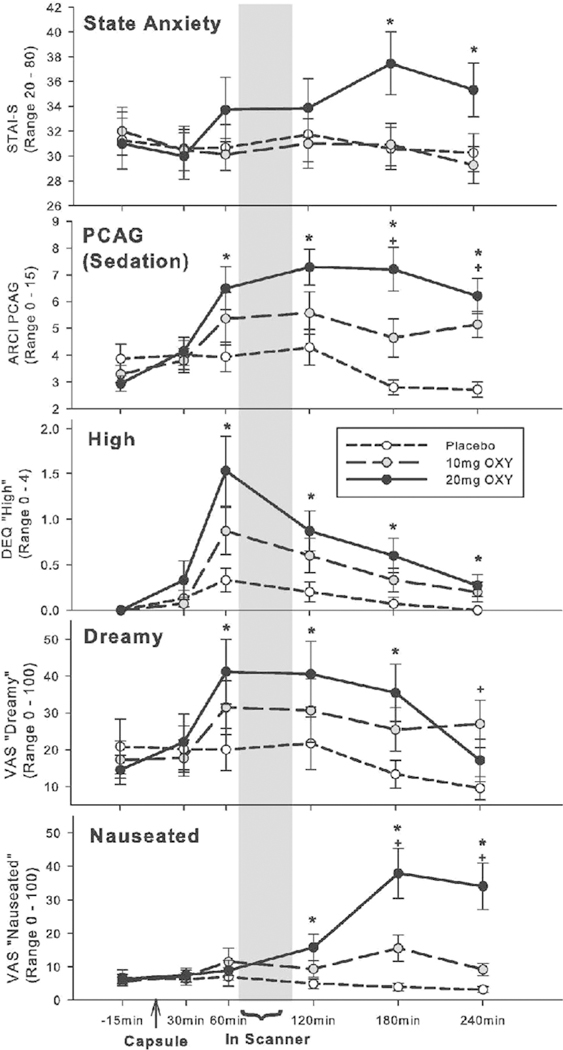
Effects of OXY on selected subjective measures across the session ► timeline. First panel: state scale of State-Trait Anxiety Scale (STAI-S); second panel from top: Addiction Research Center Pentobarbital-Chlorpromazine-Alcohol Group (ARCI PCAG), commonly used as a measure of sedative effects; third panel: Drug Effects Questionnaire (DEQ) ratings of “I feel high”; fourth panel: Visual Analog Scale (VAS) ratings of “I feel dreamy”; fifth panel: VAS ratings of “I feel nauseated”; * p<0.05 difference 20 mg versus placebo; +p<0.05 difference 10 mg versus placebo. Error bars represent standard error of the mean (SEM)
Cardiovascular effects
OXY did not affect systolic blood pressure, diastolic blood pressure, heart rate, or oxygen saturation (all p<0.05).
fMRI tasks
Behavioral results
The participant excluded from the fMRI analyses due to movement did not have atypical behavioral responses; thus, this participant’s data was included in behavioral analyses, giving N=15. On postscan measures of valence (pleasantness vs unpleasantness), participants rated positive pictures as more pleasant and negative pictures as more unpleasant (Emotion main effect: F[2, 28]=99.84, p<0.001, n2p=0.89), but the drug did not alter valence ratings (Drug main effect: F[2, 28] = 1.29, p=0.29, n2p=0.08; Drug×Emotion interaction: F[4, 56]=0.53, p=0.71, n2p=0.04). Participants rated both negative and positive pictures as more arousing than neutral pictures, as is typical for IAPS pictures (Emotion main effect: F[2, 28] = 19.51, p<0.001, n2p=0.58), but drug did not alter arousal ratings (Drug main effect: F[2, 28]=0.68, p=0.51, n2p=0.05; Drug×Emotion interaction: F[4, 56]=0.29, p=0.88, n2p=0.02). One participant had missing behavioral data for the EFMT, leaving N=14. Participants identified fear and happiness more accurately than anger (Emotion main effect: F[2, 26] = 17.14, p<0.001, n2p=0.57), but OXY did not influence identification accuracy (Drug main effect: F[2, 26] =0.11, p=0.90, n2p=0.01; Drug×Emotion interaction: F[4, 52] = 1.16, p=0.34; n2p=0.08). Finally, reaction times were faster when identifying fear and happiness compared to anger (Emotion main effect: F[2, 26] =23.10, p<0.001, n2p=0.64). OXY slowed identification times overall but did not differentially affect reaction times for any specific emotion (Drug main effect: F[2, 26] =6.64, p=0.005, n2p= 0.34; Drug×Emotion interaction: F[4, 52] = 1.47, p=0.23, n2p=0.10). Means and standard deviations by drug dose for all behavioral dependent variables are given in Table 1.
Table 1.
Means and standard deviations of behavioral outcomes by drug dose and emotion
| Emotional Picture Task | Unpleasant |
Neutral |
Pleasant |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PL | 10 mg | 20 mg | PL | 10 mg | 20 mg | PL | 10 mg | 20 mg | |
| Valence | 2.45 | 2.52 | 2.51 | 5.39 | 5.31 | 5.41 | 6.73 | 6.63 | 6.87 |
| (1.21) | (1.01) | (1.34) | (0.51) | (0.42) | (0.46) | (1.09) | (0.91) | (0.97) | |
| Arousal | 5.27 | 5.29 | 5.49 | 2.70 | 2.53 | 2.71 | 4.92 | 4.77 | 5.10 |
| (2.61) | (2.42) | (2.50) | (1.13) | (0.93) | (1.24) | (2.00) | (1.96) | (2.12) | |
| Emotional Faces Matching Task | Happy | Angry | Fearful | ||||||
| PL | 10 mg | 20 mg | PL | 10 mg | 20 mg | PL | 10 mg | 20 mg | |
| Percent correct Identifications | 88 | 91 | 88 | 81 | 82 | 82 | 88 | 92 | 88 |
| (18) | (14) | (25) | (16) | (20) | (20) | (19) | (14) | (23) | |
| Reaction time (ms) | 13,836 | 15,452 | 16,817 | 16,254 | 18,328 | 18,460 | 14,741 | 15,908 | 16,146 |
| (2,881) | (3,321) | (4,989) | (3,408) | (3,073) | (3,765) | (2,661) | (3,603) | (3,491) | |
PL placebo
Emotional Pictures Task (EPT)
As predicted, positive and negative pictures (compared to neutral) differentially activated the emotional network, indicating that this task was successful as a probe of the emotional network. Details are presented in Supplemental Materials, and we report all areas differentially activated by emotional pictures at p<0.001, uncorrected, and a 20-voxel extent, in Table 2, under “Emotion main effect.” No drug main effects or Drug×Emotion interactions were detected in either primary or secondary a priori areas. We report all areas differentially activated by Drug or the Drug×Emotion interaction at p<0.001, uncorrected, and a 20-voxel extent in Table 2.
Table 2.
Areas exhibiting effects of drug, emotion, and drug×emotion interaction during Emotional Picture Task at p<0.001, uncorrected, and cluster size of 20 voxels
| Brain region | Laterality | Volume (voxels) | F-score | MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Drug main effect | ||||||
| Cuneus | R | 7,390 | 27.06 | 18 | −96 | 32 |
| Postcentral gyrus | L | 7,864 | 24.89 | −46 | −26 | 68 |
| L | 1,256 | 19.09 | −62 | −14 | 20 | |
| R | 1,300 | 16.04 | 66 | −2 | 20 | |
| Pars triangularis | L | 53 | 12.45 | −56 | 28 | 32 |
| Thalamus | L | 63 | 11.10 | −12 | −22 | 0 |
| Fusiform gyrus | L | 280 | 10.23 | −40 | −56 | −18 |
| Lingual gyrus | R | 54 | 9.40 | 22 | −48 | −4 |
| Emotion main effect | ||||||
| Middle occipital gyrus | L | 16,281 | 92.63 | −48 | −78 | −4 |
| Postcentral gyrus | R | 2,239 | 34.14 | 44 | −24 | 62 |
| L | 68 | 10.08 | −52 | −10 | 18 | |
| Medial orbitofronal gyrus | L | 5,367 | 22.72 | −4 | 60 | −4 |
| Lateral geniculate | R | 613 | 18.69 | 22 | −26 | −4 |
| Medial frontal gyrus | R | 682 | 16.35 | 10 | 26 | 40 |
| L | 68 | 9.33 | −34 | 38 | 22 | |
| Superior frontal gyrus | L | 544 | 15.37 | 0 | −58 | 22 |
| Fusiform gyrus | L | 131 | 13.75 | −22 | −40 | −12 |
| Precentral gyrus | L | 243 | 12.82 | −48 - |
2 | 52 |
| L | 24 | 9.21 | −40 | 8 | 30 | |
| Cingulate gyrus | L | 231 | 12.35 | −18 | −28 - |
34 |
| R | 48 | 8.92 | 16 | −28 | 40 | |
| Amygdala | L | 72 | 12.04 | −24 | −4 | −16 |
| R | 66 | 11.35 | 22 | −2 | −22 | |
| Inferior parietal lobule | R | 314 | 11.40 | 48 | −54 | 58 |
| Supramarginal gyrus | L | 233 | 11.36 | 62 | −34 | 30 |
| Superior temporal gyrus | R | 99 | 10.91 | 50 | 16 | −30 |
| R | 47 | 9.64 | 32 | 20 | −28 | |
| L | 47 | 9.09 | −52 | 16 | −30 | |
| Subcallosal gyrus | L | 65 | 9.95 | −4 | 14 | −12 |
| Angular gyrus | L | 74 | 9.21 | −50 | −62 | 42 |
| Middle temporal gyrus | R | 24 | 8.77 | 56 | −18 | −12 |
| Inferior operculum | R | 20 | 8.15 | 54 | 16 | 14 |
| Drug x Emotion interaction | ||||||
| Cuneus | R | 47 | 6.65 | 16 | −86 | 46 |
| Superior temporal gyrus | L | 25 | 6.37 | −56 | 12 | −24 |
R right, L left
To examine the possibility that these null results might be due to lack of power, we extracted data from our amygdala and NAcc ROIs and calculated effect sizes for the effect of OXY on amygdala activity in response to negative pictures relative to neutral or positive stimuli, and on NAcc in response to positive and neutral pictures relative to negative stimuli. In the left (L) and right (R) amygdalae, means were in the correct direction for our hypothesis, showing less activity to unpleasant than neutral or pleasant pictures under 20 mg OXY versus placebo. For a single degree of freedom contrast representing linear effect of drug (PL<10 mg<20 mg) on unpleasant versus neutral and pleasant pictures, results in the L amygdala were F[1, 13]=0.11, p=0.741, d=0.09 and in the R amygdala were F[1, 13]=0.27, p=0.61, d=0.14. To detect effects of this size with p=0.05 and power of 0.80 in a one-tailed test would require between 318 and 763 participants (all power calculations used G*Power 3.1; Faul et al. 2007). In L and R NAcc, means were also in the correct direction, with higher activation to both pleasant and neutral pictures compared to unpleasant pictures under 20 mg OXY versus placebo. As the pattern for pleasant and neutral pictures was similar, we grouped them together for increased power. Using a single degree of freedom contrast representing linear effect of drug (PL< 10 mg<20 mg) on unpleasant versus neutral and pleasant pictures results in L NAcc of F[1, 13] = 1.47, p=0.25, d= 0.34 and in R NAcc of F[1, 13] = 1.79, p=0.20, d=0.36. To detect effects of this size with p=0.05 and power of 0.80 in a one-tailed test would require between 50 and 61 participants.
We also examined correlations between the behavioral effects of OXY on picture ratings, and activity in amygdala and NAcc. First, we constructed change scores representing the change in valence ratings for negative pictures and positive pictures from PL to 20 mg OXY and from PL to 10 mg OXY. We then constructed change scores representing the change in L and R amygdala and L and R NAcc activity from PL to 20 mg OXY and from PL to 10 mg OXY. We saw no significant relationship between change in valence ratings for negative pictures and change in amygdala activity during negative pictures at 20 mg (L amygdala r=−0.11, p=0.71; R amygdala r=−0.19, p=0.51) or at 10 mg (L amygdala r=−0.18, p=0.53; R amygdala r=0.04, p=0.89). We also saw no significant relationship between change in valence ratings for positive pictures and change in NAcc activity during positive pictures at 20 mg (L NAcc r=−0.23, p=0.43; R NAcc r=−0.31, p=0.28). There was an unexpected negative correlation between NAcc activity and ratings of positive pictures at 10 mg, but this was due to a single individual with an unusual rating pattern and was no longer significant with this individual removed (L NAcc r=−0.32, p=0.28; R NAcc r=−0.32, p=0.29).
Emotional faces matching task
When we contrasted all emotional faces versus shapes (a typical manipulation check for this task, which contains no neutral face trials; e.g., Fitzgerald et al. 2006; Labuschagne et al. 2010), the task activated emotional network areas, indicating that it successfully probed the emotional brain network. Manipulation checks are presented in Supplemental Materials, and we report all areas differentially activated by the different types of emotional pictures at p<0.001, uncorrected, and a 20-voxel extent in Table 3, under “Emotion main effect.” No drug main effect or Drug×Emotion interactions were detected in our primary a priori areas. We report all areas differentially activated by Drug or the Drug×Emotion interactions at p<0.001, uncorrected, and a 20-voxel extent in Table 3. We did see a significant Drug×Emotion interaction in one of our secondary areas of interest, the right medial OFC (28, 50, −12). Follow-up analyses conducted on 5-mm functional spheres extracted from around this peak showed that 20 mg OXY significantly decreased OFC activity to happy faces in the MOFC (t[13]= −2.26, p=0.04, d=0.60), without affecting responses to angry or fearful faces (see Fig. 3).
Table 3.
Areas exhibiting effects of drug, emotion, and drug×emotion interaction during Emotional Faces Matching Task at p<0.001, uncorrected, and cluster size of 20 voxels
| Brain region | Laterality | Volume (voxels) | F-score | MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Drug main effect | ||||||
| Lingual gyrus | L | 708 | 24.25 | −6 | −100 | −16 |
| R | 631 | 13.41 | 24 | −106 | −2 | |
| Emotion main effect | ||||||
| Postcentral gyrus | L | 70 | 15.27 | −56 | −12 | 56 |
| Lingual gyrus | L | 27 | 12.42 | −6 | −100 | −16 |
| Superior temporal gyrus | R | 342 | 11.79 | 66 | 2 | −2 |
| Insula | R | 107 | 10.19 | 52 | −22 | 14 |
| Precentral gyrus | L | 33 | 10.14 | −34 | −20 | 74 |
| R | 26 | 9.48 | 60 | 2 | 26 | |
| Middle frontal gyrus | R | 82 | 9.9 | 40 | 8 | 30 |
| R | 67 | 9.66 | 62 | 24 | 32 | |
| Middle temporal gyrus | L | 20 | 8.15 | −66 | −10 | −6 |
| Drug×Emotion interaction | ||||||
| Middle orbitofrontal gyrus | R | 74 | 7.15 | 28 | 50 | −12 |
| Superior frontal gyrus | L | 24 | 5.59 | −10 | 24 | 54 |
R right, L left
Fig. 3.
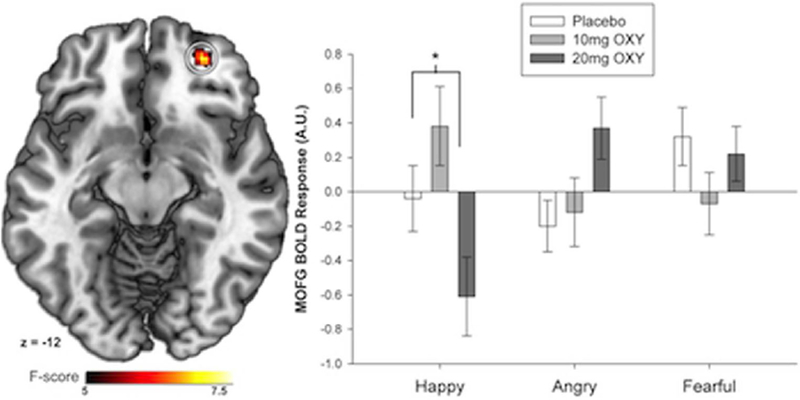
OXY (20 mg) decreased middle orbitofrontal cortex activity for happy faces, without significantly affecting activity to angry or fearful faces; *p<0.05 difference 20 mg versus placebo. Error bars represent standard error of the mean (SEM)
To examine the possibility that the null results in our primary areas of interest might be due to lack of power, we extracted data from amygdala and NAcc and calculated effect sizes for the effect of OXY on amygdala activity in response to angry and fearful faces relative to happy faces, and on NAcc activity in response to happy faces relative to neutral and negative faces. In L and R amygdala, means were in the correct direction for fear, but not anger, showing less activity under 20 mg OXY. Using a single degree of freedom contrast representing linear effect of drug (PL<10 mg<20 mg) on fearful and angry faces versus happy faces, our results in the L amygdala were F[1, 13] = 1.13, p=0.31, d=0.28 and in R amygdala were F[1, 13]=0.45, p=0.52, d=0.18. To detect effects of this size with p=0.05 and power of 0.80 in a onetailed test would require between 78 and 196 participants. In L NAcc, means were in the correct direction, showing more activity to happy faces, for the 10 mg condition only, while in the R NAcc, they were in the correct direction for 10 and 20 mg. Using a single degree of freedom contrast representing linear effect of drug (PL<10 mg<20 mg) on fearful and angry faces versus happy faces, our results in the L NAcc were F[1, 13]=<0.001, p=0.99, d=0.003 and in R NAcc were F[1,13] = 0.01, p=0.92, d=0.03. To detect effects of this size with p=0.05 and power of 0.80 in a one-tailed test would require between 8,928 and 856,591 participants.
We also examined correlations between the behavioral effects of OXY on face recognition, and activity in amygdala and NAcc. First, we constructed change scores representing the change in accuracy for angry, fearful, and happy faces from PL to 20 mg OXY and from PL to 10 mg OXY. We then constructed change scores representing the change in L and R amygdala and L and R NAcc activity from PL to 20 mg OXY and from PL to 10 mg OXY. We saw no significant relationship between change in accuracy for angry pictures and change in amygdala activity during angry pictures at 20 mg (L amygdala r=−0.09, p=0.77; R amygdala r=−0.15, p=0.62) or at 10 mg (L amygdala r=−0.31, p=0.31; R amygdala r=−0.21, p=0.49). We saw no significant relationship between change in accuracy for fearful pictures and change in amygdala activity to fearful pictures at 20 mg (L amygdala r=−0.51, p=0.08, R amygdala r=−0.44, p=0.13) or at 10 mg (L amygdala r=−0.22, p=0.47, R amygdala r=0.19, p=0.54). We also saw no significant relationship between change in accuracy for happy faces and change in NAcc activity during happy faces at 20 mg (L NAcc r=−0.28, p=0.35; R NAcc r=−0.40, p=0.17) or at 10 mg (L NAcc r=−0.27, p=0.36; R NAcc=−0.26, p=0.39).
Motor-visual task
OXY did not alter the effect of checker-board versus fixation trials on brain activity during the Visual-Motor Task at p<0.001, uncorrected, and a 20-voxel extent.
Discussion
Contrary to our primary hypotheses, OXY did not affect activity in amygdala or NAcc during presentation of positive and negative emotional pictures and faces nor did it produce any behavioral effects on evaluation of these emotional stimuli. OXY did affect activity in OFC, one of our secondary areas of interest, decreasing activity during happy faces (although not positive pictures). Our well-validated emotional tasks verifiably activated emotional network structures, and OXY produced clear subjective effects that were present before and after the scanning procedure. We did not see a relationship between the extent of “beneficial” changes in behavioral responses to emotional stimuli and OXY effects in our two a priori areas, the amygdala and the NAcc.
Taken together, OXY produced a mixed profile of subjective drug effects, some of which might be considered pleasant (feeling high, feeling dreamy) and others unpleasant (feeling anxious, feeling nauseated). It did not increase feelings of elation in our healthy adults. These apparent contradictory effects (e.g., feeling both dreamy and anxious) might be explained by the time course of the effects: putatively pleasant effects appeared to peak earlier in the session (closer to the scan), while anxiety and nausea was most evident later (after scanning). Most importantly, the measures of subjective effects confirm that the drug produced detectable effects that were evident both before and after the scan. These effects are consistent with previous studies of OXY in healthy adults (Zacny and Gutierrez 2003).
Our primary finding was that OXY decreased activity in response to happy faces in right OFC. The OFC is a part of the broader emotional network and is thought to be involved in regulation and self-monitoring of emotions (Beer et al. 2006). The OFC may integrate hedonic information such as the magnitude of reward and punishment with other aspects of decision making (Kringelbach 2005). It is difficult to determine the behavioral significance of the reduction in OFC activity to happy faces under 20 mg OXY, given that there were no accompanying differences in subjective response. However, for positive stimuli, activity in the OFC typically covaries with the value of the presented reward (Kringelbach 2005). Thus, a decrease in OFC activity under OXY does not appear consistent with our hypothesis that OXY would increase positive responses to pleasant stimuli such as happy faces.
There are several possible explanations for the lack of OXY effects in our primary a priori emotional network areas and the unexpected decrease in OFC activity to positive stimuli. First, it is never possible to completely rule out the possibility that there are subtle positive drug effects that would be detectable with a larger sample of subjects. However, the effect size estimates we obtained from strong tests of our hypothesis about the effects of OXY on emotional brain areas were quite small, requiring prohibitively large sample sizes to detect and seeming unlikely to significantly impact real-world outcomes. In contrast, the subjective effects of the drug were strongly evident in our sample, indicating that it was appropriately sized to detect any reasonably robust drug effects. Finally, OXY did not significantly change any cardiovascular parameters. Thus, it seems unlikely that a true effect of OXY on brain responding was masked by non-specific changes in respiratory or cardiovascular factors. We did examine the possibility that only a subsample of individuals receive “beneficial” emotional effects of OXY, but we did not see support for the hypothesis that individual differences in behavioral responding were related to individual differences in the effect of OXY on our key emotional areas. It is important to communicate negative findings like these, given the widespread and influential idea that individuals use opiates to assuage emotional pain (Martins et al. 2012). At a minimum, these results suggest that the effect of opiates on emotional processing is comparatively small, or perhaps even adverse, as suggested by our OFC findings.
A second potential explanation for these unexpected results is that OXY may differ critically from other opiate drugs. One point in favor of this argument is that we did not see strong main effects of the drug on areas previously identified as affected by other opiates under resting conditions (Becerra et al. 2006; Leppä et al. 2006; Wagner et al. 2001). In these studies morphine produced resting state increases in activity in nucleus accumbens (Becerra et al. 2006), while remifentanil increased activity in ACC using both PET (Wagner et al. 2001) and fMRI methodologies (Leppä et al. 2006). As noted in the introduction, there is some controversy over whether OXY is primarily a mu opiate agonist or whether it acts on kappa receptors (Beardsley et al. 2004; Lemberg et al. 2009; Nielsen et al. 2007; Ross and Smith 1997). The role of kappa receptors in regulating emotional responses is unclear, with some researchers arguing for a comparatively small involvement of this system in emotion (Filliol et al. 2000), while others suggest that kappa agonists have anxiogenic and depressogenic effects (Bruijnzeel 2009). If the emotional effects of kappa opioid agonists are in opposition to the mu opioid system, a mixed action of OXY on these systems might explain the inconsistent pattern observed here. To address this issue, it would be useful to test the effects of a “pure” mu agonist, such as morphine, on emotional responses during fMRI.
There were several limitations ofthis study. First, we used a limited range of OXY doses that were slightly below or at the therapeutic dose for analgesia. We chose these doses because they produce few adverse events in healthy volunteers. It is possible that higher doses of OXY might produce more pronounced effects on emotional functioning (although such doses would also likely produce greater non-specific effects as well, such as increased nausea and vomiting). Secondly, as noted above, by examining only healthy normal volunteers, we may have excluded individuals at higher risk for OXY abuse and dependence, including individuals with mood disorders and regular smokers. There is reason to believe that the addictive effects of opioids might be more pronounced in these at-risk populations (Martins et al. 2012; Zacny et al. 2013). Further, effects of OXY may be different individuals with more prior exposure. Typically, opioids produce a “mixed” profile in drug-naive volunteers but primarily positive effects in drug-abusing volunteers (Walsh et al. 2008). It is unclear whether this is due to preexisting differences in population or changes that occur over repeated OXY exposures. Third, OXY might show a higher abuse liability and more positive effects via an alternate route of administration from that tested here, such as intranasal (although oral is the most popular route of administration among individuals abusing OXY; Butler et al. 2011). Fourth, our emotion system probes included only tasks with visual stimuli, and tasks with different demands, such as emotional recall, may engage different parts of the emotion system that we were not able to observe here (Phan et al. 2002). Last, our study did not address non-emotional pain, so it is still possible that the effects of OXY on physical pain may contribute to OXY abuse and dependence.
In summary, the effects we observed, including increases in anxiety, combined with the lack of “beneficial” effects on neural systems involved in emotional processing, suggest that healthy adults are unlikely to be motivated to use OXY due to desirable emotional effects. It may be that the effects of OXY (and possibly opiates in general) on emotional functioning are smaller than most have anticipated, or it may be that OXY has specific pharmacological actions that differ from other opiates. These findings should inform future studies on the emotional effects of opioid agents and suggest the utility of examining alternate opioid analgesics (such as morphine) or alternate subject samples with more risk factors for opiate abuse.
Supplementary Material
Acknowledgments
This study was supported by a National Institute of Health-National Institute on Drug Abuse Grant R03DA024197 awarded to K. Luan Phan.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-014-3592-4) contains supplementary material, which is available to authorized users.
All authors declare that they have no conflicts of interest.
Contributor Information
Margaret C. Wardle, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, 5841 S. Maryland Ave., MC 3077, Chicago, IL 60637, USA
Daniel A. Fitzgerald, Department of Psychiatry, University of Illinois at Chicago, Chicago, IL, USA
Michael Angstadt, Department of Psychiatry and Neuroscience Program, University of Michigan, Ann Arbor, MI, USA.
Christine A. Rabinak, Department of Psychiatry and Neuroscience Program, University of Michigan, Ann Arbor, MI, USA
Harriet de Wit, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, 5841 S. Maryland Ave., MC 3077, Chicago, IL 60637, USA.
K. Luan Phan, Mental Health Service Line, Jesse Brown VA Medical Center, Chicago, IL, USA.
References
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL (1997) Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 84:120–126 [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS (2004) Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12:163–172 [DOI] [PubMed] [Google Scholar]
- Becerra L, Harter K, Gilberto Gonzalez R, Borsook D (2006) Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 103:208–216 [DOI] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H (2009) Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 207:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT (2006) Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci 18:871–879 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL (2011) Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med 12:657–667 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW (2009) kappa-Opioid receptor signaling and brain reward function. Brain Res Rev 62:127–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH (2011) Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR (1985) ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Depend 15:229–238 [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND (2006) Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81:103–107 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191 [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HWD, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL (2000) Mice deficient for [delta]- and [mu]-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25:195–200 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (1996) Strutured clinical interview for DSM-IV axis I disorders. Biometrics Research Department, New York [Google Scholar]
- Fischman MW, Foltin RW (1991) Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 86:1563–1570 [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL (2006) Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage 30:1441–1448 [DOI] [PubMed] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, Ingvar M, Lindefors N, Petrovic P (2008) Emotional perception modulated by an opioid and a chole-cystokinin agonist. Psychopharmacology (Berl) 197:295–307 [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoom M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE (2002) A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 115:137–143 [DOI] [PubMed] [Google Scholar]
- Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Panksepp J, Malcolm-Smith S, Thomas K, Stein DJ, van Honk J (2013) Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology 38:166–170 [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH (1980) Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 71:275–279 [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM (1988) Opiate modulation of separation-induced distress in non-human primates. Brain Res 440:285–292 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6:691–702 [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ (2010) Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35:2403–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (1999) International affective picture system (IAPS): technical manual and affective ratings NIMH Center for the study of emotion and attention. University of Florida, Gainesville [Google Scholar]
- Lemberg KK, Heiskanen TE, Kontinen VK, Kalso EA (2009) Pharmacology of oxycodone: does it explain why oxycodone has become a bestselling strong opioid? Scand J Pain 1(Supplement 1): S18–S23 [Google Scholar]
- Leppä M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, Rosenberg PH, Aronen HJ, Kalso E (2006) Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage 31:661–669 [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1988) Anatomy of CNS opioid receptors. Trends Neurosci 11:308–314 [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971) Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, an methylphenidate in man. Clin Pharmacol Ther 12:245–258 [DOI] [PubMed] [Google Scholar]
- Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL (2012) Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med 42:1261–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Boyd CJ, Teter CJ (2007) Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict Behav 32:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Boyd CJ (2013) Medical use, medical misuse, and nonmedical use of prescription opioids: Results from a longitudinal study. Pain 154:708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT (2007) Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain 132:289–300 [DOI] [PubMed] [Google Scholar]
- Oertel BG, Preibisch C, Wallenhorst T, Hummel T, Geisslinger G, Lanfermann H, Lötsch J (2008) Differential opioid action on sensory and affective cerebral pain processing. Clin Pharmacol Ther 83: 577–588 [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Vilberg T, Bishop P, DeEskinazi F (1981) Endogenous opioids and social behavior. Neurosci Biobehav Rev 4:473–487 [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia—imaging a shared neuronal network. Science 295:1737–1740 [DOI] [PubMed] [Google Scholar]
- Petrovic P, Pleger B, Seymour B, Klöppel S, De Martino B, Critchley H, Dolan RJ (2008) Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. J Neurosci 28:10509–10516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348 [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H (2008) Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 28:2313–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilapil C, Welner S, Magnan J, Zamir N, Quirion R (1986) Mu opioid receptor binding sites in human brain. NIDA Res Monogr 75:319–322 [PubMed] [Google Scholar]
- Rabinak C, Sripada C, Angstadt M, Wit H, Phan KL (2012) Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. J Neural Transm 119:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross FB, Smith MT (1997) The intrinsic antinociceptive effects of oxycodone appear to be κ-opioid receptor mediated. Pain 73:151–157 [DOI] [PubMed] [Google Scholar]
- Schweiger D, Stemmler G, Burgdorf C, Wacker J (2013) Opioid receptor blockade and warmth-liking: effects on interpersonal trust and frontal asymmetry. Soc Cogn Affect Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacob GA (1983) Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL (2011) Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage 55:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Anderson J, Baumgartner R, Coimbra A, Schwarz AJ, Pendse G, Wallin D, Nutile L, Bishop J, George E, Elman I, Sunkaraneni S, Maier G, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Becerra L, Borsook D (2012) Modulation of CNS pain circuitry by intravenous and sublingual doses of buprenorphine. Neuroimage 59:3762–3773 [DOI] [PubMed] [Google Scholar]
- Wagner KJ, Willoch F, Kochs EF, Siessmeier T, Tölle TR, Schwaiger M, Bartenstein P (2001) Dose-dependent regional cerebral blood flow changes during remifentanil infusion in humans: a positron emission tomography study. Anesthesiology 94:732–739 [DOI] [PubMed] [Google Scholar]
- Wagner KJ, Sprenger T, Kochs EF, Tölle TR, Valet M, Willoch F (2007) Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology 106:548–556 [DOI] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP (1998) Subjective, psychomotor, and analgesic effects of oral codeine and morphine in healthy volunteers. Psychopharmacology (Berl) 140:191–201 [DOI] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP (1999) Subjective, psychomotor, and physiological effects of cumulative doses of opioid μ agonists in healthy volunteers. J Pharmacol Exp Ther 289:1454–1464 [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR Jr (2008) The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend 98: 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I (2002) Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage 16:999–1014 [DOI] [PubMed] [Google Scholar]
- Zacny J, Gutierrez S (2003) Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology (Berl) 170:242–254 [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Zaragoza JG, de Wit H (1992) Subjective and behavioral responses to intravenous fentanyl in healthy volunteers. Psychopharmacology (Berl) 107:319–326 [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Flemming D, Coalson DW, Thompson WK (1994) A dose–response analysis of the subjective, psychomotor and physiological effects of intravenous morphine in healthy volunteers. J Pharmacol Exp Ther 268:1–9 [PubMed] [Google Scholar]
- Zacny JP, Apfelbaum SM, Perkins KA (2013) Modulating roles of smoking status and sex on oxycodone-induced nausea and drug liking. Exp Clin Psychopharmacol 21:103–111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


