Abstract
Synapsins are neuronal phosphoproteins that fine-tune synaptic transmission and suppress seizure activity. Synapsin II (SynII) deletion produces epileptic seizures and overexcitability in neuronal networks. Early studies in primary neuronal cultures have shown that SynII deletion results in a delay in synapse formation. More recent studies at hippocampal slices have revealed increased spontaneous activity in SynII knockout (SynII(−)) mice. To reconcile these observations, we systematically re-examined synaptic transmission, synapse formation, and neurite growth in primary hippocampal neuronal cultures. We find that spontaneous glutamatergic synaptic activity was suppressed in SynII(−) neurons during the initial developmental epoch (7 days in vitro, DIV) but was enhanced at later times (12 and18 DIV). The density of synapses, transmission between connected pairs of neurons, and the number of docked synaptic vesicles were not affected by SynII deletion. However, we found that neurite outgrowth in SynII(−) neurons was suppressed during the initial developmental epoch (7 DIV) but enhanced at subsequent developmental stages (12 and18 DIV). This finding can account for the observed effect of SynII deletion on synaptic activity. To test whether the observed phenotype resulted directly from the deletion of SynII we expressed SynII in SynII(−) cultures using an adeno-associated virus (AAV). Expression of SynII at 2 DIV rescued the SynII deletion-dependent alterations in both synaptic activity and neuronal growth. To test whether the increased neurite outgrowth in SynII(−) observed at DIV 12 and18 represents an overcompensation for the initial developmental delay or results directly from SynII deletion we performed “late expression” experiments, transfecting SynII(−) cultures with AAV at 7 DIV. The late SynII expression suppressed neurite outgrowth at 12 and 18 DIV to the levels observed in control neurons, suggesting that these phenotypes directly depend on SynII. These results reveal a novel developmentally regulated role for SynII function in the control of neurite growth.
Keywords: Synapse, neurite, sEPSC, sIPSC, synaptic vesicle
Introduction
Synapsins are a family of neuronal phosphoproteins that reversibly associate with synaptic vesicles (Cesca et al 2010, Fassio et al 2011b, Hilfiker et al 1999). Synapsins regulate clustering and mobilization of synaptic vesicles (Bykhovskaia 2011, Shupliakov et al 2011), the time course of transmitter release (Hilfiker et al 1998, Medrihan et al 2013) and neuronal development (Valtorta et al 2011). Converging basic and clinical work has convincingly demonstrated that deficiency of synapsins isoforms I and II (SynI and SynII) can produce epilepsy and autism predisposition (Fassio et al 2011b, Giovedi et al 2014). Genetic epidemiology studies have identified human mutations in SynI gene associated with syndromic epilepsy (Garcia et al 2004, Giannandrea et al 2013, Goodship et al 2003) and also a contribution of SynII to sporadic predisposition to epilepsy (Cavalleri et al 2007, Prasad et al 2014) and autism (Corradi et al 2014). Experiments in genetically modified animals have shown that deletion of either the SynI or SynII genes results in a strong epileptic phenotype (Etholm & Heggelund 2009, Li et al 1995, Rosahl et al 1995) and significant impairments in social behaviors (Greco et al 2013), and both phenotypes are more pronounced in animals lacking SynII isoform (SynII(−)) (Etholm et al 2012, Greco et al 2013).
It is not yet clear how synapsin deletion causes these pathological conditions. Extensive evidence suggests that synapsin deficiency produces neuronal overexcitability in the hippocampus and cortex (Boido et al 2010), which may be associated with excitation/inhibition imbalance (Chiappalone et al 2009, Farisello et al 2013, Gitler et al 2004). Consistent with these ideas we have previously shown that spontaneous glutamatergic transmission is enhanced in the CA1 region of the hippocampus in SynII(−) mice, while GABAergic transmission is suppressed (Feliciano et al 2013).
Previous work has shown that the epileptic phenotype caused by synapsin deficiency is strongly grounded in development, as epilepsy symptoms in synapsin-deleted mice are not manifested until the age of two or three months (Etholm et al 2013, Etholm et al 2012, Etholm & Heggelund 2009). These considerations have led to the idea that the seizure activity in synapsin deficient brain may be influenced by developmental changes that occur in neuronal networks, in addition to the excitation/inhibition imbalance in synaptic transmission (Ketzef & Gitler 2014, Ketzef et al 2011). Thus, the activity of synapsins in neuronal development may play a critical role in the maintenance of normal neuronal excitability and suppression of seizure activity.
Synapsins promote neuronal growth and maturation in both vertebrates and invertebrates (Valtorta et al 2011). Synapsin deficiency is associated with decreased neuronal outgrowth and synaptogenesis, while synapsin expression or phosphorylation reverses these deficits (Ferreira et al 1995, Han et al 1991, Kao et al 2002, Lu et al 1996, Schaeffer et al 1994, Valtorta et al 1995, Vasin et al 2014). It is likely that the developmental defects in the synapsin deficient brain affect the progress of neuronal overexcitability and the associated brain malfunction. However, the relationship between the developmental defects and abnormal excitability in synapsin deficient neurons has not yet been explored. In the present study we investigated the structure and activity of developing hippocampal neuronal cultures derived from SynII(−) mice.
Materials and Methods
Animals
Mice heterozygotes for the Syn2 targeted mutation were purchased from The Jackson Laboratory (strains B6;129S-Syn2tm1Sud/J and B6). Homozygote Syn II(−) and wild type (WT) lines were derived from breeding heterozygotes. Genotypes of all the breeders were independently confirmed using a commercial genotyping service (Transnetyx). Animals were kept under standard conditions on a 12h dark/light cycle. The husbandry conditions were identical for all the strains under the study. All the experiments were performed in accordance with the guidelines of the Animal Care and Use Committee (IACUC) of the Wayne State University School of Medicine (WSU SOM) and the National Institutes of Health of the US Public Health Service. All the procedures were approved by the WSU SOM IACUC (Protocol # A 06–04-15).
Dissociated hippocampal cultures
Cell cultures of mice hippocampal neurons were prepared according to standard protocols (Kaech & Banker 2006). Briefly, a astrocyte feeding layer was cultured and plated on poly-D-lysine/collagen-coated glass cover slips in Dulbecco’s modified Eagle Medium supplemented with 10% fetal bovine serum and N-2 supplement. Hippocampal neurons were collected from newborn mice pups (0–3 days), dissociated, and then plated on top of an astrocyte-feeding layer at a density of 40,000 cells/cm2. Experiments were performed on cultures from 7, 12 and 18 DIV in Neurobasal Medium supplemented with B27. All media and supplements were purchased from Thermofisher/Life Technologies.
In every experiment, we used 2–5 coverslips from 2–3 cultures. For each experimental group, 2–4 animals were used for each culture. Coverslips were selected based on cell density (40,000 cells/mL) and overall health of the neurons. From each culture, healthy coverslips with the designated cell density were randomly subdivided for experiments at 7, 12 and 18 DIV. Microscopy analysis, as well as patch-clamp recordings, were performed only for the cells that had a distinct pyramidal shape typical for CA1 neurons.
Electrophysiology
The recording chamber was continuously perfused with an ACSF solution composed of (in mM): 120.0 NaCl, 2.5 KCl, 1.3 MgSO4, 1.0 KH2PO4, 2.5 CaCl2, 24.0 NaHCO3, 10.0 dextrose, 50 sucrose. The ACSF solution was bubbled with a mixture of 95% O2 and 5% CO2 and then glass coverslips containing cultured cells were submerged in the recording chamber.
Whole-cell recordings were performed with the glass pipette of ∼3 MΩ resistance. Recordings were acquired using a Multiclamp 700B amplifier and PClamp10.0 software (Molecular Devices) and digitized at 20kHz. Recordings of spontaneous excitatory and inhibitory postsynaptic currents (sEPSCs and sIPSCs, respectively) in the absence of blockers were performed using the electrode filled with a potassium gluconate-based intracellular solution containing (in mM): 135 K-gluconate, 0.1 CaCl2, 2.0 MgCl2, 2.0 Na-ATP, 1.0 EGTA, 10 HEPES. Under these recording conditions, ECl was ∼−90 mV and cells were held at −15 mV to allow a clear distinction between sEPSCs and sIPSCs. In all the intracellular solution, pH was adjusted to 7.35 with KOH, and the osmolarity was adjusted to 280 mOsm by sucrose.
To record EPSCs selective, GABAergic currents were blocked with Picrotoxin (Ptx, 50 µM, Sigma Aldrich). In these experiments, recording pipettes were filled with cesium-based solution composed of (in mM): 117.5 CH3CsO3S, 1.0 MgCl2 6 H2O, 10.0 Hepes, 5.0 EGTA, 15.5 CsCl, 8.0 NaCl, 4.0 MgATP, 5.0 Qx-314-bromide. Holding potential was maintained at −60 mV. To record miniature EPSCs (mEPSCs), TTX (1µM, Sigma Aldrich) was added to ACSF external solution.
Paired double patch recordings of evoked EPSCs (eEPSCs) were performed using pipettes filled with a K-gluconate solution (in mM): 135.0 K-gluconate, 0.1 CaCl2, 2.0 MgCl2, 2.0 Na-ATP, 1.0 EGTA, 10 HEPES. Holding potential of the stimulated cell was kept at −70 mV, with a stimulation intensity of 0.9 mA and the length of the depolarizing pulse of 1–5 ms.
Immunohistochemistry
Neuronal cultures were fixed for 30 minutes with 4% paraformaldehyde in phosphate-buffered saline (PBS), then permeabilized in PBS containing 0.1% triton (PBST) for 10 minutes and then rinsed trice with PBST with 10 minute intervals between rinses. The cells were then exposed to the primary antibodies (diluted in PBST) overnight at 4°C. Finally, the cultures were rinsed in PBST and incubated with secondary antibodies for 2 h at a room temperature with 10% goat serum (Sigma Aldrich). Mouse monoclonal anti-Glutamate Receptor Interacting Protein 1 (GRIP-1 (F-2), Santa Cruz Biotech, 1:200 dilution), rabbit anti-Synaptophysin (SYP (H-93), Santa Cruz Biotech, 1:100 dilution) were used as primary antibodies. Goat anti-mouse IgG conjugated to Texas Red (Santa Cruz Biotech, 1:100 dilution), and goat anti-rabbit IgG (H+L) conjugated to Alexa Fluor 488 (Thermofisher/Life Technologies, 1:100 dilution) were used as secondary antibodies. Epifluorescence images were acquired using a Olympus BX61W microscope equipped with an X-Cite 120 LED lamp (Lumen Dynamics), and sCMOS pco.edge camera (PCO) using a 60X objective (Olympus).
Fluorescent dye injection
Coverslips containing cultured cells were submerged in a chamber continuously perfused with ACSF bubbled with a mixture of 95% O2 and 5% CO2. Patch pipettes were filled with 15µM Lucifer Yellow (Sigma Aldrich) diluted in the cesium-based internal solution. Neurons were filled with the dye for one minute under visual control. This loading time was found to be optimal, and further loading did not enhance spreading the dye. After a neuron was filled with the dye, the patch pipette was carefully removed. The neuron was imaged within one minute after the dye loading. The center of a neuron was positioned in the center of the field of view (350 µM diameter), the image was acquired, and all the neurites within this field of view were quantified.
Electron microscopy
Cultured neurons were fixed with 2.5% glutaraldehyde, 4% paraformaldehyde in 90 mM sodium cacodylate buffer containing 0.02 mM CaCl2 , pH adjusted to 7.2–7.4, in the microwave (Pelco Biowave) at 35°C for 2 min at 150W and then at room temperature for 15 min. Then the cells were washed twice with 90 mM sodium cacodylate buffer for 10 min and postfixed in 1% KFeCN and then 1% osmium tetroxide OsO4, each for 30 min. After each incubation the cells were washed twice with 90 mM sodium cacodylate buffer for 10 min. En bloc staining was done with 2% aqueous solution of uranyl acetate UO2(CH3OCO)2.2H2O for 1 h and washed twice with distilled water for 10 min. The preparations were dehydrated through a graded series of acetone (50%, 70%, 90% and 100%) and embedded in a 1:1 mixture of EMBed-812 and SPURR (Electron Microscopy Sciences). Ultrathin sections (50nm) were cut with a Leica Ultracut ultramicrotome, mounted on copper slot Formvar-coated grids and imaged with a JEM 100CX transmission electron microscope (JEOL) equipped with AMT 4 MP digital camera.
Viral transfection
An adeno-associated virus (AAV1.hSyn.SYN2-Turbo-GFP.WPRE.hGh) was custom made by Penn Vector Core to contain AAV serotype 1 and hSyn promoter to drive expression of GFP-tagged SYNIIin neurons (Karra, D., & Dahm, R., 2010; Royo et al., 2008), with WPRE and hGh poly A sequences. The GFP-tagged Syn2 (variant IIa) vector was commercially obtained from Origene (CAT#: MG225317).
Neuronal cultures from cells lacking Synapsin II (SynII(−)) at 2 days in vitro (DIV) were exposed to the vector AAV1.hSyn.SYN2-Turbo-GFP.hGh at a moderate dose of 2.5 × 1010 gene copies (GC) per milliliter (Royo et al., 2008), diluted in D-PBS at 37°C, for ~24 hours. After one day, the media containing AAV was exchanged for fresh media. For “late expression” experiments, the exposure to AAV was performed at 7 DIV. GFP fluorescence served as a marker for the gene expression.
Data analysis
The electrical recordings were analyzed using the Clampfit software (Molecular Devices), and in-house QUANTAN software (Bykhovskaia 2008). The NeuronJ plugin for Image J (Fiji implementation) was used to quantify neuronal arborization. Immunohistochemistry experiments were analyzed using Photoshop CS6 (Adobe). Statistical analyses were performed using Prism software (GraphPad). ANOVA followed by the Tukey post-hoc test was employed for multiple comparisons. Interevent cumulative histograms were also compared employing Kolmogorov-Smirnov (K-S) test. The power analysis was performed, and the sample size was adjusted to yield 1-β > 0.9.
Results
Previous studies have shown that deletion or suppression of SynII reduces neurite outgrowth and synaptogenesis in cultured neurons (Ferreira et al 1995, Ferreira et al 1994, Valtorta et al 2011). However, the functional implications of this suppression have not been defined. To address this question, we investigated activity of SynII(−) neurons during development in vitro.
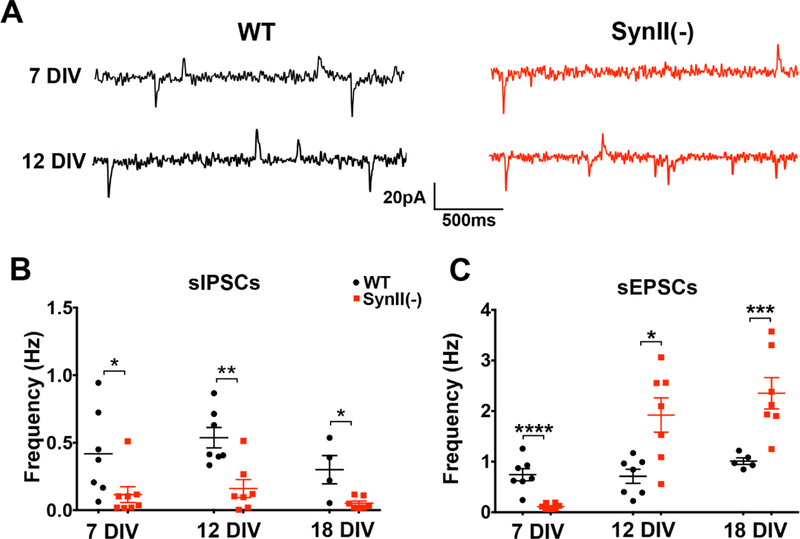
First, we asked how SynII deletion affects spontaneous excitatory and inhibitory transmission at different developmental stages. We recorded spontaneous activity from pyramidal neurons under conditions which allowed for the separation of sEPSCs from sIPSCs in the absence of receptor blockers (Fig. 1A). Consistent with the demonstrated role of synapsins in promoting inhibitory synaptic transmission (Gitler et al 2004), we found that the sIPSC frequency was uniformly reduced in SynII(−) cultures at 7–18 DIV (Fig. 1 B). In contrast, glutamatergic transmission was differentially affected by SynII deletion at different developmental stages. At 7 DIV, the frequency of sEPSCs was significantly reduced in SynII(−) cultures (0.74±0.12 Hz in WT versus 0.11±0.0.2 Hz in SynII(−), Fig. 1C). This result is in line with the developmental delay in synapse formation for SynII(−) cultured neurons reported in earlier studies (Ferreira et al 1995, Ferreira et al 1994). Interestingly, however, SynII(−) cultures demonstrated an increased sEPSC frequency at 12 and 18 DIV (from 0.71±0.14 Hz at 12 DIV and 1.01±0.06 Hz at 18 DIV in WT to 1.92±0.34 Hz at 12 DIV and 2.35±0.31 Hz at 18 DIV in SynII(−), Fig. 1 C).
Fig. 1.
Excitatory and inhibitory spontaneous transmission in SynII deleted neurons at different developmental stages in vitro. A. Representative recordings showing sEPSCs (downward polarity) and sIPSCs (upward polarity) recorded at – 15 mV holding potential. B. Spontaneous inhibitory transmission is uniformly reduced in SynII(−) neurons. C. In SynII(−) neurons, spontaneous excitatory transmission is reduced at the initial developmental stage (7 DIV) but enhanced at subsequent developmental stages (12 and18 DIV). * p<0.05; * <0.01; *** p<0.005, **** p<0.001.
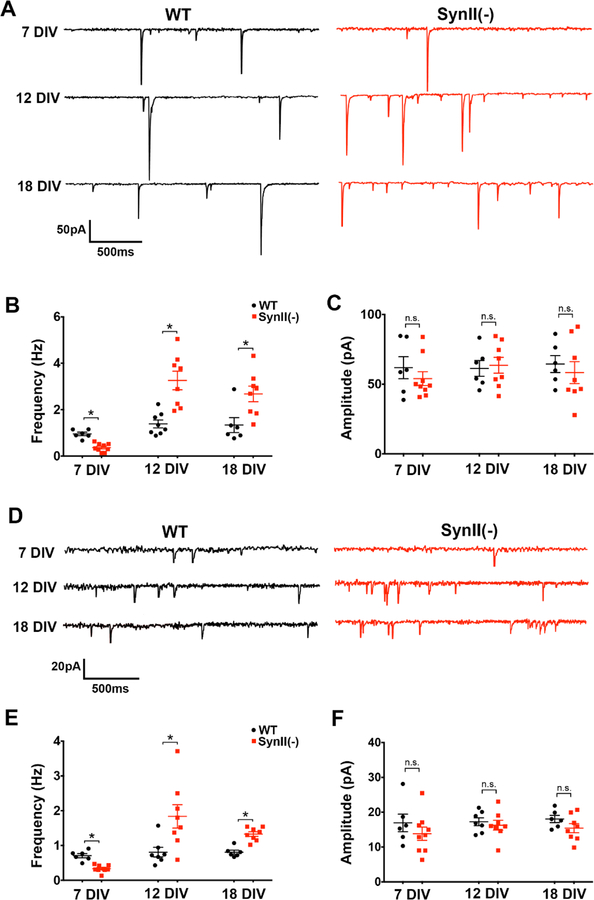
The increase in sEPSC frequency observed at 12 and 18 DIV (Fig. 1 C) is consistent with the increased spontaneous glutamatergic transmission observed at hippocampal slices derived from SynII(−) mice (Feliciano et al 2013), which was shown to be secondary to the reduced inhibitory transmission. We asked therefore whether the increase in sEPSC frequency at cultured SynII(−) neurons (Fig. 1 C) may also be secondary to the reduced GABAergic transmission in SynII(−) neurons (Fig. 1 B). To test this possibility, we recorded sEPSCs in the presence of the Ptx (50 µM) to block inhibitory synaptic transmission (Fig. 2A). We found that the effect of SynII deletion remained unchanged (Fig. 2 B), that is to say, sEPSC frequency was decreased in SynII(−) neurons at 7 DIV (0.95 ± 0.08 Hz in WT versus 0.34±0.05 Hz in SynII(−) ), but increased at both 12 and 18 DIV (1.34±0.17 Hz and 1.34±0.32 Hz in WT versus 3.26±0.40 Hz and 2.68±0.34 Hz in SynII(−), respectively, Fig 2 B). These alterations in the sEPSC frequency were not associated with any changes in the sEPSC amplitude (Fig. 2 C).
Fig. 2.
Glutamatergic spontaneous transmission in SynII(−) neurons is decreased at the initial developmental stage (7 DIV) and promoted at subsequent stages (12–18 DIV). A. Representative traces showing sEPSCs recorded in the presence of Ptx at −60 mV holding potential. B. In SynII(−) neurons, sEPSC frequency is significantly decreased at 7 DIV and significantly increased at 12 and 18 DIV. C. SynII deletion does not alter sEPSC amplitudes. D. Representative traces showing mEPSCs recorded in the presence of TTX. E. mEPSC frequency is significantly decreased at 7 DIV and significantly increased at 12 and 18 DIV in SynII(−) neurons. F. SynII deletion does not alter mEPSC amplitudes. * p<0.05
To further explore the effect of SynII deletion on spontaneous glutamatergic synaptic transmission, we next recorded mEPSCs in the presence of TTX (Fig. 2 D). The effect of SynII deletion on mEPSC frequency was similar to the effect on sEPSC frequency, namely the mEPSC frequency was reduced in SynII(−) neurons at 7 DIV and increased at 12 and 18 DIV (Fig. 2 E). These changes were not associated with any alterations in mEPSC amplitudes (Fig. 2 F). Thus, SynII deletion inhibits glutamatergic transmission at the initial developmental stages (7 DIV) and promotes it at subsequent stages.
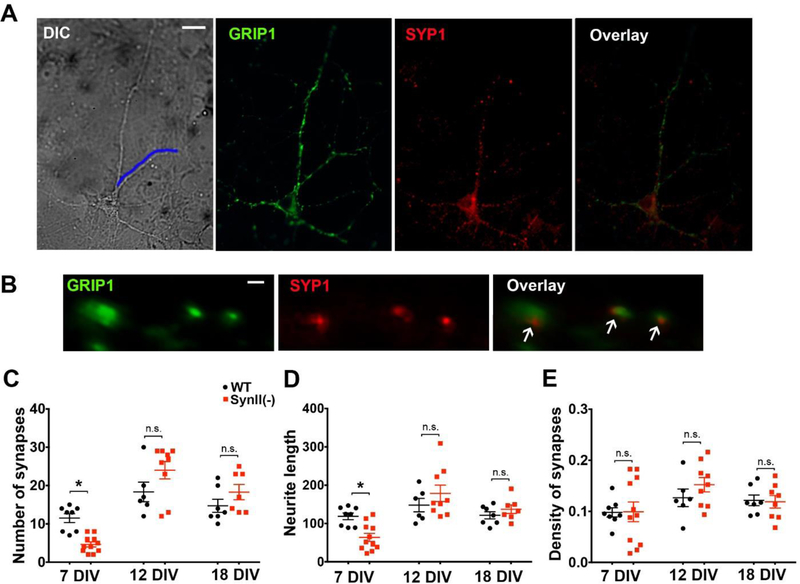
The decreased glutamatergic transmission in SynII(−) neurons at 7 DIV may be associated with the suppressed synaptogenesis. To test this possibility, and also to investigate synaptogenesis at subsequent developmental stages, we quantified synaptic puncta at SynII(−) neurons. We counted the number of the spots immunopositive for the presynaptic marker Synaptophysin 1 (SYP1) and for the postsynaptic marker Glutamate Receptor Interacting Protein 1 (GRIP1) and reconstructed neurite length using DIC imaging (Fig 3 A,B). We found that at 7 DIV the neurite length and the total number of synapses per neurite were reduced in SynII(−) neurons, although the synaptic density was not affected (Fig 3 C–E). However, at subsequent time points 12 and18 DIV, the number of synaptic puncta per neurite (Fig 3 C), the neurite length (Fig 3 D), and synaptic puncta density (Fig. 3 E), were unchanged in SynII(−) cultures with respect to WT controls, in agreement with an earlier study (Gitler et al 2004).
Fig. 3.
Synaptogenesis in SynII(−) neurons is suppressed at the initial developmental stage but not altered at subsequent developmental stages. A. DIC image and immunofluorescence in a pyramidal neuron. Blue line at the DIC image outlines the length of a neurite . Scale bar: 10 µm. B. Synapses are identified by colocalization of SYP1 and GRIP1. Scale bar: 1 µm: C. The number of synapses per neurite is decreased in SynII(−) neurons at 7 DIV but unaltered at subsequent developmental stages. D. The neurite length is decreased in SynII(−) neurons at 7 DIV but unaltered at subsequent developmental stages. E. The density of synapses is unaffected in SynII(−) neurons. * p<0.05
These results agrees with the developmental delay in synaptogenesis in SynII(−) neurons and could account for the decrease in glutamatergic transmission observed in SynII(−) neurons at 7 DIV (Fig. 1, 2). However, these results also suggest that an effect of SynII deletion on synaptogenesis alone cannot account for the increase in synaptic activity at DIV 12–18.
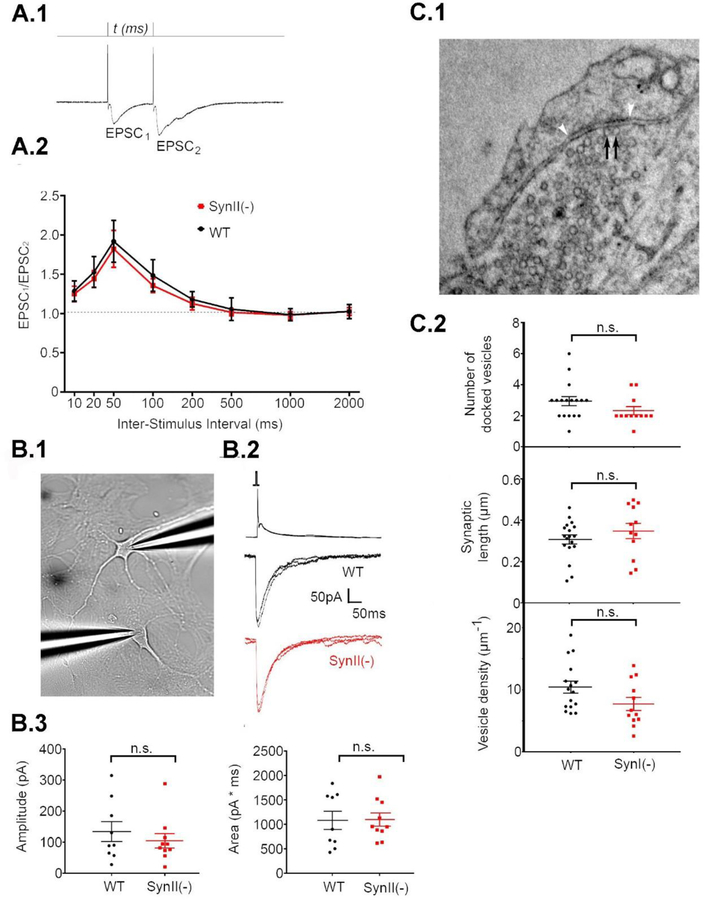
Next, we tested whether changes in the release properties of single synapses could explain the enhanced sEPSC activity observed in SynII(−) cultures by comparing transmitter release and quantifying vesicle docking at 12 DIV. First, we quantified pair-pulse facilitation (PPF), a measure of release probability, and found that it was not altered at SynII(−) synapses (Fig 4A). This result argues against changes in release properties in the Syn II(−) cultured neurons. Next, to rigorously assess synaptic function, we conducted paired double-patch recordings from connected excitatory neurons (Fig 4 B.1,2). We found that neither the amplitude nor the charge (area) of the paired recording eEPSC were affected by SynII deletion (Fig 4 B.3) suggesting again that that basal glutamatergic transmission between single connected neurons is not altered.
Fig 4.
SynII deletion does not affect synaptic strength at 12 DIV. A. PPF is not affected in SynII(−) neurons. A.1. Monitoring PPF at different time intervals. The stimulation intensity was adjusted to eliminate transmission failures for the first response, EPSC1. A.2. PPF expressed at a ratio between EPSC2 and EPSC1 amplitudes. Data collected from n=9 cells (3 cultures) for each genotype. B. Synaptic currents obtained by double patch recordings from connected pairs of neurons are unaffected in SynII(−) pairs. B.1. The recoding configuration. Presynaptic action potential (top traces) coupled with EPSCs (bottom traces). B.3. Neither amplitude (left panel) or area (right panel) are affected in SynII(−) neurons. C. SynII deletion does not affect the number of docked vesicles. C.1. A representative micrograph showing docked vesicles (black arrows). The synaptic density is marked by white arrowheads. C.2. The parameters describing vesicle docking, including the number of docked vesicles per synapse (top), the length of the synapse (middle), and the density of docked vesicles (bottom).
We next employed EM analysis to quantify the number of docked vesicles in glutamatergic synapses (Fig 4 C.1). Excitatory nerve terminals were identified by relatively round vesicles and asymmetric synapses. We found that none of the parameters characterizing vesicle docking, including the number of docked vesicles per synaptic contact, the length of the synaptic contact, and the density of docked vesicles was altered in SynII(−) synapses (Fig 4 C.3). Altogether, these results do not support the idea that changes in release properties at single synapses accounts for the increase in glutamatergic synaptic activity seen at 12–18 DIV in SynII(−) cultures.
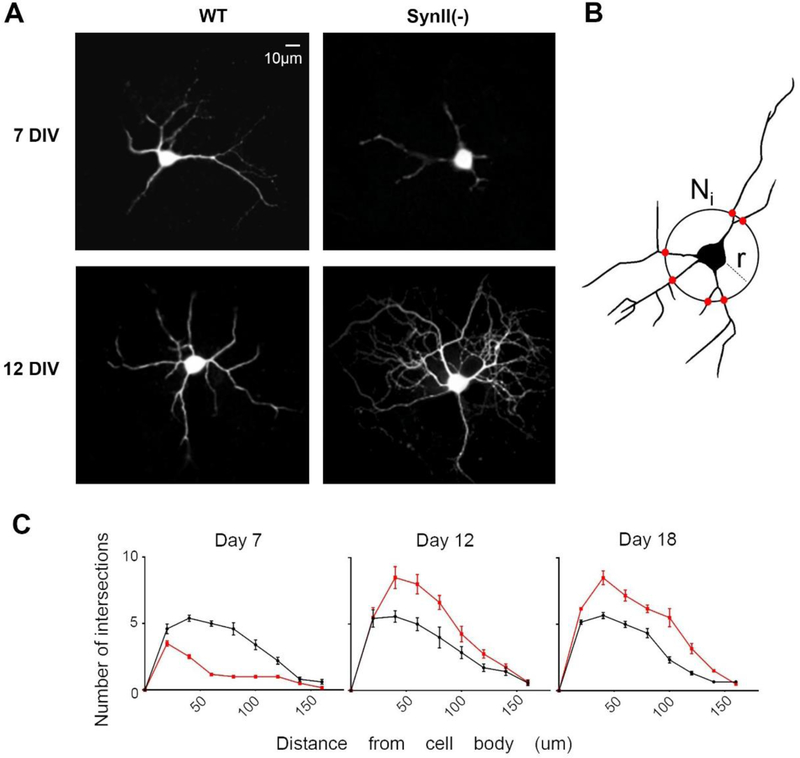
Finally, we tested whether the increase in synaptic activity could be driven by the neurite outgrowth. Visual examination after injection of Lucifer Yellow (Fig 5A) suggested that neurite branching may be reduced in SynII(−) neurons at initial developmental stages (7 DIV) and increased at subsequent developmental stages (12 DIV). To test this idea we quantitated neurite branching in SynII(−) neurons using Sholl analysis (Isaacs et al 1998, Sholl 1953) (Fig 5B). As illustrated in figure 5C, the number of intersections in SynII(−) neurons is reduced at 7 DIV but enhanced at 12–18 DIV (Fig 5 C). These results suggest that SynII(−) neurons exhibit enhanced neurite growth and arborization at 12–18 DIV when compared to WT controls.
Fig 5.
The neurite growth and arborization is inhibited in SynII neurons at 7 DIV and enhanced at 12–18 DIV. A. Representative images of WT and SynII neurons at 7 and 12 DIV. B. Sholl analysis: counting the number of intersections Ni as a function of radius r. C. The number of intersections is reduced in SynII(−) neurons at 7 DIV and increased at 12 and 18 DIV. Data collected from at least 6 cells (3 cultures) for each data point.
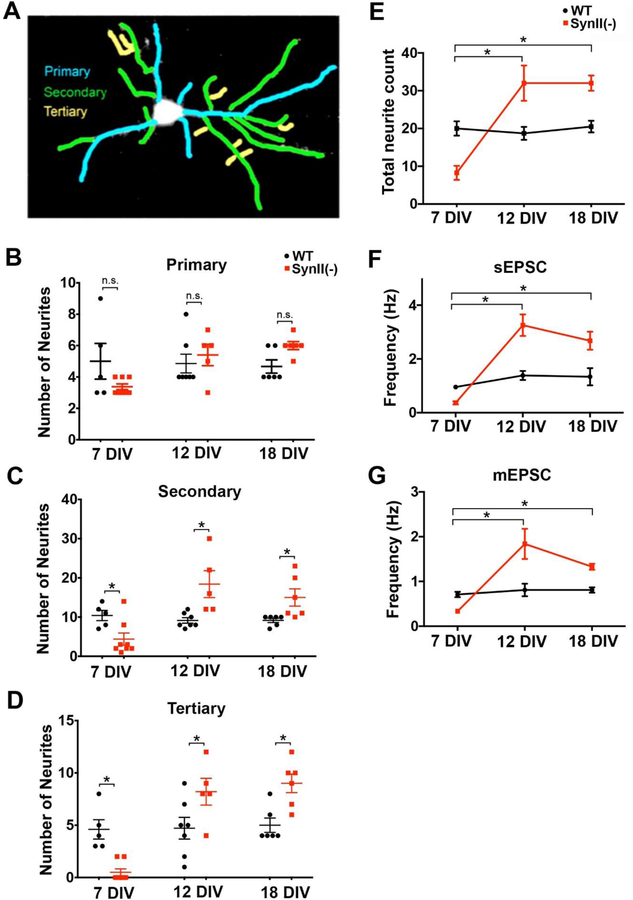
To further investigate the neurite growth and arborization, we counted the primary, secondary, and tertiary branches (Fig. 6A) in SynII(−) neurons at different developmental stages (7–18 DIV). The deletion of SynII had no detectable effect on primary neurites at either stage (Fig. 6 B). However, the numbers of secondary and tertiary neurons were significantly altered in SynII(−) neurons, and these alterations were dependent on the stage of development (Fig. 6B, C). In particular, at 7 DIV SynII deletion resulted in a significant reduction in secondary neurites (Fig. 6 C). Furthermore, tertiary neurites, while well represented in WT neurons, were almost non-existent in SynII(−) neurons (Fig. 6 D). In contrast, at 12 and 18 DIV the deletion of SynII significantly increased secondary and tertiary neurite numbers (Fig. 6 C, D). As a result, the total number of secondary and tertiary neurites (Fig. 6 C, D), as well the overall number of neurites (Fig. 6 E) was reduced at 7 DIV but increased at 12 and 18 DIV.
Fig 6.
SynII deletion affects the growth of secondary and tertiary neuronal branches. A. Quantification of primary (blue), secondary (green), and tertiary (yellow) neurites. B. Primary neurites are not affected by SynII deletion. C, D. In SynII(−) cultures, the number of secondary (C) and tertiary (D) neurites is decreased at 7 DIV but increased at 12 DIV and 18 DIV (D). E. The overall number of neurites in SynII(−) cultures is decreased at DIV7 (p = 0.0015) and increased at DIV12 and 18 (p = 0.012 and p = 0.001, respectively). F,G. The overall frequency of sEPSCs (F) and mEPSCs (G) in SynII(−) cultures is decreased at 7 DIV and increased at 12 and 18 DIV.
Since synaptic density was not affected by SynII deletion (Fig. 3), the total number of synapses per neuron would be directly dependent on neurite length and arborization. Therefore, the observed alterations in the neurite outgrowth could account for the altered glutamatergic activity in SynII(−) neurons (Fig. 6 F, G).
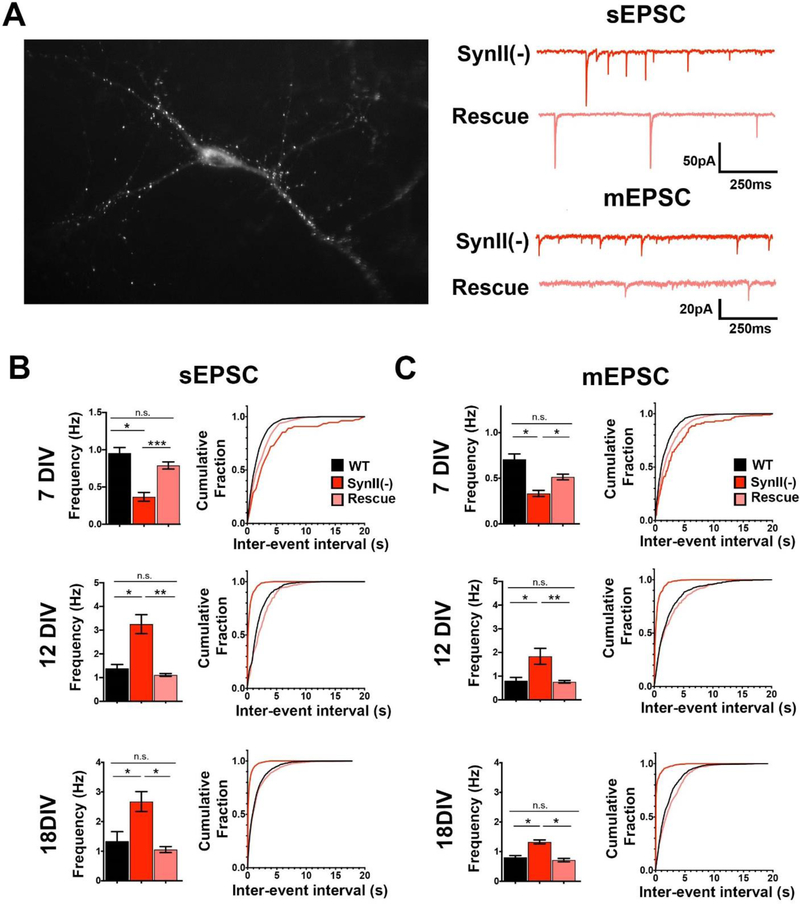
To ensure that the observed alterations in neuronal activity and neurite growth were directly produced by SynII deletion, we reinserted SynII in neuronal cultures derived from SynII(−) mice using an AAV expressing WT SynII fused to EGFP (Fig. 7A). First, we asked whether reintroduction of SynII would rescue spontaneous transmission in SynII(−) neurons. To address this question, we recorded sEPSCs and mEPSCs (Fig. 7B) from transfected CA1 pyramidal neurons in the presence of Ptx (50 µM). The average sEPSC and mEPSC frequencies and interevent intervals were compared with those obtained from WT and SynII(−) cultures (Fig. 7B, C pink bars and lines). The expression of SynII completely rescued both elevated sEPSC and mEPSC frequency at 12 and 18 DIV to the levels observed at WT. A partial rescue was also observed for the reduced activity at 7 DIV (Fig. 7 B, C top), although sEPSC and mEPSC frequencies at the rescued preparations remained slightly lower than in WT cultures (pink versus black).
Fig. 7.
Reintroducing SynII in SynII(−) neurons rescues altered synaptic activity in SynII(−) neurons. A. GFP fluorescence observed in neurons transfected with GFP-tagged SynII. . B. SynII reintroduction rescues the altered sEPSC frequency in SynII(−) cultures. C. SynII reintroduction rescues the altered mEPSC frequency in SynII(−) cultures. Frequencies of sEPSCs and mEPSCs: *p<0.05; ** p<0.01. Inter-event intervals. K-S test: WT versus SynII(−), p<0.001 for all the datasets; rescue versus SynII(−), p<0.01 for 7 DIV sEPSC and mEPSC, p<0.001 for all the other datasets; rescue versus WT, p<0.05 for 7 DIV sEPSCs and mEPSCs, p>0.05 for all the other datasets.
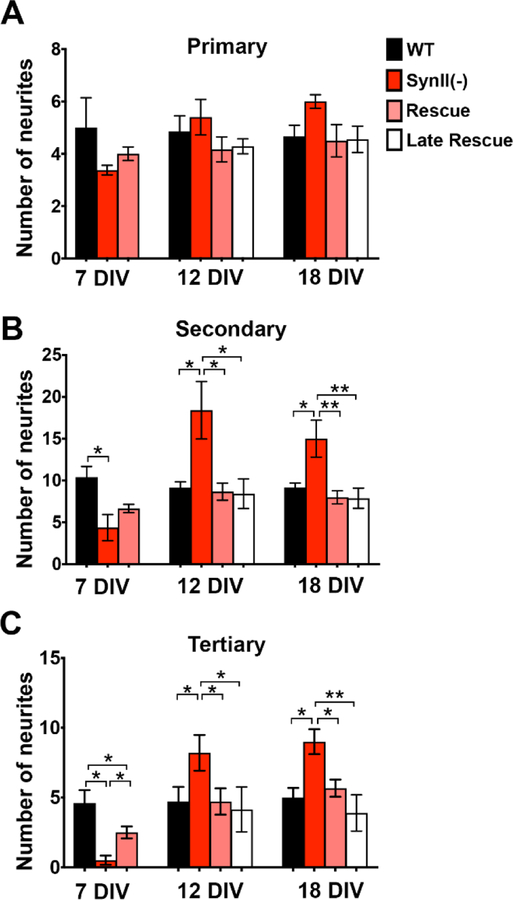
Next, we quantified the numbers of primary, secondary, and tertiary neurites in transfected neurons. As illustrated in figure 8 expression of SynII did not significantly change the number of primary neurites at 7DIV (Fig. 8A) but completely rescued the augmented growth of secondary and tertiary neurites seen at 12 and 18 DIV (Fig. 8 B, C). The rescue of the inhibited growth at 7 DIV was only partial as the number of tertiary neurites was significantly increased by SynII reintroduction, but not to the level observed in WT controls (Fig. 8 C), while the numbers of secondary neurites was increased only slightly and not significantly (Fig. 8 B). The latter result, together with the partial rescue of synaptic activity at 7 DIV (Fig. 7, B, C) could be explained by a partial gene expression at 7 DIV, which probably reached its full level by 12 DIV. Consistently, a full rescue of the neuronal growth was observed at 12 and 18 DIV (Fig. 8, B, C; 12 and 18 DIV).
Fig. 8.
Reintroducing SynII in SynII(−) neurons at either 2 DIV (Rescue) or 7 DIV (Late Rescue) rescues the enhanced neurite outgrowth in SynII(−) neurons at 12 and 18 DIV. A. The number of primary neurites is not affected by SynII reintroduction. B. SynII reintroduction at either 2 or 7 DIV rescues the increased numbers of neurites at 12 and 18 DIV to the WT level. C. SynII reintroduction at either 2 or 7 DIV rescues the increased numbers of neurites at 12 and 18 DIV to the WT level. In addition, the decreased number of tertiary neurites at 7 DIV is partially rescued. * p<0.05; ** p<0.01.
We next asked whether the enhanced neurite growth observed in SynII(−) cultures at 12–18 DIV was a direct effect of SynII deletion, or whether it represented an overcompensatory phenotype caused be the initial developmental delay. Our rescue data (Fig. 7, 8 pink bars) argues against the latter possibility, since the AAV transfection at 2 DIV produced only partial rescue of the initial developmental delay at 7 DIV, but fully rescued the enhanced outgrowth at 12 and 18 DIV. Nevertheless, to address this issue more conclusively, we performed “Late Rescue” experiments, transfecting SynII(−) neurons at 7 DIV. Notably, we found that the enhanced neurite growth and branching at 12–18 DIV was fully rescued by the delayed expression (Fig. 7, white bars). This result argues that the enhanced neurite growth and branching in SynII(−) neurons at 12–18 DIV is not produced by an overcompensation for the initial developmental delay in SynII(−) neurons, but instead directly results from SynII deletion.
Discussion
Our study demonstrated a novel, developmentally regulated function of the neuronal protein SynII in the control of neuronal growth and arborization. We show that SynII(−) neurons have a significantly increased number of secondary and tertiary neuronal branches and more extensive arborization at late developmental stages (12 and 18 DIV). In contrast, at early developmental stages (7 DIV), SynII(−) neurons have a defect in the neurite growth and arborization. We also show that SynII(−) neurons display reduced glutamatergic synaptic activity at 7 DIV but enhanced activity at 12 and 18 DIV, including an increased frequency of sEPSCs and mEPSCs.
It has been long recognized that synapsins are involved in growth, maturation, and differentiation of synaptic terminals (Valtorta et al 2011). The first evidence came from the study at differentiated glioma/neuroblastoma cells (Han et al 1991), which demonstrated that SynII overexpression promotes the formation of synaptic varicosities. Subsequently, it was shown that both synapsins promote the maturation of neuromuscular synapses (Schaeffer et al 1994, Valtorta et al 1995) and their deletion results in developmental retardation and delayed axon formation and elongation in hippocampus neurons (Ferreira et al 1994). Furtherm, it was shown that synapsins promote neurite growth in Xenopus embryonic cultures (Kao et al 2002). In line with these studies, we show here that neurite growth and arborization is compromised in SynII(−) cultured hippocampal neurons at 7 DIV. Importantly, we demonstrate that this defect is associated with a reduced glutametergic neuronal activity.
Strikingly, we also found that at subsequent developmental stages the neurite growth and arborization is enhanced in SynII(−) hippocampal cultures neurons. The mechanism of this phenomenon remains to be investigated. Since synapsins are expressed in neuronal cells as early as 3 DIV, and since synapsin levels gradually increase during development (Valtorta et al 2011), it is highly unlikely that altered expression levels of SynII could account for its reverted effect on neuronal growth at late developmental stages.
A more likely possibility is that SynII acts via the regulation of a cytomatrix remodeling. This hypothesis is supported by numerous studies demonstrating that synapsins interact with actin and other cytomatrix molecules and contribute to cytomatrix organization (Cesca et al 2010, Hilfiker et al 1999, Shupliakov et al 2011, Valtorta et al 2011). Interestingly, it was shown that polymerized actin could inhibit excessive neuronal growth and promote axonal retraction (Luo 2002). Therefore, it is a plausible hypothesis that SynII promotes neuronal growth and proliferation at initial development stages and inhibits it at later stages by stabilizing actin filaments at axonal terminal, and this in turn affects dendritic arborization.
Alternatively, SynII could control neuronal growth indirectly via modifications in neuronal networks produced by alterations in synaptic transmission, such as the decreased GABAergic release (Gitler et al 2008, Gitler et al 2004). We find this explanation unlikely, since at 7 DIV the neurite growth is inhibited in SynII(−) neurons, even though the GABAergic transmission is reduced (Fig. 1). Furthermore, a delayed transfection of SynII at 7 DIV (Fig. 8, late rescue) rapidly inhibits neuronal growth to WT levels, suggesting that SynII suppresses neuronal growth and branching directly. However, further experimentation is needed to concussively address the mechanism of the inhibition of neuronal growth by SynII.
Importantly, we demonstrated that spontaneous glutamatergic activity in SynII(−) hippocampal cultures is affected in a manner similar to the neuronal growth: sEPSC and mEPSC frequencies are inhibited at the initial developmental stages and promoted at subsequent developmental stages. It is likely that this enhanced glutamatergic activity may contribute to the developmental overexcitability and epileptic activity observed at the synapsin deficient neurons.
The association between the defects in neuronal activity and the epileptic phenotype produced by synapsin deficiency has been extensively studied (Fassio et al 2011b) but is not yet fully understood. Generation and characterization of synapsin deleted mice demonstrated that synapsin deficiency produces epileptic seizures (Li et al 1995, Rosahl et al 1995). Subsequently, a mutation in SYN1 gene has been discovered in a family with a history of epilepsy, autism, and mental retardation (Garcia et al 2004), while genetic studies identified SYN2 gene as contributing to epilepsy predisposition (Cavalleri et al 2007, Lakhan et al 2010). Finally, several loss-of-function mutations were found in the SYN1 gene in individuals with epilepsy and autism predisposition (Fassio et al 2011a).
The discovery that synapsins selectively promote GABAergic transmission (Gitler et al 2004) brought up a suggestion that the deficit in inhibition produces neuronal hyperexcitability and epileptic activity in the brain lacking synapsin. This hypothesis has received support from recent studies (Boido et al 2010, Chiappalone et al 2009, Feliciano et al 2013, Medrihan et al 2014), which have provided convincing evidence that epileptic activity in the synapsin-deficient brain is associated with a shift in the excitation/inhibition balance.
However, it has been also demonstrated that epileptic activity in synapsin deficient neurons critically depends on age-dependent modifications of neuronal networks (Ketzef & Gitler 2014, Ketzef et al 2011). Notably, the epileptic phenotype in synapsin deleted mice is age-dependent, and full epileptic seizures develop at the age of 2–3 months (Boido et al 2010, Etholm et al 2013). The delayed onset of epilepsy in synapsin deleted animals suggests that the initial deficit in inhibitory transmission may be followed by developmental alterations in the activity of neuronal networks, which lead to overexcitability and seizures (Ketzef & Gitler 2014). In support of this hypothesis, our study demonstrated that SynII deficiency selectively enhances neuronal growth and increases excitatory activity at late stages of the neuronal development. Notably, a recent study (Barbieri et al 2018) discovered that neurogenesis is inhibited in the brain of young SynII(−) mice and promoted in the brain of adult SynII(−) animals. Although the latter phenomenon could potentially result from seizure activity in adult symptomatic animals, the results of the present study argue that instead SynII deficiency promotes neuronal growth and proliferation directly at late developmental stages, and this in turn could produce epilepsy.
Acknowledgements:
Supported by the National Science Foundation grant IOS 1145010 to M.B.
References
- Barbieri R, Contestabile A, Ciardo MG, Forte N, Marte A, et al. 2018. Synapsin I and Synapsin II regulate neurogenesis in the dentate gyrus of adult mice. Oncotarget 9: 18760–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boido D, Farisello P, Cesca F, Ferrea E, Valtorta F, et al. 2010. Cortico-hippocampal hyperexcitability in synapsin I/II/III knockout mice: age-dependency and response to the antiepileptic drug levetiracetam. Neuroscience 171: 268–83 [DOI] [PubMed] [Google Scholar]
- Bykhovskaia M 2008. Making quantal analysis more convenient, fast, and accurate: user-friendly software QUANTAN. J Neurosci Methods 168: 500–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaia M 2011. Synapsin regulation of vesicle organization and functional pools. Semin Cell Dev Biol 22: 387–92 [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Weale ME, Shianna KV, Singh R, Lynch JM, et al. 2007. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case-control study. Lancet Neurol 6: 970–80 [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. 2010. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 91: 313–48 [DOI] [PubMed] [Google Scholar]
- Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, et al. 2009. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb Cortex 19: 1422–39 [DOI] [PubMed] [Google Scholar]
- Corradi A, Fadda M, Piton A, Patry L, Marte A, et al. 2014. SYN2 is an autism predisposing gene: loss-of-function mutations alter synaptic vesicle cycling and axon outgrowth. Hum Mol Genet 23: 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etholm L, Bahonjic E, Heggelund P. 2013. Sensitive and critical periods in the development of handling induced seizures in mice lacking synapsins: differences between synapsin I and synapsin II knockouts. Experimental neurology 247: 59–65 [DOI] [PubMed] [Google Scholar]
- Etholm L, Bahonjic E, Walaas SI, Kao HT, Heggelund P. 2012. Neuroethologically delineated differences in the seizure behavior of synapsin 1 and synapsin 2 knock-out mice. Epilepsy Res 99: 252–9 [DOI] [PubMed] [Google Scholar]
- Etholm L, Heggelund P. 2009. Seizure elements and seizure element transitions during tonic-clonic seizure activity in the synapsin I/II double knockout mouse: a neuroethological description. Epilepsy Behav 14: 582–90 [DOI] [PubMed] [Google Scholar]
- Farisello P, Boido D, Nieus T, Medrihan L, Cesca F, et al. 2013. Synaptic and extrasynaptic origin of the excitation/inhibition imbalance in the hippocampus of synapsin I/II/III knockout mice. Cereb Cortex 23: 581–93 [DOI] [PubMed] [Google Scholar]
- Fassio A, Patry L, Congia S, Onofri F, Piton A, et al. 2011a. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum Mol Genet 20: 2297–307 [DOI] [PubMed] [Google Scholar]
- Fassio A, Raimondi A, Lignani G, Benfenati F, Baldelli P. 2011b. Synapsins: from synapse to network hyperexcitability and epilepsy. Semin Cell Dev Biol 22: 408–15 [DOI] [PubMed] [Google Scholar]
- Feliciano P, Andrade R, Bykhovskaia M. 2013. Synapsin II and Rab3a cooperate in the regulation of epileptic and synaptic activity in the CA1 region of the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 33: 18319–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Han HQ, Greengard P, Kosik KS. 1995. Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proceedings of the National Academy of Sciences of the United States of America 92: 9225–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Kosik KS, Greengard P, Han HQ. 1994. Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science 264: 977–9 [DOI] [PubMed] [Google Scholar]
- Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, et al. 2004. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet 41: 183–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannandrea M, Guarnieri FC, Gehring NH, Monzani E, Benfenati F, et al. 2013. Nonsense-mediated mRNA decay and loss-of-function of the protein underlie the X-linked epilepsy associated with the W356x mutation in synapsin I. PLoS One 8: e67724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovedi S, Corradi A, Fassio A, Benfenati F. 2014. Involvement of synaptic genes in the pathogenesis of autism spectrum disorders: the case of synapsins. Front Pediatr 2: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Cheng Q, Greengard P, Augustine GJ. 2008. Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. The Journal of neuroscience : the official journal of the Society for Neuroscience 28: 10835–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, et al. 2004. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience 24: 11368–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship J, Garcia CC, Blair HJ, Seager M, Coulthard A, et al. 2003. Deficiency of synapsin I, a synaptic vesicle protein, causes epilepsy. J Med Genet 40: S21–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Manago F, Tucci V, Kao HT, Valtorta F, Benfenati F. 2013. Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res 251: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HQ, Nichols RA, Rubin MR, Bahler M, Greengard P. 1991. Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature 349: 697–700 [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. 1999. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354: 269–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Schweizer FE, Kao HT, Czernik AJ, Greengard P, Augustine GJ. 1998. Two sites of action for synapsin domain E in regulating neurotransmitter release. Nat Neurosci 1: 29–35 [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Hanbauer I, Jacobowitz DM. 1998. A method for the rapid analysis of neuronal proportions and neurite morphology in primary cultures. Experimental neurology 149: 464–7 [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. 2006. Culturing hippocampal neurons. Nat Protoc 1: 2406–15 [DOI] [PubMed] [Google Scholar]
- Kao HT, Song HJ, Porton B, Ming GL, Hoh J, et al. 2002. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci 5: 431–7 [DOI] [PubMed] [Google Scholar]
- Ketzef M, Gitler D. 2014. Epileptic synapsin triple knockout mice exhibit progressive long-term aberrant plasticity in the entorhinal cortex. Cereb Cortex 24: 996–1008 [DOI] [PubMed] [Google Scholar]
- Ketzef M, Kahn J, Weissberg I, Becker AJ, Friedman A, Gitler D. 2011. Compensatory network alterations upon onset of epilepsy in synapsin triple knock-out mice. Neuroscience 189: 108–22 [DOI] [PubMed] [Google Scholar]
- Lakhan R, Kalita J, Misra UK, Kumari R, Mittal B. 2010. Association of intronic polymorphism rs3773364 A>G in synapsin-2 gene with idiopathic epilepsy. Synapse 64: 403–8 [DOI] [PubMed] [Google Scholar]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, et al. 1995. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 92: 9235–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Czernik AJ, Popov S, Wang T, Poo MM, Greengard P. 1996. Expression of synapsin I correlates with maturation of the neuromuscular synapse. Neuroscience 74: 1087–97 [DOI] [PubMed] [Google Scholar]
- Luo L 2002. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol 18: 601–35 [DOI] [PubMed] [Google Scholar]
- Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F. 2013. Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun 4: 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrihan L, Ferrea E, Greco B, Baldelli P, Benfenati F. 2014. Asynchronous GABA Release Is a Key Determinant of Tonic Inhibition and Controls Neuronal Excitability: A Study in the Synapsin II−/−Mouse. Cereb Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad DK, Shaheen U, Satyanarayana U, Prabha TS, Jyothy A, Munshi A. 2014. Association of GABRA6 1519 T>C (rs3219151) and Synapsin II (rs37733634) gene polymorphisms with the development of idiopathic generalized epilepsy. Epilepsy Res 108: 1267–73 [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, et al. 1995. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375: 488–93 [DOI] [PubMed] [Google Scholar]
- Schaeffer E, Alder J, Greengard P, Poo MM. 1994. Synapsin IIa accelerates functional development of neuromuscular synapses. Proceedings of the National Academy of Sciences of the United States of America 91: 3882–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87: 387–406 [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Haucke V, Pechstein A. 2011. How synapsin I may cluster synaptic vesicles. Semin Cell Dev Biol 22: 393–9 [DOI] [PubMed] [Google Scholar]
- Valtorta F, Iezzi N, Benfenati F, Lu B, Poo MM, Greengard P. 1995. Accelerated structural maturation induced by synapsin I at developing neuromuscular synapses of Xenopus laevis. Eur J Neurosci 7: 261–70 [DOI] [PubMed] [Google Scholar]
- Valtorta F, Pozzi D, Benfenati F, Fornasiero EF. 2011. The synapsins: multitask modulators of neuronal development. Semin Cell Dev Biol 22: 378–86 [DOI] [PubMed] [Google Scholar]
- Vasin A, Zueva L, Torrez C, Volfson D, Littleton JT, Bykhovskaia M. 2014. Synapsin regulates activity-dependent outgrowth of synaptic boutons at the Drosophila neuromuscular junction. The Journal of neuroscience : the official journal of the Society for Neuroscience 34: 10554–63 [DOI] [PMC free article] [PubMed] [Google Scholar]