Abstract
The root of Chuanminshen violaceum is used as an important edible and medicinal plant in China. However, its leaves are generally considered byproducts, and therefore do not have a use. Thus, the phenolic compounds in the methanolic extracts (CVLMs) and the chemical characteristics of crude polysaccharides (CVLPs) from the leaves of C. violaceum and their in vitro antioxidant activities were explored. The results showed that chlorogenic acid and rutin were the major individual phenolic compounds in the leaves, which ranged from 1.22 ± 0.03 to 2.87 ± 0.04 mg/g DW, and from 2.25 ± 0.04 to 4.03 ± 0.05 mg/g DW, respectively. Meanwhile, the extraction yields of CVLPs from the leaves ranged from 4.73% to 5.41%. The CVLPs consisted of mannose, rhamnose, galacturonic acid, glucose, galactose, and arabinose, suggesting the existence of pectic polysaccharides. Furthermore, both CVLMs and CVLPs exhibited strong antioxidant activities. Chlorogenic acid and rutin were major contributors to the antioxidant activities of CVLMs, and the antioxidant activities of CVLPs were closely correlated to their α-1,4-D-galactosiduronic linkages. The results are beneficial for understanding the chemical properties and in vitro antioxidant activities of CVLMs and CVLPs. The leaves of C. violaceum have potential to be developed as natural antioxidants.
Keywords: Chuanminshen violaceum, phenolic compound, polysaccharide, chemical structure, antioxidant activity
1. Introduction
Generally, oxidative stress in the human body is related to various diseases/disorders, such as metabolic, neurodegenerative, cardiovascular, mitochondrial diseases, and even cancer [1]. Recently, it has been reported that antioxidants are effective in controlling the production of free radicals, and are also helpful in detoxifying activities [1,2]. Various synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butyl hydroquinone (TBHQ) have been commonly used to reduce reactive oxygen species (ROS) damage in many fields, such as the food industry and biomedicines [3,4,5]. However, their toxic and carcinogenic side effects, shown in animal models, are affecting their acceptability from consumers [6]. Thus, due to their relatively lower toxicity and side effects, there is an increasing demand for natural antioxidants from plants, such as phenolic compounds and polysaccharides [2,7,8,9].
Chuanminshen violaceum is a species of a monotypic genus in the family Umbelliferae [10], which is mainly an economic plant in four heavily cultivated regions, including Chengdu, Langzhong, Bazhong, and Guangyuan City, Sichuan Province, China. The root of C. violaceum has been used as an edible and medicinal plant for a long time by the local people [11]. Additionally, due to the high amounts of phenolic compounds and polysaccharides, and the strong antioxidant activities [8,11], C. violaceum is considered a natural source of antioxidants. However, the applications of C. violaceum are limited to its roots, and the leaves are usually regarded as byproducts. The leaves of C. violaceum are generally used as forage for livestock by local people, or may even be simply abandoned. In fact, about a half of a C. violaceum plant is composed of leaves, which suggests a high development potential. Furthermore, to the best of our knowledge, the bioactive compounds, such as phenolic compounds and polysaccharides, from the leaves of C. violaceum have seldom been studied. Phenolic compounds and polysaccharides from natural sources exert strong antioxidant activities [2,7,8]. Therefore, the study of phenolic compounds and polysaccharides, and their related in vitro antioxidant activities, from the leaves of C. violaceum is meaningful and important. Meanwhile, it is also helpful to better understand their chemical characteristics, and to increase the possibility of their use as natural antioxidants in industrial applications.
In the present work, the phenolic compounds in the methanolic extracts and the chemical characteristics of crude polysaccharides from the leaves of C. violaceum and their in vitro antioxidant activities were explored. Furthermore, in order to better understand the structure–bioactivity relationship of CVLPs, the correlations between their in vitro antioxidant activities and their glycosidic linkages were also studied.
2. Materials and Methods
2.1. Materials and Chemicals
Four batches of leaves of C. violaceum were collected from Chengdu, Langzhong, Bazhong, and Guangyuan City, Sichuan Province, China. Then, samples were dried and milled into powders. Subsequently, the powders were passed through a 60-mesh size screen and stored at −20 °C for further analysis.
Gallic acid, rutin, 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH), chlorogenic acid, Folin–Ciocalteu reagent, 2,4,6-tri(2pyridyl)-s-triazine (TPTZ), 2,2′-azino-Bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 1-phenyl-3-methyl-5-pyrazolone (PMP), sodium nitroprusside (SNP), phosphoric acid, sulfanilamide, 6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox), and monosaccharide standards (rhamnose, mannose, galacturonic acid, glucuronic acid, galactose, glucose, xylose, and arabinose) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of Methanolic Extracts and Crude Polysaccharides from the Leaves of C. violaceum
The methanolic extracts and crude polysaccharides from the leaves of C. violaceum were extracted according to our previous methods with some modifications [2,7]. In brief, 5.0 g of sample powders were extracted with 150 mL of 80% methanol (v/v) by an ultrasound processer (480 W, 24 KHZ, Kangshijie Ultrasonic Wave Tech., Dongguan, China) at 60 °C for 80 min. Then, centrifugation at 4000× g was performed for 20 min. The residues were collected for further extraction of crude polysaccharides, and the supernatant was subsequently concentrated by a rotary evaporator at 45 °C (RE-52AA, Yarong Company, Shanghai, China). Afterwards, the methanolic extracts from the leaves of C. violaceum (CVLMs) collected from Chengdu, Langzhong, Bazhong and Guangyuan were marked as CVLM-A, CVLM-B, CVLM-C and CVLM-D, respectively. Samples were stored at −20 °C for further HPLC analysis and antioxidant activity assay.
Then, the collected residues were used to extract the crude polysaccharides in C. violaceum (CVLPs). Briefly, the residues were extracted twice by 150 mL water at 95 °C for 2 h. After centrifugation (4500× g for 20 min), the supernatant was combined and collected. The combined extracts were concentrated under a vacuum at 60 °C. Then, 3.0 mg of heat-stable α-amylase (40,000 U/g, peak enzymatic activity at 80 °C) was added into mixtures to remove the starch at 80 °C for 2 h. Subsequently, the temperature was increased to 100 °C to inactivate the enzymes. Three volumes of 95% (v/v) ethanol were used for the precipitation of the crude polysaccharides in the supernatant at 4 °C overnight. After centrifugation (4500 × g for 20 min), the precipitations were dissolved in water and freeze dried. Afterwards, the CVLPs extracted from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong, and Guangyuan were marked as CVLP-A, CVLP-B, CVLP-C and CVLP-D, respectively, and then stored at −20 °C for further analysis.
2.3. Chemical Analysis of Methanolic Extracts from the Leaves of C. violaceum (CVLMs)
2.3.1. Determination of Total Phenolic Content and Total Flavonoid Content
Total phenolic content (TPC) and total flavonoid content (TFC) of CVLM-A, CVLM-B, CVLM-C and CVLM-D were determined by calorimetry according to our previous reported methods [12]. Afterwards, the TPC and TFC of CVLMs were expressed as mg GAE/g DW and mg RE/g DW, respectively.
2.3.2. HPLC Analysis of Individual Phenolic Compounds
The HPLC analysis of individual phenolic compounds of CVLM-A, CVLM-B, CVLM-C and CVLM-D were performed by our previous reported method [2]. An Agilent 1260 series HPLC (Agilent Technologies, Palo Alto, CA, USA) equipped with a diode-array detector (DAD, Agilent Technologies, Palo Alto, CA, USA) and a ZORBAX Eclipase XDB-C18 column (250 mm × 4.6 mm, 5 µm) was utilized for the analysis of each sample. The chromatographic separation was performed by a gradient elution with 0.5% (v/v) acetic acid solution (A) and acetonitrile (B) at 25 °C at a flow rate of 0.8 mL/min. Then, 20 µL of each sample was eluted as follows: 0 min, 5% B; 5 min, 5% B; 50 min, 5–20% B; 60 min, 20–70%; 65 min, 70–5% B. Identification of the major individual phenolic compounds of CVLMs was carried out by comparing retention times and absorption spectra of commercial standards. The identified compounds were quantified by using calibration curves of the standards (chlorogenic acid and rutin). Afterwards, the content of each individual phenolic compound was expressed as milligram per gram dry weight of a C. violaceum plant (mg/g DW).
2.4. Characterization of Crude Polysaccharides from the Leaves of C. violaceum (CVLPs)
2.4.1. Chemical Composition Analysis
The contents of total polysaccharides in CVLP-A, CVLP-B, CVLP-C, and CVLP-D were determined by phenol-sulfuric methods, while the glucose was used as a standard [13]. The contents of total uronic acids in CVLPs were determined by the m-hydroxydiphenyl method, while the galacturonic acid (GalA) was used as a standard [14]. Furthermore, the contents of total proteins in CVLPs were determined by Bradford’s method, while the bovine serum albumin was used as a standard [15].
2.4.2. Determination of Molecular Weights
The molecular weights (Mw) and polydispersities (Mw/Mn) of CVLP-A, CVLP-B, CVLP-C and CVLP-D were measured by high performance size exclusion chromatography coupled with multi angle laser light scattering and a refractive index detector (HPSEC-MALLS-RID, Wyatt Technology Co., Santa Barbara, CA, USA) according to our previously reported method with minor modifications [16]. The TSK-Gel GMPWXL (300 mm × 7.8 mm) was utilized for the separation of CVLPs at 30 °C. The dn/dc value of CVLPs was selected as 0.150 mL/g according to a previous study [17]. Meanwhile, the Astra software (version 7.1.3, Wyatt Technology Co., CA, USA) was utilized for data acquisition and analysis.
2.4.3. Determination of Constituent Monosaccharides
The constituent monosaccharides of CVLP-A, CVLP-B, CVLP-C and CVLP-D were analyzed by HPLC according to our previous methods with minor modifications [7]. In brief, 4.0 mg of each sample was hydrolyzed with 2.0 M trifluoracetic acid (TFA) at 95 °C for 6 h. Subsequently, the hydrolyzates were used for PMP derivatization. Meanwhile, a standard solution, including rhamnose, mannose, glucuronic acid, glucose, galacturonic acid, galactose, xylose, and arabinose, was also derivatized by PMP. Finally, an Agilent 1260 series LC system (Agilent Technologies, Palo Alto, CA, USA) coupled with a ZORBAX Eclipse XDB-C18 column (4.6 × 250 mm i.d. 5 µm) was utilized for the analysis of PMP derivatives. The mobile phase was a mixture of phosphate buffer solution (0.1 M, pH = 6.7) and acetonitrile (83:17, v/v). The flow rate and the wavelength of DAD were set at 1.0 mL/min and 245 nm, respectively.
2.4.4. Fourier Transform Infrared (FT-IR) Analysis
The FT-IR analysis of CVLP-A, CVLP-B, CVLP-C and CVLP-D was also performed by our previously reported method [7]. The Nicolet iS 10 FT-IR Spectrometer (Thermo Fisher scientific, Waltham, MA, USA) was used to record the IR spectra of CVLPs in the frequency range of 4000–500 cm−1.
2.5. Evaluation of In Vitro Antioxidant Activities of CVLMs
The DPPH radical scavenging activities, ABTS radical scavenging activities, and ferric-reducing antioxidant powers (FRAPs) of CVLM-A, CVLM-B, CVLM-C and CVLM-D were determined according to our previously reported methods [2,12]. Methanol was used as the blank control, and Trolox was used as the positive standard. Afterwards, the DPPH radical scavenging activities, ABTS radical scavenging activities, and FRAPs of each sample were expressed as µmol Trolox equivalent per gram of dry weight of C. violaceum plant (µmol Trolox/g DW).
2.6. Evaluation of In Vitro Antioxidant Activities of CVLPs
2.6.1. Determination of In Vitro Antioxidant Activities
The ABTS radical scavenging activities and nitric oxide (NO) radical scavenging activities of CVLP-A, CVLP-B, CVLP-C and CVLP-D were also determined according to our previous method [7]. Butylated hydroxytoluene (BHT) was used as the positive control. The ABTS and NO radical scavenging activities of CVLPs were measured at five different concentrations each, and the IC50 values (mg/mL) of CVLPs were calculated based on a logarithmic regression curve [7].
Furthermore, the FRAPs of CVLP-A, CVLP-B, CVLP-C and CVLP-D were determined according to our previous method [7]. Butylated hydroxytoluene (BHT) was used as the positive control, and the FRAPs of CVLPs were expressed as the absorbance at 593 nm.
2.6.2. Effects of Partial Acid Hydrolysis and Enzymatic Degradation on the In Vitro Antioxidant Activites of CVLPs
Partial acid hydrolysis of CVLP-A was performed by a previous method with minor modifications [18]. Briefly, 20.0 mg of each sample was hydrolyzed by 1.0 M trifluoroacetic acid at 90 °C for 4 h. After hydrolysis, the partial acid hydrolysates of CVLPs were evaporated to dryness at 60 °C under vacuum, and washed by methanol to remove the TFA. The dried hydrolysates were dissolved in pure water, and subsequently freeze dried. The dried sample was marked as CVLP-A2 and stored at −20 °C for further analysis.
Furthermore, enzymatic degradation of CVLP-A was also performed by a previous method with minor modifications [18]. Briefly, 4.0 mL of each sample (5 mg/mL) was mixed with 10 mg of pectinase (1 U/mg), and incubated at 40 °C for 10 h. Subsequently, the pectinase was inactivated at 90 °C for 1 h, and the mixture was centrifuged at 4000× g for 20 min. The supernatant was freeze dried, and the dried sample was marked as CVLP-A3 and stored at −20 °C for further analysis.
Afterwards, the in vitro antioxidant activities of CVLP-A2 and CVLP-A3 were determined according to the methods of Section 2.6.1.
2.7. Statistical Analysis
All experiments were conducted in triplicate, and data were expressed as means ± standard deviations. Origin 9.0 software (OriginLab Corporation, Northampton, Mass., USA) and S.P.S.S. 11.0 software (IBM Analytics, IBM, Armonk, NY, USA) were used for statistical analysis. Statistical significances were carried out by one-way analysis of variance (A.N.O.V.A.) and Duncan’s test. Values of p < 0.05 were considered as statistically significant.
3. Results and Discussions
3.1. Major Chemical Compositions of CVLMs
The total phenolic content (TPC), total flavonoid content (TFC) and the contents of major individual phenolic compounds identified in the leaves of C. violaceum are summarized in Table 1. Results showed that the TPC and TFC in the leaves of C. violaceum collected from different regions in China ranged from 8.02 ± 0.27 to 12.55 ± 1.01 mg GAE/g DW, and from 5.36 ± 0.22 to 7.97 ± 0.31 mg RE/g DW, respectively. Meanwhile, the TPC in the leaves of C. violaceum were higher than that of C. violaceum roots (7.88 mg GAE/g DW), and other commercial tea sources such as bitter gourd, Qingke, Camellia japonica L., Lilium brownie, Siraitia grosvenorii, Agadtacge rygisa, and Amomum villosum Lour [12,19,20,21]. The results suggest that the leaves of C. violaceum are rich sources of natural phenolic compounds. Furthermore, in order to better understand the major individual phenolic compounds in methanolic extractions from the leaves of C. violaceum, the HPLC-DAD analysis was performed according to a previously reported method [2]. Chlorogenic acid and rutin were identified as two major individual phenolic compounds in CVLMs according to the HPLC analysis. Meanwhile, the contents of chlorogenic acid and rutin in CVLM-A, CVLM-B, CVLM-C and CVLM-D ranged from 1.22 ± 0.03 to 2.87 ± 0.04 mg/g DW, and from 2.25 ± 0.04 to 4.03 ± 0.05 mg/g DW, respectively. The levels of chlorogenic acid and rutin are relatively higher than those of other rich sources of natural phenolic compounds, such as Camellia japonica, Bombaxceiba, Chaenomeles sinensis, Eriobotrya japonica, and Chrysanthemum morifolium [20]. Moreover, it has been reported that chlorogenic acid and rutin are related to various bioactivities, such as antioxidant, anti-cancer, and anti-diabetic activities [22,23,24].
Table 1.
TPC, TFC and major individual phenolic compounds in CVLMs from the leaves of C. violaceum collected from different regions.
| TPC (mg GAE/g DW) |
TFC (mg RE/g DW) |
Chlorogenic Acid (mg/g DW) | Rutin (mg/g DW) |
|
|---|---|---|---|---|
| CVLM-A | 9.33 ± 0.63c | 6.32 ± 0.36b | 1.89 ± 0.03c | 2.88 ± 0.01c |
| CVLM-B | 10.43 ± 0.71b | 6.05 ± 0.29b | 2.07 ± 0.02b | 3.03 ± 0.04b |
| CVLM-C | 12.55 ± 1.01a | 7.97 ± 0.31a | 2.87 ± 0.04a | 4.03 ± 0.05a |
| CVLM-D | 8.02 ± 0.27d | 5.36 ± 0.22c | 1.22 ± 0.03d | 2.25 ± 0.04d |
TPC, total phenolic content; TFC, total flavonoid content; CVLM-A, CVLM-B, CVLM-C and CVLM-D, methanolic extractions from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively. Values represent mean ± standard deviation, and superscripts a–d differ significantly (p < 0.05) among CVLMs collected from different regions. Statistical significances were determined by A.N.O.V.A.
3.2. Chemical Characterizations of CVLPs
3.2.1. Chemical Compositions of CVLPs
The extraction yields and chemical compositions of CVLP-A, CVLP-B, CVLP-C and CVLP-D are shown in Table 2. The extraction yields of CVLPs from the leaves of C. violaceum collected from different regions in China ranged from 4.73% to 5.41%. In addition, the contents of total polysaccharides in CVLPs ranged from 80.92% to 82.08%, while few proteins were detected in CVLPs (range from 1.99% to 2.73%). The results suggested that polysaccharides were major components of CVLPs. Furthermore, the levels of total uronic acids of CVLPs ranged from 22.03% to 30.96%. The relatively high amount of uronic acids in CVLPs suggested the existence of pectic-like polysaccharides in the leaves of C. violaceum [7,16,25]. Indeed, uronic acids in natural polysaccharides are believed to correlate with their various bioactivities, such as their antioxidant activity [7,25].
Table 2.
Extraction yields, chemical compositions, molecular weights (Mw) and polydispersities (Mw/Mn) of CVLPs.
| CVLP-A | CVLP-B | CVLP-C | CVLP-D | |
|---|---|---|---|---|
| Extraction yields (%) | 5.32 ± 0.22 a | 4.73 ± 0.14 b | 5.41 ± 0.27 a | 4.99 ± 0.13 b |
| Total polysaccahrides (%) | 81.08 ± 0.22 a | 80.92 ± 0.18 a | 82.08 ± 0.32 a | 81.83 ± 0.32 a |
| Total uronic acids (%) | 30.96 ± 0.22 a | 26.62 ± 0.17 c | 28.33 ± 0.31 b | 22.03 ± 0.49 d |
| Total Proteins (%) | 2.73 ± 0.11 a | 1.99 ± 0.16 a | 2.55 ± 0.25 a | 2.01 ± 0.15 a |
| Mw (×104 Da) | ||||
| Fraction 1 | 9.45 ± (3.25%) a | 7.12 ± (3.36%) c | 8.88 ± (3.17%) b | 5.37 ± (3.25%) d |
| Fraction 2 | 1.21 ± (3.19%) a | 1.01 ± (4.11%) a | 1.15 ± (3.15%) a | 1.16 ± (3.13%) a |
| Mw /Mn (polydispersity) | ||||
| Fraction 1 | 1.43 | 2.00 | 1.79 | 1.48 |
| Fraction 2 | 1.21 | 1.04 | 1.31 | 1.27 |
CVLP-A, CVLP-B, CVLP-C and CVLP-D, crude polysaccharides extracted from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively. Values represent mean ± standard deviation, and superscripts a–d differ significantly (p < 0.05) among CVLPs collected from different regions. Statistical significances were determined by A.N.O.V.A. and Duncan’s test.
3.2.2. Molecular Weights and Constituent Monosaccharides of CVLPs
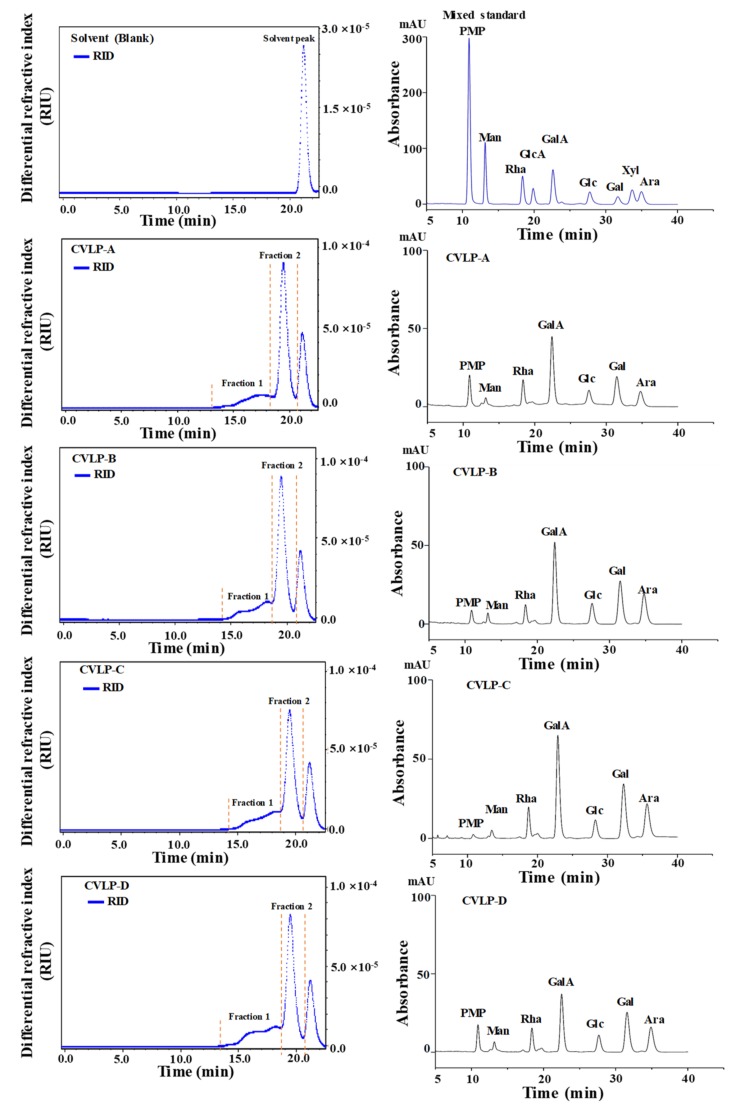
Generally, antioxidant activities of natural polysaccharides are correlated with their structural features, such as molecular weights and constituent monosaccharides [7,26]. Thus, it is necessary to analyze the structures of CVLPs, which is helpful for understanding their structure–bioactivity relationships. Therefore, the molecular weights and constituent monosaccharides of CVLPs were determined, and the results are summarized in Figure 1. As shown in Figure 1, two fractions of polysaccharides (fraction 1 and fraction 2) were detected in the CVLPs, while the fraction from 21 to 22 min was the solvent peak. Results indicated that CVLPs were heteropolysaccharides. Furthermore, Table 2 shows the detailed molecular weights of polysaccharide fraction 1 and 2 of CVLPs from the leaves of C. violaceum collected from different regions, which ranged from 5.37 × 104 to 9.45 × 104 Da, and from 1.01 × 104 to 1.21 × 104 Da, respectively. In addition, the polydispersities of polysaccharide fraction 1 and 2 ranged from 1.43 to 2.00, and from 1.04 to 1.31, respectively. Fraction 1 of CVLPs showed a relatively wider molecular weight distribution than that of fraction 2, which was in accordance with HPSEC chromatograms.
Figure 1.
High performance size exclusion chromatograms (left) and high-performance liquid chromatography profiles (right) of CVLPs. CVLP-A, CVLP-B, CVLP-C and CVLP-D, crude polysaccharides extracted from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively; PMP, 1-phenyl-3-methyl-5-pyrazolone; Man, mannose; Rha, rhamnose; GalA, galacturonic acid; Glc, glucose; Gal, galactose; Ara, arabinose.
The constituent monosaccharides of CVLPs from the leaves of C. violaceum collected from different regions were analyzed by the HPLC system. The HPLC-DAD profiles of CVLP-A, CVLP-B, CVLP-C, and CVLP-D are shown in Figure 1. Results indicated that the constituent monosaccharides of CVLPs consisted of mannose, rhamnose, galacturonic acid, glucose, galactose, and arabinose, which further confirmed that there are pectic-like polysaccharides in the leaves of C. violaceum. The molar ratios of constituent monosaccharides in CVLPs are shown in Table 3.
Table 3.
Molar ratios of constituent monosaccharides of CVLPs.
| Monosaccharides and Molar Ratios | ||||||
|---|---|---|---|---|---|---|
| Man | Rha | GalA | Glc | Gal | Ara | |
| CVLP-A | 1.00 | 3.03 | 6.54 | 4.55 | 6.39 | 3.11 |
| CVLP-B | 1.00 | 3.68 | 12.43 | 8.20 | 15.70 | 10.39 |
| CVLP-C | 1.00 | 5.29 | 14.19 | 6.45 | 17.68 | 10.48 |
| CVLP-D | 1.00 | 5.03 | 9.47 | 7.19 | 15.67 | 9.21 |
CVLP-A, CVLP-B, CVLP-C and CVLP-D, crude polysaccharides extracted from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively; Man, mannose; Rha, rhamnose; GalA, galacturonic acid; Glc, glucose; Gal, galactose; Ara, arabinose.
3.2.3. FT-IR spectra of CVLPs
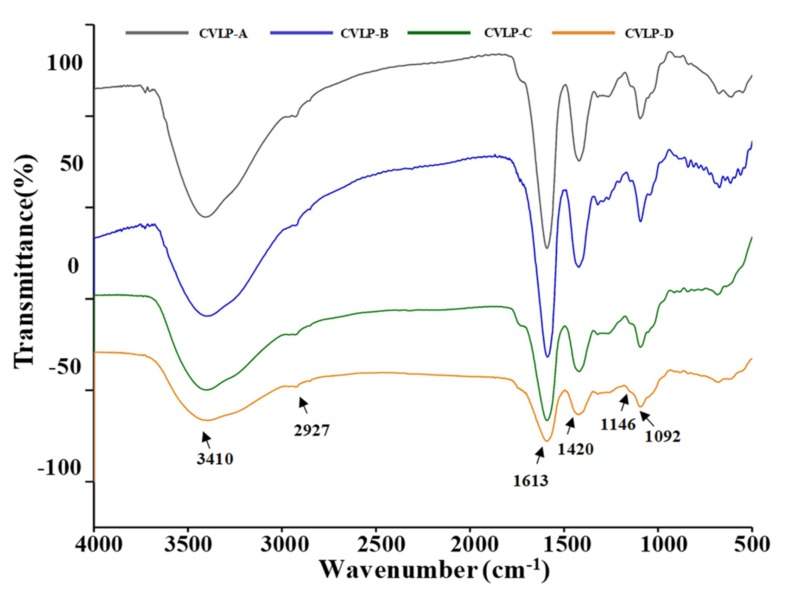
In order to investigate the chemical structures of CVLPs, the FT-IR spectrum between 4000 and 500 cm−1 was performed, and the results are shown in Figure 2. The intense and broad bands at 3410 cm−1 are characteristic of hydroxyl groups [27]. The peak at 2927 cm−1 was assigned to C–H asymmetric stretching vibration [28]. In addition, the peak at 1613 cm−1 was the C=O asymmetric stretching of –COO, which further confirmed that uronic acids existed in CVLPs [29,30]. The band at 1420 cm−1 was attributed to the bending vibration of C–H or O–H [31,32]. In addition, the band at 1146 cm−1 was the asymmetric C–O–C stretching vibration, suggesting the presence of –OCH3 [33]. No signal was detected at 1555 cm−1, which confirmed a very low amount of proteins in CVLPs [34].
Figure 2.
Fourier transform infrared (FT-IR) spectra of CVLPs. CVLP-A, CVLP-B, CVLP-C and CVLP-D, crude polysaccharides extracted from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively.
3.3. In Vitro Antioxidant Activities of CVLMs
3.3.1. In Vitro Antioxidant Activities
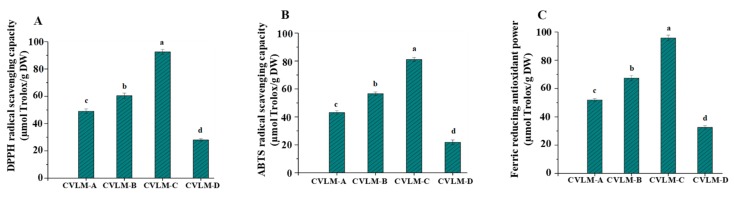
Generally, plants rich in phenolic compounds exert strong antioxidant activities [2,35,36,37]. In addition, many studies have shown that the leaves of various plants usually exhibit remarkable antioxidant activities due to their high amounts of phenolic compounds [38,39,40]. Thus, it is important to evaluate the in vitro antioxidant activities of leaves of C. violaceum. Therefore, the in vitro antioxidant activities, including DPPH radical scavenging activities, ABTS radical scavenging and FRAPs of CVLMs, in the leaves of C. violaceum collected from different regions in China were evaluated, and the results are summarized in Figure 3. As shown in Figure 3, the DPPH radical scavenging activities of CVLMs ranged from 28.02 ± 0.93 to 92.55 ± 1.81 µmol Trolox/g, the ABTS radical scavenging activities of CVLMs ranged from 21.81 ± 1.72 to 81.12 ± 1.39 µmol Trolox/g, and the FRAPs of CVLMs ranged from 32.56 ± 1.30 to 95.77 ± 2.01 µmol Trolox/g, respectively. In each test, the significantly (p < 0.05) highest in vitro antioxidant activity was found in CVLM-C, followed by lower activity in CVLM-B and CVLM-A, and the lowest activity in CVLM-D, which might be attributed to the levels of phenolic compounds in these plants [35,36,41]. The results showed that CVLMs exert strong in vitro antioxidant activities, which are higher than those of various herbs and plants, such as Astragalus membranaceus (ABTS: 9.12 ± 0.11 µmol Trolox/g), Agadtacge rygisa (ABTS: 15.07 ± 0.51 µmol Trolox/g), Amomum villosum Lour (ABTS: 67.67 ± 2.33 µmol Trolox/g), Camellia japonica L. (DPPH: 30.05 ± 2.37 µmol Trolox/g; ABTS: 17.62 ± 1.12 µmol Trolox/g; FRAP: 87.96 ± 1.28 µmol Trolox/g), Bombaxceiba (DPPH: 67.21 ± 3.82 µmol Trolox/g; ABTS: 39.09 ± 0.88 µmol Trolox/g; FRAP: 86.74 ± 0.94 µmol Trolox/g), Dianthus caryophyllus (DPPH: 51.13 ± 3.82 µmol Trolox/g; ABTS: 10.03 ± 1.05 µmol Trolox/g; FRAP: 48.55 ± 1.38 µmol Trolox/g), and Eriobotrya japonica (DPPH: 55.52 ± 4.40 µmol Trolox/g; ABTS: 50.14 ± 1.13 µmol Trolox/g; FRAP: 99.21 ± 1.03 µmol Trolox/g) [20,21]. Therefore, the high levels of antioxidant activities of CVLMs from the leaves of C. violaceum implies that they could be used as natural antioxidants in industrial applications.
Figure 3.
DPPH radical scavenging activities (A), ABTS radical scavenging activities (B) and ferric reducing antioxidant powers (C) of CVLMs. CVLM-A, CVLM-B, CVLM-C and CVLM-D, methanolic extractions from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively. Values represent mean ± standard deviation, and superscripts a–d differ significantly (p < 0.05) among CVLMs collected from different regions. Statistical significances were determined by A.N.O.V.A.
3.3.2. Correlations between In Vitro Antioxidant Activities and Phenolic Compounds of CVLMs
Pearson’s correlation coefficient analysis was carried out to investigate the relationships between the in vitro antioxidant activities and the methanolic extractions from the leaves of C. violaceum. As shown in Table 4, highly positive correlations were detected between the in vitro antioxidant activities (including DPPH radical scavenging activities, ABTS radical scavenging activities and FRAP) and TPC in the leaves of C. violaceum, and highly positive correlations were also detected between the in vitro antioxidant activities and TFC in the leaves of C. violaceum. Results were in accordance with some reports that TPC and TFC in plants are closely correlated with their in vitro antioxidant activities [22,42,43], suggesting that the phenolic compounds in the leaves of C. violaceum are the major contributors to their in vitro antioxidant activities. Furthermore, two major individual phenolic compounds (chlorogenic acid and rutin) in CVLMs were also positively correlated with their in vitro antioxidant activities. Meanwhile, results were in accordance with previous studies that chlorogenic acid and rutin were closely correlated with the in vitro antioxidant activities [42,44].
Table 4.
Pearson’s correlation coefficient among TPC, TFC, chlorogenic acid, rutin and in vitro antioxidant activities of the leaves of C. violaceum.
| TPC | TFC | Chlorogenic Acid | Rutin | DPPH | ABTS | FRAP | |
|---|---|---|---|---|---|---|---|
| TPC | 1.000 | ||||||
| TFC | 0.995 ** | 1.000 | |||||
| Chlorogenic acid | 0.953 ** | 0.984 ** | 1.000 | ||||
| Rutin | 0.954 ** | 0.968 ** | 0.994 ** | 1.000 | |||
| DPPH | 0.893 ** | 0.906 ** | 0.921 ** | 0.959 ** | 1.000 | ||
| ABTS | 0.903 ** | 0.902 ** | 0.905 ** | 0.936 ** | 0.935 ** | 1.000 | |
| FRAP | 0.892 ** | 0.900 ** | 0.914 ** | 0.976 ** | 0.944 ** | 0.959 ** | 1.000 |
TPC, total phenolic content; TFC, total flavonoid content; DPPH, DPPH radical scavenging activity; ABTS, ABTS radical scavenging activity; FRAP, ferric reducing antioxidant power; Correlation is significant at ** p < 0.01 level (tow-tailed), and the correlation relationships were analyzed by Pearson’s correlation coeffiecient analysis.
3.4. In Vitro Antioxidant Activities of CVLPs
3.4.1. In Vitro Antioxidant Activities
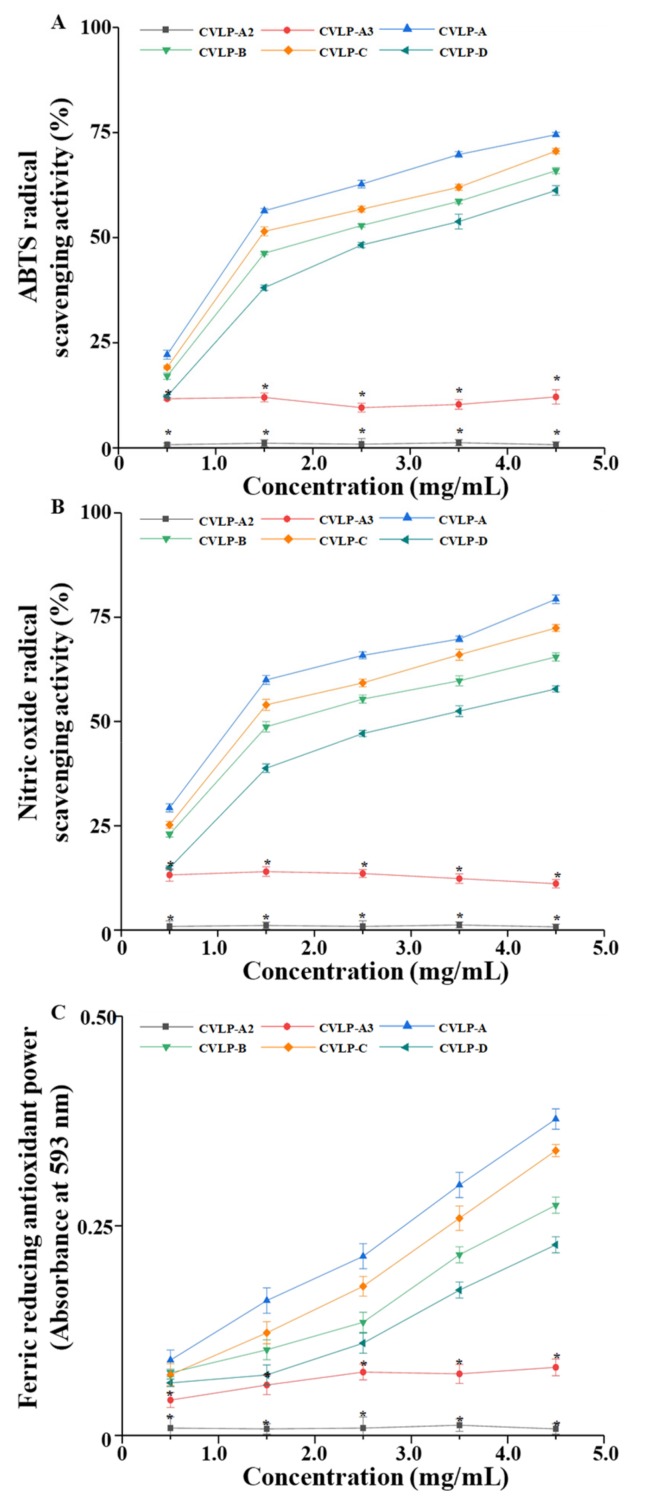
Recently, natural polysaccharides have drawn much interest as natural antioxidants due to their high antioxidant activities and low toxicity and side effects [7,8]. Previous studies have shown that polysaccharides in the roots of C. violaceum exhibit strong antioxidant activities [8,45]. However, the antioxidant activities of the leaves of C. violaceum have seldom been explored. Meanwhile, many polysaccharides extracted from leaves showed remarkable antioxidant activities [46,47,48]. Furthermore, in order to study the in vitro antioxidant activities of CVLPs and their possible structure–bioactivity relationships, it is necessary to use methods that are different from the methods used for CVLMs. Therefore, the in vitro antioxidant activities, including DPPH radical scavenging activities, NO radical scavenging activities, and FRAPs of CVLPs in the leaves of C. violaceum collected from different regions, were evaluated and are summarized in Figure 4A–C. The results showed the ABTS radical scavenging activities, NO radical scavenging activities and FRAPs of CVLPs exhibited a dose-dependent response. In addition, at the concentration of 4.5 mg/mL, the ABTS radical scavenging activities of CVLP-A, CVLP-B, CVLP-C and CVLP-D ranged from 61.22% to 74.50%. At the concentration of 4.5 mg/mL, the NO radical scavenging activities of CVLP-A, CVLP-B, CVLP-C and CVLP-D ranged from 57.82% to 79.31%. Additionally, at the concentration of 4.5 mg/mL, the FRAPs of CVLP-A, CVLP-B, CVLP-C and CVLP-D ranged from 0.22 to 0.38. As shown in Figure 4A–C, the significantly (p < 0.05) strongest in vitro antioxidant activities were found in CVLP-A, followed by weaker activities in CVLP-C and CVLP-B, and the weakest activity in CVLP-D. Indeed, the IC50 of ABTS radical scavenging activities of CVLP-A, CVLP-B, CVLP-C and CVLP-D were determined to be 1.47 mg/mL, 2.02 mg/mL, 1.84 mg/mL, and 2.80 mg/mL, respectively. In addition, the IC50 of NO radical scavenging activities of CVLP-A, CVLP-B, CVLP-C and CVLP-D were determined to be 1.18 mg/mL, 1.96 mg/mL, 1.54 mg/mL, and 2.97 mg/mL, respectively. Results further confirmed that CVLP-A exerted the strongest in vitro antioxidant activities among all tested samples, which might be attributed to its higher molecular weight and uronic acid content [3,39,40]. Furthermore, due to the existence of electrophilic groups, such as keto or aldehyde groups, in acidic polysaccharides, the liberation of hydrogen from O–H bonds is accelerated, and their radical scavenging activities are therefore improved [41]. Moreover, the IC50 of ABTS and NO radical scavenging activities of BHT (positive control) were measured to be 0.33 mg/mL and 0.27 mg/mL, respectively. Meanwhile, at the concentration of 1.0 mg/mL, the FRAP of BHT was determined to be 1.01. The results indicated that the in vitro antioxidant activities of CVLPs were lower than that of BHT. However, compared with the pectic-like polysaccharides extracted from other plants, such as Suaeda fruticosa [46], Ziziphus jujuba [49], Lycium barbarum [50], and okra [7], CVLPs showed stronger in vitro antioxidant activities, such as ABTS and NO radical scavenging activities. The results showed that CVLPs exhibited potent antioxidant activities, and have a strong potential for development as natural antioxidants for industrial applications.
Figure 4.
ABTS radical scavenging activities (A), nitric oxide (NO) radical scavenging activities (B) and ferric reducing antioxidant powers (C) of CVLPs and their acidic and enzymatic hydrolysates CVLP-A, CVLP-B, CVLP-C and CVLP-D, crude polysaccharides extracted from the leaves of C. violaceum collected from Chengdu, Langzhong, Bazhong and Guangyuan, respectively. CVLP-A2, partial acidic hydrolysates of CVLP-A; CVLP-A3, pectinase enzymatic hydrolysates of CVLP-A. Values represent mean ± standard deviation. Significant (p < 0.05) differences of ABTS radical scavenging activities, NO radical scavenging activities and FRAP between CVLP-A, CVLP-A2 and CVLP-A3 at the same concentration are shown by *; Statistical significances were determined by A.N.O.V.A. and Duncan’s test.
3.4.2. Correlations between the Glycosidic Linkage of CVLPs and Their In Vitro Antioxidant Activities
It has been reported that bioactivities, such as the antioxidant activities of natural polysaccharides, were highly correlated with their chemical structures, such as their glycosidic linkages [51,52,53]. Therefore, in order to better understand the structure–bioactivity relationship of CVLPs, the effects of glycosidic linkages on their antioxidant activities were investigated. As shown in Figure 4A–C, compared with CVLP-A, the ABTS radical scavenging activities, NO radical scavenging activities, and FRAPs of CVLP-A2 (the partial acidic hydrolysates of CVLP-A) barely existed. The results suggested that the polysaccharides fractions of CVLPs were the main contributors towards their in vitro antioxidant activities. However, considering the randomness of acidic hydrolysis of polysaccharides, the enzymatic degradation of CVLPs was performed to investigate the effects of the specific glycosidic linkages of CVLPs on their in vitro antioxidant activities. Meanwhile, according to the constituent monosaccharides of CVLPs, pectinase was selected to specifically degrade the α-1,4-D-galactosiduronic linkages in CVLPs. As shown in Figure 4A–C, compared with CVLP-A, the ABTS radical scavenging activities, NO radical scavenging activities and FRAPs of CVLP-A3 (the pectinase enzymatic hydrolysates of CVLP-A) decreased significantly, suggesting that its in vitro antioxidant activities were highly correlated with the α-1,4-D-galactosiduronic linkage of CVLPs. The results suggested that pectic-polysaccharides are the major contributors toward the in vitro antioxidant activity of CVLPs. Furthermore, the remaining in vitro antioxidant activities might be attributed to the existence of other types of polysaccharides in CVLPs [10,46]. In order to further reveal the structure–bioactivity relationships of CVLPs, the purified CVLPs will be further prepared to evaluate their structural features, and in vitro and in vivo antioxidant activities.
4. Conclusions
In this study, the major phenolic compounds in the methanolic extracts from the leaves of C. violaceum (CVLMs) collected from different regions were identified as chlorogenic acid and rutin, which ranged from 1.22 ± 0.03 to 2.87 ± 0.04 mg/g DW and from 2.25 ± 0.04 to 4.03 ± 0.05 mg/g DW, respectively. Meanwhile, the extraction yields of crude polysaccharides from the leaves of C. violaceum ranged from 4.73% to 5.41%. The CVLPs largely consisted of galacturonic acid, glucose, galactose, and arabinose, suggesting the existence of pectic-like polysaccharides. The results are beneficial for understanding the chemical properties of CVLMs and CVLPs. Furthermore, both CVLMs and CVLPs exhibited strong in vitro antioxidant activities. Chlorogenic acid and rutin were the major contributors to the in vitro antioxidant activities of CVLMs, and the in vitro antioxidant activities of CVLPs were closely correlated to their α-1,4-D-galactosiduronic linkages. The results suggest that the leaves of C. violaceum (byproducts) have great potential to be developed as natural antioxidants for industrial applications.
Author Contributions
Data curation, S.L., H.-Y.L. and D.-T.W.; Formal analysis, S.L., H.-Y.L., G.D., L.Z. and D.-T.W.; Funding acquisition, D.-T.W.; Investigation, S.L., H.-Y.L., Z.-Y.W., X.L., Y.Y. and L.Z.; Methodology, D.-T.W.; Project administration, W.Q.; Resources, X.L., Y.Y., Z.-W.C., G.D. and Q.Z.; Software, Z.-Y.W. and Q.Z.; Supervision, D.-T.W. and W.Q.; Validation, H.-Y.L. and Z.-W.C.; Writing—original draft, S.L.; Writing—review & editing, D.-T.W.
Funding
This work was supported by the Scientific Research Foundation of Sichuan Agricultural University (grant number 03120321) and the Scientific Research Fund Project of Science and Technology Department of Sichuan Province (grant number 2018JY0149).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Martins N., Barros L., Ferreira I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016;48:1–12. doi: 10.1016/j.tifs.2015.11.008. [DOI] [Google Scholar]
- 2.Li H.-Y., Yuan Q., Yang Y.-L., Han Q.-H., He J.-L., Zhao L., Zhang Q., Liu S.-X., Lin D.-R., Wu D.-T. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules. 2018;23:2957. doi: 10.3390/molecules23112957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh H.-J., Chung M.-S., Cho Y.-H., Kim J.-W., Kim D.-H., Han K.-W., Kim C.-J. Estimated daily intakes of butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tert-butyl hydroquinone (TBHQ) antioxidants in Korea. Food Addit. Contam. 2005;22:1176–1188. doi: 10.1080/02652030500195288. [DOI] [PubMed] [Google Scholar]
- 4.Dey A., Neogi S. Oxygen scavengers for food packaging applications: A Review. Trends Food Sci. Technol. 2019;90:26–34. doi: 10.1016/j.tifs.2019.05.013. [DOI] [Google Scholar]
- 5.Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Martelli G., Giacomini D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 2018;158:91–105. doi: 10.1016/j.ejmech.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Q., Lin S., Fu Y., Nie X.-R., Liu W., Su Y., Han Q.-H., Zhao L., Zhang Q., Lin D.-R., et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus) Int. J. Biol. Macromol. 2019;127:178–186. doi: 10.1016/j.ijbiomac.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Fan J., Feng H., Yu Y., Sun M., Liu Y., Li T., Sun X., Liu S., Sun M. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohydr. Polym. 2017;157:629–636. doi: 10.1016/j.carbpol.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Nirmala C., Bisht M.S., Bajwa H.K., Santosh O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018;77:91–99. doi: 10.1016/j.tifs.2018.05.003. [DOI] [Google Scholar]
- 10.Dong H., Zhang Q., Li L., Liu J., Shen L., Li H., Qin W. Antioxidant activity and chemical compositions of essential oil and ethanol extract of Chuanminshen violaceum. Ind. Crop. Prod. 2015;76:290–297. doi: 10.1016/j.indcrop.2015.04.051. [DOI] [Google Scholar]
- 11.Dong H., Lin S., Zhang Q., Chen H., Lan W., Li H., He J., Qin W. Effect of extraction methods on the properties and antioxidant activities of Chuanminshen violaceum polysaccharides. Int. J. Biol. Macromol. 2016;93:179–185. doi: 10.1016/j.ijbiomac.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 12.Lin S., Guo H., Gong J.D.B., Lu M., Lu M.Y., Wang L., Zhang Q., Wu D.T., Qin W. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018;81:69–75. doi: 10.1016/j.jcs.2018.04.001. [DOI] [Google Scholar]
- 13.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 14.Filisetticozzi T.M., Carpita N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991;197:157–162. doi: 10.1016/0003-2697(91)90372-Z. [DOI] [PubMed] [Google Scholar]
- 15.Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y., Yuan Q., Lin S., Liu W., Du G., Zhao L., Zhang Q., Lin D.-R., Liu Y.-T., Qin W., et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol. 2019;135:274–281. doi: 10.1016/j.ijbiomac.2019.05.157. [DOI] [PubMed] [Google Scholar]
- 17.Cheong K.-L., Wu D.-T., Zhao J., Li S.-P. A rapid and accurate method for the quantitative estimation of natural polysaccharides and their fractions using high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector. J. Chromatogr. A. 2015;1400:98–106. doi: 10.1016/j.chroma.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 18.Wu D.T., Cheong K.L., Deng Y., Lin P.C., Wei F., Lv X.J., Long Z.R., Zhao J., Ma S.C., Li S.P. Characterization and comparison of polysaccharides from Lycium barbarum in China using saccharide mapping based on PACE and HPTLC. Carbohydr. Polym. 2015;134:12–19. doi: 10.1016/j.carbpol.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 19.Kubola J., Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110:881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- 20.Chen G.-L., Chen S.-G., Xiao Y., Fu N.-L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018;111:430–445. doi: 10.1016/j.indcrop.2017.10.051. [DOI] [Google Scholar]
- 21.Li S., Li S.-K., Gan R.-Y., Song F.-L., Kuang L., Li H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013;51:289–298. doi: 10.1016/j.indcrop.2013.09.017. [DOI] [Google Scholar]
- 22.Riciputi Y., Diaz-De-Cerio E., Akyol H., Capanoglu E., Cerretani L., Caboni M.F., Verardo V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018;269:258–263. doi: 10.1016/j.foodchem.2018.06.154. [DOI] [PubMed] [Google Scholar]
- 23.Jeszka-Skowron M., Stanisz E., Peña M.P.D. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. LWT-Food Sci. Technol. 2016;73:243–250. doi: 10.1016/j.lwt.2016.06.018. [DOI] [Google Scholar]
- 24.Javed H., Khan M.M., Ahmad A., Vaibhav K., Ahmad M.E., Khan A., Ashafaq M., Islam F., Siddiqui M.S., Safhi M.M. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;210:340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Guo H., Yuan Q., Fu Y., Liu W., Su Y.-H., Liu H., Wu C.-Y., Zhao L., Zhang Q., Lin D.-R., et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow chrysanthemum (Coreopsis Tinctoria) Polymers. 2019;11:215. doi: 10.3390/polym11020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S., Xiong Q., Lai X., Li X., Wan M., Zhang J., Yan Y., Cao M., Lu L., Guan J. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food. Sci. Food Saf. 2016;15:237–250. doi: 10.1111/1541-4337.12161. [DOI] [PubMed] [Google Scholar]
- 27.Yu G., Duan Y., Fang G., Yan Z., Wang S. Polysaccharides from fruit calyx of Physalis alkekengi var. francheti: Isolation, purification, structural features and antioxidant activities. Carbohydr. Polym. 2009;77:188–193. [Google Scholar]
- 28.Wang R., Chen P., Jia F., Tang J., Ma F. Optimization of polysaccharides from Panax japonicus C.A. Meyer by RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2012;50:331–336. doi: 10.1016/j.ijbiomac.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Lin L., Xie J., Liu S., Shen M., Tang W., Xie M. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. Int. J. Biol. Macromol. 2017;99:665–673. doi: 10.1016/j.ijbiomac.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Liao J., Li C., Huang J., Liu W., Chen H., Liao S., Chen H., Rui W. Structure characterization of honey-processed Astragalus polysaccharides and its anti-inflammatory activity in vitro. Molecules. 2018;23:168. doi: 10.3390/molecules23010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L., Yu J.-Q., Wang X.-Y., Xu N., Liu J.-L. Microwave extraction optimization using the response surface methodology of Fructus Meliae Toosendan polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2018;118 Pt B:1501–1510. doi: 10.1016/j.ijbiomac.2018.06.172. [DOI] [PubMed] [Google Scholar]
- 32.Zheng W., Zhao T., Feng W., Wang W., Zou Y., Zheng D., Takase M., Li Q., Wu H., Yang L. Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus. Carbohydr. Polym. 2014;106:335–342. doi: 10.1016/j.carbpol.2014.02.079. [DOI] [PubMed] [Google Scholar]
- 33.Pereira P.H.F., Oliveira T.Í.S., Rosa M.F., Cavalcante F.L., Moates G.K., Wellner N., Waldron K.W., Azeredo H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016;88:373–379. doi: 10.1016/j.ijbiomac.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 34.Kpodo F.M., Agbenorhevi J.K., Alba K., Bingham R.J., Oduro I.N., Morris G.A., Kontogiorgos V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017;72:323–330. doi: 10.1016/j.foodhyd.2017.06.014. [DOI] [Google Scholar]
- 35.Peixoto C.M., Dias M.I., Alves M.J., Calhelha R.C., Barros L., Pinho S.P., Ferreira I. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018;253:132–138. doi: 10.1016/j.foodchem.2018.01.163. [DOI] [PubMed] [Google Scholar]
- 36.Pires T.C., Dias M.I., Barros L., Calhelha R.C., Alves M.J., Santos-Buelga C., Ferreira I.C. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crop. Prod. 2018;122:574–581. doi: 10.1016/j.indcrop.2018.06.046. [DOI] [Google Scholar]
- 37.Chung I.-M., Lim J.-J., Ahn M.-S., Jeong H.-N., An T.-J., Kim S.-H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alara O.R., Abdurahman N.H., Ukaegbu C.I., Azhari N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crops Prod. 2018;122:533–544. doi: 10.1016/j.indcrop.2018.06.034. [DOI] [Google Scholar]
- 39.Alara O.R., Abdurahman N.H., Ukaegbu C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants. 2018;11:12–17. doi: 10.1016/j.jarmap.2018.07.003. [DOI] [Google Scholar]
- 40.Chang Y.-L., Lin J.-T., Lin H.-L., Liao P.-L., Wu P.-J., Yang D.-J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019;289:74–83. doi: 10.1016/j.foodchem.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Stefanucci A., Zengin G., Locatelli M., Macedonio G., Wang C.-K., Novellino E., Mahomoodally M.F., Mollica A. Impact of different geographical locations on varying profile of bioactives and associated functionalities of caper (Capparis spinosa L.) Food Chem. Toxicol. 2018;118:181–189. doi: 10.1016/j.fct.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Amado I.R., Franco D., Sánchez M., Zapata C., Vázquez J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014;165:290–299. doi: 10.1016/j.foodchem.2014.05.103. [DOI] [PubMed] [Google Scholar]
- 43.López-Cobo A., Gómez-Caravaca A.M., Cerretani L., Segura-Carretero A., Fernández-Gutiérrez A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J. Food Compos. Anal. 2014;36:1–11. doi: 10.1016/j.jfca.2014.04.009. [DOI] [Google Scholar]
- 44.Nara K., Miyoshi T., Honma T., Koga H. Antioxidative activity of bound-form phenolics in potato peel. Biosci. Biotechnol. Biochem. 2006;70:1489–1491. doi: 10.1271/bbb.50552. [DOI] [PubMed] [Google Scholar]
- 45.Dong H., Zhang Q., Li Y., Li L., Lan W., He J., Li H., Xiong Y., Qin W. Extraction, characterization and antioxidant activities of polysaccharides of Chuanminshen violaceum. Int. J. Biol. Macromol. 2016;86:224–232. doi: 10.1016/j.ijbiomac.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Mzoughi Z., Abdelhamid A., Rihouey C., Le Cerf D., Bouraoui A., Majdoub H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018;185:127–137. doi: 10.1016/j.carbpol.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee P., Mukherjee S., Bera K., Ghosh K., Ali I., Khawas S., Ray B., Ray S. Polysaccharides from Thymus vulgaris leaf: Structural features, antioxidant activity and interaction with bovine serum albumin. Int. J. Biol. Macromol. 2019;125:580–587. doi: 10.1016/j.ijbiomac.2018.11.117. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Fang S., Zhou M., Shang X., Yang W., Fu X. Geographic variation in water-soluble polysaccharide content and antioxidant activities of Cyclocarya paliurus leaves. Ind. Crop. Prod. 2018;121:180–186. doi: 10.1016/j.indcrop.2018.05.017. [DOI] [Google Scholar]
- 49.Ji X., Peng Q., Yuan Y., Liu F., Wang M. Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018;108:541–549. doi: 10.1016/j.ijbiomac.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M., Wang F., Liu R., Tang X., Zhang Q., Zhang Z. Effects of superfine grinding on physicochemical and antioxidant properties of Lycium barbarum polysaccharides. LWT-Food Sci. Technol. 2014;58:594–601. doi: 10.1016/j.lwt.2014.04.020. [DOI] [Google Scholar]
- 51.Cui C., Lu J., Sun-Waterhouse D., Mu L., Sun W., Zhao M., Zhao H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT-Food Sci. Technol. 2016;73:602–608. doi: 10.1016/j.lwt.2016.07.005. [DOI] [Google Scholar]
- 52.Chen J., Zhang X., Huo D., Cao C., Li Y., Liang Y., Li B., Li L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019;215:307–315. doi: 10.1016/j.carbpol.2019.03.099. [DOI] [PubMed] [Google Scholar]
- 53.Chen L., Huang G. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. Int. J. Biol. Macromol. 2018;118:770–774. doi: 10.1016/j.ijbiomac.2018.06.148. [DOI] [PubMed] [Google Scholar]