Abstract
The association between adverse childhood experiences (ACEs) and pubertal timing has been a topic of enduring controversy. A systematic search of PubMed and Web of Science databases was undertaken to quantify the magnitude of total and specific forms of ACEs effects on early pubertal timing among girls. Our search identified 3280 records, of which 43 studies with 46 independent data sets met inclusion criteria. We estimated pooled effect sizes (Cohen’s ds) for the association between ACEs with early pubertal timing. Total ACEs was not associated with early pubertal timing. When we examined the specific types of ACEs, associations were small to medium for father absence (d = −0.40, 95% confidence interval [CI]: −0.63, −0.16) and small for sexual abuse (d = −0.13, CI: −0.17, −0.10) and family dysfunction (d = −0.08, CI: −0.11, −0.02). We identified considerable heterogeneity between estimates for almost all of the outcomes. ACEs exposure may affect female reproductive reproduction, particularly father absence, sexual abuse, and family dysfunction. We propose that future research in this area test a theoretical model linking adversity with earlier reproductive strategy, which includes early pubertal timing as a core component linking early adversity and stress physiology with poor health outcomes later in life in females.
Keywords: abuse, pubertal timing, adversity, adverse childhood experiences, meta-analysis
1. Introduction
The onset of puberty is pivotal for human growth and development [1]. However, among developed nations, there has been a secular trend of early pubertal timing over the past century, and in recent years, also in developing countries [2,3]. Early pubertal timing has been recognized as a risk factor for adverse health outcomes in adulthood including obesity, hyperinsulinemia, type-2 diabetes, hypertension, coronary heart disease, depression and early mortality [4,5,6,7,8]. Multiple inter-related social and environmental factors have been found to significantly influence pubertal timing, including nutrition and obesity, genetic factors, and general health status, race, and familial socioeconomic status (SES) [9].
Adverse childhood experiences (ACEs), defined as experiences that threaten the child’s bodily, familial, or social safety or security, classically include maltreatment and household dysfunction to more targeted experiences of bullying, exposure to crime, victimization, and economic disadvantage [10]. For the purposes of this meta-analysis, ACEs were defined as exposure to at least one significant form of the following childhood adversities: before the age of 18 years: physical abuse, sexual abuse, neglect (failure to provide and failure to supervise physically or emotionally), family dysfunction (parental death or serious illness, parental separation or divorce, parental incarceration, parental substance abuse, domestic violence, mental illness), low SES, and father absence (separation or divorce of the birth parents followed by absence of the birth father from the home).
ACEs are a major public health problem, with some estimates suggesting that about a third of the general population may be affected [11]. A growing number of studies have examined ACEs as risk factors for early pubertal timing, however, the association has been a topic of enduring controversy [3,9,12,13,14,15,16]. For example, Romans et al. [16] have found associations between father absence and early puberty whereas similar results were not observed by Henrichs et al. [9].
The life history theory provides a theoretical framework for explaining the individual differences in reproductive strategies from adolescence to adulthood, especially the role of family and ecological environment in regulating individual differences in puberty development and reproductive strategies. It attempts to explain the timing of reproductive development and events across the life span in terms of evolved strategies for distributing metabolic resources between the competing demands of growth, maintenance, and reproduction [17]. Specifically, there are environmental conditions in which accelerated maturational development is an adaptive response serving to maximize reproduction opportunities. Based on the life history theory, three middle-level life history models have been advanced to explain why ACEs may increase risk of early pubertal timing. First, the psychosocial acceleration theory, first advanced by Belsky, Steinberg, and Draper, 1991 postulates that ecological stressors in and around the family create conditions that undermine parental functioning and lower the quality of parental investment [18]. Under these conditions, parental investment is unreliable and/or not closely related to the reproductive success of offspring, girls are predicted to develop in a manner that accelerates pubertal maturation and sexual activity, and orients the individual towards unstable pair-bonds. Second, as a variant of the psychosocial acceleration theory, paternal investment theory parallels this logic but posits a more unique and central role for father and other men in the regulation of female pubertal timing, separate from the effects of other dimensions of psychosocial stress and support in the child’s environment [17]. The third theory is an extension on the psychosocial acceleration theory and paternal investment theory and is referred to as the child development theory. The child development theory describes how the composition and quality of family environments might influence the length of childhood as a developmental strategy. According to child development theory, the benefits of a longer childhood (delay puberty) are increased in high-quality family contexts that foster the development of sociocompetitive competencies, while the costs of cutting short childhood (accelerate puberty) are reduced in adverse family contexts that do not significantly facilitate these competencies. Children should be selected to take advantage of the benefits of high-quality parental investment and reduce the costs of low-quality parental investment by contingently altering the length of growth and development prior to reproductive maturity [17,19].
Activation of the hypothalamic-pituitary-adrenal (HPA) axis may be an underlying neuroendocrine mechanism linking ACEs and early pubertal timing, which governs both stress response and adrenarche, the first phase of pubertal maturation [20]. ACEs can alter the response of the HPA axis, with chronic stressful experiences resulting in hyperactivation followed by downregulation of the stress system. The attenuated cortisol profile may actually hasten the pubertal timing because the stress function of the HPA axis is dampened, allowing the cascade of pubertal hormones via the HPG axis to commence [21]. However, given the different forms of ACEs and the multiple indicators of HPA axis, more longitudinal studies are needed to understand the underlying mechanism better.
Variability in study design, methodology, subject characteristics, type of ACEs, and developmental timing limit the ability to draw definitive conclusions. The objective of this meta-analysis was to clarify the relationship between ACEs and early pubertal timing by means of a systematic examination of the literature, which may be beneficial for guiding future empirical and theoretical work in this area.
2. Materials and Methods
2.1. Search Strategy
The study was performed according to the recommendation of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [22]. A systematic database search from 1980 up to 2018 was performed on PubMed and Web of Science using the following search themes: (“ACE”, “ACEs”, “adverse childhood experiences”, “adverse childhood events”, “childhood maltreatment”, “child maltreatment”, “childhood adversity”, “child adversity”, “childhood adversities”, “child adversities”, “early adversity”, “early adversities”, “childhood physical abuse”, “child physical abuse”, “physical abuse”, “childhood sexual abuse”, “child sexual abuse”, “sexual abuse”, “childhood neglect”, “child neglect”, “childhood physical neglect”, “child physical neglect”, “physical neglect”, “child abuse”, “childhood abuse”; “child emotional abuse”, “childhood emotional abuse”, “emotional abuse”, “child emotional neglect”, “childhood emotional neglect”, “child trauma”, “childhood trauma”, “emotional neglect”, “parental incapacities”, “family incapacities”, “adverse family experiences”, “adverse family events”, “trauma”, “poverty”; “socioeconomic status”, “parental loss”, “adversity”, “early life stress”, “bullying”) combined with pubertal timing related search terms (“puberty”, “pubertal status”, “early puberty”, “early pubertal timing”, “age of menarche”, “early age of menarche”, “early maturation”, “pubertal timing”). In addition, we searched the bibliographies of the articles found on the subject [14,23]. The present analysis focused on childhood sexual abuse, physical abuse, neglect, father absence, low SES, family dysfunction. All the included studies were published in English and from peer-reviewed journals.
2.2. Inclusion Criteria and Exclusion Criteria
Selection criteria for the meta-analysis included articles exploring the association between ACEs and early pubertal timing. We excluded review articles, editorials, commentaries, and animal studies. The life history theory to pubertal timing pivots around the trade-off between the allocation of resources to physical growth versus production of offspring, which is particularly relevant to females. It has been applied more broadly and successfully to the question of female rather than male pubertal timing. In addition, there is a clear and easily assessed marker of female but not male pubertal timing: age at menarche [17]. Given the theoretical and empirical reasons, we included female participants only.
The majority of studies utilized age at menarche, Tanner staging scores, Pubertal Development Scale (PDS) and relative perceived timing questions to the evaluation of pubertal developmental. For studies using age at menarche, menarche occurring before the age of 11 years was considered as early pubertal timing, while after the age of 14 years as delayed pubertal timing. For those studies only reporting the correlation between ACEs with age at menarche, negative direction suggested that ACEs was associated with early pubertal timing. For studies using Tanner staging and the PDS, pubertal timing was then examined such that early pubertal timing were adolescents who scored two standard deviations above the mean and late pubertal timing were two standard deviations below the mean. Some studies also trichotomized pubertal timing groups, such that one standard deviation above the mean was categorized as early. Some studies used a single self-report item to assess relative perceived timing of puberty. Generally, this item was stated as follows “Compared with other boys/girls your age, would you say your physical development has developed ‘much earlier,’ ‘somewhat earlier,’ ‘about the same,’ ‘somewhat later,’ or ‘much later’ than other boys or girls your age?” Due to the considerable variation in the measurement method of pubertal timing, subgroup analysis was performed to examine significant sources of this heterogeneity [24].
2.3. Risk of Bias Assessment
Two reviewers independently assessed the methodology quality of included cohort studies using the Newcastle–Ottawa Scale (NOS) (range, 0 to 9 scores) [25]. Assessment items included the selection (four items, four scores), comparability (one item, two scores), and assessment of outcome (three items, three scores). Studies are then stratified by score: low quality (scored 0–3), medium quality (scored 4–6), and high quality (scored 7–9). The cross-sectional study quality was assessed using the Agency for Healthcare Research and Quality guidelines [26]. Studies are then stratified by score: low quality (scored 0–3), medium quality (scored 4–7), and high quality (scored 8–11). The third reviewer was involved when any disagreements existed. The quality scores did not affect studies for inclusion but were considered when performing sensitivity analysis and interpreting our research results.
2.4. Data Screening and Extraction
After duplicate citations were removed, two reviewers independently screened the titles and abstracts to identify potentially relevant citations. Then the potentially eligible studies were then screened again by full texts. The pre-designed criteria mentioned above were used to guide the entire process of screening. Subsequently, the following data were extracted from all the included studies using a pre-designed extraction form by two reviewers: (1) general information, including authors, publication year, (2) study design and specific types of ACE, (3) sample size, (4) the measure of ACEs, (5) the measure of early pubertal timing. The third reviewer was involved when any disagreements existed.
2.5. Effect Size Computation and Statistical Analyses
All analyses were carried out using the meta-analysis commands of Stata 11.0. For each study, we computed an effect size Cohen’s d [27] using the effect size calculator [28] for the association between ACEs and early pubertal timing. Cohen’s guidelines [27] were used to interpret effect sizes such that effects sizes of ds = 0.20 were considered small, ds = 0.50 were considered moderate, and ds = 0.80 were considered large. If correlations (r) or odds ratios (OR) were reported, they were converted to Cohen’s d using the formulas d = 2r/(1 − r2)1/2 [29] or d = 31/2/πlog (OR) [30], respectively. To examine the global association between ACEs and early pubertal timing, a meta-analysis was carried out on the effects extracted from studies providing a summary measure of exposure to ACEs [31]. Unadjusted values were calculated in the program, thus avoiding biases that may have arisen from adjustments to different confounders done in each study. Heterogeneity was calculated using the Q test and I2 statistic and the values of I2 metric 25%, 50%, and 75% were considered as low, medium, and high heterogeneity [32]. The effect of specific types of ACEs (i.e., sexual abuse, physical abuse, neglect, father absence, low SES, family dysfunction) were tested in this meta-analysis. Due to the large overlap, these analyses were treated as independent research syntheses and no attempt was made to statistically compare these effects using meta-regression or subgroup analyses. We did sensitivity analyses by excluding outlying studies. We explored risk of publication bias using the Egger tests when sufficient studies were included in the meta-analysis (at least 10 samples).
Planned meta-regressions and subgroup analyses were conducted to explore possible sources of heterogeneity. We did univariate analyses to test the individual association of the covariates (sample size, publication year) with pooled estimates. Subgroups were delineated using the following criteria: ACEs measure (questionnaire, interview, and combination), pubertal timing measure (age at menarche, number of early menarches, PDS, Tanner stage), original variable type (categorical, continuous and others), as well as study design (cross-sectional and longitudinal).
3. Results
3.1. Search Results
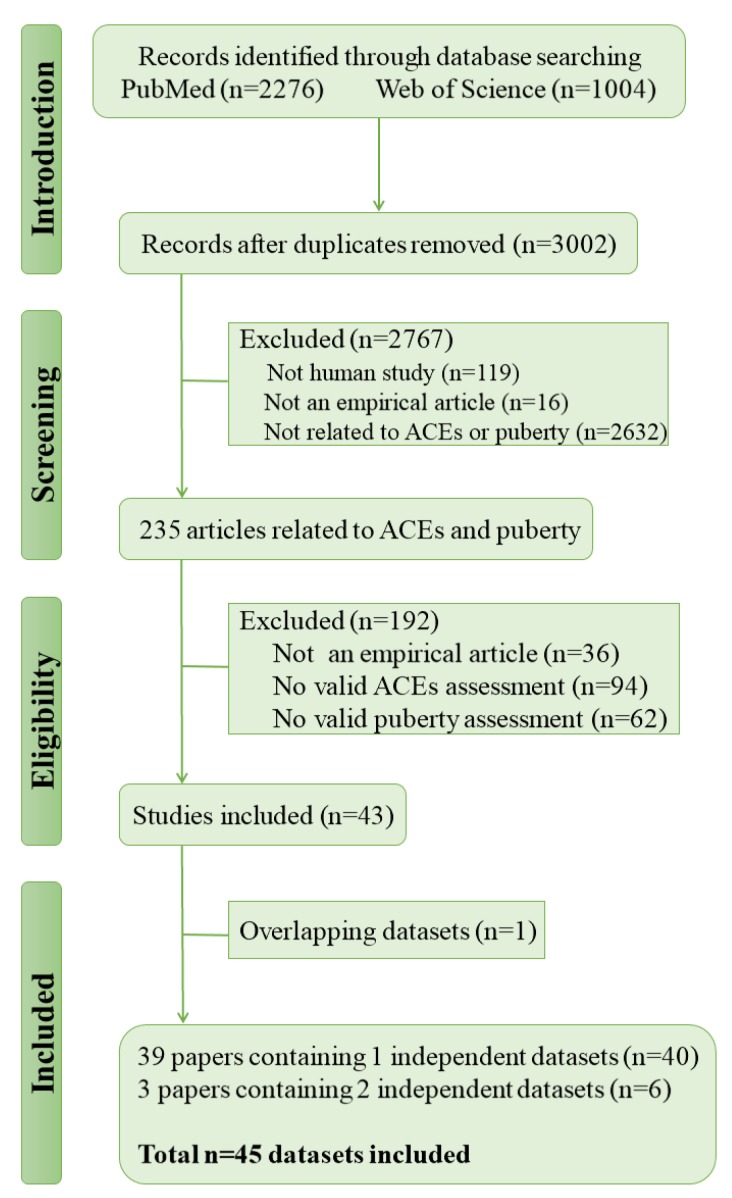
As illustrated in Figure 1, after duplicates removed, the search strategy resulted in 3002 records, of which 2767 were excluded following title and abstract, and a further 193 were excluded following review of the full text, 42 studies with 45 independent data sets met full inclusion criteria. Table 1 summarizes the characteristics of the eligible studies. 26 articles used data from cross-sectional studies and 16 used data from longitudinal studies.
Figure 1.
Flow chart of study selection.
Table 1.
Descriptive statistics and study characteristics for included studies.
| Study | Study Design | N | Age (Year) | ACEs Type | ACEs Measure | Puberty Measure |
|---|---|---|---|---|---|---|
| Abioye-Kuteyi EA (1997) [33] | C | 60 | 14.2 | low SES | questionnaire | AAM |
| Adalı T(2011) [34] | C | 2789 | 15–49 | low SES | combination | AAM |
| Al-Sahab B (2010) [35] | C | 1403 | 14–17 | low SES | questionnaire | NO. |
| Alvergne A (2008) [36] | C | 708 | 20.9 | FB | questionnaire | AAM |
| Amigo H (a) (2012) [37] | C | 127 | 8–16 | low SES | interview | AAM |
| Amigo H (b) (2012) [37] | C | 114 | 8–16 | low SES | interview | AAM |
| Asgharnia M (2009) [38] | C | 91 | 11–16 | low SES | combination | AAM |
| Barrios YV (2015) [39] | C | 1499 | 28 | PA, SA | questionnaire | NO. |
| Bleil ME (2013) [40] | C | 650 | 34.9 | FD | combination | AAM |
| Blell M (a) (2008) [41] | L | 94 | 49–51 | low SES | questionnaire | AAM |
| Blell M (b) (2008) [41] | L | 106 | 49–51 | low SES | questionnaire | AAM |
| Boynton-Jarrett R (2012) [42] | L | 4524 | 16 | PA, SA, neglect | combination | NO. |
| Boynton-Jarrett R (2013) [43] | L | 67,658 | 25–44 | abuse | questionnaire | NO. |
| Braithwaite D (a) (2009) [14] | L | 1091 | 18–19 | low SES | interview | NO. |
| Braithwaite D (b) (2009) [14] | L | 986 | 18–19 | low SES | interview | NO. |
| Buzney CD (2012) [44] | C | 70 | 19–25 | low SES | combination | AAM |
| Chavarro J (2004) [45] | C | 30 | 15−42 | low SES | questionnaire | AAM |
| Culpin I (2014) [46] | L | 3785 | 8–17 | FB | questionnaire | AAM |
| Deardorff J (2011) [23] | L | 444 | 8–10 | FB, low SES | interview | Tanner staging |
| Junqueira DLM (2003) [47] | C | 2053 | 9–19 | low SES | questionnaire | AAM |
| El Dosoky M (1997) [48] | C | 929 | 9–18 | low SES | questionnaire | AAM |
| Ersoy B (2004) [49] | C | 534 | 15.7 | low SES | combination | AAM |
| Graber JA (1995) [50] | L | 75 | 11.93 | FB, low SES | questionnaire | AAM |
| Henrichs KL (2014) [9] | C | 3288 | 45.7 | PA, SA, neglect, low SES, FB, FD | interview | NO. |
| Islam MS (2017) [3] | C | 680 | 14 | low SES | questionnaire | NO. |
| James-Todd T (2010) [51] | L | 237 | —a | low SES | questionnaire | AAM |
| Jorm AF (2004) [52] | C | 3702 | —a | PA, SA, neglect, FB | questionnaire | AAM |
| Kelly Y (2016) [53] | L | 5839 | 11.2 | low SES | interview | PDS |
| Magnus MC (2018) [54] | L | 8984 | 28.5 | Total adversity, SA, FD | questionnaire | AAM |
| Matchock RL (2006) [55] | C | 1896 | 20 | FB | questionnaire | AAM |
| Mendle J (2006) [56] | C | 1284 | 24.5 | FB, FD | interview | AAM |
| Mendle J (2016) [20] | L | 6273 | 28.7 | SA, PA, neglect | interview | AAM |
| Moffitt TE (1992) [57] | L | 416 | 15 | FB, low SES, FD | combination | AAM |
| Negriff S (2015) [15] | L | 213 | 8–13 | SA | questionnaire | PDS |
| Noll JG (2017) [12] | L | 173 | 6–16 | SA | interview | Tanner staging |
| Onat T (1995) [58] | L | 169 | 8.5–13.4 | low SES | questionnaire | AAM |
| Opare-Addo PM (2012) [59] | C | 720 | 7–17 | low SES | questionnaire | AAM |
| Romans SE (2003) [16] | C | 488 | 39.1 | low SES, PA, SA, FB | interview | NO. |
| Sun Y(2017) [60] | L | 1770 | 10–11 | low SES | questionnaires | PDS |
| Tahirovic HF (1998) [61] | C | 6077 | 8–17 | War | questionnaire | AAM |
| Tekgül N (2014) [62] | C | 61,293 | 15–49 | low SES | interview | AAM |
| Tither JM (2008) [63] | C | 136 | 16–44 | FB | questionnaire | AAM |
| Toromanović A (2015) [64] | C | 22,469 | 9–17.5 | FD | questionnaire | AAM |
| Vigil JM (2005) [65] | C | 616 | 26.9 | SA | questionnaire | AAM |
| Wise LA (2009) [66] | C | 35,330 | M = 38 | PA, SA | questionnaire | NO. |
L = longitudinal, C = cross-sectional, PA = physical abuse, SA = sexual abuse, FB = father absence, FD = family dysfunction, SES = socioeconomic status, AAM = age at menarche, NO. = number of participants with early menarche, PDS = Pubertal Development Scale. a Dashed cells indicate insufficient information available in study.
3.2. Association between Total ACEs and Early Pubertal Timing
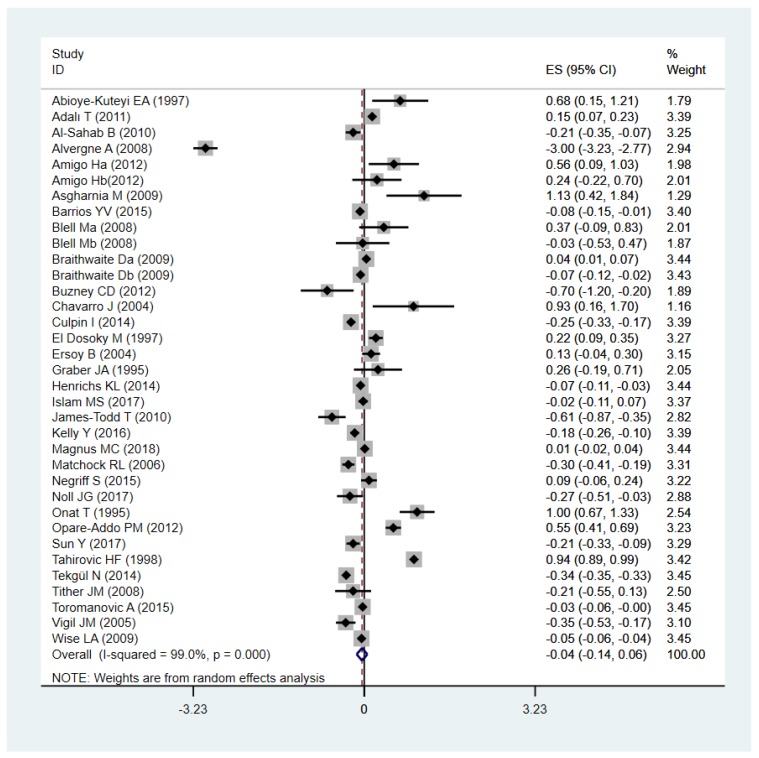
Figure 2 shows the association between total ACEs (n = 35) and early pubertal timing, with an effect of Cohen’s d: −0.04 (95% CI: −0.14, 0.06). The Q and I2 tests indicated that the association between total ACEs and early pubertal timing was statistically heterogeneous (I2 = 99%, p < 0.001), subgroup analyses were performed to examine significant sources of this heterogeneity. Egger’s test (B = 2.21, SE = 2.17, p = 0.32) showed no evidence of substantial publication bias. We examined possible changes in the effects by omitting one study at a time in the analysis but did not find any meaningful changes. Analyzing all studies except Tahirović HF et al. [61] on account of its indefinite type of ACEs, we obtained a significant results with an effect of Cohen’s d: −0.09 (95% CI: −0.17, −0.01), but the heterogeneity was still considerable (I2 = 98.3%, p < 0.001). We excluded Alvergne et al. [36], which had a huge outlier value, from the analysis. Using the random effect model, we got a pooled Cohen’s d: 0.04 (95% CI: −0.05, 0.14), suggesting that the association between total ACEs and delay puberty was still small and not significant.
Figure 2.
Forest plot of effect sizes reported as Cohen’s d (x-axis) evaluating adverse childhood experiences (ACEs) and early pubertal timing using the random effects model.
3.3. Associations between Specific Types of ACEs and Early Pubertal Timing
The pooled effect size of specific types of ACEs are presented in Table 2. Influence analyses were performed on each type of adversities, we did not find any meaningful changes. We found that the effect for sexual abuse (Cohen’s d −0.14 (95% CI −0.18, −0.11), I2 = 72.4, p < 0.001) and family dysfunction (Cohen’s d −0.08 (95% CI −0.11, −0.04), I2 = 66.9, p = 0.001) was small, while father absence (Cohen’s d −0.40 (95% CI −0.63, −0.16), I2 = 98.2, p < 0.001) was between small to medium. When we excluded Alvergne et al. [36], the effect size of father absence was reduced by nearly half (Cohen’s d −0.19 (95% CI −0.27, −0.11), I2 = 83.2, p < 0.001). We did not observe significant associations in other types of ACEs, or across total ACEs.
Table 2.
Pooled effect size of specific types of ACEs.
| Types of Adversity | K | Cohen’s d (95% CI) | I2 (%), p Value |
|---|---|---|---|
| Sexual abuse | 12 | −0.14(−0.18, −0.11) | 72.4, <0.001 |
| Physical abuse | 8 | −0.03 (−0.07,0.01) | 91.5, <0.001 |
| Neglect | 4 | 0.02 (−0.1,0.14) | 72.8, 0.011 |
| Low SES | 25 | 0.07 (−0.03,0.18) | 97.4, <0.001 |
| Father absence | 12 | −0.40 (−0.63, −0.16) | 98.2, <0.001 |
| Family dysfunction | 11 | −0.08 (−0.11,−0.04) | 66.9, 0.001 |
Data in parentheses are 95% Cis, ES = effect size (Cohen’s d), SES = socioeconomic status.
Except for physical abuse, neglect and low SES, the association between ACEs and early pubertal timing was small but significant, and the pooled effect size was highest for father absence and the least for family dysfunction. We found considerable heterogeneity between estimates for almost all of the outcomes. Heterogeneity between estimates was higher for father absence and lower for sexual abuse, relatively. Meta-regression revealed that the publication years (p = 0.083) and sample size (p = 0.661) in the primary studies did not influence the observed effect sizes.
Subgroup analyses are presented in Table 3. After subgroup analysis by ACEs measure (questionnaire, interview and combination), pubertal timing measure (age at menarche, number of early menarche, PDS, Tanner stage), original variable type (categorical, continuous and others), as well as study design (cross-sectional and longitudinal), heterogeneity between estimates is still considerable. In the subgroup analysis by pubertal timing measure, the pooled effect size was small but significant in studies measuring pubertal timing by a number of early menarche and Tanner stage. Pooled effect size of one study measuring pubertal timing by Tanner stage was Cohen’s d: −0.27 (95% CI: −0.52, −0.03) [12]. Similarly, in subgroup analysis by original variable type, the pooled effect size for categorical was small but significant.
Table 3.
Subgroup analyses of Associations between ACEs and Early Pubertal Timing.
| Moderator | Cohen’s d | 95% Confidence Interval | p-Value | I2 (%) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| ACEs measure | |||||
| questionnaire | −0.06 | −0.22 | 0.10 | 0.493 | 99.1 |
| interview | −0.07 | −0.23 | 0.09 | 0.382 | 98.7 |
| combination | 0.03 | −0.14 | 0.20 | 0.728 | 86.3 |
| Puberty measure | |||||
| No. of early menarche | −0.050 | −0.090 | −0.011 | 0.012 | 81.3 |
| PDS | −0.109 | −0.268 | 0.050 | 0.180 | 82.1 |
| Tanner staging | −0.270 | −0.515 | −0.025 | 0.031 | — |
| age at menarche | 0.013 | −0.172 | 0.199 | 0.889 | 99.3 |
| Original variable type | |||||
| categorical | −0.073 | −0.114 | −0.031 | 0.001 | 82.1 |
| continuous | −0.011 | −0.254 | 0.233 | 0.931 | 99.5 |
| others | 0.098 | −0.137 | 0.333 | 0.415 | 65.3 |
| Study design | |||||
| cross-sectional | −0.050 | −0.194 | 0.094 | 0.492 | 99.3 |
| longitudinal | −0.051 | −0.141 | 0.038 | 0.262 | 91.4 |
Cohen’s d effect sizes coded such that more negative values reflect a stronger association between early pubertal timing and ACEs. No. of early menarche = number of participants with early menarche.
4. Discussion
To our knowledge, this is the first meta-analysis to examine the association between ACEs and early pubertal timing and has extended previous studies by quantifying the effect size for early pubertal timing. Our finding suggests that sexual abuse, father absence, and growing up with challenging family circumstances were significantly associated with early pubertal timing among girls. Using Cohen’s categorization, the effect for sexual abuse and family dysfunction is small, while father absence is between small to medium. However, similar associations were not observed in other types of ACEs, or across total ACEs.
Herman-Giddens et al. [67] first reported in 1997 that child sexual abuse increased the risk of girls’ pubic hair and breast development before age 8. Subsequent studies have consistently found that sexual abuse was associated with early pubertal timing [16,42,43]. Recently, a cohort study of the Avon Longitudinal Study of Parents and Children (ALSPAC) mothers showing that total ACEs was not associated with age at menarche, while childhood sexual abuse was associated with lower age at menarche, which is consistent with our findings [54]. Sexual abuse is often regarded as the most prevalent health problem children face with the most serious array of consequences, while in our study, early pubertal timing may be more affected by father absence instead of sexual abuse.
As early as the 1980s to the 1990s, Draper and Harpending [68] and Belsky et al. [18] began to focus on the association between father absence and reproductive behaviors in adulthood, which has received increased attention over recent years. Majority of these studies have indicated a significant association between father absence and early pubertal timing. A study from a large UK birth cohort (ALSPAC) demonstrated that girls from father-absent homes had earlier menarche than girls from father-present homes [46]. The observed association between father absence and early pubertal timing has also been supported by other articles [20,23]. Moreover, the earlier that father absence occurs, the earlier girls tend to experience puberty, there is evidence to suggest that exposure to father absence during the first five years of life is more strongly associated with early menarche than father absence later in childhood [24].
It has been previously argued that the effects of age at menarche may be explained by low SES arising as a result of father absence, for example, decrease in family income, major financial problems [69]. However, in this meta-analysis, we did not find a significant association between low SES and early pubertal timing. When we excluded studies in which pubertal timing measured by Tanner stage or PDS [12,15,23,60], results suggested that girls living in low SES households may experience delayed onset of menarche (Cohen’s d: 0.12, 95% CI: 0.003, 0.25). A longitudinal study on 1091 black and 986 white girls from the three sites in the United States found that high SES was a protective factor for the risk of early menarche among white girls but opposite among black girls [14]. Given the challenges in methodology and definition of SES along with socially heterogeneous of study populations, it is difficult to draw firm conclusions concerning the relationship between low SES and early pubertal timing.
The association between family dysfunction and early pubertal timing was weak, we suspect that family dysfunction may be a proxy variable that represents a range of other family characteristics (including parental divorce, separation, mental illness, and family conflict) and have an effect similar to father absence. However, when we excluded three articles involving parental divorce or separation, the effect size was small but still significant. The effect of total ACEs on adolescent development was not significant. One explanation may be that there are great individual differences in the types, timing, duration, and severity of ACEs. Another explanation is that the association may differ depending on race/ethnicity. As demonstrated in prior research, high SES was a protective factor for the risk of early menarche among white girls but opposite among black girls [14].
Overall, despite some inconsistencies across specific forms of ACEs with early pubertal timing, these results support that ACEs exposure may affect female reproductive function. Given the association between ACEs and early pubertal timing, clinicians should sensitively enquire about past childhood experiences, and tailor support accordingly, thus resetting their trajectory for lifelong health and reducing the risk of chronic diseases and conditions.
There are also several limitations. Firstly, most of the measurements relied on retrospective self-report which is subject to recall or reporting biases. Secondly, this sample was restricted to females, and these findings cannot be extrapolated to males. In addition, the analysis revealed substantial statistical heterogeneity, which allows for less confidence in the estimated effect sizes, but is not surprising given the methodological and analytic variances in the identified studies. We cannot rule out the effect of interactions of ACEs with other factors (parental early puberty history, physical activity, etc.) in the current meta-analysis because most studies did not adjust for these interactions, or partly adjusted for these factors as possible moderators. The current meta-analysis focused on specific types of ACEs (sexual abuse, physical abuse, neglect, father absence, low SES, and family dysfunction). Nevertheless, adversity is a heterogeneous concept (including types of ACEs not considered here, such as bullying, exposure to war, and natural disasters, etc.). Although most studies measured ACEs at any point in childhood or adolescence, we were unable to obtain sufficient information about the frequency, timing, and duration of ACEs. Additionally, studies without sufficient statistical information for the computation of effects were not included in our analysis. Nevertheless, the thorough search of literature ensures a full coverage of the existing evidence and makes the results more convincing. Moreover, the large sample of the study population derived from the included studies enhances the possibility of effect detection with reasonable statistical power.
These limitations also suggest some directions for future research. Identification of distinct dimensions, duration and sensitive periods of adverse childhood experience that differentially influence pubertal development are required to uncover mechanisms that explain how childhood adversity is associated with female reproductive strategies.
5. Conclusions
In summary, our analyses indicate that father absence is more strongly related to early pubertal timing among girls, followed by sexual abuse and family dysfunction. Additional research is needed to better understand the mechanisms driving these relationships. Due to the high heterogeneity, a conclusive statement on the effect size of the association between ACEs and early pubertal timing should be made with caution.
Author Contributions
Y.S. and L.Z. contributed to the conception and design of the review. L.Z. applied the search strategy. L.Z. and D.Z. applied the selection criteria. L.Z. and D.Z. completed the assessment of risk of bias. L.Z. and Y.S. analyzed the data and interpreted data. L.Z. and Y.S. drafted this manuscript. All authors edited this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81872638] and Natural Science Foundation for Distinguished Young Scholars of Anhui Province [grant number 1908085J26].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Perry J.R., Murray A., Day F.R., Ong K.K. Molecular insights into the aetiology of female reproductive ageing. Nat. Rev. Endocrinol. 2015;11:725–734. doi: 10.1038/nrendo.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sørensen K., Mouritsen A., Aksglaede L., Hagen C.P., Mogensen S.S., Juul A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm. Res. Paediatr. 2012;77:137–145. doi: 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- 3.Islam M.S., Hussain M.A., Islam S., Mahumud R.A., Biswas T., Islam S.M.S. Age at menarche and its socioeconomic determinants among female students in an urban area in Bangladesh. Sex. Reprod. Healthc. 2017;12:88–92. doi: 10.1016/j.srhc.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Elks C.E., Ong K.K., Scott R.A., van der Schouw Y.T., Brand J.S., Wark P.A., Amiano P., Balkau B., Barricarte A., Boeing H., et al. Age at menarche and type 2 diabetes risk: The EPIC-InterAct study. Diabetes Care. 2013;36:3526–3534. doi: 10.2337/dc13-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canoy D., Beral V., Balkwill A., Wright F.L., Kroll M.E., Reeves G.K., Green J., Cairns B.J., Million Women Study Collaborators Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131:237–244. doi: 10.1161/CIRCULATIONAHA.114.010070. [DOI] [PubMed] [Google Scholar]
- 6.Charalampopoulos D., McLoughlin A., Elks C.E., Ong K.K. Age at menarche and risks of all-cause and cardiovascular death: A systematic review and meta-analysis. Am. J. Epidemiol. 2014;180:29–40. doi: 10.1093/aje/kwu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negriff S., Hillman J.B., Dorn L.D. Does competence mediate the associations between puberty and internalizing or externalizing problems in adolescent girls? J. Adolesc. Health. 2011;49:350–356. doi: 10.1016/j.jadohealth.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himes J.H. Examining the evidence for recent secular changes in the timing of puberty in US children in light of increases in the prevalence of obesity. Mol. Cell. Endocrinol. 2006;254:13–21. doi: 10.1016/j.mce.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Henrichs K.L., McCauley H.L., Miller E., Styne D.M., Saito N., Breslau J. Early menarche and childhood adversities in a nationally representative sample. Int. J. Pediatr. Endocrinol. 2014;2014:14. doi: 10.1186/1687-9856-2014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suglia S.F., Koenen K.C., Boynton-Jarrett R., Chan P.S., Clark C.J., Danese A., Faith M.S., Goldstein B.I., Hayman L.L., Isasi C.R., et al. Childhood and Adolescent Adversity and Cardiometabolic Outcomes: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e15–e28. doi: 10.1161/CIR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Aguilar-Gaxiola S., Alhamzawi A.O., Alonso J., Angermeyer M., et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noll J.G., Trickett P.K., Long J.D., Negriff S., Susman E.J., Shalev I., Li J.C., Putnam F.W. Childhood Sexual Abuse and Early Timing of Puberty. J. Adolesc. Health. 2017;60:65–71. doi: 10.1016/j.jadohealth.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Obradović J., Hipwell A. Psychopathology and social competence during the transition to adolescence: The role of family adversity and pubertal development. Dev. Psychopathol. 2010;22:621–634. doi: 10.1017/S0954579410000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braithwaite D., Moore D.H., Lustig R.H., Epel E.S., Ong K.K., Rehkopf D.H., Wang M.C., Miller S.M., Hiatt R.A. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 2009;20:713–720. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- 15.Negriff S., Blankson A.N., Trickett P.K. Pubertal Timing and Tempo: Associations with Childhood Maltreatment. J. Res. Adolesc. 2015;25:201–213. doi: 10.1111/jora.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romans S.E., Martin J.M., Gendall K., Herbison G.P. Age of menarche: The role of some psychosocial factors. Psychol. Med. 2003;33:933–939. doi: 10.1017/S0033291703007530. [DOI] [PubMed] [Google Scholar]
- 17.Ellis B.J. Timing of pubertal maturation in girls: An integrated life history approach. Psychol. Bull. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- 18.Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: And evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.2307/1131166. [DOI] [PubMed] [Google Scholar]
- 19.Ellis B.J., Essex M.J. Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 20.Mendle J., Ryan R.M., McKone K.M. Early Childhood Maltreatment and Pubertal Development: Replication in a Population-Based Sample. J. Res. Adolesc. 2016;26:595–602. doi: 10.1111/jora.12201. [DOI] [PubMed] [Google Scholar]
- 21.Ruttle P.L., Shirtcliff E.A., Armstrong J.M., Klein M.H., Essex M.J. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev. Psychobiol. 2015;57:688–704. doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Deardorff J., Ekwaru J.P., Kushi L.H., Ellis B.J., Greenspan L.C., Mirabedi A., Landaverde E.G., Hiatt R.A. Father absence, body mass index, and pubertal timing in girls: Differential effects by family income and ethnicity. J. Adolesc. Health. 2011;48:441–447. doi: 10.1016/j.jadohealth.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullsperger J.M., Nikolas M.A. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk? Psychol. Bull. 2017;143:903–938. doi: 10.1037/bul0000106. [DOI] [PubMed] [Google Scholar]
- 25.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews. [(accessed on 13 July 2018)]; Available online: http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction = displayproduct&productid = 318. [PubMed]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; New York, NY, USA: 1998. [Google Scholar]
- 28.Effect Size Calculator. [(accessed on 13 July 2018)]; Available online: https://cebcp.org/practical-meta-analysis-effect-size-calculator/standardized-mean-difference-d/means-and-standard-deviations/
- 29.Bornstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2009. [Google Scholar]
- 30.Sánchez-Meca J., Marín-Martínez F., Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol. Methods. 2003;8:448–467. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 31.Trotta A., Murray R.M., Fisher H.L. The impact of childhood adversity on the persistence of psychotic symptoms: A systematic review and meta-analysis. Psychol. Med. 2015;45:2481–2498. doi: 10.1017/S0033291715000574. [DOI] [PubMed] [Google Scholar]
- 32.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abioye-Kuteyi E.A., Ojofeitimi E.O., Aina O.I., Kio F., Aluko Y., Mosuro O. The influence of socioeconomic and nutritional status on menarche in Nigerian school girls. Nutr. Health. 1997;11:185–195. doi: 10.1177/026010609701100304. [DOI] [PubMed] [Google Scholar]
- 34.Adalı T., Koç I. Menarcheal age in Turkey: Secular trend and socio-demographic correlates. Ann. Hum. Biol. 2011;38:345–353. doi: 10.3109/03014460.2011.552891. [DOI] [PubMed] [Google Scholar]
- 35.Al-Sahab B., Ardern C.I., Hamadeh M.J., Tamim H. Age at menarche in Canada: Results from the National Longitudinal Survey of Children & Youth. BMC Public Health. 2010;10:736. doi: 10.1186/1471-2458-10-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvergne A., Faurie C., Raymond M. Developmental plasticity of human reproductive development: Effects of early family environment in modern-day France. Physiol. Behav. 2008;95:625–632. doi: 10.1016/j.physbeh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Amigo H., Vásquez S., Bustos P., Ortiz G., Lara M. Socioeconomic status and age at menarche in indigenous and non-indigenous Chilean adolescents. Cad. Saude Publica. 2012;28:977–983. doi: 10.1590/S0102-311X2012000500016. [DOI] [PubMed] [Google Scholar]
- 38.Asgharnia M., Faraji R., Sharami H., Yadak M., Oudi M. A study of menarcheal age in northern iran (rasht) Oman Med. J. 2009;24:95–98. doi: 10.5001/omj.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrios Y.V., Sanchez S.E., Nicolaidis C., Garcia P.J., Gelaye B., Zhong Q., Williams M.A. Childhood abuse and early menarche among Peruvian women. J. Adolesc. Health. 2015;56:197–202. doi: 10.1016/j.jadohealth.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bleil M.E., Adler N.E., Appelhans B.M., Gregorich S.E., Sternfeld B., Cedars M.I. Childhood adversity and pubertal timing: Understanding the origins of adulthood cardiovascular risk. Biol. Psychol. 2013;93:213–219. doi: 10.1016/j.biopsycho.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blell M., Pollard T.M., Pearce M.S. Predictors of age at menarche in the newcastle thousand families study. J. Biosoc. Sci. 2008;40:563–575. doi: 10.1017/S0021932007002696. [DOI] [PubMed] [Google Scholar]
- 42.Boynton-Jarrett R., Harville E.W. A prospective study of childhood social hardships and age at menarche. Ann. Epidemiol. 2012;22:731–737. doi: 10.1016/j.annepidem.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boynton-Jarrett R., Wright R.J., Putnam F.W., Hibert E.L., Michels K.B., Forman M.R., Rich-Edwards J. Childhood abuse and age at menarche. J. Adolesc. Health. 2013;52:241–247. doi: 10.1016/j.jadohealth.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buzney C.D., DeCaro J.A. Explanatory models of female pubertal timing: Discordances between cultural models of maturation and the recollection and interpretation of personal developmental experiences. Cult. Med. Psychiatry. 2012;36:601–620. doi: 10.1007/s11013-012-9277-8. [DOI] [PubMed] [Google Scholar]
- 45.Chavarro J., Villamor E., Narváez J., Hoyos A. Socio-demographic predictors of age at menarche in a group of Colombian university women. Ann. Hum. Biol. 2004;31:245–257. doi: 10.1080/03014460310001652239. [DOI] [PubMed] [Google Scholar]
- 46.Culpin I., Heron J., Araya R., Melotti R., Lewis G., Joinson C. Father absence and timing of menarche in adolescent girls from a UK cohort: The mediating role of maternal depression and major financial problems. J. Adolesc. 2014;37:291–301. doi: 10.1016/j.adolescence.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Junqueira Do Lago M., Faerstein E., De Souza Lopes C., Werneck G.L. Family socio-economic background modified secular trends in age at menarche: Evidence from the Pró-Saúde Study (Rio de Janeiro, Brazil) Ann. Hum. Biol. 2003;30:347–352. doi: 10.1080/0301446031000091783. [DOI] [PubMed] [Google Scholar]
- 48.El Dosoky M., Al Amoudi F. Menarcheal age of school girls in the city of Jeddah, Saudia Arabia. J. Obstet. Gynaecol. 1997;17:195–198. doi: 10.1080/01443619750113861. [DOI] [PubMed] [Google Scholar]
- 49.Ersoy B., Balkan C., Gunay T., Onag A., Egemen A. Effects of different socioeconomic conditions on menarche in Turkish female students. Early Hum. Dev. 2004;76:115–125. doi: 10.1016/j.earlhumdev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Graber J.A., Brooks-Gunn J., Warren M.P. The antecedents of menarcheal age: Heredity, family environment, and stressful life events. Child Dev. 1995;66:346–359. doi: 10.2307/1131582. [DOI] [PubMed] [Google Scholar]
- 51.James-Todd T., Tehranifar P., Rich-Edwards J., Titievsky L., Terry M.B. The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Ann. Epidemiol. 2010;20:836–842. doi: 10.1016/j.annepidem.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jorm A.F., Christensen H., Rodgers B., Jacomb P.A., Easteal S. Association of adverse childhood experiences, age of menarche, and adult reproductive behavior: Does the androgen receptor gene play a role? Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004;125:105–111. doi: 10.1002/ajmg.b.20114. [DOI] [PubMed] [Google Scholar]
- 53.Kelly Y., Zilanawala A., Sacker A., Hiatt R., Viner R. Early puberty in 11-year-old girls: Millennium Cohort Study findings. Arch. Dis. Child. 2017;102:232–237. doi: 10.1136/archdischild-2016-310475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magnus M.C., Anderson E.L., Howe L.D., Joinson C.J., Penton-Voak I.S., Fraser A. Childhood psychosocial adversity and female reproductive timing: A cohort study of the ALSPAC mothers. J. Epidemiol. Community Health. 2018;72:34–40. doi: 10.1136/jech-2017-209488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matchock R.L., Susman E.J. Family composition and menarcheal age: Anti-inbreeding strategies. Am. J. Hum. Biol. 2006;18:481–491. doi: 10.1002/ajhb.20508. [DOI] [PubMed] [Google Scholar]
- 56.Mendle J., Turkheimer E., D’Onofrio B.M., Lynch S.K., Emery R.E., Slutske W.S., Martin N.G. Family structure and age at menarche: A children-of-twins approach. Dev. Psychol. 2006;42:533–542. doi: 10.1037/0012-1649.42.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moffitt T.E., Caspi A., Belsky J., Silva P.A. Childhood experience and the onset of menarche: A test of a sociobiological model. Child Dev. 1992;63:47–58. doi: 10.2307/1130900. [DOI] [PubMed] [Google Scholar]
- 58.Onat T., Ertem B. Age at menarche: Relationships to socioeconomic status, growth rate in stature and weight, and skeletal and sexual maturation. Am. J. Hum. Biol. 1995;7:741–750. doi: 10.1002/ajhb.1310070609. [DOI] [PubMed] [Google Scholar]
- 59.Opare-Addo P.M., Stowe M., Ankobea-Kokroe F., Zheng T. Menarcheal and pubertal development and determining factors among schoolgirls in Kumasi, Ghana. J. Obstet. Gynaecol. 2012;32:159–165. doi: 10.3109/01443615.2011.638092. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y., Mensah F.K., Azzopardi P., Patton G.C., Wake M. Childhood Social Disadvantage and Pubertal Timing: A National Birth Cohort from Australia. Pediatrics. 2017;139:e20164099. doi: 10.1542/peds.2016-4099. [DOI] [PubMed] [Google Scholar]
- 61.Tahirović H.F. Menarchal age and the stress of war: An example from Bosnia. Eur. J. Pediatr. 1998;157:978–980. doi: 10.1007/s004310050981. [DOI] [PubMed] [Google Scholar]
- 62.Tekgül N., Saltık D., Vatansever K. Secular trend of menarche age in an immigrant urban city in Turkey: Izmir. Turk. J. Pediatr. 2014;56:138–143. [PubMed] [Google Scholar]
- 63.Tither J.M., Ellis B.J. Impact of fathers on daughters’ age at menarche: A genetically and environmentally controlled sibling study. Dev. Psychol. 2008;44:1409–1420. doi: 10.1037/a0013065. [DOI] [PubMed] [Google Scholar]
- 64.Toromanović A., Tahirović H. Effect of family disintegration on age at menarche. Acta Med. Acad. 2015;44:124–134. doi: 10.5644/ama2006-124.140. [DOI] [PubMed] [Google Scholar]
- 65.Vigil J.M., Geary D.C., Byrd-Craven J. A life history assessment of early childhood sexual abuse in women. Dev. Psychol. 2005;41:553–561. doi: 10.1037/0012-1649.41.3.553. [DOI] [PubMed] [Google Scholar]
- 66.Wise L.A., Palmer J.R., Rothman E.F., Rosenberg L. Childhood abuse and early menarche: Findings from the black women’s health study. Am. J. Public Health. 2009;99(Suppl 2):S460–S466. doi: 10.2105/AJPH.2008.149005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herman-Giddens M.E., Slora E.J., Wasserman R.C., Bourdony C.J., Bhapkar M.V., Koch G.G., Hasemeier C.M. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 68.Draper P., Harpending H. Father absence and reproductive strategy: An evolutionary perspective. J. Anthropol. Res. 1982;38:255–273. doi: 10.1086/jar.38.3.3629848. [DOI] [Google Scholar]
- 69.Ellis B.J., McFadyen-Ketchum S., Dodge K.A., Pettit G.S., Bates J.E. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: A longitudinal test of an evolutionary model. J. Personal. Soc. Psychol. 1999;77:387–401. doi: 10.1037/0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]