Abstract
Introduction
Community health workers (CHWs)—shown to improve access to care and reduce maternal, newborn, and child morbidity and mortality—are re-emerging as a key strategy to achieve health-related Sustainable Development Goals (SDGs). However, recent evaluations of national programmes for CHW-led integrated community case management (iCCM) of common childhood illnesses have not found benefits on access to care and child mortality. Developing innovative ways to maximise the potential benefits of iCCM is critical to achieving the SDGs.
Methods and analysis
An unblinded, cluster randomised controlled trial in rural Mali aims to test the efficacy of the addition of door-to-door proactive case detection by CHWs compared with a conventional approach to iCCM service delivery in reducing under-five mortality. In the intervention arm, 69 village clusters will have CHWs who conduct daily proactive case-finding home visits and deliver doorstep counsel, care, referral and follow-up. In the control arm, 68 village clusters will have CHWs who provide the same services exclusively out of a fixed community health site. A baseline population census will be conducted of all people living in the study area. All women of reproductive age will be enrolled in the study and surveyed at baseline, 12, 24 and 36 months. The survey includes a life table tracking all live births and deaths occurring prior to enrolment through the 36 months of follow-up in order to measure the primary endpoint: under-five mortality, measured as deaths among children under 5 years of age per 1000 person-years at risk of mortality.
Ethics and dissemination
The trial has received ethical approval from the Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry, University of Bamako. The results will be disseminated through peer-reviewed publications, national and international conferences and workshops, and media outlets.
Trial registration number
NCT02694055; Pre-results.
Keywords: child health, maternal health, cluster randomized trial, community health workers, integrated community case management, child mortality
Strengths and limitations of this study.
This is a cluster randomised controlled trial powered to detect a 25% relative difference in the incidence rate of under-five mortality between the two study arms.
The trial will generate evidence on the efficacy, cost-effectiveness and equity of door-to-door proactive case detection by community health workers on access to care and child mortality.
The intervention is designed to facilitate public sector adoption and scale-up if found to be effective.
The large geographical area and 3-year time frame leave the study vulnerable to unexpected events that may influence the extent to which the intervention can be implemented per protocol.
Changes to the health system or other contextual factors in the intervention area, such as drug stock-outs, health centre staff strikes, concurrent programme implementation by other actors, and political insecurity may be beyond the control of the study implementers.
Introduction
The vast majority of maternal, newborn and child deaths in low-income and middle-income countries are preventable. Evidence-based and cost-effective methods for prevention and treatment are available for the leading causes of death, yet many still face barriers to obtaining timely, quality and appropriate care. If community-based interventions, such as the treatment of malaria with artemisinin compounds, oral rehydration solution for childhood diarrhoea, oral antibiotics for pneumonia, nutritional interventions during pregnancy and hand washing with soap, were scaled to achieve 90% coverage in high-burden countries before 2020, an estimated 6.9 million maternal and child deaths could be averted.1
Integrated community case management (iCCM) of common childhood illnesses entails a package of services to diagnose, treat and refer children under 5 with malaria, diarrhoea, pneumonia or moderate malnutrition, delivered by community health workers (CHWs).2 CCM of common childhood illnesses has been shown to improve access to care3–5 and treatment adherence,3 6 and reduce mortality due to malaria,7 diarrhoea,3 8 9 pneumonia,3 10 11 as well as all causes.7 9 10
Many countries in sub-Saharan Africa have adopted iCCM as an evidence-based strategy to improve child health.12 13 However, the expected benefits of iCCM have not been realised in all contexts.14–19 Several recent evaluations of national iCCM programmes did not find impacts on care seeking or child mortality, in part, study authors conclude, due to low demand for CHW services.20–23 These national programmes shared certain design and implementation features that may have contributed to the lack of overall effects by not addressing barriers to care, such as user fees for services, lack of frequent and dedicated CHW supervision for quality assurance, and community care provision exclusively (or primarily) for patients that seek care from a fixed health site. As more countries commit to scaling up CHW-led healthcare systems, it is critical that we understand how to best design and implement iCCM and CHW services more broadly, in order to bring about their full potential.
To address this need, we designed a cluster randomised controlled trial to test door-to-door proactive case detection by CHWs compared with a conventional approach to iCCM service delivery, which relies on patient-initiated care seeking. In both arms of the trial, CHWs will provide an integrated package of child, reproductive and maternal health services, primary health centres (PHCs) will be reinforced in infrastructure and capacity, and user fees will be removed at all levels of care. The difference between the intervention (ProCCM) arm and the control (iCCM) arm is the proactive versus conventional approach to the delivery of community-based services. The comparator was chosen to isolate and assess the effects of one design feature of CHW service delivery: proactive case detection.
The ProCCM approach is designed to overcome additional social, structural and health system barriers that may impede or lead to delayed access, even under a community-based comprehensive iCCM approach. At a systems level, these include the direct and indirect costs of care, including distance to care. At the household level, lack of resources, mistrust in the healthcare system and complex familial decision-making dynamics due to in part to gender inequality can contribute to delays in reaching care.24 25 By proactively seeking out patients and linking community members to the healthcare system, ProCCM is designed to reduce the time from onset of condition to utilisation of health services, including direct provision of comprehensive primary care services for all household members, ultimately reducing mortality.
Methods and analyses
Study aims and hypothesis
Our cluster randomised controlled trial aims to:
Estimate the effect of adding door-to-door proactive case detection by CHWs to an enhanced iCCM intervention on under-five child mortality; we hypothesise that, after 36 months, the relative difference in the incidence rate of under-five mortality between the two study arms will be greater than 25%.
Estimate the effect of adding door-to-door proactive case detection by CHWs to an enhanced iCCM intervention on utilisation of reproductive, maternal and child health services.
Evaluate the ProCCM intervention model, compared with the iCCM control model, in terms of cost-effectiveness, equity and affordability at scale.
Study site
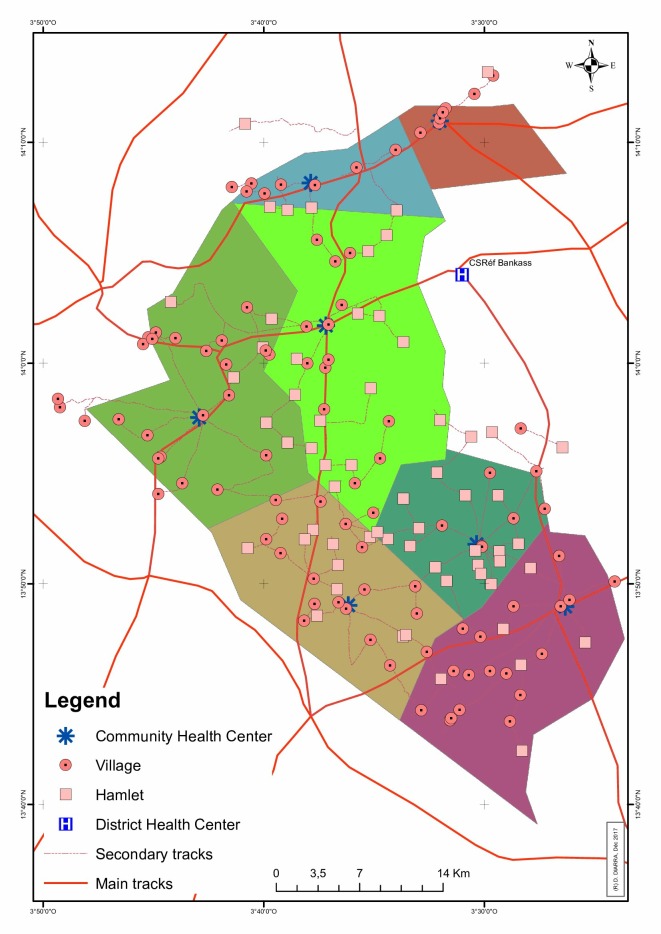
The trial will be conducted in the Bankass health district of the Mopti region in eastern Mali, approximately 600 km east of the nation’s capital, Bamako. The district has a 2016 population of approximately 300 000 people and is served by a public secondary referral hospital located in Bankass, the largest town in the district.26 Within the Bankass health district, the study is being conducted in 7 (of 22) health catchment areas: Dimbal, Doundé, Ende, Kani Bozon, Koulongon, Lessagou and Soubala (figure 1). The study area has a 2016 population of approximately 100 000 people.26 Each health catchment area is served by a PHC operated by the Ministry of Health.
Figure 1.
Map of study area; colours indicate the seven health catchment areas within which the trial is being conducted.
Study design
This is an unblinded, pragmatic, cluster randomised controlled trial, with 69 village clusters in the intervention arm and 68 village clusters in the comparison arm. Clusters are randomised to receive either enhanced iCCM from stationary CHW(s) serving patients exclusively at a community health site (control) as per Mali’s national iCCM strategy,27 or ProCCM from CHW(s) conducting daily proactive case-finding home visits in addition to serving patients at a community health site. Only the intervention arm will receive door-to-door proactive case detection by CHWs, including doorstep care and home-based follow-up.
Intervention
Local community members—female candidates encouraged—who can read and write in French will be recruited, trained, supervised and supported as CHWs from the village cluster in which they will work. CHW coverage will be based on Mali’s national iCCM strategy, which recommends one CHW for a population of 700 in the southern region where the study area is situated.27 Clusters, therefore, may have one or multiple resident CHWs, depending on the size of the cluster population. Clusters with less than 200 people and within 3 km of another cluster assigned to the same study arm will share a CHW, provided there is no geographic barrier (ie, river) between the two clusters and no linguistic barrier for the CHW.
In both arms, CHWs will provide a comprehensive set of primary care services, including iCCM in accordance with national and international standards,2 as well as maternal and reproductive health for women of reproductive age (see table 1 for a full description of the CHW package of care). CHW services will include counselling, diagnostics, treatment, referral to reinforced PHCs and follow-up care. CHWs will be required to be on call, available to receive and care for patients who seek them out, 24 hours per day, 7 days per week. CHWs will receive a salary circa minimum wage (FCFA40 000 per month), and user fees will be removed for all CHW and referral services for all patients in the study area. A detailed description of the entire health system strengthening intervention in both arms is provided in the online supplementary document.
Table 1.
Community health worker (CHW) package of care, provided at the patient’s doorstep (intervention arm) or at the CHW’s health site (both arms)
| CHW services | Description |
| Diagnosis and treatment of malaria, all ages* |
|
| iCCM of common childhood illnesses* |
|
| Detection of pregnancy |
|
| Family planning services* |
|
| Accompaniment or referral to PHC for danger signs, all ages* |
|
| Follow-up care |
|
| Newborn assessment* |
|
| Health promotion and disease prevention* |
|
*These services are also offered by conventional CHWs in the Malian context, according to the Ministry of Health’s policy on CHW care.27
iCCM, integrated community case management; IUD, intrauterine device; PHC, primary health centre.
bmjopen-2018-027487supp001.pdf (81.3KB, pdf)
Control arm: conventional CHW service delivery
In clusters assigned to the control arm, CHWs will be stationed at a community health site to provide the comprehensive package of primary care services for at least 4 hours per day, 6 days per week, available to receive patients seeking care. The community health site is at the cluster level and separate from the PHC.
Intervention arm: proactive CHW service delivery
In clusters assigned to the intervention arm, CHW(s) will be trained and deployed to conduct proactive case finding, door-to-door home visits for at least 2 hours each day, 6 days a week, with the goal of visiting each household at least two times each month. During the home visit, CHWs will screen all household members for recent illness or symptoms and provide services at the home, including follow-up for sick children and adults, pregnant women, newborns and postpartum mothers. In addition to home visits, ProCCM CHWs will provide care at their community health site for at least 2 hours a day, 6 days per week, according to a calendar shared with the community. At the health site, CHWs will provide the same services as those offered by CHWs in the control arm to care-seeking patients.
Cluster definition and randomisation
In order to identify distinct clusters, a field team visited all villages and hamlets in the study area and collected global positioning system (GPS) coordinates at the public space where community-wide meetings, announcements and festivities are held. GPS coordinates were mapped and the cardinal distances between neighbouring villages and hamlets were calculated. Villages and hamlets 1 km or less from each another were grouped into clusters, resulting in 160 individual villages and hamlets grouped into 137 unique clusters. A cluster definition based in geographical reality rather than administrative delineation helps to mitigate against contamination.
Clusters located 1.0 or more km from a PHC were stratified by health catchment area and distance to the nearest PHC (1.0–5.0 km vs more than 5.0 km). The cut-off point of 5.0 km was defined in accordance with national iCCM guidelines,27 which deploys CHWs to deliver iCCM services only in communities greater than 5.0 km from a PHC. An additional stratum included all villages where the PHC was located to ensure balanced assignment of PHC villages across arms. Within each stratum, clusters were randomly assigned to the control or treatment arm using a computer-generated random number. Randomisation was conducted by a member of the research team based in the USA who did not have any involvement in CHW recruitment or participant enrolment. Trial statisticians will remain blinded to cluster allocation until the end of the trial.
Sample size and primary and secondary endpoints
Primary endpoint
The primary endpoint is under-five mortality, measured as deaths among children under 5 years of age per 1000 person-years at risk of mortality. In Mopti, the region of the study site, the 10-year under-five mortality rate (U5MR) was 111 deaths per 1000 live births during 2012–2013 Demographic and Health Survey (DHS), which is higher than the national U5MR.28 Since the 2013 DHS, intermittent prophylactic therapy in children for malaria has been rolled out across the region. As intermittent preventive treatment in children is associated with a risk ratio of all-cause under-five mortality of 0.66 in areas of seasonal transmission of malaria,29 we estimate that baseline U5MR in the area of the intervention will be 111*0.66=72.6/1000.
The sample size for the trial was based on this primary endpoint, derived using methods for cluster randomised trials30 in which each cluster was treated as an observation and the cluster-level outcome was defined as the U5MR per person-years at risk. We used a negative binomial model to simulate the number of deaths among children under 5. According to 2014 national population estimates adjusted for 2016 using a 2.2% annual growth rate,26 the seven health catchment areas encompassed a population of 103 848 inhabitants. Assuming that 20% of the population was children aged 0–59 months and 22% was women aged 15–49, we calculated a mean of 152 children and 167 women per cluster. Person-years at risk were calculated assuming 3 years of prospective study follow-up with 10% attrition based on experience with previous trials in Mali.31 32 We used a coefficient of variation of k=0.2930 to model the extra variation due to clustering (1/k2 is the size parameter in the negative binomial model). With these parameters, the trial will be able to detect a relative difference of 25% (alpha=0.05, two-tailed test) in the under-five mortality incidence between treatment and control arms with 81.8% power after 36 months.
Secondary endpoints
We will also estimate the effect of the intervention on a number of secondary endpoints:
Infant mortality (deaths per 1000 live births among children aged 0–11 months).
Newborn mortality (deaths per 1000 live births among children aged 0–28 days).
Pregnancy-related mortality ratio (number of deaths among women while pregnant or within 42 days of delivery or termination per 100 000 live births per year) if there is sufficient and robust data to do so.
Receipt of oral rehydration therapy and zinc within 24 hours of diarrhoea onset among children under 5.
Receipt of diagnostic testing and/or effective treatment for malaria within 24 hours of fever onset among children under 5.
Evaluation by a qualified provider within 24 hours of symptom onset among children under 5 with cough and/or fast breathing.
Receipt of three or more doses of sulfadoxine–pyrimethamine as intermittent preventive treatment during a woman’s most recent pregnancy.
Enrolment in antenatal care (ANC) with a skilled provider in the first trimester during a woman’s most recent pregnancy.
Completing four or more ANC consultations with a skilled provider during a woman’s most recent pregnancy.
Use of a modern method of contraception among women of reproductive age.
Inclusion criteria
Any individual in the study area at any point during the study period, including visitors, is eligible to receive the health services offered through the intervention. Only permanent residents of the study area are eligible to be included in the household survey. All women aged 15–49 permanently residing in the study area at baseline who provide consent or assent and report no foreseeable plans to leave the study area are eligible to participate in the women’s questionnaire of the household survey—the data source used for the measurement of primary and secondary endpoints. Women who did not meet the inclusion criteria at baseline but who become newly eligible during the course of the study are invited to participate at follow-up household survey rounds.
Sources of data
The effects of the ProCCM model of service delivery, compared with the iCCM model, for the primary and secondary endpoints will be assessed using data from three sources: (1) household surveys, (2) the CHW mobile application and (3) facility records.
Household surveys
A household survey will be administered to all eligible women at baseline (prior to the launch of the intervention), and 12, 24 and 36 months after the intervention start. Surveyors will not be members of the villages they survey, nor will they be members of the intervention healthcare delivery staff. All surveyors will be female, as the survey tool contains sensitive questions regarding contraception and reproductive health. The survey includes a household roster, which may be completed by the female head of household, and a questionnaire administered to consenting or assenting women of reproductive age (15–49).
The household survey instrument was adapted from the Mali DHS and designed in Open Data Kit, which permits real-time quality and completeness control on data collection. The women’s questionnaire will include a full birth history to capture all live births, which will then be updated during each of the follow-up survey rounds. To track maternal mortality, the survey will record all household deaths occurring the previous year, with additional information on timing of death (during pregnancy, childbirth, after childbirth) for women of reproductive age. The survey also captures detailed information on household and individual sociodemographic characteristics, access and utilisation of reproductive and maternal healthcare, and care-seeking behaviours and investments for recently ill children under 5. Follow-up household survey rounds will add new household members to the study cohort (eg, due to births, migration) and record absences due to out-migration or death. Surveyors will attempt to contact each eligible woman up to three additional times if she is absent at the first visit.
CHW mobile application data
CHWs in both study arms will be equipped with an Android smartphone and trained to use a mobile application to track services rendered. The app is also designed to be a job aid with integrated data validation and prompts to guide the CHW through the appropriate case management protocol. Population census data collected at baseline, including individual unique identifiers and demographic information, will be prepopulated into the CHW application so that each CHW can access the records of families in his/her service delivery zone. During each encounter with a prospective patient, the CHW will either identify the individual in the application or register newborns, new arrivals or visitors, before selecting the appropriate form in the application for the specific health concern (eg, malaria case management). The types of actions displayed under a patient’s profile are linked to her sex and age (eg, pregnancy follow-up is displayed only for women aged 15–49). The application will also alert the CHW of upcoming tasks related to patient follow-up, with an action calendar for 24-hour follow-up available starting at midnight each day.
Facility data
Each PHC will be equipped with five laptop computers, and the physician-in-chief, midwife, pharmacist, vaccine administration technician and receptionist will be trained in data collection on an Electronic Medical Records (EMR) system. Population census data collected at baseline will be imported into the EMR system, including individual unique identifiers and basic demographic information. When attending a PHC, patients will present first to reception, where their medical records will be identified using their unique identifier, name, family and/or village information. During the patient consultation, the service provider will record patient health information (ie, diagnostic tests, results, treatment, posology) in both the EMR and in the paper facility registers, the source documents of the Malian Ministry of Health and required by law. Referral by a CHW will be recorded.
Analytical plan
Analyses of the primary and secondary endpoints will estimate intention-to-treat (ITT) effects.
Analysis of primary endpoint
Using data collected prospectively in the 12, 24 and 36 months follow-up household surveys, we will test for the difference in the incidence of deaths among children under 5 across treatment and control arms using a Poisson regression model with cluster-level random effects, controlling for household distance to PHC (less than 5 km vs 5 km or more). Children surveyed at baseline will contribute person-years of exposure from the start date of the trial’s intervention launch; children born during the trial will contribute person-years of exposure beginning at birth. Children who enter the trial after baseline will contribute person-years of exposure beginning at the household survey interview date in which they are enrolled. All children included in the analysis will contribute person-years through the date of their death, or are right censored on their fifth birthday or the end date of the trial, whichever comes first. The coefficient of interest with be the incidence rate ratio estimated on a dichotomous variable that indicates the child’s residence in a treatment versus control cluster. We will control for the non-constant risk of mortality in early childhood by controlling for age (in months) constant over time, and will control for any individual-level characteristics that are unbalanced at baseline. To estimate mortality, a child’s date of birth, date of interview, vital status at interview, and if applicable, date of death are required. We will replicate the procedures for missing mortality data used in the DHS, described in detail elsewhere.33
Analysis of secondary endpoints
The same modelling approach will be used to estimate ITT effects for secondary endpoints (excluding the covariate for child’s age); regression analyses will test the significance of the regression coefficient on the treatment assignment variable. Linking functions will be chosen based on the type of outcome variable analysed (ie, logit for dichotomous outcomes). If 10% or fewer observations have missing secondary outcome data, we will drop observations from analysis; otherwise, we will determine and apply sample weights to estimates derived from the complete sample of observations. For any secondary endpoints that differ significantly by arm at baseline, we will use a difference-in-differences estimation approach to account for this difference.
Per-protocol estimates
ITT estimates will be compared with estimates from a per-protocol analysis of primary and secondary outcomes. Our per-protocol analysis will estimate the effects of the intervention only for households that received the ProCCM CHW services according to the intervention protocol. This will be defined as households, which report they have received two or more visits from a CHW in the month preceding the household survey for each year they participated in the survey, regardless of treatment assignment. Finally, exploratory analyses will be conducted to assess the existence and magnitude of heterogeneous treatment effects according to village population size and household wealth.
Cost-effectiveness analysis
Cost-effectiveness analysis (CEA) compares different programme alternatives in terms of their cost-effectiveness ratio, which can be thought of as the average cost per unit of impact or benefit (eg, cost per life year saved). In most cases, CEA is used to determine whether or not a new alternative policy is better than the status quo, or whether the extra cost is worth the extra benefit. In such cases, the incremental cost-effectiveness ratio (ICER) is used, which takes the ratio between the incremental costs of the new programme with respect to the status quo, to the incremental benefits of the new programme with respect to the status quo. We will perform an ICER analysis to evaluate the relative cost-effectiveness of the ProCCM model with respect to the enhanced iCCM (control) model.
We will calculate the total economic costs of both programmatic models, which will reflect the monetary value of programme and household resources used to deliver and access services, respectively. From the programme perspective, these will include personnel and other recurrent costs such as drugs, laboratory tests and other inputs used to provide services. These data will come from three sources: (1) the CHW mobile application, which reflects all services and supplies used by CHWs for service provision; (2) PHC EMR, which include the services rendered at the PHC and resources will be valued at prices paid by the Ministry of Health; and (3) programme records, including CHW’s time and value of work time vis-à-vis salaries. From the household perspective, costs include time used to access health services, valued at their opportunity costs (ie, time lost from work), as well as out-of-pocket expenses such as paying for drugs or health services. These data will be obtained from the household survey, which asks about out-of-pocket expenditures, time spent accessing services and earnings from paid work.
Patient and public involvement
The study was designed and implemented in partnership with national, district and local health officials of the Malian Ministry of Health. Bankass health district was chosen in consultation with the Ministry of Health for three reasons: (1) healthcare utilisation (prenatal and curative consultations) was low and under-five mortality was high; (2) there were no overlapping interventions by other non-governmental organisations at the time or intended for the period of the trial and (3) local authorities were highly engaged and interested in collaborating on study implementation. Research questions and outcome measures were also chosen in consultation, to answer questions of key concern to government partners for informing the design of the national strategic plan for iCCM scale-up, including whether the intervention is equitable, cost-effective and affordable at scale. Community consultation and permission will be sought prior to trial commencement in meetings with representatives of the village clusters, such as village chiefs and their advisories, politico-administrative authorities, religious leaders and representatives of women’s and youth associations. Representatives will then communicate with community members via open public meetings. Once the study has terminated, results will be disseminated to participants via dissemination workshops at all levels of local, regional, and national representation.
Ethics and dissemination
The University of California, San Francisco exempted secondary analysis of the trial data from ethical approval. External monitoring of the study will be assured by a Clinical Research Associate (CRA) external to the trial team. Any substantial protocol amendments or deviations, or any unintended effects of trial interventions or conduct, will be submitted to the Ethics Committee and records reviewed by the CRA.
Surveyors will obtain informed consent from all household survey respondents prior to enrolment in the trial, or from the respondent’s parent or guardian if she is a minor. Identifying information (ie, proper name, phone number) will be stored separately from the survey data, linked by the registration ID. Access to identifying information will be restricted to the data collection and management team; trial statisticians and other external collaborators will access only de-identified data.
An independent Data Safety and Monitoring Board (DSMB) will provide oversight throughout the trial. The DSMB will oversee participant safety and evaluate interim results to determine if the trial should be stopped early. Interim analyses of the primary endpoint (under-five mortality) will be performed at 12 and 24 months, estimated using data from the first and second follow-up household surveys. The DSMB will terminate the study early if a 50% relative difference in under-five mortality is detected after 12 months (statistical significance at p<0.001) or a 35% relative difference in under-five mortality after 24 months (p<0.001), a stopping rule more stringent than Haybittle-Peto stopping rules.34 35 At the end of the trial period, or if the trial is terminated early, all participating villages will receive the care with the condition identified in the superior study arm.
Trial results will be published in peer-reviewed journals following the International Committee of Medical Journal Editors guidelines. Findings will be disseminated via conferences and workshops with national and international stakeholders in community-based healthcare delivery including researchers, policy-makers and practitioners. De-identified data will be made publicly available after the conclusion of the trial and publication of the main effects.
Discussion
Supported by the emergence of global health guidelines and the accumulation of rigorous evidence on the efficacy of iCCM, countries across sub-Saharan Africa are scaling up iCCM to improve child health.12 13 Yet, the most recent evaluations of national iCCM programmes suggest further improvements in the delivery of iCCM programmes are necessary to reduce under-five mortality.20–23 Because the core design and implementation of CHW services vary across health systems, their optimal features must be identified and evaluated for iCCM to realise its full potential. This includes identifying how financing mechanisms, health system integration, packages and delivery of care, and CHW recruitment, training, supervision and compensation relate to care outcomes where CHWs are deployed as front-line health workers. The current trial aims to address one of these gaps by testing door-to-door proactive case detection by CHW against a conventional CHW service delivery approach on reducing under-five mortality risk. The results of the trial will, thus, be pertinent to policy-makers and implementers to determine how CHWs may be better deployed for amplifying public health impact.
The current study was designed and will be implemented in partnership with the Mali Ministry of Health to facilitate adoption of lessons learnt and scale-up in the public sector if the intervention is found to be effective. In addition to the primary objective related to CHW service delivery mechanisms, secondary objectives explore questions of key concern to ministerial partners for informing the design of the national strategic plan for iCCM scale-up, including whether the intervention is equitable, cost-effective, affordable at scale. The intervention itself is designed to be scalable as the planning and implementation of the intervention was executed in partnership with the Ministry of Health and district health officials, including operating through government PHCs. Findings from this study could have important policy implications for CHW-led iCCM scale up across sub-Saharan Africa.
Limitations
The large geographical area and 3-year time frame leave the study open to a number of potential confounding effects. Although contingency measures have been put into place for various situations that may arise, unexpected events may occur that influence the extent to which the study can be implemented per protocol. CHWs may have avenues for interacting with each other outside the structures of the intervention which may lead to contamination. Changes to the health system or other contextual factors in the intervention area, such as drug stock-outs, health centre staff strikes, concurrent programme implementation by other actors, and political insecurity may be beyond the control of the study implementers. However, close partnership with national and local health authorities during study preparation will enable us to proactively track these events, implement contingency steps and/or otherwise document them for later sensitivity analyses of the trial’s effects.
Trial status
The household baseline survey was carried out from December 2016 to February 2017. Health facility improvements, CHW trainings and provider trainings were completed by December 2016. Implementation of the intervention including the removal of user fees began in February 2017.
Supplementary Material
Acknowledgments
We are grateful to Aminata dite Nene Konipo, Seydou Sidibé, and Yacouba Samaké of Muso for their roles in preparation and execution of baseline data collection, implementing the intervention and assuring adherence to protocol. We thank Dansiné Diarra for the GPS mapping that allowed us to generate our clusters, and for providing figure 1. We thank the community-based and facility-based health workers and their supervisors for their role in implementation. We are grateful to the Malian Ministry of Health, representatives from Community Health Associations at each PHC site, village leaders and the communities of the Bankass District for their collaboration.
Footnotes
Contributors: CW, KK, BP, JL, ET and ADJ drafted the original study protocol with input from ABC, DD, YK, SS, NP, SB-A and MF. MF conducted the sample size calculations. CW and ET prepared the protocol manuscript. All authors reviewed and provided feedback on the final version of the manuscript.
Funding: The trial is funded with resources received by Muso though unrestricted funding as well as dedicated research funding from Child Relief International Foundation, Grand Challenges Canada, Johnson & Johnson Foundation and USAID Development Innovation Ventures. Child Relief International Foundation serves as the nonlegal sponsor of the trial.
Competing interests: ABC, ADJ, CW, DD, KK and YK declare grants from Child Relief International Foundation and USAID Development Innovation Ventures.
Ethics approval: Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry, University of Bamako (2016/03/CE/FMPOS).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
Map disclaimer: The depiction of boundaries on the map(s) in this article do not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
References
- 1. Chou VB, Friberg IK, Christian M, et al. Expanding the population coverage of evidence-based interventions with community health workers to save the lives of mothers and children: an analysis of potential global impact using the Lives Saved Tool (LiST). J Glob Health 2017;7:020401 10.7189/jogh.07.020401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young M, Wolfheim C, Marsh DR, et al. World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. Am J Trop Med Hyg 2012;87:6–10. 10.4269/ajtmh.2012.12-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das JK, Lassi ZS, Salam RA, et al. Effect of community based interventions on childhood diarrhea and pneumonia: uptake of treatment modalities and impact on mortality. BMC Public Health 2013;13:S29 10.1186/1471-2458-13-S3-S29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalyango JN, Alfven T, Peterson S, et al. Integrated community case management of malaria and pneumonia increases prompt and appropriate treatment for pneumonia symptoms in children under five years in Eastern Uganda. Malar J 2013;12:340 10.1186/1475-2875-12-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mubiru D, Byabasheija R, Bwanika JB, et al. Evaluation of integrated community case management in eight districts of Central Uganda. PLoS One 2015;10:e0134767–13. 10.1371/journal.pone.0134767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalyango JN, Rutebemberwa E, Karamagi C, et al. High adherence to antimalarials and antibiotics under integrated community case management of illness in children less than five years in eastern Uganda. PLoS One 2013;8:1–8. 10.1371/journal.pone.0060481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomised trial. Lancet 2000;356:550–5. 10.1016/S0140-6736(00)02580-0 [DOI] [PubMed] [Google Scholar]
- 8. Munos MK, Walker CL, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol 2010;39(Suppl 1):i75–i87. 10.1093/ije/dyq025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker CL, Black RE. Zinc for the treatment of diarrhoea: effect on diarrhoea morbidity, mortality and incidence of future episodes. Int J Epidemiol 2010;39(Suppl 1):i63–i69. 10.1093/ije/dyq023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sazawal S, Black RE. Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis 2003;3:547–56. 10.1016/S1473-3099(03)00737-0 [DOI] [PubMed] [Google Scholar]
- 11. Theodoratou E, Al-Jilaihawi S, Woodward F, et al. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol 2010;39(Suppl 1):i155–i171. 10.1093/ije/dyq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rasanathan K, Muñiz M, Bakshi S, et al. Community case management of childhood illness in sub-Saharan Africa - findings from a cross-sectional survey on policy and implementation. J Glob Health 2014;4:020401 10.7189/jogh.04.020401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boschi-Pinto C, Labadie G, Dilip TR, et al. Global implementation survey of Integrated Management of Childhood Illness (IMCI): 20 years on. BMJ Open 2018;8:1–9. 10.1136/bmjopen-2017-019079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Druetz T, Siekmans K, Goossens S, et al. The community case management of pneumonia in Africa: a review of the evidence. Health Policy Plan 2015;30:253–66. 10.1093/heapol/czt104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutebemberwa E, Kadobera D, Katureebe S, et al. Use of community health workers for management of malaria and pneumonia in urban and rural areas in eastern Uganda. Am J Trop Med Hyg 2012;87:30–5. 10.4269/ajtmh.2012.11-0732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Druetz T, Ridde V, Kouanda S, et al. Utilization of community health workers for malaria treatment: results from a three-year panel study in the districts of Kaya and Zorgho, Burkina Faso. Malar J 2015;14:71 10.1186/s12936-015-0591-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boone P, Elbourne D, Fazzio I, et al. Effects of community health interventions on under-5 mortality in rural Guinea-Bissau (EPICS): a cluster-randomised controlled trial. Lancet Glob Health 2016;4:e328–e335. 10.1016/S2214-109X(16)30048-1 [DOI] [PubMed] [Google Scholar]
- 18. Mukanga D, Tiono AB, Anyorigiya T, et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: a multi-country cluster randomized trial. Am J Trop Med Hyg 2012;87:21–9. 10.4269/ajtmh.2012.11-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yansaneh AI, Moulton LH, George AS, et al. Influence of community health volunteers on care seeking and treatment coverage for common childhood illnesses in the context of free health care in rural Sierra Leone. Trop Med Int Health 2014;19:1466–76. 10.1111/tmi.12383 [DOI] [PubMed] [Google Scholar]
- 20. Amouzou A, Hazel E, Shaw B, et al. Effects of the integrated Community Case Management of Childhood Illness Strategy on Child Mortality in Ethiopia: A Cluster Randomized Trial. Am J Trop Med Hyg 2016;94:596–604. 10.4269/ajtmh.15-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amouzou A, Kanyuka M, Hazel E, et al. Independent Evaluation of the integrated Community Case Management of Childhood Illness Strategy in Malawi Using a National Evaluation Platform Design. Am J Trop Med Hyg 2016;94:574–83. 10.4269/ajtmh.15-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munos M, Guiella G, Roberton T, et al. Independent Evaluation of the Rapid Scale-Up Program to Reduce Under-Five Mortality in Burkina Faso. Am J Trop Med Hyg 2016;94:584–95. 10.4269/ajtmh.15-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hazel E, Bryce J, The Iip-Jhu iCCM Evaluation Working Group. On Bathwater, Babies, and Designing Programs for Impact: Evaluations of the Integrated Community Case Management Strategy in Burkina Faso, Ethiopia, and Malawi. Am J Trop Med Hyg 2016;94:568–70. 10.4269/ajtmh.94-3intro1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colvin CJ, Smith HJ, Swartz A, et al. Understanding careseeking for child illness in sub-Saharan Africa: a systematic review and conceptual framework based on qualitative research of household recognition and response to child diarrhoea, pneumonia and malaria. Soc Sci Med 2013;86:66–78. 10.1016/j.socscimed.2013.02.031 [DOI] [PubMed] [Google Scholar]
- 25. Johnson A, Goss A, Beckerman J, et al. Hidden costs: the direct and indirect impact of user fees on access to malaria treatment and primary care in Mali. Soc Sci Med 2012;75:1786–92. 10.1016/j.socscimed.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 26. District Sanitaire de Bankass Ministère de l’Hygiène et Santé Publique. Microplanification des Associations de Santé Communautaire, 2016. [Google Scholar]
- 27. Ministère de la Santé et l’Hygiène Publique. Plan Stratégique National des Soins Essentiels dans la Communauté, 2014. [Google Scholar]
- 28. Cellule de Planification et de Statistique de Mali, Institut National de la Statistique de Mali, Centre d’Études et d’Information Statistique de Mali, et al. Mali Enquête Démographique et de Santé (EDSM V) 2012-2013. 2014. http://dhsprogram.com/pubs/pdf/FR286/FR286.pdf
- 29. Meremikwu MM, Donegan S, Sinclair D, et al. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev 2012:CD003756 10.1002/14651858.CD003756.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayes RJ, Moulton LH. Cluster randomised trials. 2 edn Boca Raton: Chapman and Hall/CRC, 2017. [Google Scholar]
- 31. Diakite OS, Maiga OM, Kayentao K, et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the prevention of malaria during pregnancy in mali: a randomized controlled trial. Clin Infect Dis 2011;53:215–23. 10.1093/cid/cir374 [DOI] [PubMed] [Google Scholar]
- 32. Kayentao K, Kodio M, Newman RD, et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis 2005;191:109–16. 10.1086/426400 [DOI] [PubMed] [Google Scholar]
- 33. The DHS Program. Guide to DHS statistics, 2018. [Google Scholar]
- 34. Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793–7. 10.1259/0007-1285-44-526-793 [DOI] [PubMed] [Google Scholar]
- 35. Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977;35:1–39. 10.1038/bjc.1977.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027487supp001.pdf (81.3KB, pdf)