Abstract
Fibrosis is involved in the pathogenesis of kidney diseases. We previously discovered that Rosa roxburghii fruit (Cili) possesses antifibrosis property in chronic renal disease, but the mechanisms are unknown. We hypothesized that Cili might prevent fibrosis development through mediating TGF-β/Smads signaling, which is known to be involved in renal fibrosis. This study aimed to confirm the effects of freeze-dried Cili powder in a rat model of unilateral ureteral obstruction (UUO) and examine TGF-β/Smads signaling. Rats were randomized to (n=12/group): sham operation, UUO, UUO with losartan, UUO with moderate Cili dose (3 g/kg/d), and UUO with high Cili dose (6 g/kg/d). The rats were sacrificed after 14 days of treatment. Collagen deposition was tested using Masson's staining. TGF-β/Smads signaling was examined by qRT-PCR, western blot, and immunohistochemistry. Rats in the UUO group showed excessive deposition of collagen in kidney interstitium, accompanied with high levels of renal 8-hydroxy-2′-deoxyguanosine, renal malondialdehyde, blood urea nitrogen (BUN), serum creatinine (Scr), and proteinuria (all P<0.05). Cili powder efficiently alleviated the pathological changes and oxidative stress in the kidneys of UUO rats, and decreased BUN, Scr and proteinuria (all P<0.05). Cili powder also inhibited the upregulation of TGFB1, TGFBR1, TGFBR2, SMAD2, and SMAD3 and reversed the downregulation of SMAD7 in obstructed kidneys (mRNA and protein) (all P<0.05). In summary, the results suggest that Cili freeze-dried powder effectively prevents renal fibrosis and impairment in UUO rats, which is associated with the inhibition of oxidative stress and TGF-β1/Smads signaling.
1. Introduction
Renal fibrosis is a consequence of progressive kidney diseases such as chronic infection, obstruction of the ureters, hypertension, and diabetic nephropathy [1, 2]. Renal fibrosis is characterized by tubulointerstitial fibrosis and glomerulosclerosis, both caused by the excessive deposition of collagens and extracellular matrix (ECM) components. Although the kidney can regenerate after acute injury, renal fibrosis is usually irreversible [3].
Transforming growth factor-beta (TGF-β)/Smads signaling plays a pivotal role in renal fibrosis [4–6]. Elevated TGFβ1 and downstream Smad3 and Smad2 in the kidney activate profibrotic genes and mediate renal fibrosis [5]. On the other hand, Smad7 suppresses renal fibrosis by altering the expression of TGF-β/Smad3 through microRNAs [7]. Inhibition of the TGF-β1/Smad2/3 pathway alleviates kidney injury and renal fibrosis [8, 9].
The traditional Chinese medicine (TCM) is based on a personalized approach aiming to restore the balance between Yin and Yang [10, 11]. There are hundreds of TCM formulation against chronic kidney diseases (CKD) [12, 13]. Some TCM components were studied (including Astragalus, Angelica sinensis, Rheum plantarum, Radix bupleuri, Cordyceps sinensis, and Tripterygium wilfordii [11]) and their effects include anti-inflammation, antioxidation, antifibrosis, anticoagulation, and immune system modulation [13, 14]. On the other hand, some TCM components can be toxic to the kidney [11, 15], hence the importance of characterizing them carefully.
Rosa roxburghii is a member of the rose family and is a wild plant from the Guizhou Province and distributed in south China. In TCM, the fruit of Rosa roxburghii (named Cili or CL) improves immune response, enhances digestive ability, and possesses antiaging effects [16]. Chemical analysis revealed that Cili is rich in antioxidants including superoxide dismutase (SOD), vitamin C, vitamin E, and flavonoids [17, 18]. Cili has a long history in China and has been proven to possess antioxidant [19, 20], antiatherosclerosis [21], antitumor [22], and radioprotective [23] effects. Nevertheless, the exact molecular mechanisms responsible for these beneficial effects are not well understood.
Our preliminary data showed that freeze-dried Cili powder improved renal fibrosis and indexes of oxidative stress in 90 patients with stages 3-4 renal failure [24], but the exact mechanisms responsible for these effects of Cili on kidney diseases are unknown. We hypothesized that Cili could prevent the development of renal fibrosis through mediating TGF-β/Smads signaling, which is known to be involved in renal fibrosis [4–6]. Therefore, this study established a renal fibrosis rat model induced by unilateral ureteral obstruction (UUO) in order to confirm the effects of freeze-dried Cili powder on renal fibrosis and examine TGF-β/Smads signaling.
2. Materials and Methods
2.1. Animals
Sixty specific pathogen-free- (SPF-) grade male Sprague-Dawley rats (140-160 g) were provided by Shanghai Sippr/BK Laboratory Animal Ltd., Co. (Shanghai, China; certificate number: DA942C48-72F76A1E-466ABC83-FC6974). The rats were kept at the Experimental Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, under controlled temperature (25°C), humidity (45-55%), and light (12 h light/dark cycle). The animals were allowed free access to food (standard chow) and water. All rats were kept one week before experiments. All experimental procedures were performed according to the local Animal Care Committee's guidelines.
2.2. Unilateral Ureteral Obstruction (UUO) Modeling and Treatment
According to the principles of sample size estimation in Chinese medicine pharmacology research [25], “When using small animals (mice or rats) to carry out the experiments and comparing categorical data among 3-5 groups, the number of animals in each group should be no smaller than 8-10”. Some other studies with a similar methodology also used 8-10 rats/group [26, 27]. Being prudent, a sample size of 12 rats/group was used in the present study. The rats were randomized to (n=12/group): sham operation group (Sham), UUO group (UUO), UUO rats treated with losartan (UUO+losartan), UUO rats treated with moderate dose of Cili freeze-dried powder (UUO+CL moderate), and UUO rats that received high dose of Cili freeze-dried powder (UUO+CL high). Rats that underwent UUO and were treated with resveratrol were used as a positive control group.
UUO was performed as previously described [28]. Briefly, animals were anesthetized with 2% pelltobarbitalum natricum (0.6-0.8 mL/rat). The left kidney was exposed and separated through a 2-3 cm flank incision. The left ureter was ligated at two locations with 4-0 silk and cut between the two ligatures. In the sham-operated rats, the left ureter was separated but not ligated or cut.
All rats were treated on the next day after successful modeling. The conversion formula for the drug dose in rats (in g/kg) is [clinical dose in humans, in g/kg]×6.2 [29]. Our previous study found that the equivalent dose for patients with chronic renal failure at stages 3-4 was about 40 g/day of freeze-dried powder of Rosa roxburghii fruit (Pharmacy Department, The First Affiliated Hospital, Guiyang College of Traditional Chinese Medicine) by oral administration [24]. The reference human body weight is about 60 kg [29]. Therefore, the equivalent dose for rats using this kind of Cili freeze-dried powder should be 40 g/60 kg × 6.2 =4 g/kg. The content of active ingredients (flavonoids) is twice higher in commercially available Cili freeze-dried powder (GuizhouJincili Technology Development Co., Ltd., Guizhou, China) compared with home-made preparation, due to optimized manufacturing processes [30]. Hence, the equivalent dose for rats using the commercially available Cili freeze-dried powder is 2 g/kg. In the present study, the dose of Cili freeze-dried powder (Guizhou Jincili Technology Development Co., Ltd., Guizhou, China) was 3 g/kg/d for the UUO+CL moderate group (1.5 times the equivalent dose) and 6 g/kg/d for the UUO+CL high group (three times the equivalent dose). The rats in the sham and UUO groups were given the same volume of sterile saline. Losartan (losartan potassium, lot: L037309; Merck Frost, Montreal, Canada) was given by oral gavage at 10 mg/kg/d (20% concentration). All experimental procedures and care were performed at the same time, once per day, for 14 days. According to previous studies [31, 32], early renal fibrosis is observed in UUO rats from 3 days after surgery, most of the quantitative pathological changes occur about 7 days after surgery, and obvious renal fibrosis is observed on the 14th day after surgery. Therefore, 14 days of treatment was used for the prevention of renal fibrosis in the present study. According to similar studies [33, 34], 14 days of traditional Chinese medicine intervention in UUO model rats showed treatment effectiveness.
The rats were sacrificed on day 14 after gavage under general anesthesia with 3.5% isoflurane. Both kidneys were collected, rinsed with ice-cold saline, dissected and frozen in liquid nitrogen or fixed in 5% PBS-buffered formalin, rinsed with phosphate buffer, dehydrated using a graded series of ethanol and xylene, and embedded in paraffin.
2.3. Histopathology
Paraffin sections (4 μm thick) were stained with hematoxylin and eosin (HE) for histopathological examination. Kidney fibrosis was evaluated by Masson's trichrome staining, as described previously [35]. The amount of collagen-positive (blue) pixels in the stained sections was quantified by automatic analysis using the Image-Pro Plus 6 software (Media Cybernetics, Rockville, MD, USA). Eight random fields on each section were selected for analysis. The area of interest (AOI) was acquired based on the total amount of stained tissue per high-power field. Integrated optical density (IOD) of blue pixels was determined and the percentage of blue pixels in the AOI was automatically calculated.
2.4. Immunohistochemistry
Paraffin-embedded kidneys were sectioned at 4 μm. The sections were air-dried, dewaxed, and rehydrated in graded ethanol and phosphate buffered saline (PBS). Endogenous peroxidase activity was blocked using 3% hydrogen peroxide and the sections were blocked with 10% goat serum. The primary antibodies were anti-TGF-β1(1:200; Boster Bioengineering Co., Wuhan, China), anti-TGF-β1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Smad2 (1:200; #ab40855; Abcam, Cambridge, MA, USA), anti-Smad7 (1:200; #BA1499, Boster Bioengineering Co., Wuhan, China), and anti-Smad3 (1:1000; #ab40854, Abcam, Cambridge, MA, USA). All sections were subsequently incubated with the respective secondary antibodies at room temperature for 1 h. Peroxidase-labeled polymer and diaminobenzidine (DAB) were used to visualize staining. Three to five sections of each specimen were used for immunohistochemistry. A qualitative analysis was first performed; according to the location of the specific protein in the control group (e.g., the cortical part), the same location was analyzed in the treatment groups. Semiquantitative analysis was then conducted using eight randomly selected nonoverlapping fields (×400) from each section. The quantitative analysis was conducted using Image Pro Plus 6.0.
2.5. Measurement of Oxidative Stress Markers
Rat kidneys were minced and added to normal saline [for 8-hydroxy-2′-deoxyguanosine (8-OHdG) measurement, the solution was PBS (pH 7.4) instead] using a ratio of weight (g):volume (ml) of 1:9. The samples were homogenized on ice and centrifuged at 2750 rpm for 10 min, and the supernatant was collected. The protein concentration was determined using the Coomassie bright blue kit (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). SOD activity was determined using the xanthine oxidase method. The samples were incubated with the substrate, enzyme, and buffer mixture at 37°C for 20 min, according to the manufacturer's recommendations (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). The reaction product was detected at 450 nm. The results were expressed as units per milligram of protein (U/mg protein). MDA amount was determined using the thiobarbituric acid (TBA) method. The samples were incubated with the reaction buffer at 95°C for 40 min, according to the manufacturer's recommendations (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). The MDA-TBA product was detected at 535 nm. The results were expressed as nmol per milligram of protein (nmol/mg protein). 8-OHdG amount was determined using competitive ELISA (No. H165; Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).The microplates were read at 450 nm.
2.6. Quantitative Real-Time PCR
Total RNA was isolated from frozen kidney samples and subjected to reverse transcription to obtain cDNA. Quantitative real-time PCR was performed using the SYBR Green PCR Kit (Qiagen, Venlo, The Netherlands) on a LightCycler® 480 System (Roche Molecular Systems, Pleasanton, CA, USA). GAPDH was used as internal reference. The RT-PCR primers are listed in Table 1. The PCR conditions were (1) 95°C for 30 s and (2) 40 cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 20 s.
Table 1.
Primers for quantitative RT-PCR.
| Gene | Primer | Sequence (5′->3′) | Size (bp) |
|---|---|---|---|
| GAPDH | Forward | CATGAGAAGTATGACAACAGCCT | 113 |
| Reverse | AGTCCTTCCACGATACCAAAGT | ||
| SMAD2 | Forward | GCCGCCCGAAGGGTAGAT | 164 |
| Reverse | TTCTGTTCTCCACCACCTGC | ||
| SMAD7 | Forward | GGTGCGTGGTGGCATACT | 144 |
| Reverse | GCTGACTCTTGTTGTCCGAAT | ||
| TGFBR1 | Forward | GCAATGGGCTTAGTATTCTGG | 76 |
| Reverse | AAGGCAACTGGTAGTCTTCGTG | ||
| TGFBR2 | Forward | TGTGGAGGAAGAACGACAAGA | 78 |
| Reverse | GAGTGAAGCCGTGGTAGGTG | ||
| TGFB1 | Forward | CATGGAGCTGGTGAAACGGAAG | 74 |
| Reverse | GACTGGCGAGCCTTAGTTTGGAC | ||
| SMAD3 | Forward | CGATGTCCCCAGCACACAATAAC | 82 |
| Reverse | TAGTAGGAGATGGAGCACCAAAAGG |
2.7. Western Blot
Western blot analyses were performed using antibodies against TGF-βR1 (1:50; Wuhan Boster Bio-Engineering Limited Company, Wuhan, China), TGF-βR2 (1:50; Wuhan Boster Bio-Engineering Limited Company, Wuhan, China), Smad7 (1:200; #BA1399; Wuhan Boster Bio-Engineering Limited Company, Wuhan, China), SIRT1 (1:200; #ab110304; Abcam, Cambridge, MA, USA), Smad2 (1:200; #ab40855; Abcam, Cambridge, MA, USA), Smad3 (1:200; #ab40854; Abcam, Cambridge, MA, USA), TGF-β1 (1:500; #ab92486; Abcam, Cambridge, MA, USA), and GAPDH (1:10,000; Abcam, Cambridge, MA, USA).
2.8. Biochemistry
The serum levels of blood urea nitrogen (BUN) and creatinine (Scr) were detected with assay kits from the Nanjing Jiancheng Bioengineering Institute (C013-1 for BNU and C011-1 for creatinine; Nanjing, China), according to the manufacturer's instructions. Twenty-four-hour urine samples were collected using stainless steel metabolic cages at indicated time points. Urine was centrifuged at 12,000 ×g for 15 min at 4°C and the supernatant was used for detection. Urine protein (Upro) concentrations were determined using a urinary protein assay kit (C035-2; Nanjing Jiancheng Bioengineering Institute; Nanjing, China).
2.9. Statistical Analyses
All data were verified using the Kolmogorov-Smirnov test and were found to follow the normal distribution. The results were expressed as means ± standard deviation. The differences among the groups were analyzed using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post hoc test. All analyses were performed using SPSS 18.0 (IBM, Armonk, NY, USA). The differences were considered statistically significant at P<0.05 (two-sided).
3. Results
3.1. Analysis of the Freeze-Dried Powder of Rosa roxburghii
In order to ensure the consistency among batches of freeze-dried powder of Rosa roxburghii, samples were sent to and tested by the China National Analytical Center in Guangzhou (China). The results showed that the flavonoids content was the same (0.4%) for all three batches, while the polysaccharides contents were 5.5%, 6.2%, and 5.7%, respectively (mean of 5.8%). Table 2 presents the exact content of some nutrients. SOD activity was 2.88 × 104 U/g.
Table 2.
Nutrient content of freeze-dried powder of Rosa roxburghii.
| Item | Value | Unit |
|---|---|---|
| Aspartate | 8.3 | mg/100g |
| Glutamate | 10 | |
| Serine | 13.1 | |
| Glycine | 88 | |
| Threonine | 11.2 | |
| Histidine | 4.4 | |
| Alanine | 58.9 | |
| Arginine | 147 | |
| Tyrosine | 7.4 | |
| Valine | 15.3 | |
| Methionine | 2 | |
| Phenylalanine | 3.6 | |
| Isoleucine | 7.6 | |
| Leucine | 3.4 | |
| Lysine | 11.5 | |
| Proline | 6.2 | |
| All amino acids | 398 | |
| Total flavonoids | 361 | mg/100g |
| Vitamin C | 6.8 | mg/g |
| Vitamin P | 0.346 | |
| Superoxide dismutase activity | 2.88 × 104 | U/g |
3.2. Cili Administration Inhibits the Pathological Changes in Kidney after UUO
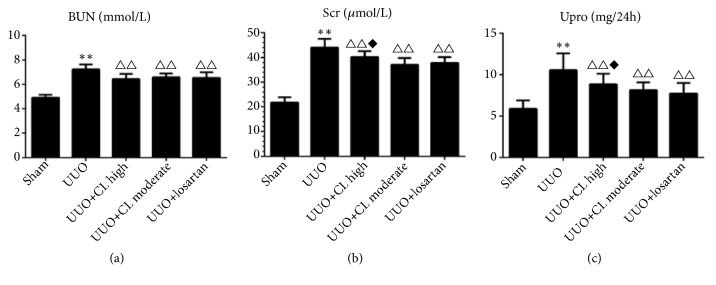
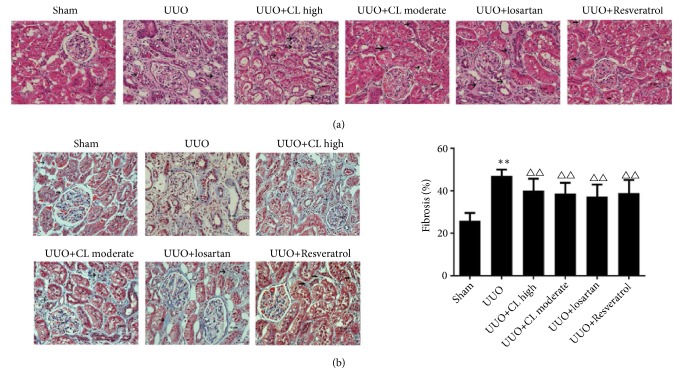
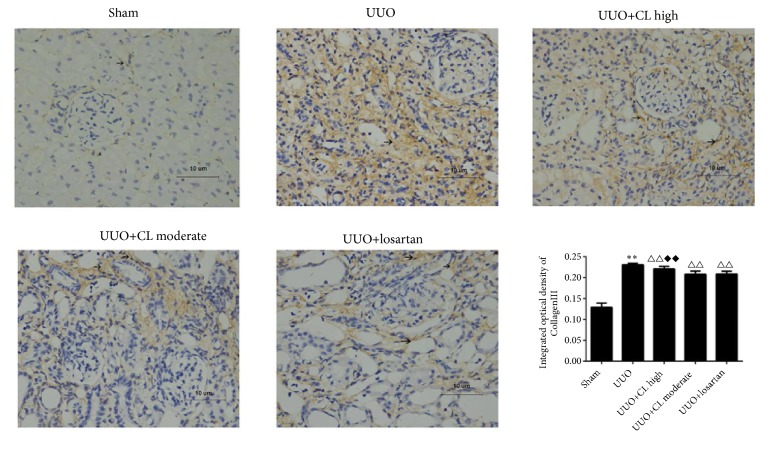
The markers of kidney damage (BUN, serum creatinine, and 24 h urine protein) were higher in the UUO group than in the sham group (+46.9%, +84.3%, and 49.3%, all P<0.05) but were decreased after treatment with CL moderate dose (-9.8%, -12.5%, and -22.1%, resp., all P<0.01) or losartan (-11.1%, -15.3%, and -24.0%, all P<0.01) (Figure 1). Backing those results of kidney function markers, compared to the sham-operated kidneys, the obstructed kidneys from the UUO group showed tubular congestion, dilatation, and interstitial expansion (Figure 2(a)), which are pathological changes associated with kidney injury. The moderate dose of Cili freeze-dried powder (CL moderate), as well as losartan, attenuated the pathological changes induced by UUO (Figure 2(a)). In addition, collagen synthesis was high in the interstitium of kidneys from the UUO group (Figure 2(b)). The level of fibrosis was increased by UUO (+278.5%, P<0.01), but it was prevented by CL moderate dose (-16.2%, P<0.01), losartan (-22.9%, P<0.01), and resveratrol (-∗∗∗%, P<0.01) (Figure 2(b)). This was confirmed by immunohistochemistry for collagen III (Figure 3). Taken together, those results suggest that Cili could prevent renal fibrosis after UUO, with similar effects to those of losartan and resveratrol, which are renal protective agents.
Figure 1.
Cili alleviates the functional changes in kidneys after unilateral ureteral obstruction (UUO). Sixty rats were randomized to five groups: sham operation, UUO, UUO with losartan (UUO+losartan), UUO with moderate Cili dose (3 g/kg/d) (UUO+CL moderate), and UUO with high Cili dose (6 g/kg/d) (UUO+CL high). Biochemical markers indicative of kidney functions were measured, including (a) blood urea nitrogen (BUN), (b) serum creatinine (Scr), and (c) urine protein (Upro) levels. N=12/group. The results are expressed as mean ± standard deviation (SD). ∗∗P<0.01 vs. the sham group; △△P<0.01 vs. the UUO group; ◆P<0.05 vs. the losartan group.
Figure 2.
Cili alleviates the morphological changes in kidneys after unilateral ureteral obstruction (UUO). Sixty rats were randomized to six groups: sham operation, UUO, UUO with losartan (UUO+losartan), UUO with moderate Cili dose (3 g/kg/d) (UUO+CL moderate), UUO with high Cili dose (6 g/kg/d) (UUO+CL high), and UUO with resveratrol (UUO+Resveratrol). (a) Hematoxylin and eosin (H&E) staining of kidneys was performed. Lesions such as glomerular hypertrophy and vacuolation of renal tubular epithelial cells are indicated by arrows. (b) Masson's staining of kidneys and quantification of the fibrosis area. Scale bar: 10 μm. N=12/group. The results are expressed as mean ± standard deviation (SD). ∗∗P<0.01 vs. the sham group; △△P<0.01 vs. the UUO group.
Figure 3.
Cili attenuates collagen deposition in kidneys after UUO. Sixty rats were randomized to five groups: sham operation, UUO, UUO with losartan (UUO+losartan), UUO with moderate Cili dose (3 g/kg/d) (UUO+CL moderate), and UUO with high Cili dose (6 g/kg/d) (UUO+CL high). Immunohistochemistry for collagen III in kidneys and quantification. Scale bar: 10 μm. N=12/group. The results are expressed as mean ± SD. ∗∗P<0.01 vs. the sham group; △△P<0.01 vs. the UUO group; ◆◆P<0.01 vs. the losartan group.
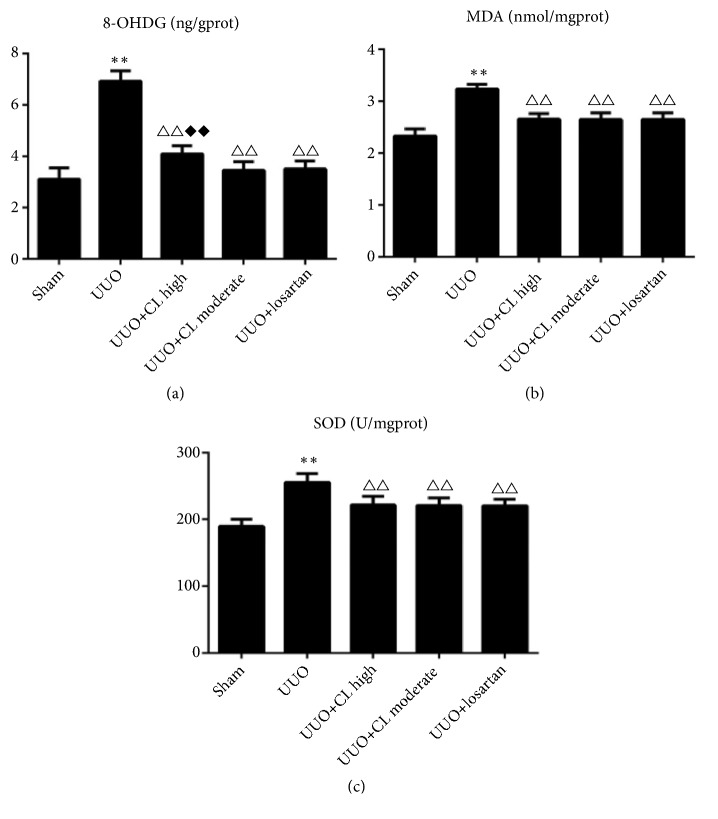
3.3. Cili Powder Protects the Obstructed Kidneys from Severe Oxidative Stress
To determine the oxidative stress in the kidneys, the levels of 8-OHdG, MDA, and SOD were measured. The levels of 8-OHdG, MDA, and SOD were all increased by UUO modeling (all P<0.01) (Figure 4). Cili (both moderate and high doses) and losartan decreased the 8-OHdG, MDA, and SOD levels compared with the UUO group (all P<0.01) (Figure 4). The decrease of 8-OHdG of the UUO+CL high dose group was a little smaller than that of the UUO+losartan group (P<0.001) (Figure 4). Those results suggest that Cili prevented oxidative stress development after UUO, in a similar way as in the losartan group.
Figure 4.
Cili improves the markers of oxidative stress in kidneys after UUO. Sixty rats were randomized to five groups: sham operation, UUO, UUO with losartan (UUO+losartan), UUO with moderate Cili dose (3 g/kg/d) (UUO+CL moderate), and UUO with high Cili dose (6 g/kg/d) (UUO+CL high). (a) Renal 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels. (b) Renal malondialdehyde (MDA) levels. (c) Renal superoxide dismutase (SOD) levels. N=12/group. The results are expressed as mean ± SD. ∗∗P<0.01 vs. the sham group; △△P<0.01 vs. the UUO group; ◆◆P<0.01 vs. the losartan group.
3.4. Cili Powder Alleviates Renal Fibrosis Which Is Associated with the Inhibition of TGF-β/Smads Signaling
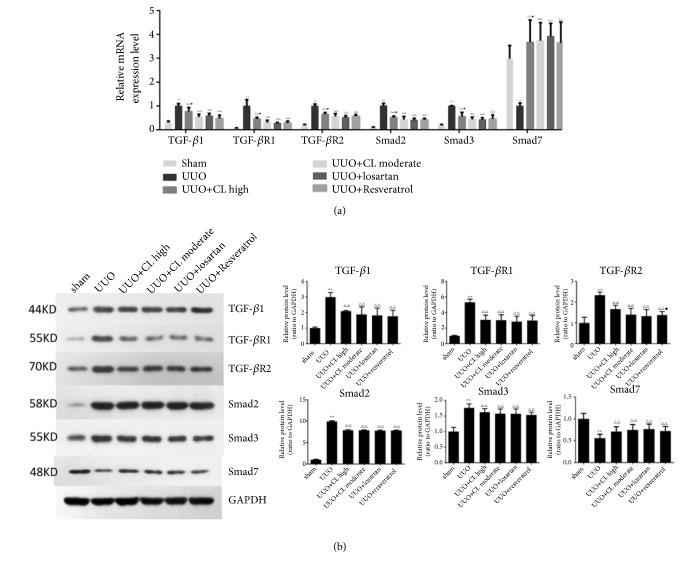
Because the TGF-β/Smads pathway plays a pivotal role in renal fibrosis, the effects of Cili powder on the mRNA and protein expressions of TGF-β, TGF-β receptors, and Smads were investigated in the kidney. In the UUO group, the mRNA levels of TGFB1, TGFBR1, TGFBR2, SMAD2, and SMAD3 were significantly upregulated (P<0.01), while the protective SMAD7 was decreased (P<0.01) (Figure 5(a)). Treatment with losartan, resveratrol, or Cili powder efficiently prevented the changes in TGFB1, TGFBR1, TGFBR2, SMAD2, SMAD3, and SMAD7 (Figure 5(a)) (P<0.01 vs. UUO without treatment).
Figure 5.
Cili modulates the mRNA and protein expressions of proteins involved in the TGF-β/Smads pathway in kidneys after UUO. Sixty rats were randomized to six groups: sham operation, UUO, UUO with losartan (UUO+losartan), UUO with moderate Cili dose (3 g/kg/d) (UUO+CL moderate), UUO with high Cili dose (6 g/kg/d) (UUO+CL high), and UUO with resveratrol (UUO+resveratrol). (a) Renal expression of the TGFB1, TGFBR1, TGFBR2, SMAD2, SMAD3, and SMAD7 genes detected by quantitative RT-PCR. GAPDH was used as an internal control, and the relative mRNA level of each gene was normalized to the mRNA levels in the UUO group. (b) Renal expressions of the TGFB1, TGFBR1, TGFBR2, SMAD2, SMAD3, and SMAD7 proteins detected by western blot. N=12/group. The results are expressed as mean ± SD. ∗∗P<0.01 vs. the sham group;△P<0.05 and △△P<0.01 vs. the UUO group; ◆P<0.05 vs. the losartan group.
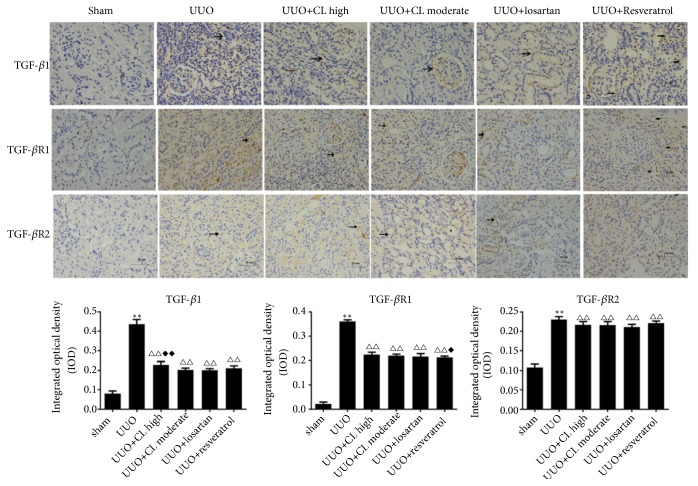
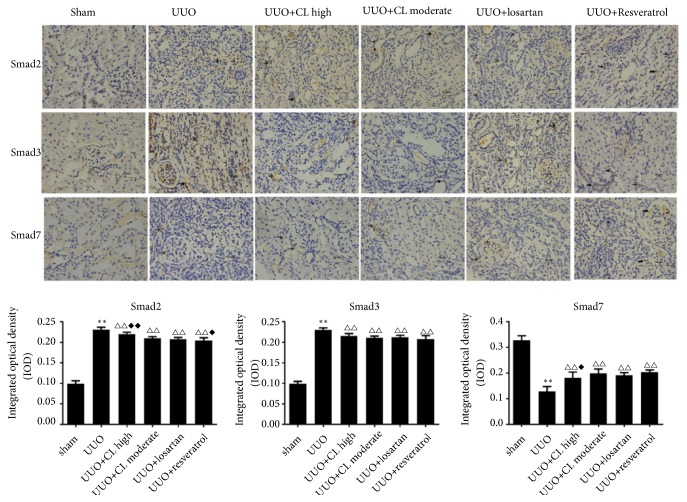
At the protein level, Cili moderate and high dose, losartan, and resveratrol inhibited the expression of TGF-β1, TGF-βR1, TGF-βR2, and Smad3, but high dose of Cili powder did not show a better antagonistic effect on the expression of any TGF-β/Smad proteins (Figure 5(b)). According to immunohistochemistry, Cili, losartan, and resveratrol inhibited the expression of TGF-β1, TGF-βR1, and Smad3, while maintaining Smad7 expression in obstructed kidneys (Figures 6 and 7), which is consistent with the RT-PCR results. Taken together, the results suggest that Cili powder improved renal fibrosis, possibly through the inhibition of the TGF-β/Smads pathway.
Figure 6.
Cili decreases the protein expression of members of the TGF-β signaling pathway in kidneys after UUO. Representative immunohistochemistry images of key proteins in the TGF-β signaling in kidneys are shown with quantification. Scale bar: 10 μm. Positive staining is indicated by arrows. N=12/group. The results are expressed as mean ± SD. ∗∗P<0.01 vs. the sham group; △△P<0.01 vs. the UUO group; ◆◆P<0.01 vs. the losartan group.
Figure 7.
Cili decreases the protein expression of Smad2 and Smad3 and increases the expression of Smad7 in kidneys after UUO. Representative immunohistochemistry images of key proteins in the Smads signaling in kidneys are shown with quantification. Scale bar: 10 μm. Positive staining is indicated by arrows. N=12/group. The results are expressed as mean ± SD. ∗∗P<0.01 vs. the sham group; △△P<0.01 vs. the UUO group; ◆P<0.05 vs. the losartan group.
4. Discussion
Since fibrosis is involved in the pathogenesis of kidney diseases [1, 2], since Cili possesses antifibrosis properties [16, 19, 20], and since our preliminary data showed that freeze-dried Cili powder improved renal fibrosis and indexes of oxidative stress in 90 patients with stages 3-4 renal failure [24], we hypothesized that Cili could prevent the development of renal fibrosis in rat models of UUO through TGF-β/Smads signaling, which is known to be involved in renal fibrosis [4–6]. Therefore, this study aimed to confirm the effects of freeze-dried Cili powder on renal fibrosis and examine the TGF-β/Smads signaling. The results showed that Cili freeze-dried powder could effectively prevent the phenotype of renal fibrosis in UUO rat models. The beneficial effects may result from the inhibition of oxidative stress and TGF-β1/Smads signaling pathway. Therefore, we accepted the research hypothesis.
The incidence of chronic kidney diseases is increasing, and current treatments are limited to angiotensin II receptor blockers and angiotensin converting enzyme inhibitors. Losartan is an angiotensin II receptor inhibitor and can inhibit interstitial fibrogenesis by modulating nitric oxide synthase (NOS) isoforms and cyclooxygenase-2 (COX-2) expression [36], decreasing oxidative stress in the kidneys [37, 38] and inhibiting MCP1 and TGF-β1 expression in rats with UUO [39]. Resveratrol is a well-known kidney protective agent through potent reactive oxygen species scavenging abilities [40]. Resveratrol has been shown to protect against renal injury in models of diabetic nephropathy, drug-induced injury, aldosterone-induced injury, ischemia/reperfusion injury, sepsis, and UUO [40]. Therefore, losartan and resveratrol were selected as positive controls for the prevention of renal fibrosis in the UUO model.
Cili, used as a dietary supplement, has been shown to enhance the antioxidant status [17–20]. Unlike losartan, the effects of Cili extract on chronic renal diseases are still poorly understood, which limits its wide application. We previously used freeze-dried Cili powder (oral administration) to treat 90 patients with stages 3-4 chronic renal failure [24]. Therapeutic effects were achieved based on the evaluation of syndrome improvement, renal fibrosis indexes, and oxidative stress indexes. The present findings in rats confirmed the effects of Rosa roxburghii fruit extracts in attenuating the development of renal fibrosis in mammals. In the present study, 8-OHdG and MDA in the UUO kidneys were increased as indicatives of the renal oxidative stress induced upon injury, which could be significantly reduced by the administration of Cili freeze-dried powder. In addition, there was a higher level of SOD in the UUO kidneys as compared to the normal ones, suggesting the endogenous regulation to counteract the renal oxidative stress. Cili freeze-dried powder restored the counterregulatory rise of renal SOD in UUO rats, possibly because the antioxidant effects have been achieved. The effects observed with Cili were comparable to those of resveratrol, a potent antioxidant [40].
Notably, high-dose Cili did not result in better efficacy in preventing renal fibrosis in the UUO rat model, as revealed by the markers of kidney function, histological examination, collagen III expression, and oxidative stress. We supposed that, apart from supplying more antioxidants, high-dose Cili powder may also put a heavier excretion load on the obstructed kidney, as observed with some TCM preparations [11], limiting its efficacy at high doses, where benefits are limited by harms. A similar example is that high-dose vitamin C can induce hyperoxaluric nephropathy and progressive renal failure [41]. Therefore, since Cili is not a pure compound but a mixture of several compounds with different biological effects, a comprehensive analysis of each of the major or biologically significant Cili component will contribute to a better understanding of the effects of Cili on renal diseases.
Reactive oxygen species are important mediators of kidney damage and fibrosis, but they are not the only factors involved; many others are involved, including the various players (genes, proteins, and miRNAs) of the HIF, TGF-β, Notch, PKC/ERK, PI3K/Akt, NF-κB, and Ang II/ROS pathways [42]. The present results provided some clues for the mechanisms underlying the effects, with a focus on the TGFβ1/Smads pathway. The positive control resveratrol was also shown to inhibit renal fibrosis through the TGFβ1/Smads pathway [43], supporting the present study. As the dysregulation of the TGFβ1/Smads pathway is central in the progression of renal fibrosis [44, 45], any intervention that could modulate this pathway should play important roles in the management of the condition. Additional parameters (e.g., leukocyte infiltration [46], fibroblasts, and apoptosis [47]) need to be taken into account and to be investigated in the future. A better understanding of the mechanisms involved in renal fibrosis could lead to improved treatments in those patients.
A potential limitation of the present study was that Cili was given concomitantly with UUO. Hence, the effects observed in the present study were more preventive effects than treatment effects. Future studies should first establish renal fibrosis after UUO and then observe the effects of Cili on the kidneys. In addition, the involvement of TGF-β1/Smads signaling pathway will need to be confirmed using knock-out models and/or siRNAs. Furthermore, a number of other pathways are involved in renal fibrosis (e.g., HIF, TGF-β, Notch, PKC/ERK, PI3K/Akt, NF-κB, and Ang II/ROS pathways [42]) and those pathways should be explored in order to gain a more comprehensive understanding of the effects of Cili on renal fibrosis.
5. Conclusions
In conclusion, the results demonstrate that Cili freeze-dried powder could effectively prevent renal fibrosis and injury in UUO rat models, suggesting the underlying mechanisms may involve the inhibition of oxidative stress and TGF-β1/Smads signaling.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81460728).
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper
References
- 1.Al-Bayati M. A., Xie Y., Mohr F., Margolin S. B., Giri S. N. Effect of pirfenidone against vanadate-induced kidney fibrosis in rats. Biochemical Pharmacology. 2002;64(3):517–525. doi: 10.1016/S0006-2952(02)01213-3. [DOI] [PubMed] [Google Scholar]
- 2.Yang H.-C., Zuo Y., Fogo A. B. Models of chronic kidney disease. Drug Discovery Today: Disease Models. 2010;7(1-2):13–19. doi: 10.1016/j.ddmod.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen E. P. Fibrosis causes progressive kidney failure. Medical Hypotheses. 1995;45(5):459–462. doi: 10.1016/0306-9877(95)90221-x. [DOI] [PubMed] [Google Scholar]
- 4.Jung K.-J., Min K.-J., Park J.-W., Park K. M., Kwon T. K. Carnosic acid attenuates unilateral ureteral obstruction-induced kidney fibrosis via inhibition of Akt-mediated Nox4 expression. Free Radical Biology & Medicine. 2016;97:50–57. doi: 10.1016/j.freeradbiomed.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Lan H. Y. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. International Journal of Biological Sciences. 2011;7(7):1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B., Mu J., Gong Y., et al. Brd4 inhibition attenuates unilateral ureteral obstruction-induced fibrosis by blocking TGF-β-mediated Nox4 expression. Redox Biology. 2017;11:390–402. doi: 10.1016/j.redox.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung A. C. K., Dong Y., Yang W., Zhong X., Li R., Lan H. Y. Smad7 suppresses renal fibrosis via altering expression of TGF-β/Smad3-regulated microRNAs. Molecular Therapy. 2013;21(2):388–398. doi: 10.1038/mt.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi S., Zou Y., Togao O., et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. The Journal of Biological Chemistry. 2011;286(10):8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y. B. Y., Qu X., Caruana G., Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102–107. doi: 10.1016/j.diff.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z., Liang A. New theoretical study on the biological origins of Chinese medicinal herbs. Zhongguo Zhong Yao Za Zhi. 1995;20(5):261–318. [PubMed] [Google Scholar]
- 11.Zhong Y., Deng Y., Chen Y., Chuang P. Y., Cijiang He J. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney International. 2013;84(6):1108–1118. doi: 10.1038/ki.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L. D., Jing Y. Z., Li Y. O. Integrating traditional and Western medicine to research and develop Chinese new drugs. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16(5):301–304. [PubMed] [Google Scholar]
- 13.Li X. M., Wang H. Y. Chinese herbal medicine in the treatment of chronic kidney disease. Advances in Chronic Kidney Disease. 2005;12(3):276–281. doi: 10.1016/j.ackd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Wojcikowski K., Johnson D. W., Gobe G. Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. Journal of Laboratory and Clinical Medicine. 2006;147(4):160–166. doi: 10.1016/j.lab.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Bagnis C. I., Deray G., Baumelou A., Le Quintrec M., Vanherweghem J. L. Herbs and the kidney. American Journal of Kidney Diseases. 2004;44(1):1–11. doi: 10.1053/j.ajkd.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Li S. Y., Huang X. E., Cui J. J., Zhao T., Zhang H. Inhibition of tumor growth in vitro by a combination of extracts from Rosa roxburghii Tratt and Fagopyrum cymosum. Asian Pacific Journal of Cancer Prevention. 2012;13(5):2409–2414. doi: 10.7314/apjcp.2012.13.5.2409. [DOI] [PubMed] [Google Scholar]
- 17.An H. M., Liu M., Yang M., Fan W. G. Analysis of main organic acid compositions in Rosa roxburghii Tratt. Scientia Agricultura Sinica. 2011;2011(10):p. 15. [Google Scholar]
- 18.Liu M., Zhang Q., Zhang Y., Lu X., Fu W., He J. Chemical analysis of dietary constituents in rosa roxburghii and rosa sterilis fruits. Molecules. 2016;21(9):p. 1204. doi: 10.3390/molecules21091204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rensburg C. J., Erasmus E., Loots D. T., et al. Rosa roxburghii supplementation in a controlled feeding study increases plasma antioxidant capacity and glutathione redox state. European Journal of Nutrition. 2005;44(7):452–457. doi: 10.1007/s00394-005-0555-x. [DOI] [PubMed] [Google Scholar]
- 20.van der Westhuizen F. H., van Rensburg C. S., Rautenbach G. S., et al. In vitro antioxidant, antimutagenic and genoprotective activity ofRosa roxburghii fruit extract. Phytotherapy Research. 2008;22(3):376–383. doi: 10.1002/ptr.2330. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C., Liu X., Qiang H., et al. Inhibitory effects of rosa roxburghii tratt juice on in vitro oxidative modification of low density lipoprotein and on the macrophage growth and cellular cholesteryl ester accumulation induced by oxidized low density lipoprotein. Clinica Chimica Acta. 2001;313(1-2):37–43. doi: 10.1016/S0009-8981(01)00647-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Liu Z., Liu J., Liu L., Zhang E., Li W. Inhibition of metastasis and invasion of ovarian cancer cells by crude polysaccharides from rosa roxburghii tratt in vitro. Asian Pacific Journal of Cancer Prevention. 2015;15(23):10351–10354. doi: 10.7314/APJCP.2014.15.23.10351. [DOI] [PubMed] [Google Scholar]
- 23.Xu P., Zhang W.-B., Cai X.-H., et al. Flavonoids of Rosa roxburghii Tratt act as radioprotectors. Asian Pacific Journal of Cancer Prevention. 2014;15(19):8171–8175. doi: 10.7314/apjcp.2014.15.19.8171. [DOI] [PubMed] [Google Scholar]
- 24.Zhan J. H., Guo Y. X. Clinical study of cili powder on oxidative stress of CKD 3-4 patients with dampness accumulation associated with spleen kidney deficiency. Chinese Journal of Experimental Traditional Medical Formulae. 2014;20(23):224–226. [Google Scholar]
- 25.Chen Q. Research Methods in Pharmacology of Chinese Materia Medica. 3rd. Beijing, China: People’s Medical Publishing House; 2011. [Google Scholar]
- 26.Wang L., Ma J., Guo C., et al. Danggui buxue tang attenuates tubulointerstitial fibrosis via suppressing nlrp3 inflammasome in a rat model of unilateral ureteral obstruction. BioMed Research International. 2016;2016:12. doi: 10.1155/2016/9368483.9368483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Ma M., Zhang X., et al. Ethanol extract of gardenia fruit alleviates renal interstitial fibrosis induced by unilateral ureteral obstruction in rats. Experimental and Therapeutic Medicine. 2017;14(2):1381–1388. doi: 10.3892/etm.2017.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chevalier R. L., Forbes M. S., Thornhill B. A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney International. 2009;75(11):1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 29.Nair A. B., Jacob S. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S. M., Zhang Q. Q., Zhao J. Experimental study on the influence of the freeze-drying process on the quality of seabuckthorn fruit. Food Research and Development. 2011;32(1):65–67. [Google Scholar]
- 31.Ohashi R., Shimizu A., Masuda Y., et al. Peritubular capillary regression during the progression of experimental obstructive nephropathy. Journal of the American Society of Nephrology. 2002;13(7):1795–1805. doi: 10.1097/01.ASN.0000018408.51388.57. [DOI] [PubMed] [Google Scholar]
- 32.Efrati S., Berman S., Chachashvili A., et al. Rosiglitazone treatment attenuates renal tissue inflammation generated by urinary tract obstruction. Nephrology. 2009;14(2):189–197. doi: 10.1111/j.1440-1797.2008.01032.x. [DOI] [PubMed] [Google Scholar]
- 33.Jiang M., Wang L., Cao A., et al. HuangQi decoction improves renal tubulointerstitial fibrosis in mice by inhibiting the Up-regulation of Wnt/β-catenin signaling pathway. Cellular Physiology and Biochemistry. 2015;36(2):655–669. doi: 10.1159/000430128. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Wang B., Du F., et al. Epigallocatechin-3-gallate attenuates unilateral ureteral obstruction-induced renal interstitial fibrosis in mice. Journal of Histochemistry & Cytochemistry. 2015;63(4):270–279. doi: 10.1369/0022155414568019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen A. H. Masson’s trichrome stain in the evaluation of renal biopsies: an appraisal. American Journal of Clinical Pathology. 1976;65(5):631–643. doi: 10.1093/ajcp/65.5.631. [DOI] [PubMed] [Google Scholar]
- 36.Manucha W., Oliveros L., Carrizo L., Seltzer A., Vallés P. Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney International. 2004;65(6):2091–2107. doi: 10.1111/j.1523-1755.2004.00643.x. [DOI] [PubMed] [Google Scholar]
- 37.Manucha W., Carrizo L., Ruete C., Molina H., Vallés P. Angiotensin II type I antagonist on oxidative stress and heat shock protein 70 (HSP 70) expression in obstructive nephropathy. Cellular and Molecular Biology. 2005;51(6):547–555. doi: 10.1170/T662. [DOI] [PubMed] [Google Scholar]
- 38.Wolf G., Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney International. 2005;67(3):799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y.-Y., Xu A.-P., Zhou S.-S., Fu J.-Z., Du H. Effect of losartan on renal expression of monocyte chemoattractant protein-1 and transforming growth factor-β(1) in rats after unilateral ureteral obstruction. Journal of Southern Medical University. 2011;31(8):1405–1410. [PubMed] [Google Scholar]
- 40.Kitada M., Koya D. Renal protective effects of resveratrol. Oxidative Medicine and Cellular Longevity. 2013;2013:7. doi: 10.1155/2013/568093.568093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon J. M., Chun B. J. The efficacy of high doses of vitamin C in patients with paraquat poisoning. Human & Experimental Toxicology. 2010;30(8):844–850. doi: 10.1177/0960327110385633. [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Ning X., Li R., et al. Signalling pathways involved in hypoxia-induced renal fibrosis. Journal of Cellular and Molecular Medicine. 2017;21(7):1248–1259. doi: 10.1111/jcmm.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., Qu X., Ricardo S. D., Bertram J. F., Nikolic-Paterson D. J. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. The American Journal of Pathology. 2010;177(3):1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding Y., Choi M. E. Regulation of autophagy by TGF-β: emerging role in kidney fibrosis. Seminars in Nephrology. 2014;34(1):62–71. doi: 10.1016/j.semnephrol.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L., Yang T., Lu D., et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomedicine & Pharmacotherapy. 2018;101:670–681. doi: 10.1016/j.biopha.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 46.Jung K. J., Kim J., Park Y. K., Yoon Y. R., Park K. M. Wen-pi-tang-Hab-Wu-ling-san reduces ureteral obstructive renal fibrosis by the reduction of oxidative stress, inflammation, and TGF-beta/Smad2/3 signaling. Food and Chemical Toxicology. 2010;48(2):522–529. doi: 10.1016/j.fct.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Xu P., Cai X., Zhang W., et al. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2(Ca2+)/Caspase-3/PARP-1 pathway. Apoptosis. 2016;21(10):1125–1143. doi: 10.1007/s10495-016-1270-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.