Abstract
Plants, unlike animals, have developed a unique system in which they continue to form organs throughout their entire life cycle, even after embryonic development. This is possible because plants possess a small group of pluripotent stem cells in their meristems. The shoot apical meristem (SAM) plays a key role in forming all of the aerial structures of plants, including floral meristems (FMs). The FMs subsequently give rise to the floral organs containing reproductive structures. Studies in the past few decades have revealed the importance of transcription factors and secreted peptides in meristem activity using the model plant Arabidopsis thaliana. Recent advances in genomic, transcriptomic, imaging, and modeling technologies have allowed us to explore the interplay between transcription factors, secreted peptides, and plant hormones. Two different classes of plant hormones, cytokinins and auxins, and their interaction are particularly important for controlling SAM and FM development. This review focuses on the current issues surrounding the crosstalk between the hormonal and genetic regulatory network during meristem self-renewal and organogenesis.
Keywords: Arabidopsis thaliana, shoot apical meristem, floral meristem, auxin, cytokinin, WUSCHEL, CLAVATA, AGAMOUS
1. Introduction
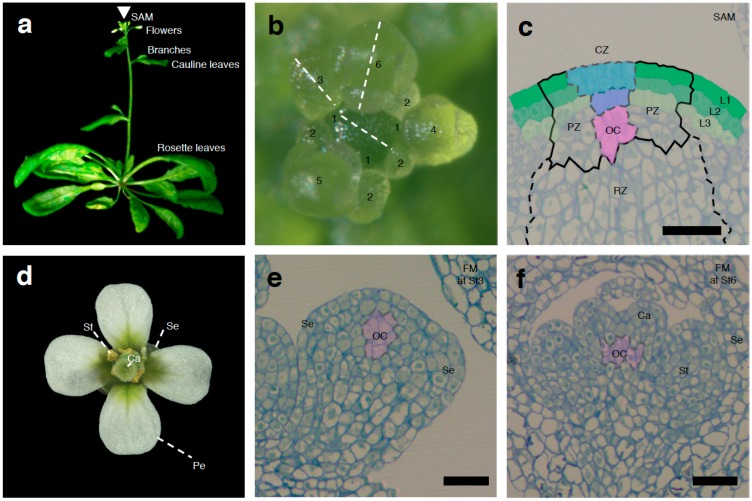
Throughout their entire life cycle, plants possess a small group of pluripotent stem cells in their meristems [1,2,3]. The shoot apical meristem (SAM) at the top of the plant is responsible for postembryonic growth and gives rise to plant aerial structures (Figure 1a) [4]. To sustain proper continuous growth, the SAM maintains the balance between self-renewal of stem cells and cell recruitment for lateral organ formation. Stem cells in the SAM produce daughter cells, which remain stem cells or become differentiated. The SAM also establishes phyllotaxy, i.e., the arrangement of lateral organs along the stem [5,6,7,8,9]. (Figure 1b). In Arabidopsis thaliana, the SAM produces rosette leaves during the vegetative phase (Figure 1a). Just after the floral transition, a few cauline leaves and branches form from the SAM [10] (Figure 1a). During the reproductive phase, flowers are produced from the SAM (Figure 1a). The phase-specific activities of the SAM determine plant architecture (Figure 1a). The SAM is divided into the outer tunica and corpus. The outer layer is composed of epidermal (L1) and subepidermal (L2) layers (Figure 1c). L1 and L2 are single cell layers that divide anticlinally to the plane of the tissue surface. By contrast, the corpus, or inner L3, is a collection of cells whose division occurs in all planes [11]. The SAM harbors a set of stem cells within the central zone (CZ) surrounded by the peripheral zone (PZ) (Figure 1c). Cells located in the CZ divide slowly, while cells located in the PZ divide rapidly (Figure 1c). The balance of cell division in the CZ and PZ determines organ size and number. The PZ is active in the production of lateral organs. The rib zone (RZ) provides multipotent cells to form stem cells, which support the SAM. The CZ acts as a reservoir of stem cells. CZ activity is maintained by the underlying organizing center (OC). The CZ and OC partially overlap and form the stem cell niche (Figure 1c) [12,13].
Figure 1.
Morphology of the shoot apical meristem (SAM) and floral meristems (FM). (a) Side view of an Arabidopsis plant. Aerial tissues form from the SAM, which is located at the top of the plant (arrowhead). Thirty-day-old plants are shown. Lateral organs such as rosette leaves, cauline leaves, branches, and flowers form from the SAM. (b) Overhead view of the SAM. Numbers indicate floral stages [57]. The longitudinal sections shown in (c,e,f) were obtained by cutting across the SAM, stage 3 FM and stage 6 FM along the dashed lines, respectively. Thirty-day-old SAMs and FMs are shown. (c) Organization of the SAM showing functional zones and cell layers. The central zone (CZ) consists of stem cells (blue) and the organizing center (OC) (pink). PZ and RZ are the peripheral and rib zones, respectively. Epidermal (L1) and subepidermal (L2) layers are shown in green and light green, respectively. Scale bar = 20 µm. (d) Top view of an Arabidopsis flower at stage 13. A flower consists of four sepals, six stamens, four petals, and two carpels. A flower from a 30-day-old plant is shown. (e) Top view of the FM at stage 3. Scale bar = 20 µm. (f) Top view of the FM at stage 6. Organization of the FM showing functional zones and cell layers. The OC, which exhibits weak WUS expression only at stage 3 and early stage 6, is shown in pink. Scale bar = 20 µm.
The WUSCHEL (WUS) transcription factor and CLAVATA (CLV) ligand–receptor system are key determinants of meristematic activity in the SAM [14,15,16,17,18]. Mutations in the WUS gene result in premature termination of the SAM after a few organs have formed [16]. WUS, which is expressed in the OC, controls biological processes through the transcriptional regulation of downstream target genes related to meristem growth, cell division, and hormonal signaling [19,20,21,22]. In particular, WUS specifies stem cell identity, partially through the direct activation of CLV3. On the other hand, the loss-of-function CLV3 mutant aberrantly accumulates stem cells in the SAM [23]. CLV3, which is expressed in the CZ, encodes a founding member of the CLAVATA3/ESR-RELATED (CLE) family of small peptides. The CLV3 peptide binds to the CLV receptor proteins [24]. CLV1 encodes a receptor kinase containing an extracellular leucine-rich repeat domain and an intracellular kinase domain. CLV2 is similar to CLV1 but lacks a cytoplasmic kinase domain [12,25,26]. CLV1 forms homodimers, while CLV2 forms heterodimers with the receptor-like cytoplasmic kinase CORYNE (also known as SUPPRESSOR OF LLP1 2 (SOL2)) for signal transduction [12,27,28,29,30]. CLV signaling itself represses WUS expression to restrict its spatial expression domain [12,13]. The negative feedback loop between stem cells and the OC mediated by these two proteins ensures stem cell homeostasis in the SAM and indefinite organ formation (Figure 1d). Components of the CLV-WUS negative feedback pathway are well conserved in model and crop plants [31,32]. In rice (Oryza sativa), FON2-LIKE CLE PROTEIN1 (FCP1) and FCP2, which encode CLV3-related CLE proteins, play key roles in vegetative SAM maintenance [33]. FCP1 and FCP2 act redundantly to repress WUSCHEL RELATED HOMEOBOX4 (WOX4) expression [33]. In maize (Zea mays), ZmFCP1 also acts as a ligand for the receptor kinase FASCIATED EAR3 (FEA3) [34,35]. ZmFCP1 and FEA3 negatively regulate ZmWUS1 expression to maintain reproductive SAM activity [34,35]. Thus, molecular evidence indicates that the CLV-WUS pathway is critical for SAM maintenance in higher plants.
During the reproductive phase, SAMs give rise to floral meristems (FMs) in a regular, geometric fashion. FMs also contain stem cell populations during their early growth stages. The balance between the rates of stem cell proliferation and differentiation in the FM is pivotal for the proper formation of flower organs, such as sepals, petals, stamens, and carpels (Figure 1d) [36]. Floral organ patterning is determined by the combined actions of homeotic genes [37,38]. Sepals, stamens, carpels, and petals become visible in order (Figure 1d–f). Like the SAM, FM activity is also regulated by WUS activity during floral organ formation [16]. Mutations in WUS also result in premature termination of the FM. The regulatory principles that determine stem cell homeostasis in the SAM and FM are largely similar to each other. Like the SAM, the FM is also maintained by the CLV–WUS pathway in higher plants. In rice, FLORAL ORGAN NUMBER1 (FON1) and FON2 control FM sizes [39,40,41]. FON1 encodes an ortholog of the CLV1 receptor, whereas FON2 encodes a CLV3-like protein. These two proteins act in the same pathway for FM maintenance [39,40]. In tomato, the FACIATED AND BRANCHED (FAB) receptor and the SlCLV3 (ligand) signaling pathway also control FM size [42]. In Arabidopsis, the SAM is indeterminate and the FM is determinate, while SAMs can also be determinate in many species and can vary within a single plant species [6,43,44,45]. Precocious FM termination leads to the formation of fewer floral organs. By contrast, delayed FM termination leads to increased numbers of floral organs. Thus, the timing of FM termination is crucial for the production of a fixed number of organs, including the female structure, the gynoecium. This termination is controlled by multiple gene regulatory networks. The precise control of the termination of the FM by multiple factors ensures stem cell homeostasis in the FM and the formation of determinate floral organs.
Plant hormones (also known as phytohormones) are signaling molecules that influence a variety of physiological processes. Phytohormones are classified into several different groups depending on their chemical structures [46,47]. Plant hormones are synthesized in one location and move to other locations in the plant. Hormones trigger many biological and cellular processes in locally targeted cells, such as seed dormancy, growth, metabolism, organ formation, reproduction, and stress responses [48,49,50,51,52,53]. Plant hormone biosynthesis, transport, perception, signal transduction, and downstream effects coordinate the hormonal control of cell division, growth, and differentiation.
Recent studies of the regulators of the SAM and FM have revealed that plant hormones contribute to the fine-tuning of meristem maintenance and organ formation. Cytokinin and auxin are two core plant hormones that function in the SAM and FM regulatory network. Cytokinins are required for cell division in meristematic tissues, whereas auxins promote organ formation, growth, and differentiation. Cytokinins and auxins often work together in multiple organs, tissues, and cells [46]. Although these two hormones were originally considered to be antagonists, recent studies have revealed their synergistic interactions as well [54,55,56]. In this review, we discuss recent findings on the molecular basis of SAM and FM regulation, with an emphasis on the roles of cytokinin and auxin.
2. Hormonal Control of Meristematic and Primordial Fate in the SAM
2.1. The Role of Cytolonin in Specifying Meristem Fate in the SAM
2.1.1. Cytokinin and Its Role in Specifying the Fate of the SAM
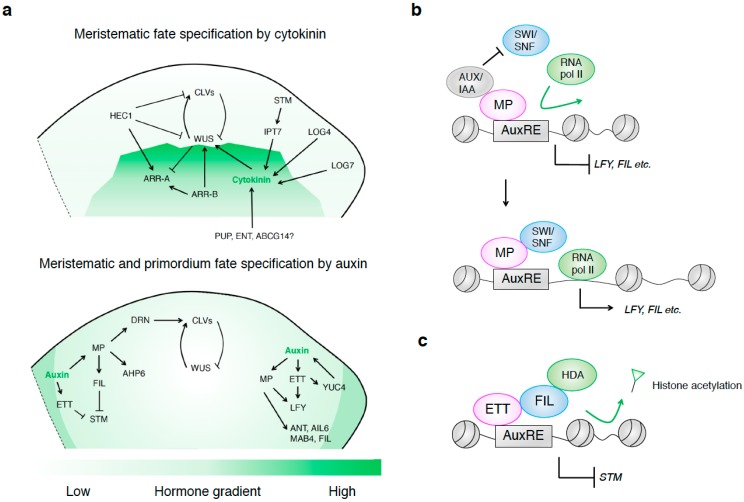
Cytokinin, which triggers cell division, was first discovered more than 50 years ago. This plant hormone controls every aspect of plant growth and development, including meristem function, vascular development, stress responses, and senescence. Cytokinin maintains the stem cell population in the SAM. In Arabidopsis, high levels of cytokinin signaling induce ectopic WUS expression and lead to stem cell fate in the surrounding cells (Figure 2a). This finding suggests that cytokinin is sufficient for the induction of WUS and the specification of stem cell fate. WUS levels are regulated by two different cytokinin-dependent pathways: CLV-dependent and CLV-independent pathways [58]. Cytokinins are transported from root to shoot and accumulate in the OC of the SAM [59]. Cytokinin activity is further fine-tuned by cytokinin biosynthesis, degradation, transport, and signaling [60,61]. Tissue-specific transcription factors and environmental signals also contribute to this activity [62].
Figure 2.
Hormonal control of the SAM to specify meristematic and primordium fate. (a) Top: Cytokinin-mediated SAM regulatory network for meristematic fate specification. Bottom: Auxin-mediated SAM regulatory network for meristematic and floral primordium fate specification. Green indicates hormonal gradients. The arrows represent the activation of gene expression, while the flat arrows represent its repression. (b) Chromatin state switch for primordium specification by the ARF5/MP complex in response to auxin. The arrows represent the activation of gene expression, while the flat arrows represent its repression. (c) Chromatin state switch for primordium specification by ARF3 and the FIL complex. The arrows represent the activation of gene expression, while the flat arrows represent its repression.
2.1.2. Cytokinin Biosynthesis Controls WUS Expression in the SAM
Cytokinin biosynthesis in Arabidopsis begins with the addition of a prenyl group to the N6 position of adenosine diphosphate/adenosine triphosphate (ADP/ATP). This reaction is catalyzed by adenosine phosphate-isopentenyltransferase (IPT) proteins [63]. Arabidopsis contains 11 IPT homologs [64,65], which are differentially expressed to produce cytokinins in a tissue-specific manner. In the SAM, IPT7 expression is activated by another pluripotency factor, SHOOT MERISTEMLESS (STM) (Figure 2a) [66]. IPT7 is localized to the mitochondria [63,67]. The resulting N6-isopentenyladenine (iP) ribotides produced via IPTs are subsequently converted to trans-zeatin (tZ—the most abundant form of cytokinin in plants) via hydroxylation of the isoprenoid side chain [68,69]. Another group of enzymes, LONELY GUY (LOG) cytokinin nucleoside 5′-monophosphate phosphoribohydrolases, contribute to the production of cytokinin [70,71]. LOG genes were originally identified in rice [70]. Arabidopsis contains nine LOG genes (LOG1 to LOG9) [71]. Among these, LOG4 and LOG7 are expressed in the L1 layer of the SAM and floral primordia, respectively [72]. The cytokinin pathway also contributes to WUS expression in response to environmental conditions, such as changes in light and nitrate availability (Figure 2a) [73,74,75]. CYTOKININ OXIDASE5 (CKX5) and CKX6 catalyze the irreversible degradation of cytokinin. CKX5 and CKX6 repress WUS expression via the degradation of cytokinin in the dark [74]. Cytokinins also mediate stem cell size through WUS expression in response to nutritional status. Grafting experiments revealed that cytokinin precursors function as long-distance signals to control SAM size and WUS expression [75]. Cytokinin accumulation is adjusted accordingly based on these environmental inputs. The resulting cytokinin functions as a systemic signal to the SAM and determines WUS expression and stem cell activity. Thus, cytokinin biosynthesis is mediated by both multiple enzymes and environmental inputs and governs WUS expression in the SAM.
2.1.3. Cytokinin Transport and its Role in Specifying the Fate of the SAM
Cytokinin transport is mediated by three types of cytokinin transporters: purine permeases (PUPs), equilibrative nucleoside transporters (ENTs), and ATP-binding cassette G (ABCG) transporters [76,77,78,79,80,81]. Arabidopsis contains 23 PUP and 8 ENT family members [76,77]. PUP1, PUP2, PUP14, and ENT1 control cytokinin uptake, as revealed in a yeast expression system [76,82,83]. On the other hand, the ABCG14 transporter coordinates cytokinin export [80,81,84,85,86]. The ABCG transporter gene family, comprising 28 genes, plays diverse roles in cytokinin transport [86,87]. Although ABCG14 is mainly expressed in roots, a mutation in ABCG14 leads to reduced shoot growth [80,81]. ABCG14 localizes to the plasma membrane and transports tZ, as revealed by a tracer experiment [81]. Phenotypic and molecular experiments suggest that long-distance cytokinin transport is essential for the regulation of SAM activity. Whether these transporters contribute to the establishment of cytokinin activity at the OC of the SAM has not yet been addressed (Figure 2a).
2.1.4. Another Feedback Loop between Cytokinin Signal Transduction and WUS Specifies SAM Fate
Cytokinin signaling is mediated by three ARABIDOPSIS HISTIDINE KINASE (AHK) receptors, AHK2, AHK3, and AHK4 (also known as CYTOKININ RESPONSE1), through a multistep His–Asp phosphorelay similar to that found in bacterial two-component signaling systems. AHK receptors contain both histidine kinase and receiver domains. Upon sensing cytokinin, the AHKs autophosphorylate at a His residue and transfer the phosphate to an aspartate residue on the receiver domain. Subsequently, this phosphate is transferred to a histidine residue on authentic histidine-containing phospho-transmitters (AHPs) [88,89,90,91,92]. This phosphorylation step allows the AHPs to be translocated from the cytoplasm to the nucleus to activate Arabidopsis response regulators (ARRs). To date, 22 ARR genes have been identified. Typical ARRs are categorized into type A or type B [93,94]. Type-B ARRs are transcriptional activators that promote the cytokinin response, whereas most type-A ARRs are transcriptional repressors [89,95]. Type-B ARRs, such as ARR1, ARR10, and ARR12, activate WUS expression by directly binding to its promoter [96,97,98,99,100]. The type-B ARR binding cis-element located in the promoter region of WUS plays primary roles in the activation of this gene by ARR1, ARR10, and ARR12 (Figure 2a) [98]. WUS represses the expression of type-A ARR genes ARR5, ARR6, ARR7, and ARR15 (Figure 2a) [101]. WUS binds directly to the ARR7 and ARR15 promoters. Thus, WUS possesses another feedback loop controlling its expression pattern in the SAM.
2.1.5. WUS and HEC Compete for Shared Cytokinin Signal Transduction Genes to Specify SAM Fate
The interplay between WUS–CLV and cytokinin is fine-tuned by tissue-specific transcription factors. The basic helix-loop-helix (bHLH) transcription factor HECATE1 (HEC1) is directly repressed by high concentrations of WUS protein (Figure 2a) [101,102,103,104]. HEC1 physically interacts with other bHLH transcription factors, such as HEC2 and HEC3. Consistent with this role in repression, HEC1 is expressed throughout the SAM except in the OC, where WUS highly accumulates. Ectopic expression of HEC1 in the OC interferes with the maintenance of the SAM. HEC1 represses CLV3 expression and activates type-A ARR7 and ARR15 expression (Figure 2a). Shared target genes of HEC1 and WUS, such as ARR7 and ARR15, are regulated in an opposite manner. How this competitive regulation by HEC1 and WUS is regulated remains to be elucidated. Multiple feedback systems mediated by hormonal components and transcription factors act in parallel to control meristematic fate and WUS expression in the SAM. A feedback circuit-driven regulatory mechanism is a common strategy for reliable, irreversible cell fate determination [105,106,107,108].
2.2. The Role of Auxin in Specifying Meristem and Primordium Fate in the SAM
2.2.1. AUXIN RESPONSE FACTORs Specify Meristem and Primordium Fate in the SAM
Auxin controls almost every aspect of plant growth and development, including cell division, elongation, and differentiation, with important effects on the final shapes and functions of plant cells and tissues. During SAM development, a classic role of auxin is to specify organ primordium fate in the PZ of the SAM. Mutants in components of auxin biosynthesis, transport, and signaling exhibit naked inflorescences lacking flowers, even though the earlier products from the SAM, such as rosette leaves, are generally present (Figure 2a) [109,110,111,112]. Recent evidence suggests that in addition to specifying organ primordium fate, auxin specifies meristematic fate in the SAM (Figure 2a). AUXIN RESPONSE FACTORs (ARFs) are transcription factors that play roles in specifying these identities [113,114]. To date, 23 ARF genes have been identified in Arabidopsis. ARFs function as transcription factors by binding to auxin-responsive elements (AuxREs) in the promoters of their target genes [113,114]. Individual ARFs control distinct developmental processes. Recent studies have identified downstream genes of ARFs, which play key roles in SAM development.
2.2.2. MP/ARF5 Specifies Meristem and Primordium Fate in the SAM
Among ARF transcription factors, ARF5 (also known as MONOPTEROS (MP)) plays a key role in specifying meristematic and primordium fate by orchestrating gene expression [114,115,116,117,118]. MP is a canonical ARF (class A) that modulates auxin signaling [119,120]. MP dimerizes with AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) repressor proteins in the absence of auxin [115,116,117,118]. In the nucleus, auxin is perceived by TRANSPORT INHIBITOR RESISTANT1 (TIR1)/AUXIN SIGNALING F-BOX proteins [121,122]. These F-box proteins form the substrate recognition subunits of SKP-CULLIN-box (SCF) ubiquitin ligases [123]. Upon sensing auxin, Aux/IAA proteins are degraded through the action of SCFTIR1 ubiquitin ligase. MP activity is subsequently derepressed and triggers the expression of many auxin response genes. MP proteins are present at low levels in the CZ and at high levels in the PZ of the SAM [124,125]. Consistent with this accumulation pattern, MP modulates different downstream target genes in the CZ and PZ. To control meristematic fate in the CZ, MP directly represses ARR7/ARR15 and activates AHP6 through the regulation of cytokinin homeostasis (Figure 2a) [91,126]. AHP6 establishes inhibitory fields of cytokinin signaling to define organ initiation sites [91]. ARR7 and ARR15 integrate cytokinin and auxin signals and relay them to the WUS–CLV network [126]. MP also represses the expression of DORNRÖSCHEN/ENHANCER OF SHOOT REGENERATION1 (DRN/ESR1) to promote CLV3 expression (Figure 2a) [116,117,118,127,128]. Whether other transcription factors are required for the transcriptional activation of CLV3 by DRN at the CZ of the SAM has not yet been addressed.
To specify primordium fate in the PZ, MP directly activates the auxin transporter gene PIN-FORMED1 (PIN1), the floral meristem identity gene LEAFY (LFY), the organ size regulatory genes AINTEGUMENTA (ANT) and AINTEGUMENTA-LIKE6 (AIL6), and the abaxial identity gene FILAMENTOUS FLOWER (FIL) [129,130,131,132,133,134,135]. The MP homodimer might bind to the LFY promoter via an AuxRE variant for MP homodimer binding [136]. MP also induces the expression of MACCHI-BOU4 (MAB4) family genes in the PZ to control basipetal auxin transport (Figure 2a) [137]. The target specificity of MP occurs in a zone-dependent manner, perhaps due to threshold levels of MP protein, different affinities of MP binding sites, and/or chromatin-mediated regulation of gene expression. In fact, the expression of several MP targets, which specify primordium identity, requires the activity of Switch/Sucrose Non-Fermentable (SWI/SNF) family chromatin remodelers [138,139,140,141]. In the presence of auxin, MP interacts with SWI/SNF chromatin remodelers to open up the promoter regions of downstream target genes for their activation [141]. This structural change in chromatin allows additional transcription factors and/or general transcriptional machinery (Figure 2b). Hence, MP coordinates fate specification in the meristem and primordium in response to auxin in the SAM. The crosstalk between hormonal transcription factors and epigenetic regulators plays prominent roles in fate specification.
2.2.3. ETT/ARF3 and ARF4 Switch Off Meristematic Cell Fate in the PZ of the SAM
ARF3 (also known as ETTIN (ETT)) and ARF4 are noncanonical class B ARFs that lack a domain for interaction with Aux/IAA proteins [113,142,143]. Both ETT and ARF4 are highly expressed in the PZ of the SAM [144]. ETT shares redundant functions with ARF4 during plant development. Unlike MP, an auxin-dependent interaction between ETT and process-specific transcription factors determines the transcriptional activity of ETT [144]. The molecular nature of the interaction between ETT and auxin remains to be addressed. To specify primordium fate, an ETT-FIL dimer directly represses the expression of the pluripotency factor gene STM through histone deacetylation and negatively regulates the STM target, IPT7 (Figure 2a,c) [145]. Since FIL is a direct target of MP, MP also indirectly helps specify primordium fate by terminating the meristem (Figure 2a) [145]. The organogenic program that terminates the meristem is conserved between plants and animals. Furthermore, genome-wide analyses identified direct targets of ETT whose expression is ETT- and auxin-dependent. ETT directly controls the expression of LFY and the auxin biosynthetic gene YUCCA4 (YUC4) in an auxin-dependent manner (Figure 2a) [144,146,147]. Like MP, ETT might also specify primordium fate via LFY in the PZ [144]. Furthermore, ETT might provide direct positive feedback regulation of auxin biosynthesis by activating YUC4 during primordium fate specification. The proper control of gene activation and repression by auxin-dependent transcription factors switches the fates of meristematic and primordium cells.
3. Hormonal Control of the Termination of Meristematic Fate in the FM for Subsequent Organogenesis
3.1. The Roles of Cytokinin and Auxin in FM Development and Subsequent Organ Differentiation
Unlike the SAM, the FM is determinate, and its meristematic fate must be terminated. WUS ensures the maintenance of the stem cell pool in the SAM and FM. The MADS-box transcription factor AGAMOUS (AG) plays central roles in early stages of FM development and the termination of the meristematic fate of the FM during later stages of development [36,45,148,149,150,151]. In addition to mutations in AG, ectopic WUS expression beyond stage 6 is sufficient to trigger indeterminacy of the FM. AG is expressed throughout FM formation beginning at stage 3 of flower development and terminates FM activity at stage 6 of flower development by regulating gene expression. Recent studies have shown that AG influences cytokinin and auxin homeostasis during both the earlier and later stages of FM development. After FM termination, cytokinin and auxin homeostasis play key roles in gynoecium formation.
3.2. AG Controls Auxin and Cytokinin Levels during FM Formation
During the early stages of FM formation, AG controls FM activity by maintaining proper hormone levels. AG modulates ETT expression partially though GIANT KILLER (GIK), which encodes an AT-HOOK MOTIF CONTAINING NUCLEAR LOCALIZED (AHL) protein [152,153]. AHL family proteins interact with each other to form homo- or heterodimer complexes. AHLs also interact with other components, such as DOMAIN OF UNKNOWN FUNCTION296 (DUF296) [154]. The availability of many different forms of AHL protein complexes might enable the fine-tuning of downstream genes of AG in a spatiotemporal manner. ETT then directly represses the expression cytokinin biosynthetic genes IPT3, IPT5, and IPT7 and mediates cell-cycle-related gene expression [155]. AG also activates another downstream target, SUPERMAN (SUP), which encodes a transcriptional repressor with a C2H2 zinc-finger DNA-binding domain and an EAR repression motif [156,157,158,159]. SUP downregulates the expression of YUC1 and YUC4 to decrease local auxin biosynthesis. SUP interacts with histone modification enzymes to deposit negative histone marks, thereby silencing gene expression [160,161,162]. Proper hormone levels are required for maintaining FM activity and floral organ formation [162].
3.3. Proper Control of Cytokinin Homeostasis Promotes Cell Proliferation in the Terminating FM
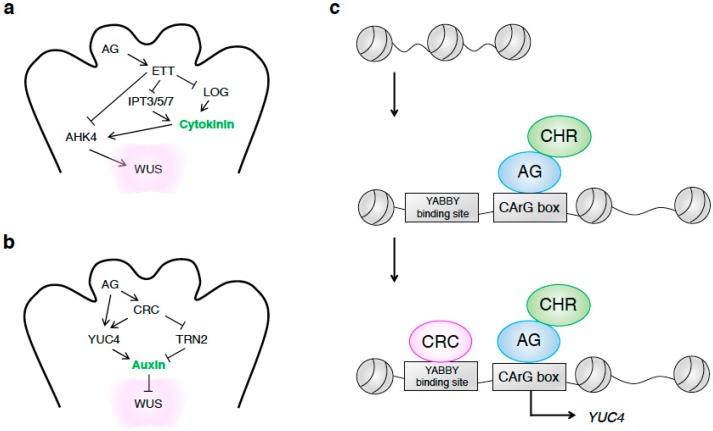
During FM termination, AG controls cytokinin homeostasis [155]. Exogenous cytokinin treatment enhances the FM indeterminacy of ag mutants [155]. This phenotype is enhanced in cytokinin-treated ag ett double mutants, suggesting that ETT genetically interacts with cytokinin to regulate FM determinacy. ETT integrates AG and auxin inputs to repress cytokinin activity. ETT negatively controls cytokinin biosynthesis. Similar to its activity during FM formation, ETT directly represses the expression of the cytokinin biosynthetic genes IPT3, IPT5, and IPT7 during FM termination (Figure 3a) [155]. In addition, ETT indirectly represses the expression of several LOG genes to fine-tune cytokinin levels [155]. In addition to controlling cytokinin levels, ETT controls the level of cytokinin signaling. ETT binds to the AHK4 promoter to repress its expression (Figure 3a) [155]. AuxREs play key roles in the direct binding of ETT to IPT7 and AHK4 [155]. The role of auxin downstream of ETT adds multiple layers of complexity to the role of cytokinin in regulating FM termination to provide cells for subsequent gynoecium formation.
Figure 3.
Hormonal control of the termination of floral meristematic fate. (a) Cytokinin-mediated meristematic fate termination in the FM at stage 6 of flower development. (b) Auxin-mediated primordium fate specification in the FM. The terminating OC is shown in pale pink. The arrows represent the activation of gene expression, while the flat arrows represent its repression. (c) Chromatin state switch for termination of floral meristematic fate by CRC and the AG complex. The arrow represents the activation of gene expression.
3.4. Proper Control of Auxin Homeostasis Terminates Meristematic Fate in the FM
During FM termination, AG also activates its direct target, CRABS CLAW (CRC), to enable FM determinacy via auxin (Figure 3b) [163,164,165,166]. AG binds to the CRC promoter and promotes its expression. CRC belongs to the YABBY family of transcription factors, which contain a zinc finger and a helix-loop-helix domain. CRC expression begins during stage 6 of flower development and localizes to the abaxial region of the developing carpels [157]. In addition to AG, CRC increases auxin levels through the direct activation of YUC4 [167]. AG interacts with the SWI/SNF chromatin remodelers and evicts well-positioned nucleosomes (Figure 3c) [141,168,169,170]. The pioneer activity of AG, together with SWI/SNF chromatin remodelers, opens up the promoter region of YUC4 from stage 3 of flower development (Figure 3c) [167]. Subsequently, CRC associates the YUC4 promoter near the AG binding site (Figure 3c). The feed-forward activation of YUC4 via the synergistic interaction between AG and CRC specifies primordium cell fate. Consistent with this finding, mutations in multiple YUC genes, including YUC4, induce FM indeterminacy. CRC also represses another direct target, TORNADO2 (TRN2) (also known as TETERASPANIN1 or EKEKO), which encodes a transmembrane protein of the tetraspanin family [171,172,173,174]. Tetraspanins interact with numerous partner proteins to control multiple cellular processes, such as cell adhesion, cell fusion, and intracellular membrane trafficking. This transcriptional repression of TRN2 by CRC partially contributes to the creation of auxin maxima by interfering with auxin transport [175]. How TRN2 affects auxin accumulation remains to be addressed. These two direct CRC targets, YUC4 and TRN2, act in parallel to repress WUS expression for FM determinacy and subsequent organ formation (Figure 3b) [171]. The sequential actions of transcription factors control auxin homeostasis in the FM for subsequent carpel formation.
3.5. Synergistic Interaction between Auxin and Cytokinin Promotes Gynoecium Formation
Although it is well known that auxin and cytokinin act antagonistically during plant growth and development, several studies on the molecular mechanism between these phytohormones have focused on their synergistic activity, which maximizes their effects during gynoecium formation [54,55,56]. Unlike the SAM, in carpels, the auxin and cytokinin accumulation patterns partially overlap after FM termination. SPATULA (SPT), a bHLH transcription factor, induces cytokinin accumulation in the carpel margin via an unknown mechanism [163,176,177,178]. SPT activates the expression of the auxin biosynthesis gene TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1) and the auxin efflux carrier gene PIN3 [177,179,180,181]. This expression change also promotes auxin accumulation in the apical region of the carpel. One remaining question that needs to be addressed is how one transcription factor mediates the activities of two antagonistic hormones in the same manner. The target specificity of SPT could be due to the presence of cofactors and/or the different affinities of SPT binding sites. Since most transcription factors that regulate gynoecium development are categorized into only four classes based on mRNA distribution [182], threshold regulation at the protein level could also be important. SPT forms multiple complexes with transcription factors, such as HEC and INDEHISCENT (IND) [163,176,183]. Proteomic-based studies of protein interactions might help reveal cofactors of SPT that function in response to auxin or cytokinin.
4. Concluding Remarks and Future Perspectives
The robust establishment of the SAM and FM is primarily controlled by conserved secreted peptides and master transcriptional regulators. In the past five years, plant stem cell researchers have identified various interactions between these key factors and phytohormones using a candidate gene approach or omics data. Phytohormones help determine when and where these factors function. The spatiotemporal fine-tuning of these factors by phytohormones appears to be regulated by complex multilayered networks of hormonal components, since hormones affect not only transcription, but also the epigenetic state, protein stability/protein–protein interactions, and metabolic rates of meristematic cells. However, in practice, most omics data are available only for bulk samples rather than single cells. Furthermore, technologies to integrate multilayer omics data are not yet well established. To elucidate these networks, multi-omics data at the single-cell level are needed. The integration of multilayered omics data via high-resolution readouts across hierarchies will provide new insights into phytohormone-mediated meristem activities.
Acknowledgments
The authors would like to thank Hitomi Ichiwaka for sharing the photographs of tissue sections photos and Sachi Ando for editing the first draft of this manuscript.
Abbreviations
| SAM | Shoot apical meristem |
| FM | Floral meristem |
| L1 | layer 1 |
| L2 | layer 2 |
| L3 | layer 3 |
| CZ | central zone |
| PZ | peripheral zone |
| WUS | WUSCHEL |
| CLV3 | CLAVATA3 |
| CLE | CLAVATA3/ESR-RELATED |
| CRN | CORYNE |
| SOL2 | SUPPRESSOR OF LLP1 2 |
| FCP1 | FON2-LIKE CLE PROTEIN1 |
| FCP2 | FON2-LIKE CLE PROTEIN2 |
| WOX4 | WUSCHEL RELATED HOMEOBOX 4 |
| ZmFCP1 | ZmFON2-LIKE CLE PROTEIN1 |
| FEA3 | FASCIATED EAR3 |
| ZmWUS1 | Zm WUSCHEL1 |
| FON1 | FLORAL ORGAN NUMBER1 |
| FON2 | FLORAL ORGAN NUMBER2 |
| SlCLV3 | SlCLAVATA3 |
| FAB | FACIATED AND BRANCHED |
| ADP | adenosine diphosphate |
| ATP | adenosine triphosphate |
| IPT | ISOPENTENYLTRANSFERASE |
| STM | SHOOT MERISTEMLESS |
| iP | isopentenyladenine |
| tZ | trans-zeatin |
| LOG | LONELY GUY |
| PUP | purine permease |
| ENT | equilibrative nucleoside transporter |
| ABCG | ATP-binding cassette G |
| AHK | ARABIDOPSIS HISTIDINE KINASE |
| ARR | Arabidopsis response regulators |
| AHP | authentic histidine-containing phospho-transmitter |
| HEC1 | HECATE1 |
| ARF | AUXIN RESPONSE FACTOR |
| AuxRE | auxin-responsive element |
| MP | MONOPTEROS |
| Aux/IAA | AUXIN/INDOLE-3-ACETIC ACID |
| DRN | DORNRÖSCHEN |
| ESR1 | ENHANCER OF SHOOT REGENERATION1 |
| PIN1 | PIN-FORMED1 |
| LFY | LEAFY |
| ANT | AINTEGUMENTA |
| AIL6 | AINTEGUMENTA-LIKE6 |
| FIL | FILAMENTOUS FLOWER |
| MAB4 | MACCHI-BOU4 |
| AG | AGAMOUS |
| GIK | GIANT KILLER |
| SUP | SUPERMAN |
| CRC | CRABS CLAW |
| YUC4 | YUCCA4 |
| TRN2 | TORNADO2 |
| SPT | SPATULA |
| TAA1 | TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 |
| PIN3 | PIN-FORMED3 |
| IND | INDEHISCENT |
Author Contributions
Writing—original draft preparation, Z.H.L. and N.Y.; writing—review and editing, Z.H.L., T.H., N.Y., and T.I.; visualization, T.H. and N.Y.; supervision, N.Y. and T.I.; project administration, N.Y. and T.I.; funding acquisition, T.H., N.Y., and T.I.
Funding
This research was funded by JSPS, grant number 19J00658 to T.H., Japan Science and Technology Agency ‘Precursory Research for Embryonic Science and Technology’ (JPMJPR15QA), JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (No. 18H04782), JSPS KAKENHI Grant-in-Aid for Scientific Research B (No. 18H02465), and the LOTTE Foundation to N.Y., and JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (No. 18H04839, 19H04865), JSPS KAKENHI Grant-in-Aid for Scientific Research A (No. 15H02405), Grant-in-Aid for challenging Exploratory Research (No. 18K19342) to T.I.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Barton M.K. Cell type specification and self renewal in the vegetative shoot apical meristem. Curr. Opin. Plant Biol. 1998;1:37–42. doi: 10.1016/S1369-5266(98)80125-8. [DOI] [PubMed] [Google Scholar]
- 2.Laux T. The stem cell concept in plants: A matter of debate. Cell. 2003;113:281–283. doi: 10.1016/S0092-8674(03)00312-X. [DOI] [PubMed] [Google Scholar]
- 3.Sun B., Ito T. Regulation of floral stem cell termination in Arabidopsis. Front. Plant Sci. 2015;6:17. doi: 10.3389/fpls.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miwa H., Kinoshita A., Fukuda H., Sawa S. Plant meristems: CLAVATA3/ESR-related signaling in the shoot apical meristem and the root apical meristem. J. Plant Res. 2009;122:31–39. doi: 10.1007/s10265-008-0207-3. [DOI] [PubMed] [Google Scholar]
- 5.Itoh J.I., Kitano H., Matsuoka M., Nagato Y. Shoot organization genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice. Plant Cell. 2000;12:2161–2174. doi: 10.1105/tpc.12.11.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt D., Kuhlemeier C. Plant architecture. EMBO Rep. 2002;3:846–851. doi: 10.1093/embo-reports/kvf177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giulini A., Wang J., Jackson D. Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature. 2004;430:1031–1034. doi: 10.1038/nature02778. [DOI] [PubMed] [Google Scholar]
- 8.Mandel T., Moreau F., Kutsher Y., Fletcher J.C., Carles C.C., Eshed Williams L. The ERECTA receptor kinase regulates Arabidopsis shoot apical meristem size, phyllotaxy and floral meristem identity. Development. 2014;141:830–841. doi: 10.1242/dev.104687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y., Wagner D. Plant inflorescence architecture: The formation, activity, and fate of axillary meristems. CSH Perspect Biol. 2019:a034652. doi: 10.1101/cshperspect.a034652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Li J. Molecular basis of plant architecture. Annu. Rev. Plant Biol. 2008;59:253–279. doi: 10.1146/annurev.arplant.59.032607.092902. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher J.C. Shoot and floral meristem maintenance in Arabidopsis. Annu. Rev. Plant Biol. 2002;53:45–66. doi: 10.1146/annurev.arplant.53.092701.143332. [DOI] [PubMed] [Google Scholar]
- 12.Carles C.C., Fletcher J.C. Shoot apical meristem maintenance: The art of dynamic balance. Trends Plant Sci. 2003;8:394–401. doi: 10.1016/S1360-1385(03)00164-X. [DOI] [PubMed] [Google Scholar]
- 13.Adibi M., Yoshida S., Weijers D., Fleck C. Centering the organizing center in the Arabidopsis thaliana shoot apical meristem by a combination of cytokinin signaling and self-organization. PLoS ONE. 2016;11:e0147830. doi: 10.1371/journal.pone.0147830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark S.E., Running M.P., Meyerowitz E.M. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development. 1993;119:397–418. doi: 10.1242/dev.119.2.397. [DOI] [PubMed] [Google Scholar]
- 15.Clark S.E., Running M.P., Meyerowitz E.M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development. 1995;121:2057–2067. [Google Scholar]
- 16.Laux T., Mayer K.F.X., Berger J., Jürgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122:87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- 17.Mayer K.F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/S0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 18.Schoof H., Lenhard M., Haecker A., Mayer K.F.X., Jürgens G., Laux T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/S0092-8674(00)80700-X. [DOI] [PubMed] [Google Scholar]
- 19.Lenhard M., Jürgens G., Laux T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development. 2002;129:3195–3206. doi: 10.1242/dev.129.13.3195. [DOI] [PubMed] [Google Scholar]
- 20.Yadav R.K., Girke T., Pasala S., Xie M., Reddy G.V. Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc. Natl. Acad. Sci. USA. 2009;106:4941–4946. doi: 10.1073/pnas.0900843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busch W., Miotk A., Ariel F.D., Zhao Z., Forner J., Daum G., Suzaki T., Schuster C., Schultheiss S.J., Leibfried A., et al. Transcriptional control of a plant stem cell niche. Dev. Cell. 2010;18:849–861. doi: 10.1016/j.devcel.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Aichinger A., Kornet N., Friedrich T., Laux T. Plant stem cell niches. Annu. Rev. Plant Biol. 2012;63:615–636. doi: 10.1146/annurev-arplant-042811-105555. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher J.C., Brand U., Running M.P., Simon R., Meyerowitz E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science. 1999;283:1911–1914. doi: 10.1126/science.283.5409.1911. [DOI] [PubMed] [Google Scholar]
- 24.Ohyama K., Shinohara H., Ogawa-Ohnishi M., Matsubayashi Y. A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat. Chem. Biol. 2009;5:878–880. doi: 10.1038/nchembio.182. [DOI] [PubMed] [Google Scholar]
- 25.Jeong S., Trotochaud A.E., Clark S.E. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell. 1999;11:1925–1933. doi: 10.1105/tpc.11.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiers M., Ku K.L., Liu C.M. CLE peptide ligands and their roles in establishing meristems. Curr. Opin. Plant Biol. 2007;10:39–43. doi: 10.1016/j.pbi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Mitchum M.G., Wang X., Davis E.L. Diverse and conserved roles of CLE peptides. Curr. Opin. Plant Biol. 2008;11:75–81. doi: 10.1016/j.pbi.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Miwa H., Betsuyaku S., Iwamoto K., Kinoshita A., Fukuda H., Sawa S. The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant Cell Physiol. 2008;49:1752–1757. doi: 10.1093/pcp/pcn148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butenko M.A., Vie A.K., Brembu T., Aalen R.B., Bones A.M. Plant peptides in signalling: Looking for new partners. Trends Plant Sci. 2009;14:255–263. doi: 10.1016/j.tplants.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita A., Betsuyaku S., Osakabe Y., Mizuno S., Nagawa S., Stahl Y., Simon R., Yamaguchi-Shinozaki K., Fukuda H., Sawa S. RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development. 2010;137:3911–3920. doi: 10.1242/dev.048199. [DOI] [PubMed] [Google Scholar]
- 31.Somssich M., Je B.I., Simon R., Jackson D. CLAVATA-WUSCHEL signaling in the shoot meristem. Development. 2016;143:3238–3248. doi: 10.1242/dev.133645. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher J.C. The CLV-WUS stem cell signaling pathway: A roadmap to crop yield optimization. Plants. 2018;7:87. doi: 10.3390/plants7040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohmori Y., Tanaka W., Kojima M., Sakakibara H., Hirano H.Y. WUSCHEL-RELATED HOMEOBOX4 is involved in meristem maintenance and is negatively regulated by the CLE gene FCP1 in rice. Plant Cell. 2013;25:229–241. doi: 10.1105/tpc.112.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Je B.I., Gruel J., Lee Y.K., Bommert P., Arevalo E.D., Eveland A.L., Wu Q., Goldshmidt A., Meeley R., Bartlett M., et al. Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat. Genet. 2016;48:785–791. doi: 10.1038/ng.3567. [DOI] [PubMed] [Google Scholar]
- 35.Je B.I., Xu F., Wu Q., Liu L., Meeley R., Gallagher J.P., Corcilius L., Payne R.J., Bartlett M.E., Jackson D. The CLAVATA receptor FASCIATED EAR2 responds to distinct CLE peptides by signaling through two downstream effectors. Elife. 2018;7:e35673. doi: 10.7554/eLife.35673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun B., Xu Y., Ng K.H., Ito T. A Timing Mechanism for Stem Cell Maintenance and Differentiation in The Arabidopsis Floral Meristem. Genes Dev. 2009;23:1791–1804. doi: 10.1101/gad.1800409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowman J.L., Smyth D.R., Meyerowitz E.M. Genetic interactions among floral homeotic genes. Development. 1991;112:1–20. doi: 10.1242/dev.112.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Coen E.S., Meyerowitz E.M. The war of the whorls: Genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 39.Suzaki T., Sato M., Ashikari M., Miyoshi M., Nagato Y., Hirano H.Y. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development. 2004;131:5649–5657. doi: 10.1242/dev.01441. [DOI] [PubMed] [Google Scholar]
- 40.Suzaki T., Toriba T., Fujimoto M., Tsutsumi N., Kitano H., Hirano H.Y. Conservation and diversification of meristem maintenance mechanism in Oryza sativa: Function of the FLORAL ORGAN NUMBER2 gene. Plant Cell Physiol. 2006;47:1591–1602. doi: 10.1093/pcp/pcl025. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida H., Nagato Y. Flower development in rice. J. Exp. Bot. 2011;62:4719–4730. doi: 10.1093/jxb/err272. [DOI] [PubMed] [Google Scholar]
- 42.Xu C., Liberatore K.L., MacAlister C.A., Huang Z., Chu Y.H., Jiang K., Brooks C., Ogawa-Ohnishi M., Xiong G., Pauly M., et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 2015;47:784–792. doi: 10.1038/ng.3309. [DOI] [PubMed] [Google Scholar]
- 43.Mizukami Y., Ma H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell. 1992;71:119–131. doi: 10.1016/0092-8674(92)90271-D. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz G., Theres K. Genetic control of branching in Arabidopsis and tomato. Curr. Opin. Plant Biol. 1999;2:51–55. doi: 10.1016/S1369-5266(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 45.Sun B., Looi L.S., Guo S., He Z., Gan E.S., Huang J., Xu Y., Wee W.Y., Ito T. Timing mechanism dependent on cell division is invoked by Polycomb eviction in plant stem cells. Science. 2014;343:1248559. doi: 10.1126/science.1248559. [DOI] [PubMed] [Google Scholar]
- 46.Kende H., Zeevaart J.A.D. The five “classical” plant hormones. Plant Cell. 1997;9:1197–1210. doi: 10.1105/tpc.9.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bari R., Jones J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 48.Nemhauser J.L., Hong F., Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 50.Rubio V., Bustos R., Irigoyen M.L., Cardona-López X., Rojas-Triana M., Paz-Ares J. Plant hormones and nutrient signaling. Plant Mol. Biol. 2009;69:361–373. doi: 10.1007/s11103-008-9380-y. [DOI] [PubMed] [Google Scholar]
- 51.Santner A., Calderon-Villalobos L.I., Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009;5:301–307. doi: 10.1038/nchembio.165. [DOI] [PubMed] [Google Scholar]
- 52.Pieterse C.M.J., van der Does D., Zamioudis C., Leon-Reyes A., van Wees S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 53.Wasternack C., Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolters H., Jurgens G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009;10:305–317. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- 55.El-Showk S., Ruonala R., Helariutta Y. Crossing paths: Cytokinin signalling and crosstalk. Development. 2013;140:1373–1383. doi: 10.1242/dev.086371. [DOI] [PubMed] [Google Scholar]
- 56.Schaller G.E., Bishopp A., Kieber J.J. The Yin-Yang of Hormones: Cytokinin and Auxin interactions in plant development. Plant Cell. 2015;27:44–63. doi: 10.1105/tpc.114.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smyth D.R., Bowman J.L., Meyerowitz E.M. Early flower development in Arabidopsis. Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon S.P., Chickarmane V.S., Ohno C., Meyerowitz E.M. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA. 2009;106:16529–16534. doi: 10.1073/pnas.0908122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chickarmane V.S., Gordon S.P., Tarr P.T., Heisler M.G., Meyerowitz E.M. Cytokinin signaling as a positional cue for patterning the apical– basal axis of the growing Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA. 2012;109:4002–4007. doi: 10.1073/pnas.1200636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakakibara H. Cytokinin biosynthesis and regulation. Vitam. Horm. 2005;72:271–287. doi: 10.1016/S0083-6729(05)72008-2. [DOI] [PubMed] [Google Scholar]
- 61.Werner T., IKöllmer I., Bartrina I., Holst K., Schmülling T. New insights into the biology of cytokinin degradation. Plant Biol. 2006;8:1–12. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- 62.Werner T., Schmülling T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Sakakibara H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- 64.Kakimoto T. Identification of plant biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltranferases. Plant Cell Physiol. 2001;42:677–685. doi: 10.1093/pcp/pce112. [DOI] [PubMed] [Google Scholar]
- 65.Takei K., Sakakibara H., Sugiyama T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001;276:26405–26410. doi: 10.1074/jbc.M102130200. [DOI] [PubMed] [Google Scholar]
- 66.Yanai O., Shani E., Dolezal K., Tarkowski P., Sablowski R., Sandberg G., Samach A., Ori N. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr. Biol. 2005;15:1566–1571. doi: 10.1016/j.cub.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 67.Ghosh A., Shah M.N.A., Jui Z.S., Saha S., Fariha K.A., Islam T. Evolutionary variation and expression profiling of Isopentenyl transferase gene family in Arabidopsis thaliana L. and Oryza sativa L. Plant Gene. 2018;15:15–27. doi: 10.1016/j.plgene.2018.06.002. [DOI] [Google Scholar]
- 68.Takei K., Yamaya T., Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J. Biol. Chem. 2004;279:41866–41872. doi: 10.1074/jbc.M406337200. [DOI] [PubMed] [Google Scholar]
- 69.Osugi A., Sakakibara H. Q&A: How do plants respond to cytokinins and what is their importance? BMC Biol. 2015;13:102. doi: 10.1186/s12915-015-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurakawa T., Ueda N., Maekawa M., Kobayashi K., Kojima M., Nagato Y., Sakakibara H., Kyozuka J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- 71.Kuroha T., Tokunaga H., Kojima M., Ueda N., Ishida T., Nagawa S., Fukuda H., Sugimoto K., Sakakibara H. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell. 2009;21:3152–3169. doi: 10.1105/tpc.109.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gruel J., Landrein B., Tarr P., Schuster C., Refahi Y., Sampathkumar A., Hamant O., Meyerowitz E.M., Jönsson H. An epidermis-driven mechanism positions and scales stem cell niches in plants. Sci. Adv. 2016;2:e1500989. doi: 10.1126/sciadv.1500989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau O.S., Deng X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010;13:571–577. doi: 10.1016/j.pbi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Pfeiffer A., Janocha D., Dong Y., Medzihradszky A., Schöne S., Daum G., Suzaki T., Forner J., Langenecker T., Rempel E., et al. Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. Elife. 2016;5:e17023. doi: 10.7554/eLife.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landrein B., Formosa-Jordan P., Malivert A., Schuster C., Melnyk C.W., Yang W., Turnbull C., Meyerowitz E.M., Locke J.C.W., Jönsson H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA. 2018;115:1382–1387. doi: 10.1073/pnas.1718670115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillissen B., Burkle L., Andre B., Kuhn C., Rentsch D., Brandu B., Frommer W.B. A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell. 2000;12:291–300. doi: 10.1105/tpc.12.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bürkle L., Cedzich A., Döpke C., Stransky H., Okumoto S., Gillissen B., Kühn C., Frommer W.B. Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J. 2003;34:13–26. doi: 10.1046/j.1365-313X.2003.01700.x. [DOI] [PubMed] [Google Scholar]
- 78.Wormit A., Traub M., Flörchinger M., Neuhaus H., Möhlmann T. Characterization of three novel members of the Arabidopsis thaliana equilibrative nucleoside transporter (ENT) family. Biochem. J. 2004;383:19–26. doi: 10.1042/BJ20040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun J., Hirose N., Wang X., Wen P., Xue L., Sakakibara H., Zuo J. Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in planta. J. Integr. Plant Biol. 2005;47:588–603. doi: 10.1111/j.1744-7909.2005.00104.x. [DOI] [Google Scholar]
- 80.Ko D., Kang J., Kiba T., Park J., Kojima M., Do J., Kim K.Y., Kwon M., Endler A., Song W.Y., et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. USA. 2014;111:7150–7155. doi: 10.1073/pnas.1321519111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang K., Novak O., Wei Z., Gou M., Zhang X., Yu Y., Yang H., Cai Y., Strnad M., Liu C.J. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 2014;5:3274. doi: 10.1038/ncomms4274. [DOI] [PubMed] [Google Scholar]
- 82.Li J., Wang D. Cloning and in vitro expression of the cDNA encoding a putative nucleoside transporter from Arabidopsis thaliana. Plant Sci. 2000;157:23–32. doi: 10.1016/S0168-9452(00)00261-2. [DOI] [PubMed] [Google Scholar]
- 83.Cedzich A., Stransky H., Schulz B., Frommer W.B. Characterization of cytokinin and adenine transport in Arabidopsis cell cultures. Plant Physiol. 2008;148:1857–1867. doi: 10.1104/pp.108.128454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kudo T., Kiba T., Sakakibara H. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 2010;52:53–60. doi: 10.1111/j.1744-7909.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- 85.Girke C., Daumann M., Niopek-Witz S., Möhlmann T., Kerr I. Nucleobase and nucleoside transport and integration into plant metabolism. Front. Plant Sci. 2014;5:443. doi: 10.3389/fpls.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borghi L., Kang J., Ko D., Lee Y., Martinoia E. The role of ABCG-type ABC transporters in phytohormone transport. Biochem. Soc. Trans. 2015;43:924–930. doi: 10.1042/BST20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang J., Song W., Hong D., Ko D., Yamaoka Y., Jang S., Yim S., Lee E., Khare D., Kim K., et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant. 2016;9:338–355. doi: 10.1016/j.molp.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 88.Sakai H., Honma T., Aoyama T., Sato S., Kato T., Tabata S., Oka A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science. 2001;294:1519–1521. doi: 10.1126/science.1065201. [DOI] [PubMed] [Google Scholar]
- 89.Mason M.G., Mathews D.E., Argyros D.A., Maxwell B.B., Kieber J.J. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell. 2005;17:3007–3018. doi: 10.1105/tpc.105.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang I., Sheen J., Müller B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012;63:353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- 91.Besnard F., Refahi Y., Morin V., Marteaux B., Brunoud G., Chambrier P., Rozier F., Mirabet V., Legrand J., Lainé S., et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature. 2014;505:417–421. doi: 10.1038/nature12791. [DOI] [PubMed] [Google Scholar]
- 92.Besnard F., Rozier F., Vernoux T. The AHP6 cytokinin signaling inhibitor mediates an auxin-cytokinin crosstalk that regulates the timing of organ initiation at the shoot apical meristem. Plant Signal. Behav. 2014;9:e28788. doi: 10.4161/psb.28788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brandstatter I., Kieber J.J. Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell. 1998;10:1009–1020. doi: 10.1105/tpc.10.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Imamura A., Hanaki N., Nakamura A., Suzuki T., Taniguchi M., Kiba T., Ueguchi C., Sugiyama T., Mizuno T. Compilation and characterization of Arabidopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol. 1999;40:733–742. doi: 10.1093/oxfordjournals.pcp.a029600. [DOI] [PubMed] [Google Scholar]
- 95.To J.P.C., Haberer G., Ferreira F.J., Deruère J., Mason M.G., Schaller G.E., Alonso J.M., Ecker J.R., Kieber J.J. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signalling. Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meng W.J., Cheng Z.J., Sang Y.L., Zhang M.M., Rong X.F., Wang Z.W., Tang Y.Y., Zhang X.S. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017;29:1357–1372. doi: 10.1105/tpc.16.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J., Tian C., Zhang C., Shi B., Cao X., Zhang T.Q., Zhao Z., Wang J.W., Jiao Y. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell. 2017;29:1373–1387. doi: 10.1105/tpc.16.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang T.Q., Lian H., Zhou C.M., Xu L., Jiao Y., Wang J.W. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell. 2017;29:1073–1087. doi: 10.1105/tpc.16.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zubo Y., Blakley I.C., Yamburenko M., Worthen J.M., Street I., Franco-Zorrilla J.M., Zhang W., Hill K., Solano R., Kieber J.J., et al. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2017;114:E5995–E6004. doi: 10.1073/pnas.1620749114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie M., Chen H., Huang L., O’Neil R.C., Shokhirev M.N., Ecker J.R. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat. Commun. 2018;9:1604. doi: 10.1038/s41467-018-03921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leibfried A., To J.P.C., Busch W., Stehling S., Kehle A., Demar M., Kieber J.J., Lohmann J.U. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- 102.Gremski K., Ditta G., Yanofsky M.F. The HECATE genes regulate female reproductive tract development in Arabidopsis thaliana. Development. 2007;134:3593–3601. doi: 10.1242/dev.011510. [DOI] [PubMed] [Google Scholar]
- 103.Schuster C., Gaillochet C., Medzihradszky A., Busch W., Daum G., Krebs M., Kehle A., Lohmann J.U. A regulatory framework for shoot stem cell control integrating metabolic, transcriptional, and phytohormone signals. Dev. Cell. 2014;28:438–449. doi: 10.1016/j.devcel.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 104.Gaillochet C., Stiehl T., Wenzl C., Ripoll J.J., Bailey-Steinitz L.J., Li L., Pfeiffer A., Miotk A., Hakenjos J., Forner J., et al. Control of plant cell fate transitions by transcriptional and hormonal signals. Elife. 2017;6:1–30. doi: 10.7554/eLife.30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao X.Y., Su Y.H., Cheng Z.J., Zhang X.S. Cell fate switch during in vitro plant organogenesis. J. Integr. Plant Biol. 2008;50:816–824. doi: 10.1111/j.1744-7909.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 106.MacArthur B.D., Ma’ayan A., Lemischka I.R. Systems biology of stem cell fate and cellular reprogramming. Nat. Rev. Mol. Cell Biol. 2009;10:672–681. doi: 10.1038/nrm2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi Y., Zhao X., Hsieh J., Wichterle H., Impey S., Banerjee S., Neveu P., Kosik K.S. MicroRNA regulation of neural stem cells and neurogenesis. J. Neurosci. 2010;30:14931–14936. doi: 10.1523/JNEUROSCI.4280-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heidstra R., Sabatini S. Plant and animal stem cells: Similar yet different. Nat. Rev. Mol. Cell Biol. 2014;15:301–312. doi: 10.1038/nrm3790. [DOI] [PubMed] [Google Scholar]
- 109.Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 110.Sassi M., Vernoux T. Auxin and self-organization at the shoot apical meristem. J. Exp. Bot. 2013;64:2579–2592. doi: 10.1093/jxb/ert101. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y., Jiao Y. Auxin and above-ground meristems. J. Exp. Bot. 2018;69:147–154. doi: 10.1093/jxb/erx299. [DOI] [PubMed] [Google Scholar]
- 112.Banasiak A., Biedron M., Dolzblasz A., Berezowsk M.A. Ontogenetic changes in auxin biosynthesis and distribution determine the organogenic activity of the shoot apical meristem in pin1 mutants. Int. J. Mol. Sci. 2019;20:180. doi: 10.3390/ijms20010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pekker I., Alvarez J.P., Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17:2899–2910. doi: 10.1105/tpc.105.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vidaurre D.P., Ploense S., Krogan N.T., Berleth T. AMP1 and MP antagonistically regulate embryo and meristem development in Arabidopsis. Development. 2007;134:2561–2567. doi: 10.1242/dev.006759. [DOI] [PubMed] [Google Scholar]
- 115.Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. Aux/IAA proteins repress expression of reporter genes containing catural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chandler J.W., Cole M., Flier A., Grewe B., Werr W. The AP2 transcription factors DORNROSCHEN and DORNROSCHEN-LIKE redundantly control Arabidopsis embryo patterning via interaction with PHAVOLUTA. Development. 2007;134:1653–1662. doi: 10.1242/dev.001016. [DOI] [PubMed] [Google Scholar]
- 117.Cole M., Chandler J., Weijers D., Jacobs B., Comelli P., Werr W. DORNROESCHEN is a direct target of the auxin response factor MONOPTEROS in the Arabidopsis embryo. Development. 2009;136:1643–1651. doi: 10.1242/dev.032177. [DOI] [PubMed] [Google Scholar]
- 118.Krogan N.T., Marcos D., Weiner A.I., Berleth T. The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol. 2016;212:42–50. doi: 10.1111/nph.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reed J.W. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001;6:420–425. doi: 10.1016/S1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- 120.Rademacher E.H., Möller B., Lokerse A.S., Llavata-Peris C.I., van den Berg W., Weijers D. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J. 2011;68:597–606. doi: 10.1111/j.1365-313X.2011.04710.x. [DOI] [PubMed] [Google Scholar]
- 121.Dharmasiri N., Dharmasiri S., Weijers D., Lechner E., Yamada M., Hobbie L., Ehrismann J.S., Jürgens G., Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 122.Kepinski S., Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 123.Skowyra D., Craig K., Tyers M., Elledge S., Harper J. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/S0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 124.Hardtke C.S., Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao Z., Andersen S.U., Ljung K., Dolezal K., Miotk A., Schultheiss S.J., Lohmann J.U. Hormonal control of the shoot stem-cell niche. Nature. 2010;465:1089–1092. doi: 10.1038/nature09126. [DOI] [PubMed] [Google Scholar]
- 126.Denay G., Chahtane H., Tichtinsky G., Parcy F. A flower is born: An update on Arabidopsis floral meristem formation. Curr. Opin. Plant Biol. 2017;35:15–22. doi: 10.1016/j.pbi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Kirch T., Simon R., Grunewald M., Werr W. The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell. 2003;15:694–705. doi: 10.1105/tpc.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luo L., Zeng J., Wu H., Tian Z., Zhao Z. A Molecular Framework for Auxin-Controlled Homeostasis of Shoot Stem Cells in Arabidopsis. Mol. Plant. 2018;11:819–913. doi: 10.1016/j.molp.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 129.Weigel D., Alvarez J., Smyth D.R., Yanofsky M.F., Meyerowitz E.M. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-N. [DOI] [PubMed] [Google Scholar]
- 130.Elliott R.C., Betzner A.S., Huttner E., Oakes M.P., Tucker W.Q.J., Gerentes D., Perez P., Smyth D.R. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sawa S., Watanabe K., Goto K., Kanaya E., Morita E.H., Okada K. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 1999;13:1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krizek B. AINTEGUMENTA and AINTEGUMENTA-LIKE6 act redundantly to regulate Arabidopsis floral growth and patterning. Plant Physiol. 2009;150:1916–1929. doi: 10.1104/pp.109.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamaguchi N., Wu M.F., Winter C.M., Berns M.C., Nole-Wilson S., Yamaguchi A., Coupland G., Krizek B.A., Wagner D. A molecular framework for auxin-mediated initiation of flower primordia. Dev. Cell. 2013;24:271–282. doi: 10.1016/j.devcel.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 134.Yamaguchi N., Wu M.F., Winter C.M., Wagner D. LEAFY and polar auxin transport coordinately regulate Arabidopsis flower development. Plants. 2014;3:251–265. doi: 10.3390/plants3020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wakeel A., Ali I., Khan A.R., Wu M., Upreti S., Liu D., Liu B., Gan Y. Involvement of histone acetylation and deacetylation in regulating auxin responses and associated phenotypic changes in plants. Plant Cell Rep. 2018;37:51–59. doi: 10.1007/s00299-017-2205-1. [DOI] [PubMed] [Google Scholar]
- 136.Boer D.R., Freire-Rios A., van den Berg W.A.M., Saaki T., Manfield I.W., Kepinski S., López-Vidrieo I., Franco-Zorrilla J.M., de Vries S.C., Solano R., et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell. 2014;156:577–589. doi: 10.1016/j.cell.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 137.Furutani M., Nakano Y., Tasaka M. MAB4-induced auxin sink generates local auxin gradients in Arabidopsis organ formation. Proc. Natl. Acad. Sci. USA. 2014;111:1198–1203. doi: 10.1073/pnas.1316109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wagner D., Meyerowitz E.M. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 2002;12:85–94. doi: 10.1016/S0960-9822(01)00651-0. [DOI] [PubMed] [Google Scholar]
- 139.Bezhani S., Winter C., Hershman S., Wagner J.D., Kennedy J.F., Kwom C.S., Pfluger J., Su Y., Wagner D. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell. 2007;19:403–416. doi: 10.1105/tpc.106.048272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Farrona S., Hurtado L., March-Diaz R., Schmitz R.J., Florencio F.J., Turck F., Amasino R.M., Reyes J.C. Brahma is required for proper expression of the floral repressor FLC in Arabidopsis. PLoS ONE. 2011;6:e17997. doi: 10.1371/journal.pone.0017997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu M.F., Yamaguchi N., Xiao J., Bargmann B., Estelle M., Sang Y., Wagner D. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. Elife. 2015;4:e09269. doi: 10.7554/eLife.09269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hunter C., Willmann M.R., Wu G., Yoshikawa M., de la Luz Gutierrez-Nava M., Poethig S.R. Trans-acting siRNA-mediated repression of ETTIN and ARF4 regulates heteroblasty in Arabidopsis. Development. 2006;133:2973–2981. doi: 10.1242/dev.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simonini S., Deb J., Moubayidin L., Stephenson P., Valluru M., Freire-Rios A., Sorefan K., Weijers D., Friml J., Østergaard L. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 2016;30:2286–2296. doi: 10.1101/gad.285361.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simonini S., Bencivenga S., Trick M., Østergaard L. Auxin-induced modulation of ETTIN activity orchestrates gene expression in Arabidopsis. Plant Cell. 2017;29:1864–1882. doi: 10.1105/tpc.17.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chung Y., Zhu Y., Wu M.F., Simonini S., Kuhn A., Armenta-Medina A., Jin R., Østergaard L., Gillmor C.S., Wagner D. Auxin Response Factors promote organogenesis by chromatin-mediated repression of the pluripotency gene SHOOTMERISTEMLESS. Nat. Commun. 2019;10:886. doi: 10.1038/s41467-019-08861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng Y., Dai X., Zhao Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng Y., Dai X., Zhao Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell. 2007;19:2430–2439. doi: 10.1105/tpc.107.053009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yanofsky M.F., Ma H., Bowman J.L., Drews G.N., Feldmann K.A., Meyerowitz E.M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- 149.Lenhard M., Bohnert A., Jürgens G., Laux T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell. 2001;105:805–814. doi: 10.1016/S0092-8674(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 150.Lohmann J.U., Hong R.L., Hobe M., Busch M.A., Parcy F., Simon R., Weigel D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell. 2001;105:793–803. doi: 10.1016/S0092-8674(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 151.Payne T., Johnson S.D., Koltunow A.M. KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development. 2004;131:3737–3749. doi: 10.1242/dev.01216. [DOI] [PubMed] [Google Scholar]
- 152.Zhao J., Favero D.S., Qiu J., Roalson E.H., Neff M.M. Insights into the evolution and diversification of the AT-hook motif nuclear localized gene family in land plants. BMC Plant Biol. 2014;14:266. doi: 10.1186/s12870-014-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ng K.H., Yu H., Ito T. AGAMOUS controls GIANT KILLER, a multifunctional chromatin modifier in reproductive organ patterning and differentiation. PLoS Biol. 2009;7:e1000251. doi: 10.1371/journal.pbio.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao J., Favero D.S., Peng H., Neff M.M. Arabidopsis thaliana AHL family modulates hypocotyl growth redundantly by interacting with each other via the PPC/DUF296 domain. Proc. Natl. Acad. Sci. USA. 2013;110:E4688–E4697. doi: 10.1073/pnas.1219277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang K., Wang R., Zi H., Li Y., Cao X., Li D., Guo L., Tong J., Pan Y., Jiao Y., et al. AUXIN RESPONSE FACTOR3 regulates floral meristem determinacy by repressing cytokinin biosynthesis and signaling. Plant Cell. 2018;30:324–346. doi: 10.1105/tpc.17.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bowman J.L., Sakai H., Jack T., Weigel D., Mayer U., Meyerowitz E.M. SUPERMAN, a regulator of floral homeotic genes in Arabidopsis. Development. 1992;114:599–615. doi: 10.1242/dev.114.3.599. [DOI] [PubMed] [Google Scholar]
- 157.Sakai H., Medrano L.J., Meyerowitz E.M. Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries. Nature. 1995;378:199–203. doi: 10.1038/378199a0. [DOI] [PubMed] [Google Scholar]
- 158.Kazan K. Negative regulation of defence and stress genes by EAR-motif-containing repressors. Trends Plant Sci. 2006;11:109–112. doi: 10.1016/j.tplants.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 159.Prunet N., Yang W., Das P., Meyerowitz E.M., Jack T.P. SUPERMAN prevents class B gene expression and promotes stem cell termination in the fourth whorl of Arabidopsis thaliana flowers. Proc. Natl. Acad. Sci. USA. 2017;114:7166–7171. doi: 10.1073/pnas.1705977114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu L., Shen W.H. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr. Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 161.Xiao J., Wagner D. Polycomb repression in the regulation of growth and development in Arabidopsis. Curr. Opin. Plant Biol. 2015;23:15–24. doi: 10.1016/j.pbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 162.Xu Y., Prunet N., Gan E.S., Wang Y., Stewart D., Wellmer F., Huang J., Yamaguchi N., Tatsumi Y., Kojima M., et al. SUPERMAN regulates floral whorl boundaries through control of auxin biosynthesis. EMBO J. 2018;37:e97499. doi: 10.15252/embj.201797499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alvarez J., Smyth D.R. CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development. 1999;126:2356–2375. doi: 10.1242/dev.126.11.2377. [DOI] [PubMed] [Google Scholar]
- 164.Bowman J.L., Smyth D.R. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126:2387–2396. doi: 10.1242/dev.126.11.2387. [DOI] [PubMed] [Google Scholar]
- 165.Eshed Y., Baum S.F., Bowman J.L. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell. 1999;99:199–209. doi: 10.1016/S0092-8674(00)81651-7. [DOI] [PubMed] [Google Scholar]
- 166.Lee J.Y., Baum S.F., Alvarez J., Patel A., Chitwood D.H., Bowman J.L. Activation of CRABS CLAW in the nectaries and carpels of Arabidopsis. Plant Cell. 2005;17:25–36. doi: 10.1105/tpc.104.026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamaguchi N., Huang J., Tatsumi Y., Abe M., Sugano S.S., Kojima M., Takebayashi Y., Kiba T., Yokoyama R., Nishitani K., et al. Chromatin-mediated feed-forward auxin biosynthesis in floral meristem determinacy. Nat. Commun. 2018;9:5290. doi: 10.1038/s41467-018-07763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huanca-Mamani W., Garcia-Aguilar M., Leon-Martinez G., Grossniklaus U., Vielle-Calzada J.P. CHR11, a chromatin-remodeling factor essential for nuclear proliferation during female gametogenesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2005;102:17231–17236. doi: 10.1073/pnas.0508186102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li G., Zhang J., Li J., Yang Z., Huang H., Xu L. Imitation Switch chromatin remodeling factors and their interacting RINGLET proteins act together in controlling the plant vegetative phase in Arabidopsis. Plant J. 2012;72:261–270. doi: 10.1111/j.1365-313X.2012.05074.x. [DOI] [PubMed] [Google Scholar]
- 170.Li G., Liu S., Wang J., He J., Huang H., Zhang Y., Xu L. ISWI proteins participate in the genome-wide nucleosome distribution in Arabidopsis. Plant J. 2014;78:706–714. doi: 10.1111/tpj.12499. [DOI] [PubMed] [Google Scholar]
- 171.Cnops G., Neyt P., Raes J., Petrarulo M., Nelissen H., Malecina N., Luschnig C., Tietz O., Ditengou F., Palme K., et al. The TORNADO1 and TORNADO2 genes function in several patterning processes during early leaf development in Arabidopsis thaliana. Plant Cell. 2006;18:852–866. doi: 10.1105/tpc.105.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chiu W.H., Chandler J., Cnops G., Van Lijsebettens M., Werr W. Mutations in the TORNADO2 gene affect cellular decisions in the peripheral zone of the shoot apical meristem of Arabidopsis thaliana. Plant Mol. Biol. 2007;63:731–744. doi: 10.1007/s11103-006-9105-z. [DOI] [PubMed] [Google Scholar]
- 173.Boavida L.C., Qin P., Broz M., Becker J.D., McCormick S. Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo- and heterodimers when expressed in Yeast. Plant Physiol. 2013;163:696–712. doi: 10.1104/pp.113.216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang F., Muto A., Van de Velde J., Neyt P., Himanen K., Vandepoele K., Van Lijsebettens M. Functional analysis of the Arabidopsis TETRASPANIN gene family in plant growth and development. Plant Physiol. 2015;69:2200–2214. doi: 10.1104/pp.15.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yamaguchi N., Huang J., Xu Y., Tanoi K., Ito T. Fine-tuning of auxin homeostasis governs the transition from floral stem cell maintenance to gynoecium formation. Nat. Commun. 2017;8:1125. doi: 10.1038/s41467-017-01252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heisler M.G., Atkinson A., Bylstra Y.H., Walsh R., Smyth D.R. SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development. 2001;128:1089–1098. doi: 10.1242/dev.128.7.1089. [DOI] [PubMed] [Google Scholar]
- 177.Reyes-Olalde J.I., Zúñiga-Mayo V.M., Marsch-Martinez N., de Folter S. Synergistic relationship between auxin and cytokinin in the ovary and the participation of the transcription factor SPATULA. Plant Signal. Behav. 2017;12:e1376158. doi: 10.1080/15592324.2017.1376158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reyes-Olalde J.I., Zúñiga-Mayo V.M., Serwatowska J., Chavez Montes R.A., Lozano-Sotomayor P., Herrera-Ubaldo H., Gonzalez-Aguilera K.L., Ballester P., Ripoll J.J., Ezquer I., et al. The bHLH transcription factor SPATULA enables cytokinin signaling, and both activate auxin biosynthesis and transport genes at the medial domain of the gynoecium. PLoS Genet. 2017;13:e1006726. doi: 10.1371/journal.pgen.1006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van Gelderen K., van Rongen M., Liu A., Otten A., Offringa R. An INDEHISCENT-controlled auxin response specifies the separation layer in early Arabidopsis fruit. Mol. Plant. 2016;9:857–869. doi: 10.1016/j.molp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 180.Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heldstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 181.Mashiguchi K., Tanaka K., Sakai T., Sugawara S., Kawaide H., Natsume M., Hanada A., Yaeno T., Shirasu K., Yao H., et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reyes-Olalde J.I., de Folter S. Control of stem cell activity in the carpel margin meristem (CMM) in Arabidopsis. Plant Reprod. 2019;32:123–136. doi: 10.1007/s00497-018-00359-0. [DOI] [PubMed] [Google Scholar]
- 183.Girin T., Paicu T., Stephenson P., Fuentes S., Körner E., O’Brien M., Sorefan K., Wood T.A., Balanzá V., Ferrándiz C., et al. INDEHISCENT and SPATULA interact to specify carpel and valve margin tissue and thus promote seed dispersal in Arabidopsis. Plant Cell. 2011;23:3641–3653. doi: 10.1105/tpc.111.090944. [DOI] [PMC free article] [PubMed] [Google Scholar]