Abstract
Objective
Individuals with immunocompromised (IC) conditions are at a higher risk of developing herpes zoster (HZ) than IC-free individuals. This study assessed the healthcare resource utilisation (HCRU) burden and costs, of HZ in IC and IC-free individuals ≥18 years of age (YOA).
Methods
We conducted an observational retrospective study in a cohort of IC (n=621 588) and IC-free (n=621 588) individuals, matched by age, gender and General Practitioner practice region, contributing to the Clinical Practice Research Datalink database from 2000 to 2012 and linked to the Hospital Episode Statistics inpatient data. HCRU (ie, primary and secondary care consultations, hospital inpatient stays and treatment prescriptions) was analysed from 7 days before to: (1) 30, (2) 365 days after the HZ diagnosis date for individuals with (1) HZ only (no postherpetic neuralgia (PHN)) and (2) individuals with HZ and PHN only. Healthcare costs were computed by multiplying the number of units of resources used by the unit costs, summed across all HCRU categories to obtain a total cost per subject. Values were expressed in 2014 UK pound sterling (£) and presented for HZ cases overall, stratified by age (ie, 18–49, 50–59, 60–69, 70–79 and ≥80 YOA) and IC status.
Results
The percentage of HZ cases requiring hospitalisation was higher in IC individuals (2.7% vs 0.4% in IC and IC-free individuals aged 18–49 YOA, respectively and 9.5% vs 7.5% in IC and IC-free individuals aged ≥80 YOA, respectively). Similarly, HZ-related mean treatment costs per subject were higher in IC individuals (£189 vs £104 in IC and IC-free individuals aged 18–49 YOA, respectively and £557 vs £401 in IC and IC-free individuals aged ≥80 YOA, respectively). Costs varied considerably by IC condition.
Conclusions
Individuals with IC conditions, have a high burden of HZ, associated with an increased risk of HZ and high HZ-related healthcare costs.
Keywords: herpes zoster, postherpetic neuralgia, immunocompromised, hospitalisation, healthcare burden, herpes zoster treatment
Strengths and limitations of this study.
The study is an observational retrospective descriptive study presenting the healthcare resource utilisation and costs associated with herpes zoster (HZ) in both immunocompromised (IC) and IC-free populations aged ≥18 years of age in England.
The IC population included 621 588 individuals who were registered in the Clinical Practice Research Datalink from January 2000 to March 2012 with ≥12 month follow-up before being diagnosed with any of the selected 16 IC conditions and matched to the Hospital Episode Statistics database by age, gender and practice location to extract the IC-free population (n=621 588).
The particularity of this study is that the design allowed calculation of IC condition prevalence rates, HZ incidence rates and occurrence of HZ-related healthcare utilisation and costs at individual level in the same predefined population(s).
This key study will provide data to be used in economic analyses to evaluate the value of vaccination in reducing the burden of HZ in IC populations.
A limitation of the study is that the diagnoses were derived from administrative codes, which are recognised to be subject to miscoding or under-coding and are not validated against medical charts.
Introduction
Varicella zoster virus cell-mediated immunity (VZV-CMI) inhibits the development of herpes zoster (HZ).1 Therefore, if for any reason VZV-CMI declines, the risk of HZ increases. Reasons for VZV-CMI decline can include, increasing age and immune suppression. VZV-CMI is not optimal in individuals with immunocompromised (IC) conditions and the age-specific incidence and severity of HZ greatly increases in IC patients due to underlying illness (eg, Human immunodeficiency virus infection) or immunosuppressive therapies for autoimmune disease, malignancy, or organ transplantation.2
The incidence and severity of HZ is marked with an increase in people ≥50 years of age (YOA) due to an age-related decline in immunity (ARDI). In the United Kingdom (UK) the incidence of HZ rises from 7.1 per 1000 person-years (PY) among 60–64 year olds to 12.2 per 1000 PY among individuals aged ≥85 YOA.3 Further to the impact of ARDI, a study by Forbes et al in 2014 investigated the increased risk for HZ in the UK population, associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus; and chronic conditions such as diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease and asthma.2 In addition to the increased risk of HZ in the various IC conditions these populations also experience increased severity of disease. In a study in Canada, Drolet et al reported that individuals with an impaired immune status had HZ severity of illness scores, as measured by the Zoster Brief Pain Inventory, which were twice as high as individuals with normal immune function.4 5 In a study in the USA, Yawn et al reported that although 8% of HZ cases were in individuals who were immunocompromised, these individuals represented 23.8% of the total HZ-related costs.6 The increase in healthcare costs was associated with higher rates of postherpetic neuralgia (PHN) and non-pain complications in this group of individuals.6
This study aims to estimate the healthcare resource utilisation of HZ in selected IC populations and in an IC-free (ie, immunocompetent) population aged ≥18 YOA in England. The clinical burden of disease epidemiological results of the study are reported elsewhere,7 and may be summarised as follows: the prevalence of IC conditions increased from 7.6% in individuals aged 18–44 YOA to 42.2% in individuals aged ≥80 YOA; the incidence rate of HZ in the IC cohort was 3.5/1000 PY in individuals aged 18–49 YOA increasing to 12.6/1000 PY in individuals aged ≥80 YOA. In this manuscript, we focus on the healthcare resource utilisation and costs associated with HZ in both IC and IC-free populations.
Methods
The study was conducted as an observational retrospective descriptive study (e-track number: 201615), in a cohort of eligible matched IC and IC-free populations (aged ≥18 YOA). The IC population included individuals who were registered in the Clinical Practice Research Datalink (CPRD) from January 2000 to March 2012 with ≥12 month follow-up before being diagnosed with any of the selected 16 IC conditions (see online supplementary material). The CPRD IC population cohort was linked to the Hospital Episode Statistics (HES) database and matched, using a 1:1 ratio, to a cohort of CPRD-HES linked IC-free population (n=621 588), by age, gender and practice location.8 Individuals with a missing date of IC diagnosis were excluded from the study population. Clinical diagnoses were based on READ codes used in CPRD and with the International Classifications of Diseases-10th revision (ICD-10) codes in the HES database.
bmjopen-2018-023502supp001.pdf (475.5KB, pdf)
The study protocol was approved by the Independent Scientific Advisory Committee (ISAC) for the Medicines and Healthcare Products Regulatory Agency database research (ISAC protocol number 14_222R). The study was conducted in accordance with all applicable regulatory requirements, with the Guidelines for Good Pharmacoepidemiology Practices,9 all applicable patient privacy requirements and the guiding principles of the Declaration of Helsinki.
The matched IC and IC-free cohorts were followed up from the index date until the earliest of the following events: transfer out of the practice date, the last GP practice collections date, death date or the end of the study.7 Healthcare resource data associated with an incident HZ episode during the study follow-up were extracted for IC and matched IC-free CPRD-HES-linked individuals. Only reported records (resource utilisation) with available event dates during the individuals’ eligibility period and those that occurred 7 days before the initial HZ onset date, up to 365 days after the initial HZ onset date, were extracted. Consequently, individuals who recorded the first PHN event date after 365 days post HZ event date were classified as not having PHN.
Patient and public involvement
This is a retrospective database analysis carried out following ethical committee approval. No patient or the public was involved in the study design or in the recruitment or the conduct of this study. No specific dissemination of study results to participants was done. However, we provided a lay language summary contextualising the results and potential clinical research relevance and impact in figure 1.
Figure 1.
Lay language summary.
Data sources
Data were extracted from the following sources: (1) CPRD GOLD 2014Q3: Consultation, Clinical, Therapy and Referral datasets; (2) HES Inpatient 2013Q3: HES_DIAGNOSIS_EPI dataset; (3) HES Outpatient data (Set 9): Appointment and clinical datasets. Healthcare resource utilisation was defined as: HZ-treatment related prescribed medications; Consultations and care provided by General Practitioners (GPs) or others in the GP practice); HES secondary care outpatient visits (HES outpatient events); and HES inpatient hospitalizations (HES inpatient events).
For each patient, healthcare costs stratified by subcategory of interest (HES Inpatient Hospitalizations; HES Outpatient consultations/visits; CPRD Ambulatory Visits; CPRD Other Ambulatory Visits; CPRD Prescriptions) were computed by multiplying units of resource use by their unit costs. These were then summed over all resource use categories to obtain a total cost for each patient. Values were expressed in 2014 UK pound sterling (£).
Healthcare resource costs
For each patient, the cost of each prescription was calculated by merging the product code, package type and prescribed quantity with the associated standard package size and unit cost. The unit cost of a product in a prescription instance (ie, one distinct record in the CPRD therapy) was calculated using the cost described in the British National Formulary (BNF), 2015 (as listed price if included or indicative price based on price in BNF).
Ambulatory visits included consultations with GPs and nurses in primary or community care. Visits included consultations at the practice or at the home of the patient, during working hours and out of hours. Consultations for which no clinical intervention was recorded were not included in the cost estimate for GP practice related healthcare utilisation, for example: information technology data migration, administrative recording of received information. Administrative resource use in primary care was considered, including time on the phone, writing reports, referrals, etc. A referral to secondary care noted in a patient’s record, per se, was not allocated the cost of the secondary care appointment. The most conservative option for the cost per unit as included in the Personal Social Services Research Unit (PSSRU) Costs of Health and Social Care, 2014 were applied eg, GP consultation costs excluded qualification, direct staff care and travel costs.10 Where specific costs for 2013/14 were not available, 2012/13 costs, were adjusted by applying the Hospital and Community Health Services inflation index.10 Administration costs were based on unit costs as stated in the PSSRU, 2014.
Inpatient hospitalizations related to HZ were derived from HES data. Hospital Outpatient resource utilisation concerned HZ related referrals for non-inpatient hospital consultations, derived from the HES Outpatient data. Additionally, visits to the Accident and Emergency (A&E) department in hospitals were also recorded and costed. Inpatient hospitalisation costs were based on the average cost per episode using HES data for 2013/14 (calculated from the total average payment by result spell cost and the average number of episodes per spell). Hospital outpatient costs were sourced from National Tariff costs (2014) for specific consultant led outpatient consultations; conservative costs were allocated that is wherever applicable costs for first attendance by a single professional appointment were used.11 Costs allocated to A&E visits were based on the cost of a category 3 investigation with category 1–3 treatment.11 Only events related to HZ were costed out. Resources related to HZ complications were considered using ICD-10 Code B020.
No costs were assigned to Referrals, Sick leave or Nursing home care/admission entries in CPRD. Further details, including information on the IC populations included, ICD-10 codes for HZ and PHN, and unit healthcare costs are provided in the online supplementary material, specifically in online supplementary tables 1 to 4.
Results
The CPRD-HES-linked matched IC and IC-free population cohorts (n=621 588 each) included approximately 44% males and 56% females with a mean age of approximately 56 years. The age distribution of matched cohorts was: 18–44 YOA (28.8%), 45–49 YOA (7.1%), 50–59 YOA (17.2%), 60–64 YOA (9.9%), 65–69 YOA (9.4%), 70–79 YOA (16.6%) and ≥80 YOA (11.01%).
The proportion of inpatient hospital admissions by age group for the CPRD-HES-linked matched IC and IC-free cohorts over the time periods of 7 days prior to 90 days post initial HZ onset (Panel A) or 7 days prior to 365 days post initial HZ onset (Panel B) are presented in figure 2. Hospital admissions over the longer follow-up period of 7 days prior to 365 days post initial HZ onset (Panel B) were similar to those of the shorter follow-up period (Panel A) over all age groups. The percentage of HZ cases hospitalised were higher in IC individuals (eg, in Panel B 2.7% vs 0.4% in IC and IC-free individuals aged 18–49 YOA, respectively and 9.5% vs 7.5% in IC and IC-free individuals aged ≥80 YOA, respectively). Multiple HZ-related hospital visits were reported for some individuals. As such, table 1 presents the mean number of healthcare resources used by IC Status, Age Group and Analysis period. The mean number of hospitalizations per HZ case for the 365-day analysis was, 0.035 and 0.005 in IC and IC-free individuals aged 18–49 YOA, respectively and 0.173 and 0.115 in IC and IC-free individuals aged ≥80 YOA, respectively. A similar pattern of higher healthcare resource utilisation with increasing age and in IC individuals was observed for all resources for which costs were assigned. A similar mean number of sick leave certificates were observed between the IC and the IC-free cohorts with the mean decreasing with age. Nursing home care/admissions were only recorded for individuals aged ≥70 YOA in CPRD.
Figure 2.
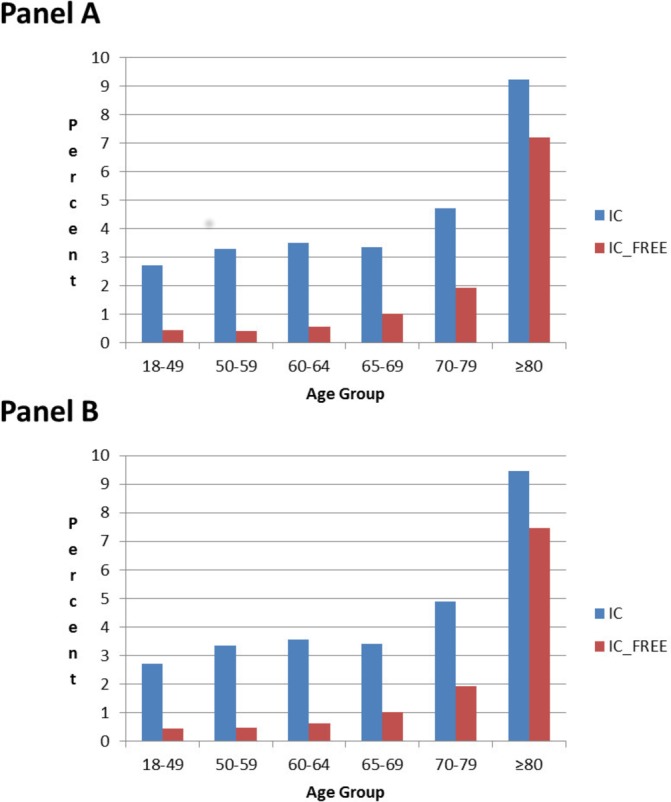
Inpatient hospital admission by HES-linked matched IC or IC-free cohort over the time periods: 7 days prior to 90 days post initial HZ onset (Panel A) and 7 days prior to 365 days post initial HZ onset (Panel B). For HZ individuals without PHN: data from 7 days prior to 30 days post HZ onset included. For HZ individuals with PHN: data from 7 days prior until the following time periods after HZ onset included—90 days (Panel A) and 365 days (Panel B). HES, Hospital Episode Statistics; HZ, herpes zoster; IC, immunocompromised; PHN, postherpetic neuralgia.
Table 1.
Mean number of healthcare resources by IC status, age group and analysis period
| IC cohort | IC-free cohort | |||
| 90 day | 365 day | 90 day | 365 day | |
| HES Hospital admission | ||||
| 18–49 | 0.035 | 0.035 | 0.005 | 0.005 |
| 50–59 | 0.042 | 0.046 | 0.006 | 0.007 |
| 60–64 | 0.053 | 0.055 | 0.009 | 0.010 |
| 65–69 | 0.049 | 0.050 | 0.014 | 0.014 |
| 70–79 | 0.072 | 0.076 | 0.029 | 0.030 |
| ≥80 | 0.163 | 0.173 | 0.108 | 0.115 |
| HES Outpatient consultation | ||||
| 18–49 | 0.095 | 0.116 | 0.041 | 0.045 |
| 50–59 | 0.086 | 0.122 | 0.062 | 0.086 |
| 60–64 | 0.136 | 0.180 | 0.065 | 0.078 |
| 65–69 | 0.146 | 0.217 | 0.085 | 0.108 |
| 70–79 | 0.165 | 0.267 | 0.113 | 0.181 |
| ≥80 | 0.173 | 0.313 | 0.149 | 0.231 |
| CPRD Ambulatory visits | ||||
| 18–49 | 2.816 | 3.168 | 2.186 | 2.360 |
| 50–59 | 3.334 | 4.175 | 2.466 | 2.907 |
| 60–64 | 3.733 | 5.081 | 2.598 | 3.115 |
| 65–69 | 4.089 | 6.009 | 2.774 | 3.610 |
| 70–79 | 4.534 | 6.959 | 3.413 | 4.767 |
| ≥80 | 4.881 | 7.422 | 3.811 | 5.367 |
| CPRD Other ambulatory visits | ||||
| 18–49 | 0.319 | 0.411 | 0.155 | 0.170 |
| 50–59 | 0.433 | 0.623 | 0.218 | 0.277 |
| 60–64 | 0.545 | 0.885 | 0.251 | 0.360 |
| 65–69 | 0.607 | 1.064 | 0.330 | 0.454 |
| 70–79 | 0.686 | 1.251 | 0.417 | 0.722 |
| ≥80 | 0.860 | 1.616 | 0.668 | 1.183 |
| CPRD Prescriptions (all treatments) | ||||
| 18–49 | 1.247 | 1.363 | 0.890 | 0.931 |
| 50–59 | 1.670 | 1.994 | 1.143 | 1.227 |
| 60–64 | 1.969 | 2.602 | 1.379 | 1.489 |
| 65–69 | 2.129 | 2.894 | 1.473 | 1.717 |
| 70–79 | 2.310 | 3.295 | 1.814 | 2.347 |
| ≥80 | 2.405 | 3.743 | 1.844 | 2.575 |
| CPRD Referrals* | ||||
| 18–49 | 0.018 | 0.020 | 0.011 | 0.012 |
| 50–59 | 0.021 | 0.026 | 0.018 | 0.022 |
| 60–64 | 0.031 | 0.040 | 0.020 | 0.024 |
| 65–69 | 0.031 | 0.044 | 0.015 | 0.023 |
| 70–79 | 0.033 | 0.054 | 0.031 | 0.047 |
| ≥80 | 0.040 | 0.065 | 0.029 | 0.048 |
| CPRD Sick leave* | ||||
| 18–49 | 0.162 | 0.175 | 0.155 | 0.161 |
| 50–59 | 0.156 | 0.178 | 0.173 | 0.182 |
| 60–64 | 0.060 | 0.069 | 0.080 | 0.087 |
| 65–69 | 0.017 | 0.017 | 0.008 | 0.008 |
| 70–79 | 0.001 | 0.001 | 0.002 | 0.003 |
| ≥80 | 0.000 | 0.000 | 0.000 | 0.000 |
| CPRD Nursing home care/admission* | ||||
| 18–69 | 0.000 | 0.000 | 0.000 | 0.000 |
| 70–79 | 0.001 | 0.001 | 0.001 | 0.001 |
| ≥80 | 0.004 | 0.004 | 0.003 | 0.003 |
Costs were assigned for HES hospital admission, HES outpatient consultation, CPRD ambulatory Visits, CPRD other ambulatory visits, CPRD prescriptions.
*No costs were assigned for CPRD Referrals, CPRD sick leave, CPRD Nursing home care/admission.
CPRD, Clinical Practice Research Datalink; HES, Hospital Episode Statistics; IC, immunocompromised.
Figure 3 and table 2 present the overall healthcare costs by CPRD-HES-linked matched IC cohort and age group for the analysis period 7 days prior to 365 days post initial HZ onset. The costs increase with age and are consistently higher in the IC cohort compared with the IC-free cohort. Although the absolute cost difference between IC and IC-free individuals increases with age from £85.5 in individuals aged 18–49 YOA to £156.1 in individuals aged ≥80 YOA the relative difference is higher in younger individuals (ie, 75.8%–99.2% in <70 YOA) compared with older individuals (ie, 38.9%–57.6% in ≥70 YOA). It is also noteworthy that the means are consistently higher than medians, and as is common for healthcare cost data, the distribution is skewed to the right. See online supplementary table 5 and online supplementary figures 1 and 2 provide additional data on healthcare Costs for the analysis period 7 days prior to 90 days post initial HZ onset.
Figure 3.
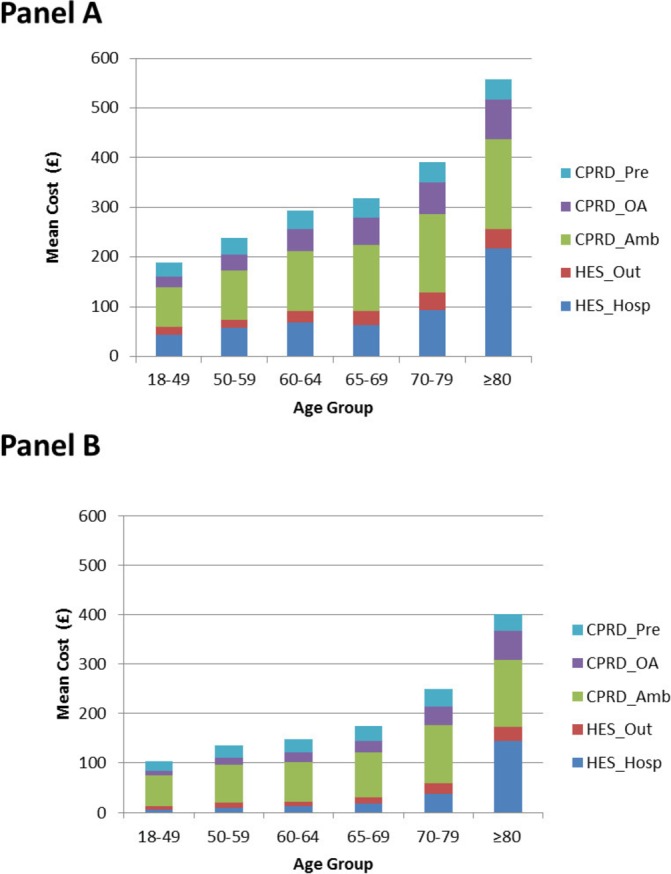
Healthcare costs by HES-linked matched IC (Panel A) and IC-free cohort (Panel B) for the analysis period 7 days prior to 365 days post initial HZ onset. For HZ individuals without PHN: data from 7 days prior to 30 days post HZ onset included. For HZ individuals with PHN: data from 7 days prior until 365 days after HZ onset. £, 2014 UK pound sterling; CPRD, Clinical Practice Research Datalink; CPRD_Amb, CPRD Ambulatory Visits; CPRD_OA, CPRD Other Ambulatory Visits; CPRD_Pre, CPRD Prescriptions; HES, Hospital Episode Statistics; HES_Hosp, HES Hospital admission; HES_Out, HES Outpatient consultation; HZ, herpes zoster; IC, immunocompromised; PHN, postherpetic neuralgia.
Table 2.
Mean cost (£) of healthcare resource utilisation by IC status, age group and analysis period*
| Age groups (YOA) | Statistic | Mean cost (£) | |||
| IC cohort | IC-free cohort | ||||
| 90 day | 365 day | 90 day | 365 day | ||
| 18–49 | Mean | 173.3 | 189.3 | 98.2 | 103.8 |
| Median, SE | 86.1, 6.03 | 86.9, 6.81 | 59.6, 3.06 | 62.0, 3.35 | |
| 50–59 | Mean | 199.0 | 237.8 | 118.9 | 135.3 |
| Median, SE | 106.6, 6.37 | 108.8, 9.05 | 74.6, 3.72 | 74.8, 4.68 | |
| 60–64 | Mean | 236.2 | 294.2 | 126.8 | 147.7 |
| Median, SE | 120.2, 9.39 | 124.1, 12.01 | 78.9, 4.48 | 80.9, 5.81 | |
| 65–69 | Mean | 241.6 | 317.4 | 145.5 | 174.4 |
| Median, SE | 132.2, 8.52 | 140.0, 11.25 | 87.9, 4.64 | 90.7, 5.97 | |
| 70–79 | Mean | 289.6 | 391.7 | 189.8 | 248.6 |
| Median, SE | 154.2, 7.21 | 163.9, 10.11 | 108.8, 4.97 | 113.6, 6.88 | |
| ≥80 | Mean | 427.0 | 557.1 | 319.7 | 401.0 |
| Median, SE | 176.2, 13.12 | 188.6, 17.05 | 143.0, 11.20 | 154.0, 13.63 | |
*Post initial HZ onset.
£, 2014 UK pound sterling; HZ, herpes zoster; IC, immunocompromised; SE, Standard Error; YOA, years of age.
Figure 4 presents the overall healthcare costs by each IC condition in the CPRD-HES-linked matched IC and IC-free cohort by age group for the analysis period 7 days prior to 365 days post initial HZ onset. For all IC conditions, the costs were higher than those for the IC-free group, in particular for the hematopoietic stem cell transplantation (HSCT), haematological malignancies and solid organ transplantations (SOT) conditions. In general, there was a similar trend of increasing costs with increasing age-groups. A few outliers were observed due to small sample sizes. For example, only 3 and 8 individuals aged ≥70 YOA were included in the HIV and HSCT groups, respectively. Similarly, in total only 207 and 271 individuals with autoimmune thyroiditis and SOT were included, respectively.
Figure 4.
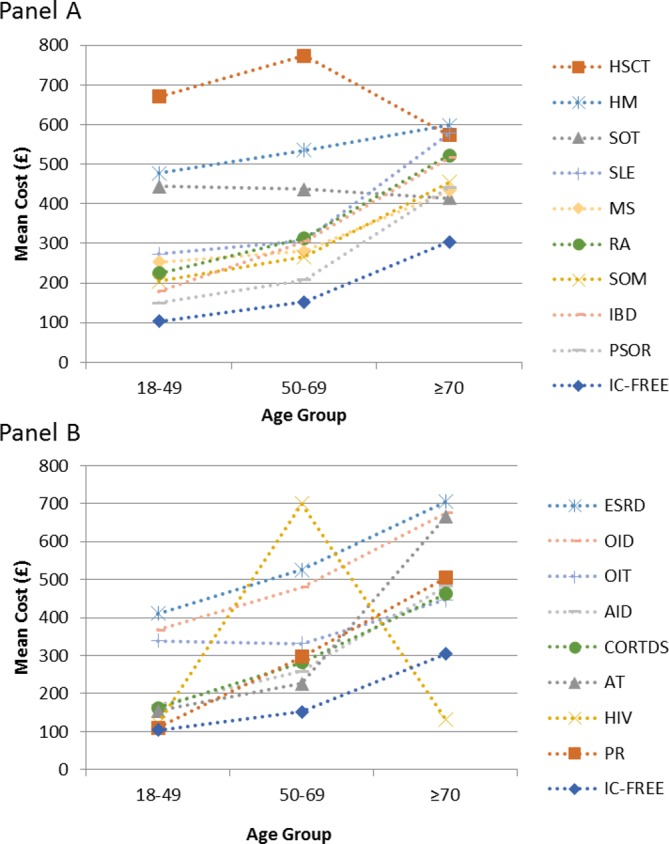
Healthcare costs for each IC condition in the HES-linked matched IC and IC-free cohort by age group for the analysis period 7 days prior to 365 days post initial HZ onset. For HZ individuals without PHN: data from 7 days prior to 30 days post HZ onset included. For HZ individuals with PHN: data from 7 days prior until 365 days after HZ onset. £, 2014 UK pound sterling; AID, autoimmune diseases; AT, autoimmune thyroiditis; CORTDS, corticosteroid exposure; ESRD, end-stage renal disease; HES, Hospital Episode Statistics; HIV, human immunodeficiency virus; HM, haematological malignancies; HSCT, hematopoietic stem cell transplantation; HZ, herpes zoster; IBD, inflammatory bowel syndrome; IC, immunocompromised; MS, multiple sclerosis; OID, other immunodeficiency; OIT, other immunosuppressive therapy; PHN, postherpetic neuralgia; PR, polymyalgia rheumatica; PSOR, psoriasis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SOM, solid organ malignancies; SOT, solid organ transplantations.
Table 3 presents the mean healthcare costs by IC status and HZ complication status. The mean healthcare costs were approximately 4 to 5 times higher for individuals with PHN for the analysis period 7 days prior to 365 days compared with individuals with HZ only. Similarly, mean healthcare costs were approximately 2 to 4 times higher for individuals with HZ complications compared with individuals with HZ only.
Table 3.
Mean cost (£) of healthcare resource utilisation by IC status, age group, analysis period and HZ complication status
| Age groups (YOA) | HZ only* | PHN day 90† | PHN day 365‡ | HZ-Comp§ | |
| Mean cost (£), IC | |||||
| 18–49 | Mean | 156.6 | 302.4 | 746.6 | 573.3 |
| Median, SE | 81.8, 5.60 | 194.7, 33.83 | 465.7, 77.58 | 176.5, 87.70 | |
| 50–59 | Mean | 168.1 | 468.0 | 998.9 | 562.6 |
| Median, SE | 93.9, 5.05 | 262.8, 49.39 | 588.8, 89.42 | 226.0, 89.23 | |
| 60–64 | Mean | 190.8 | 538.7 | 1135.5 | 780.5 |
| Median, SE | 108.8, 7.59 | 297.7, 51.60 | 688.2, 76.50 | 226.4, 175.40 | |
| 65–69 | Mean | 195.6 | 489.3 | 1064.3 | 551.8 |
| Median, SE | 109.6, 8.04 | 305.4, 31.80 | 738.9, 50.39 | 204.9, 104.01 | |
| 70–79 | Mean | 228.9 | 540.4 | 1200.2 | 847.5 |
| Median, SE | 129.0, 6.18 | 324.5, 27.02 | 808.4, 44.67 | 337.4, 111.20 | |
| ≥80 | Mean | 307.6 | 779.5 | 1536.0 | 1396.4 |
| Median, SE | 148.6, 11.11 | 384.3, 40.82 | 937.3, 64.54 | 516.5, 151.71 | |
| Mean cost (£), IC-free | |||||
| 18–49 | Mean | 91.6 | 216.1 | 391.9 | 246.4 |
| Median, SE | 54.9, 2.67 | 137.7, 39.25 | 261.9, 48.41 | 106.6, 58.73 | |
| 50–59 | Mean | 106.8 | 262.8 | 540.8 | 275.1 |
| Median, SE | 72.0, 2.76 | 208.2, 25.72 | 391.3, 45.62 | 118.1, 124.73 | |
| 60–64 | Mean | 114.1 | 270.5 | 556.1 | 192.7 |
| Median, SE | 74.6, 4.34 | 191.9, 23.69 | 409.4, 46.78 | 78.1, 56.06 | |
| 65–69 | Mean | 123.7 | 287.7 | 595.5 | 592.6 |
| Median, SE | 80.9, 3.45 | 202.4, 22.35 | 440.2, 37.43 | 229.5, 179.85 | |
| 70–79 | Mean | 149.2 | 388.2 | 813.7 | 511.5 |
| Median, SE | 88.7, 3.93 | 248.6, 21.27 | 546.1, 34.81 | 232.9, 89.19 | |
| ≥80 | Mean | 242.0 | 607.4 | 1182.8 | 1046.5 |
| Median, SE | 121.3, 9.08 | 310.5, 41.96 | 726.9,1 58.46 | 341.0, 149.46 | |
*Individuals with HZ only (ie, without PHN and complications): includes only costs 7 days prior to 30 days post initial HZ onset.
†Individuals with HZ and PHN: includes only costs 7 days prior to 90 days post initial HZ onset.
‡Individuals with HZ and PHN: includes costs 7 days prior to 365 days post initial HZ onset.
§Individuals with HZ and complications but no PHN: includes only costs 7 days prior to 30 days post initial HZ onset.
£: 2014 UK pound sterling; HZ,herpes zoster; HZ-Comp, HZ and complications with no PHN; IC, immunocompromised; PHN, postherpetic neuralgia; SE, Standard Error; YOA, years of age.
Online supplementary table 6 presents the non-HZ related hospital inpatient stay for the period 7 days to 365 days post initial-HZ onset. The mean number of non-HZ related hospitalisations were consistently higher in IC patients compared with and IC-free patients and increased with age.
Discussion
In this study, we presented the healthcare resource utilisation and costs associated with HZ in both IC and IC-free populations using large electronic health record databases in the UK. An important feature of this study was that the design enabled the calculation of IC condition prevalence rates, HZ incidence rates and occurrence of HZ-related healthcare utilisation and costs at individual level in the same predefined population(s), see Yanni et al for further detail on epidemiological outcomes.7 In this study, every effort was made to include only resources directly related to HZ. For example, only hospitalised patients were included who had an ICD-10 HZ diagnosis identified in the HES database. Similarly, only medications potentially related to HZ treatment were included (see online supplementary tables 2 and 4). HZ-related mean treatment costs per patient were higher in IC individuals (£189 vs £104 in IC and IC-free individuals aged 18–49 YOA, respectively increasing to £557 vs £401 in IC and IC-free individuals aged ≥80 YOA, respectively).
Previous studies of healthcare costs of HZ in the UK, included a small study, which estimated the mean healthcare costs per HZ patient, from an National Health Services perspective, of £85.6 and £400.9 in individuals aged <65 YOA and ≥65 YOA, respectively.12 A later UK study that used the HES and the health improvement network databases, estimated the mean cost of treating a HZ patient to be £65.5 in the first month of diagnosis, with patients aged ≥70 YOA having a mean cost of £83 in the first month and £15.80 in months 2 and 3.13 The costs of treating individuals with PHN were much higher, that is, mean cost per patient was estimated to be £921 in all individuals and £909.60 in individuals aged ≥70 YOA.13 Another study evaluated mean healthcare costs (excluding hospitalisation costs) to be £75.63 per HZ patient with mean direct costs for treating PHN episodes (PHN pain occurring or persisting for 3 months) of £340.04.14 These values augmented with hospitalisation costs were used as inputs in a cost-effectiveness model evaluating a HZ vaccine using the population of England and Wales.3 The costs estimated by van Hoek et al are consistent with the values estimated in our study for IC-free individuals by age group.3
In a previous study, mean prescription costs per HZ patient were reported to be £40.52.14 In our study, the mean prescription costs per HZ patient ranged from £19.7 to £40.8 depending on the age group, IC status and analysis period included. Our study aimed to include only medications considered to be directly related to HZ; that is, excluded medications that may be linked to IC conditions (eg, aspirin, analgesic creams as they could be used primarily to reduce pain from other conditions). This restriction and the introduction of generic versions of medications such as acyclovir, gabapentin (and derivatives of gabapentin) which resulted in lower prices, contributed to the reduced overall medication costs reported in this study.
Many studies on HCRU and costs include a number of days prior to diagnosis, for example, 14 or 21 days, as there may be a delay in diagnosis and HCRU may be used prior to diagnosis.6 15 In this analysis, costs of HZ only cases were assessed during the period 7 days prior to 30 days post HZ onset, although it is recognised that HZ episodes can last for longer. The costs of PHN were analysed over two time-periods, ie, (1) 7 days prior to 90 days post HZ onset and (2) 7 days prior to 365 days post HZ onset. The rationale for the time periods studied was that using analysis period 1 alone could lead to an underestimation of PHN costs whereas using analysis period 2 only could overestimate these costs. The most frequently used definition of PHN is: pain persisting or appearing at least 90 days following rash onset. The median duration of PHN has been reported to be 10.3 and 12.9 months in individuals aged ≤69 and ≥70 YOA respectively,16 and is likely to be longer in individuals who are immunocompromised.5
The healthcare costs associated with PHN and complications were higher than those for individuals with HZ only. However, as reported elsewhere, when considering the overall cost of disease at a population level, the overall healthcare-associated cost is higher for HZ only.17 This is primarily a result of the higher incidence rates of HZ only.
Few studies have investigated healthcare resource utilisation and costs in IC individuals. Schroder et al carried out a study using the German Pharmacoepidemiological Research Database, which consists of claims data from four statutory health insurances.18 They reported that during the quarter of the HZ diagnosis or during the two following quarters, 10% of all HZ patients with an IC condition were hospitalised (with a HZ diagnosis), whereas among IC-free HZ patients, 4.2% were hospitalised. White et al reported that in their study using the US Market Scan Research Database, direct medical costs were nearly twice as high in IC patients compared with IC-free patients.19 Li et al carried out a study using the US Truven Health MarketScan Commercial and Medicare Supplemental Insurance databases.15 They concluded that patients with the studied IC conditions (ie, HIV, SOT, bone marrow or stem cell transplant and cancer) had significantly higher healthcare utilisation and cost when developing HZ than their comparable matches without HZ. Insurance databases include not only the healthcare resource utilisation but also costs. In the CPRD and HES Databases only the resource utilisation is captured. As such the overall costs need to be calculated by assigning unit costs to the resource utilisation. There are advantages however of using the CPRD and HES in that the databases offer more diversity than might be observed using insurance databases, the latter of which may be somewhat limited by bias associated with factors such as age, race and income. A strength of the CPRD database is that it is considered to be broadly representative of the characteristics of patients and GP practices in the UK.20 21
This study has several limitations. Diagnoses were derived from administrative codes, which are recognised to be subject to miscoding or under-coding and are not validated against medical charts.22 Increasing healthcare resource utilisation and cost is likely to be related to increased severity of IC conditions. In a study, Schroder et al categorised individuals as low IC and high IC.18 However, insufficient details are recorded in the CPRD and HES databases to allow adequate definition of patients’ severity of immunosuppression for example, laboratory parameters, immunosuppressive medication details such as chemotherapy. In addition, many IC individuals had prescriptions that included more than one immunosuppressing medicine. In this study we selected 16 IC conditions in our definition of an IC population but perhaps other researchers would select different IC conditions. As such our study is exploratory in nature and was not intended to be definitive.
Conclusion
Immunosuppression is known to be associated with an increased risk of HZ in the UK.2 7 In this descriptive analysis, involving a large representative national data source, the results suggest that individuals with IC conditions were associated with higher HZ related healthcare utilisation and costs than IC-free individuals.6 7 15 The results from this study could be used in economic analyses to evaluate the value of vaccination in reducing the burden of HZ in these populations.
Supplementary Material
Acknowledgments
The authors would like to thank Emmanuelle Espié, Emad Yanni, Morgane Guinnec, François Haguinet for their contribution to the study. They would also like to thank the Business & Decision Life Sciences platform for editorial assistance and coordination, on behalf of GSK. Gregory Collet coordinated manuscript development and editorial support. Kathleen Daly provided editing support. This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone. Part of the data have been previously published as an abstract (Curran al., 2017) and presented at ISPOR-EU (2017) International Society for Pharmacoeconomics and Outcomes Research - 20th Annual European Congress.
Footnotes
Contributors: VB, AEG, YEH, GF, MH and DC participated in the conception and design of the study. VB, AEG, YEH, GF and MH participated in the collection or generation of the study data. VB, AEG and YEH performed the study. AEG, YEH, MH and DC contributed to the material. VB, AEG, YEH, GF, MH and DC were involved in the analysis or interpretation of the data. All named authors provided substantial intellectual and scientific input during the manuscript development, critically reviewing the content, revising the manuscript and giving final approval before submission. The work described was carried out in accordance with the ICMJE recommendations for conducting, reporting, editing and publishing scholarly work in medical journals. All authors had full access to the data and gave final approval before submission. The corresponding author was responsible for submission of the publication.
Funding: GlaxoSmithKline Biologicals SA was the funding source and was involved in all study (GSK study identifier: e-track number: 201615) activities and overall data management (collection, analysis and interpretation). GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript.
Competing interests: VB, MH and DC are employees of the GSK group of companies. DC and MH hold shares in the GSK group of companies. AEG and YEH have nothing to disclose. GF was employed by the GSK group of companies between 2012 and Feb 2015, during which the study was designed and implemented. Later, as an employee of P-95 epidemiology and pharmacovigilance, GF provided contracted consultancy services to the GSK group of companies for this and other GSK-sponsored studies. P-95 provides contracted services to the GSK group of companies, beyond the scope of this study.
Ethics approval: Approval was obtained from the Clinical Practice Research Datalink Independent Scientific Advisory Committee (14_222R).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data used in this study are presented in the manuscript, references to the original material are provided. Please contact the corresponding author shall you require any additional information.
Author note: AEG and YEH were consultant for GSK, Wavre, Belgium at the time of the study. AEG is currently at F. Hoffmann-La Roche Ltd., Basel, Switzerland and YEH is currently at Accord Research s.r.o., Prague, Czech Republic.
Patient consent for publication: Not required.
References
- 1. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010;51:197–213. 10.1086/653605 [DOI] [PubMed] [Google Scholar]
- 2. Forbes HJ, Bhaskaran K, Thomas SL, et al. Quantification of risk factors for herpes zoster: population based case-control study. BMJ 2014;348:g2911 10.1136/bmj.g2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hoek AJ, Gay N, Melegaro A, et al. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009;27:1454–67. 10.1016/j.vaccine.2008.12.024 [DOI] [PubMed] [Google Scholar]
- 4. Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004;5:344–56. 10.1016/j.jpain.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 5. Drolet M, Brisson M, Schmader K, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain 2010;11:1211–21. 10.1016/j.jpain.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 6. Yawn BP, Itzler RF, Wollan PC, et al. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009;84:787–94. 10.4065/84.9.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanni EA, Ferreira G, Guennec M, et al. Burden of herpes zoster in 16 selected immunocompromised populations in England: a cohort study in the Clinical Practice Research Datalink 2000-2012. BMJ Open 2018;8:e020528 10.1136/bmjopen-2017-020528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant 2016;22:557–63. 10.1016/j.bbmt.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. International Society for Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practices (GPP). 2015. https://www.pharmacoepi.org/resources/guidelines_08027.cfm
- 10. Curtis LPSSRU. Unit costs of health & social care 2014. 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014/
- 11. National Tariff Payment System. Annex 5A national prices 2015. 2014. https://www.gov.uk/government/publications/national-tariff-payment-system-2014-to-2015
- 12. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of Herpes Zoster: a prospective population based study. Vaccine 2006;24:1308–14. 10.1016/j.vaccine.2005.09.026 [DOI] [PubMed] [Google Scholar]
- 13. Taieb V, Schwarzbard J, Butt T, et al. The Epidemiological and Cost Burden of Herpes Zoster (Hz) and Post-Herpetic Neuralgia (Phn) in the Uk. Value Health 2015;18:A589 10.1016/j.jval.2015.09.1512 [DOI] [Google Scholar]
- 14. Gauthier A, Breuer J, Carrington D, et al. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 2009;137:38–47. 10.1017/S0950268808000678 [DOI] [PubMed] [Google Scholar]
- 15. Li Q, Chen SY, Burstin SJ, et al. Cost of Herpes Zoster in Patients With Selected Immune-Compromised Conditions in the United States. Open Forum Infect Dis 2016;3:ofw067 10.1093/ofid/ofw067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore L, Remy V, Martin M, et al. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010;8:7 10.1186/1478-7547-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gater A, Uhart M, McCool R, et al. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health 2015;15:193 10.1186/s12889-015-1514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schröder C, Enders D, Schink T, et al. Incidence of herpes zoster amongst adults varies by severity of immunosuppression. J Infect 2017;75:207–15. 10.1016/j.jinf.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 19. White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009;27:781–92. 10.2165/11317560-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 20. Campbell J, Dedman DJ, Eaton SC, et al. Is the CPRD GOLD population comparable to the U.K. population? Pharmacoepidemiol Drug Saf 2013;22:280. [Google Scholar]
- 21. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pierik JG, Gumbs PD, Fortanier SA, et al. Epidemiological characteristics and societal burden of varicella zoster virus in the Netherlands. BMC Infect Dis 2012;12:110 10.1186/1471-2334-12-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023502supp001.pdf (475.5KB, pdf)