Abstract
Coenzyme Q10 (Q10) plays an important role in mammals for energy production in the mitochondria, and as a potent antioxidant. Oxidation ratio (% oxidized in relation to total Q10) has been proposed as an important biomarker. A sensitive and reproducible HPLC-ECD method was developed for determination of reduced and oxidized Q10 in canine plasma and heart tissue. Chromatographic separation was achieved in 10 min using a Waters Nova-pak C18 column and a mobile phase with lithium perchlorate in ethanol/methanol/2-propanol. The validation showed satisfying results. Excellent linear correlation was found (r2 > 0.9997), intra- and inter-day precisions were below 6.5% (n = 5) and recoveries were between 89 and 109% (n = 5). Sensitivity stated as Lower Limit of Quantification (LLOQ) was 10 nM. Acceptable stability of both extracted and un-extracted samples was observed. The plasma concentration range of total Q10 was found to be between 0.64 and 1.24 µg/mL. Comparison with a developed LC-MS/MS method showed a correlation of r = 0.85 for reduced Q10 and r = 0.60 for oxidized Q10 (N = 17). However, average results were around 30% lower for ubiquinol using the LC-MS/MS method as compared with the HPLC-ECD analysis. The two methods are therefore not considered to be interchangeable.
Keywords: coenzyme Q10, oxidative stress, congestive heart failure, HPLC, LC-MS/MS
1. Introduction
Q coenzymes are ubiquitous in eukaryotic cells. Chemically, they consist of a benzoquinone ring coupled to between 6 and 10 isoprenoid units, depending on species [1,2]. Coenzyme Q10 (Q10) is prevalent in mammals, including dogs, and exists in a reduced and oxidized form, i.e., ubiquinol (CoQ10H2) and ubiquinone (CoQ10) [1,3]. Q10 is involved in the electron transport chain, needed to increase the proton gradient in the membrane in order to synthetize ATP and CoQ10H2 plays an important role as antioxidant preventing lipid peroxidation and oxidative impairment of DNA [4,5]. A shift in oxidation ratio (i.e., % oxidized in relation to total Q10) is an indication of increased oxidative stress [3,6]. The heart contains particularly high concentrations of Q10 implying that the heart is highly energy-demanding [7]. It could therefore be speculated that the heart is more vulnerable to mitochondrial dysfunction possibly resulting in cardiovascular disease. Several reports have shown a correlation between Q10 deficiency and cardiovascular incidents [8,9]. On that basis, Q10 has been given as supplement for humans in order to investigate its positive effects on cardiovascular disease. Results from a newer clinical study, where Q10 supplement was given as add-on therapy to patients suffering from congestive heart failure, showed a reduction in the relative risk of cardiovascular death of 43% [10].
In order to study the mechanism by which Q10 may improve cardiac health in vivo, the use of a relevant comparator model, e.g., the dog, is an obvious option. When assessing the functional status of Q10, the total concentration of Q10 or oxidation ratio is often determined in plasma. In plasma, Q10 is mostly found as CoQ10H2. However, as the oxidation ratio in plasma is highly affected by dietary uptake and daily rhythm, it may be more relevant to measure the concentration of Q10 or oxidation ratio in relevant peripheral tissue [11]. Sample preparation and subsequent analysis is critical for determination of oxidation ratio due to instability of the reduced form of Q10 [12,13]. Historically, high-performance liquid chromatography coupled with ultraviolet detection (HPLC-UV) has been used for quantification of the total Q10 concentration [7,14]. In order to investigate the oxidation ratio, HPLC coupled to electrochemical detection (HPLC-ECD) has been used extensively, since oxidation ratio may be determined by performing reduction or oxidation in the electrochemical cell after chromatographic separation [13,15,16,17,18]. In recent years, liquid chromatography–mass spectrometry (LC-MS) and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) have become important tools in quantitative bio-analysis due to their high sensitivity and selectivity, and several publications have demonstrated the applicability of LC-MS and LC-MS/MS for Q10 quantification and evaluation of oxidation ratio in biological samples [19,20,21,22].
In order to pave the road for future experimental and clinical studies using the dog as comparator model of human congestive heart failure, the overall aim of this study was to develop a fast and sensitive HPLC-ECD method for simultaneous quantification of the reduced and oxidized form of Q10—i.e., ubiquinol and ubiquinone—in canine plasma and heart tissue. Analysis of heart tissue poses additional challenges as compared to other tissues due to its hard and fibrous nature, and the Q10 oxidation ratio in heart tissue has only been scarcely described in the literature [21,23,24,25]. Initially, optimization of sample preparation for heart tissue and plasma was performed, before development of an HPLC-ECD method. Further objectives were: (i) to perform validation of the developed HPLC-ECD method regarding linearity, sensitivity, precision, and accuracy by taking the current FDA bio-analytical guideline into account [26]; and (ii) to compare the HPLC-ECD method with a developed LC-MS/MS method and discuss benefits and drawbacks of using the two detection principles for quantification of total Q10 concentration and evaluation of oxidation ratio.
2. Materials and Methods
2.1. Chemicals and Materials
Ubiquinone (purity > 98%), butylated hydroxytoluene (BHT), 1-propanol, 2-propanol, 96% ethanol, methanol, hexane, lithium perchlorate (LiPerChl·3H20), and ammonium acetate were purchased from Merck (Darmstadt, Germany). Ubiquinol (USP reference standard) was obtained from Sigma Aldrich, now Merck (Darmstadt, Germany). For homogenization of heart tissue, Lysing Matrix A (2 & 4.5 mL) and Lysing Matrix S (2 mL) was purchased from MP Biomedicals (Eschwege, Germany). 5- and 7-mm stainless steel beads were obtained from Qiagen (Manchester, UK). Coenzyme Q10-[2H9] (CoQ10-d9, purity > 97%) used as internal standard (IS) in the LC-MS/MS analysis was obtained from IsoSciences (Ambler, PA, USA). Stock solutions of CoQ10, CoQ10H2 and CoQ10-d9 were prepared in hexane at 1000 µM and kept at −80 °C.
2.2. Biological Samples
Plasma samples were obtained from client-owned dogs associated with the University of Copenhagen’s surveillance program for myxomatous mitral valve disease [27]. Plasma samples were used for optimization and validation of the HPLC-ECD method and for comparison of the HPLC-ECD and LC-MS/MS methods. All samples were collected following informed consent from the owner and with ethical approval from the Danish Animal Experimentation Inspectorate (Approval no. 2016-15-0201-01074). Canine blood was collected in K3-EDTA tubes. Centrifugation at 3000× g at 4 °C for 10 min was performed to obtain plasma. Additionally, heart tissue sample was obtained from two dogs from the same program undergoing elective euthanasia. Plasma and heart tissue was processed as described in detail below and stored at −80 °C until analysis.
2.3. HPLC-ECD
An Ultimate 3000 HPLC system (Thermo Scientific, Waltham, MA, USA) was used for the HPLC-ECD method. The chromatographic separation was achieved with a Waters Nova-pak C18 column with the dimensions 150 × 3.9 mm (4 µm, 60 Å) employing a mobile phase consisting of 20 mM LiPerChl ·3H20 dissolved in ethanol/methanol/2-propanol (75:16.7:8.3, v/v/v). Electrochemical detection was performed with a RS6011 ultra-analytical cell (Thermo Scientific, Waltham, MA, USA) set at 500 mV as determined by a hydrodynamic voltammogram. The guard cell RS6020 was set at −600 mV prior to the analytical cell to reduce all compounds eluting from the column.
2.4. LC-MS/MS
LC-MS/MS quantification was performed on a Waters 2795 separation module hyphenated with a Micromass Quattro micro API mass spectrometer with an ESI ionization source (Waters Corp., Milford, MA, USA). The chromatographic conditions from the HPLC-ECD method were used for the LC-MS/MS system with the following adaptations: LiPerChl·3H2O was exchanged with 2 mM ammonium acetate and the proportions of the mobile phase components ethanol/methanol/2-propanol were changed to (76:16.9:7.1, v/v/v). The same HPLC column was used, although with an inner diameter of 2.1 mm. The MS system was employed in positive ionization mode using the following parameters (partly adapted from Tang et al. [22]): Capillary voltage: 2.75 kV; cone voltage 30 V; source temperature: 120 °C; and desolvation temperature: 400 °C. Nitrogen was used as desolvation gas (700 L·h−1) and cone gas (60 L·h−1). Argon was used for collision-induced dissociation. The collision energy was 25 V. Dwell time was 1 s. To exclude salts from entering into the MS, a solvent delay of 4 min was applied. Multiple reaction monitoring (MRM) was used for quantification of CoQ10 and CoQ10H2 as [M + NH4]+ with the following transitions from precursor to product ions: CoQ10: m/z 881 → 197. CoQ10H2: m/z 883 → 197. CoQ10-d9: 890 → 206. MassLynx software, version 4.1, was used for acquisition and analysis of the data (Waters Corp., Milford, MA, USA).
2.5. Optimization of Extraction Procedure
2.5.1. Plasma
Extraction was tested with the solvents 1-propanol, 2-propanol, and hexane:ethanol (50:50, v/v) in different ratios (2:1, 3:1, 4:1, and 5:1) in relation to plasma.
2.5.2. Heart Tissue
Several factors considered to affect homogenization were tested for their potential influence: Extraction solvents (1-propanol, 2-propanol, hexane:ethanol (50:50, v/v), beads (5 mm stainless steel beads, 7 mm stainless steel beads, Matrix A and Matrix S), amount of tissue (22 mg, 45 mg, 60 mg, 75 mg and 270 mg), and homogenization time (45 s, 60 s, 120 s). Furthermore, different homogenization methods were tested: Homogenization with a Potter-Elvehjem apparatus (IKA Labortechnik, Staufen, Germany), a FastPrep-24TM (MP Biomedicals, Eschwege, Germany) or mortar and pistil. The effect of freeze-drying and treatment with liquid nitrogen of the heart tissue prior to homogenization was also evaluated. The replicate number was at least three in all optimization experiments.
2.6. Final Extraction Procedures
2.6.1. Plasma
75 µL of 1-propanol and 10 µL BHT (10 mg/mL in 96% ethanol) was added to 25 µL of canine plasma kept on dry ice. The solution was mixed for 2 min on a shaker placed at 4 °C followed by centrifugation at 16,000× g in 2 min at 4 °C. The supernatant was analyzed immediately. For LC-MS analysis, 2 µL of a 10 µM Coenzyme Q10-[2H9] dissolved in hexane was added before the addition of 1-propanol and BHT.
2.6.2. Heart Tissue
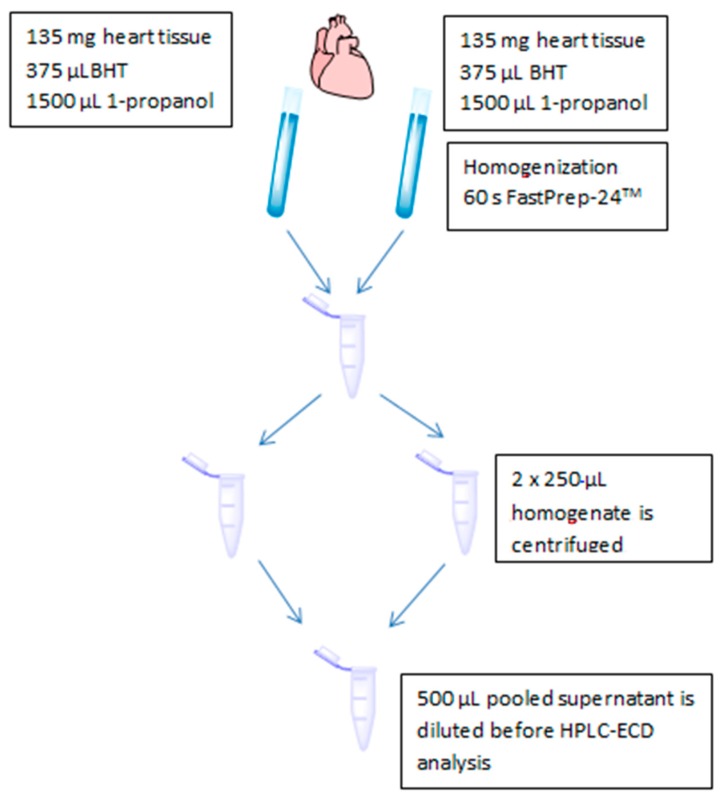
The sample preparation for heart tissue is shown in Figure 1. Around 2 × 135 mg of tissue was cut off from a canine heart kept on dry ice; the amounts were weighed exactly and thereafter transferred to two 4.5 mL homogenization tubes containing lysing matrix A, 375 µL BHT (10 mg/mL in 96% ethanol) and 1500 µL 1-propanol. The homogenization tubes were sealed and homogenized for 60 s with an oscillation frequency of 50 Hz on the FastPrep-24. The resulting homogenates were centrifuged using 16,000× g at 4 °C for 2 min. Clear supernatants from two homogenizations were pooled and analyzed immediately.
Figure 1.
Schematic overview of heart tissue sample preparation before quantification of reduced and oxidized Q10 with HPLC-ECD.
2.7. Validation of the HPLC-ECD Method
2.7.1. Calibration Curves and Sensitivity
Before preparation of calibration curves, the 1000 µM solution of CoQ10 and CoQ10H2 prepared in hexane was further diluted to 100 µM with 96% ethanol. The CoQ10 stock solution was further diluted 1:1 with 96% ethanol before measurement on a spectrophotometer at 275 nm (ε = 14240). CoQ10H2 was measured without further dilution at 290 nm (ε = 4010). The spectrophotometric measurements were performed to determine the exact concentrations [28]. Working calibration solutions of CoQ10 and CoQ10H2 at 10 µM in 96% ethanol were used for preparation of the calibration curves. Eight-point calibration curves from 10 to 1000 nM in 96% ethanol were used for both the HPLC-ECD and LC-MS/MS methods. LLOQ was determined as the concentration giving a signal-to-noise ratio (S/N ratio) of 10:1. The precision was investigated at LLOQ by diluting plasma with PBS buffer and homogenate with extraction solution to the expected LLOQ and analyzing the prepared sample five times. Upper Limit of Quantification (ULOQ) was considered to be equal to the highest concentration in the calibration curve at 1000 nM.
2.7.2. Precision and Accuracy
Precision (intra- and inter-assay) and accuracy was estimated in the same set up. 25 µL of canine plasma and around 2 × 135 mg of canine heart tissue were analyzed on three consecutive days (n = 5) as is, and with the addition of 25%, 50%, and 75% of CoQ10 and CoQ10H2. Precision was acceptable, if the coefficient of variation, CV%, was within 15%, although a CV% of 20% was acceptable at LLOQ. Accuracy was expressed as % recovery. The requirement for accuracy was a CV% of less than 15%.
2.7.3. Stability
Stability at 4 °C mimicking storage in a cooled auto sampler was investigated for extracted canine plasma and heart tissue every second hour for 8 h. Furthermore, the effect of BHT on the stability of Q10 in plasma was evaluated. Extracted samples were tested at −20 °C in up to 4 days. Long-term stability at −80 °C of un-extracted plasma and heart tissue was evaluated for 2 months. A deviation of no more than ± 15% of the nominal concentration was accepted.
2.7.4. Data Analysis
Concentration ranges of CoQ10H2 and CoQ10 are expressed as average ± SD. Regarding precision, the variation was stated as CV%, whereas accuracy was expressed as % recovery of measured concentrations from the expected concentration plus the added concentration of calibration standard. Oxidation ratio was calculated as the concentration of oxidized Q10 in relation to the total concentration of Q10. Student’s t-test was applied for comparison of two groups. Single factor ANOVA was used for comparison of more than two groups. Correlation plots, Bland–Altman plots and statistical calculations were performed in GraphPad Prism 6.0 (GraphPad Prism software, La Jolla, CA, USA). Pearson’s r is stated for the correlation plots. Bias ± standard deviation (SD) and 95% limits of agreement are derived from the Bland–Altman plots. A p-value of less than 0.05 was considered to be significant.
2.8. Comparison of HPLC-ECD with LC-MS/MS
Twenty samples of canine plasma were analyzed with the developed HPLC-ECD method. During the same time frame, comparative data on the 20 samples were collected using the LC-MS/MS method. The integrity of the samples was therefore considered to be the same for both analyses. Data from three dogs were omitted due to an IS response in the LC-MS/MS analysis of almost half of the average response. No additional samples were available for re-analysis. Typical equations for calibration curves obtained using the LC-MS/MS system: ubiquinol: y = 55.4x + 2.12, r2 = 0.995. Ubiquinone: y = 26.1x + 2.56, r2 = 0.994. Weighing was not applied.
3. Results
3.1. Extraction of Q10 from Canine Plasma and Heart Tissue
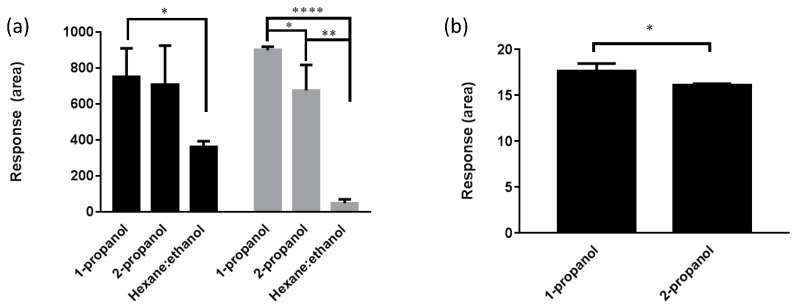
Figure 2a shows results from applying different extraction solvents for extraction of CoQ10H2 and CoQ10 in heart. Heart tissue extraction with 1-propanol resulted in higher and significantly different responses in the HPLC-ECD method as compared to hexane:ethanol (50:50, v/v) extraction for both CoQ10H2 and CoQ10 (p = 0.013 and p = 8.4 × 10−7, respectively). There was no significant difference between 1-propanol and 2-propanol regarding CoQ10H2. However, a significant difference was found between 1-propanol and 2-propanol extraction for CoQ10 in heart tissue (p = 0.045). Furthermore, the variation seemed to be lower with 1-propanol extraction. The homogenization of heart tissue was tested with different procedures; mortar and pistil, Potter-Elvehjem or using a FastPrep-24TM. The FastPrep-24TM resulted in fast and efficient homogenization of the heart tissue with CV% of less than 10%, when combined with lysing matrix A in relation to matrix S showing a variation of around 30%. Steel beads measuring 5 mm and 7 mm gave variations between 13 and 24%, respectively. It was possible to downscale the heart tissue amount to 45 mg, thereby reducing extraction volumes equally, since no significant difference was found in a single ANOVA between extraction from the tested tissue amounts (p = 0.22). Increasing homogenization time did not result in increased responses (p = 0.30). The application of freeze-drying of the heart tissue did not improve the oxidation ratio (p = 0.97) as compared to no free-drying. Finally, the application of nitrogen-frozen tissue combined with homogenization by mortar and pistil resulted in low responses for both CoQ10H2 and CoQ10 with unacceptably high variation (CV% > 60%).
Figure 2.
(a) Extraction of CoQ10H2 (▀) and CoQ10 (▀) from canine heart tissue (n = 3); (b) Extraction of CoQ10H2 (▀) from plasma (n = 3). * means p ≤ 0.05, ** means p ≤ 0.01, **** means p ≤ 0.0001.
Figure 2b shows results from testing different extraction solvents for plasma. A significant difference was found for CoQ10H2 regarding extraction with 1-propanol and 2-propanol (p = 0.038). Plasma extraction of CoQ10H2 with hexane:ethanol (50/50) was below LLOQ. Extraction of CoQ10 was below LLOQ for all solvents in the extraction experiments. Based on the above data, 1-propanol was selected as the best solvent for extraction in both heart tissue and plasma. The lowest possible extraction ratio for plasma was selected to 3:1, which was found to give acceptable responses above LLOQ in the following optimization experiments and a clear supernatant.
3.2. Validation of the HPLC-ECD Method
3.2.1. Linearity and Sensitivity
The following equations for calibration curves were typical for CoQ10H2: y = 0.0590x − 0.3076, r2 = 0.9998 and for CoQ10: y = 0.0139x – 0.0929, r2 = 0.9997. In plasma and heart tissue, reduced and oxidized Q10 demonstrated an LLOQ of 10 nM corresponding to 0.2 pmol on column. The precision at LLOQ for plasma was 2.0% for CoQ10H2 and 7.2% for CoQ10. Regarding heart tissue, the precision at 10 nM was 2.9% for CoQ10H2, and 4.0% for CoQ10. The validated linear range of the method was therefore considered to be from 10 nM to 1000 nM.
3.2.2. Precision and Accuracy
Precision and accuracy results for plasma and heart tissue can be found in Table 1a,b, respectively. Good reproducibility stated as CV% of less than 6.5% was achieved for both intra-day and inter-day variation for CoQ10H2 and CoQ10. The obtained recoveries in% for accuracy were in a range from 89 to 109% regarding both plasma and heart tissue.
Table 1.
(a) Precision (CV%) and accuracy (recovery%) for the quantification of CoQ10H2 and CoQ10 in canine plasma determined within one day (n = 5) or in three different days (n = 5). (b) Precision (CV%) and accuracy (recovery%) for the quantification of CoQ10H2 and CoQ10 in canine heart tissue determined within one day (n = 5) or in three different days (n = 5).
| (a) | |||||
| Analyte | Concentration Added (nM) | Intra-Day | Inter-Day 1 | ||
| CV% | Recovery% | CV% | Recovery% | ||
| CoQ10H2 | 40 | 1.73 | 91.9 | 1.93 | 95.0 |
| 80 | 6.00 | 98.8 | 3.53 | 93.2 | |
| 119 | 0.89 | 94.2 | 1.37 | 89.1 | |
| CoQ10 | 11 | 3.46 | 90.9 | 6.49 | 88.8 |
| 22 | 2.89 | 88.9 | 4.42 | 101 | |
| 33 | 2.06 | 86.7 | 4.24 | 109 | |
| (b) | |||||
| Analyte | Concentration Added (nM) | Intra-Day | Inter-Day 1 | ||
| CV% | Recovery% | CV% | Recovery% | ||
| CoQ10H2 | 890 | 5.48 | 89.6 | 3.75 | 90.7 |
| 1775 | 2.50 | 96.4 | 3.43 | 91.5 | |
| 2660 | 3.96 | 94.1 | 2.49 | 94.0 | |
| CoQ10 | 7516 | 4.06 | 109 | 2.90 | 107 |
| 15,033 | 4.47 | 92.1 | 3.08 | 106 | |
| 22,549 | 5.02 | 107 | 3.70 | 103 | |
1 Average of three different days (n = 15).
3.2.3. Stability
Stability results can be found in Table 2. Extracted canine plasma and heart tissue were stable for 8 h at 4 °C. Without the addition of BHT, the stability of CoQ10H2 and CoQ10 in plasma was acceptable for 6 h at 4 °C. The addition of BHT is therefore justified. The stability of CoQ10H2 and CoQ10 in plasma and heart tissue was found to be 2 days at −20 °C. CoQ10H2 and CoQ10 were stable in un-extracted canine plasma and heart tissue for at least 2 months at −80 °C.
Table 2.
Stability results of CoQ10H2 and CoQ10 in extracted canine plasma and heart tissue at 4 °C and −20 °C and un-extracted canine plasma and heart tissue at −80 °C (n = 3). Extracted canine plasma was investigated with and without the addition of the antioxidant BHT at 4 °C.
| Analyte (%) | Autosampler (4 °C, 8 H) w/BHT |
Autosampler (4 °C, 6 H) w/o BHT |
Short-Term (−20 °C, 2 d) |
Long-Term (−80 °C, 2 m) |
|||
|---|---|---|---|---|---|---|---|
| Plasma | Heart | Plasma | Plasma | Heart | Plasma | Heart | |
| CoQ10H2 | 91 | 103 | 86 | 89.9 | 94.8 | 108 | 107 |
| CoQ10 | 114 | 115 | 115 | 112 | 119 | 87 | 106 |
3.3. Comparison of HPLC-ECD with LC-MS/MS
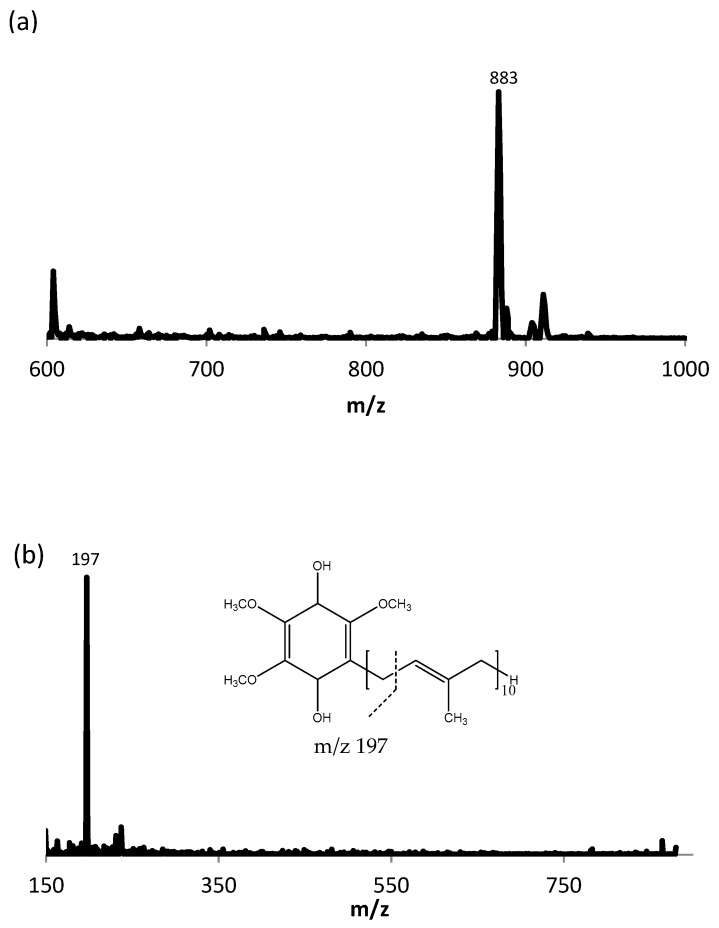
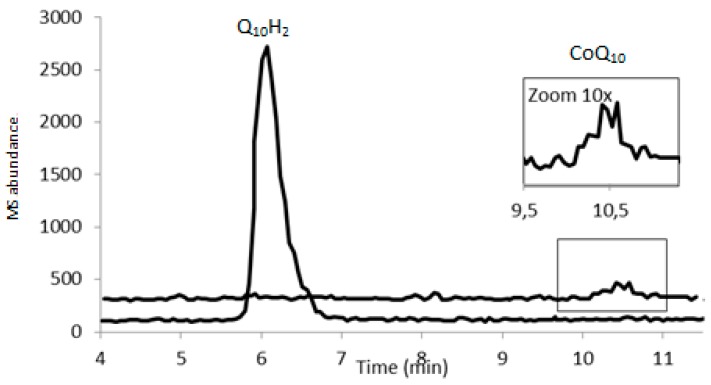
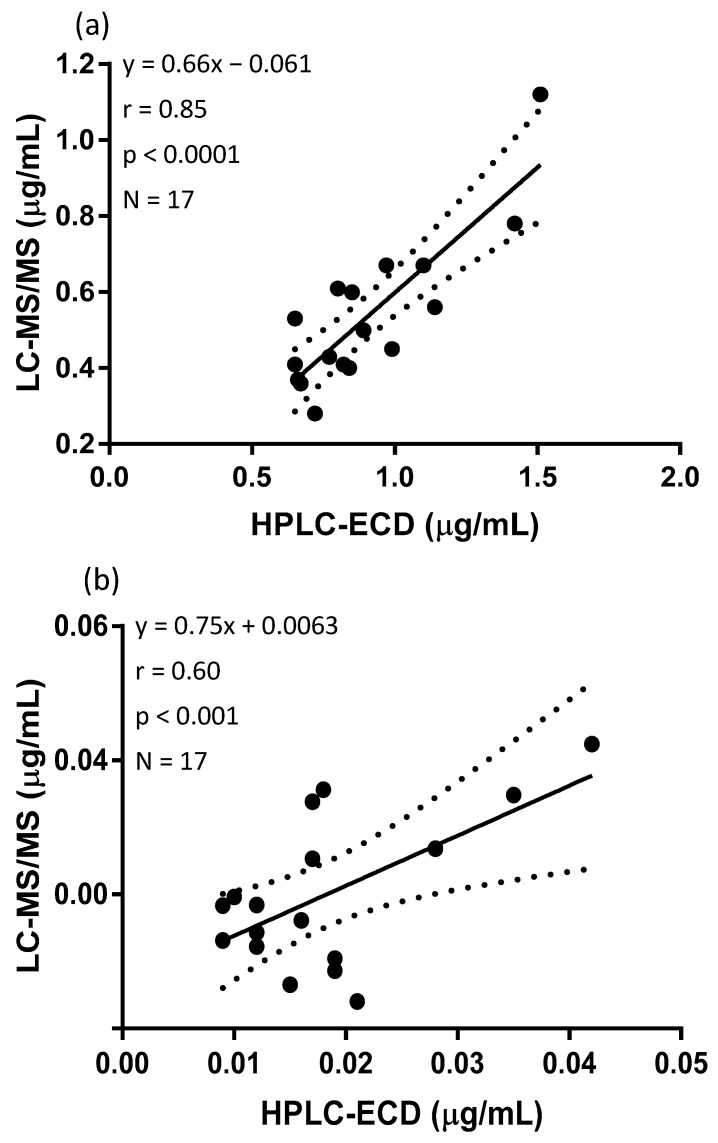
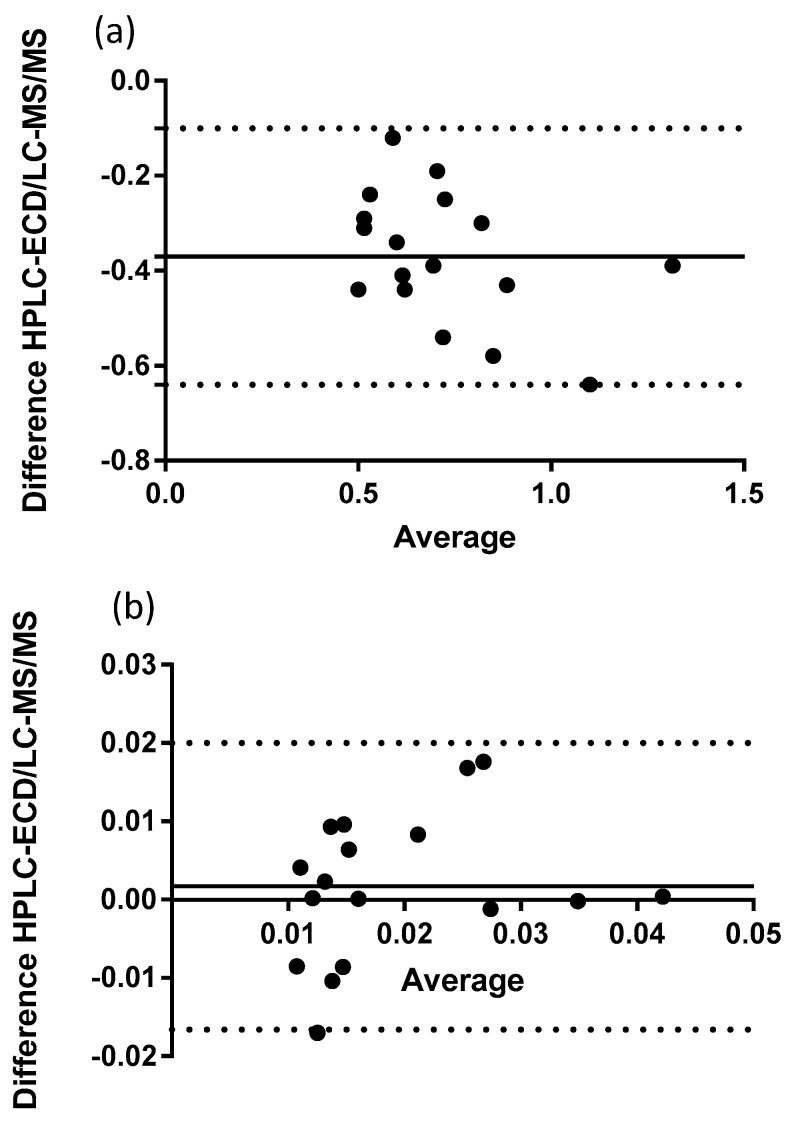
Seventeen canine plasma samples were analyzed with HPLC-ECD and LC-MS/MS. Both analytical methods were able to quantify the reduced and oxidized forms of Q10. Figure 3a shows the full scan mass spectrum of CoQ10H2 with the product ion mass spectrum of m/z of 197, Figure 3b. The MRM chromatogram traces of CoQ10H2 and CoQ10 are shown in Figure 4. CoQ10 was considered to be at the LLOQ of the mass spectrometer. The comparison showed that the correlation for CoQ10H2 regarding the two methods was acceptable, r = 0.85 and for CoQ10, r = 0.60, see Figure 5a,b, respectively. When including the samples showing low responses of the IS, the correlation for CoQ10H2 was found to be r = 0.73 and for CoQ10, r = 0.23. Bland–Altman plots are shown in Figure 6. For CoQ10H2, Figure 6b, the mean bias ± SD was found to be −0.37 ± 0.14 and the limits of agreement −0.64 to −0.10. The Bland–Altman plot for CoQ10, Figure 6b, showed a mean bias ± SD of 0.00172 ± 0.0094 and limits of agreement of −0.017 to 0.02. Both correlation and Bland–Altman plots showed that LC-MS/MS results for ubiquinol were on average around 30% lower as compared to HPLC-ECD results. The intra-day variation of the LC-MS/MS method was estimated from calibration standards to a CV% of 10.8% for CoQ10H2 and 15.0% for CoQ10. The range of total Q10 concentration was 0.64 to 1.24 µg/mL for HPLC-ECD in relation to 0.37 to 0.85 µg/mL for LC-MS/MS. The oxidation ratio was determined to 1.9% in the HPLC-ECD analysis and 3.9% using the LC-MS/MS method (p = 0.007).
Figure 3.
(a) Full scan mass spectrum of CoQ10H2, [M+NH4]+. (b) Product ion mass spectrum of CoQ10H2 (m/z of 197).
Figure 4.
Chromatogram from the MRM trace of a canine plasma sample using the LC-MS/MS method. Lower trace: CoQ10H2. Upper trace: CoQ10. The insert shows 10 times zoom of the CoQ10 peak.
Figure 5.
(a) Correlation of CoQ10H2 and (b) correlation of CoQ10 in canine plasma analyzed by HPLC-ECD and LC-MS/MS. The dotted line corresponds to 95% confidence limits.
Figure 6.
(a) Bland–Altman plot of ubiquinol (N = 17) and (b) Bland–Altman plot of ubiquinone (N = 17). The continuous line is the bias. The dotted lines are the 95% limits of agreement.
4. Discussion
The present study shows optimization and validation of an analytical method for quantification of CoQ10H2 and CoQ10 in canine plasma and heart tissue using HPLC-ECD. As expected, obtaining a homogeneous solution of heart tissue was somewhat challenging. Lysing matrix A containing garnet matrix and a zirconium banded satellite demonstrated the best results in terms of variation. In addition, these materials are considered to have acceptable compatibility with electrochemical detection due to their inert nature. This is in contrast to stainless steel beads that could potentially leak metal ions, and thereby have a negative impact on the S/N ratio of the electrochemical detector or increase post-sampling oxidation. Extraction of tissue amounts between 45 and 270 mg were found to be acceptable. 1-propanol was selected as the best extraction solvent. The usability of 1-propanol for Q10 extraction in plasma is in line with previous studies [13,29]. Niklowitz et al. tested extraction of CoQ10H2 and CoQ10 from different swine tissues—including the heart—and found 2-propanol to be the best extraction solvent [23]. However, they did not test 1-propanol. In our study, 1-propanol was significantly better than 2-propanol.
Satisfactory results were obtained during validation of the HPLC-ECD method. Intra-day and inter-day precisions for plasma were comparable with previously published data [22,24,30]. Regarding heart tissue, Pandey et al. [21] measured reduced and oxidized Q10 in heart tissue of mice, and found CV% of 17% and 14%, respectively. Tang et al. [24] reported a CV% for heart tissue for both forms of Q10 to 20%. Our CV% for reduced and oxidized Q10 in heart tissue of less than 6% is therefore markedly better than previously published methods. There was a tendency that recoveries in the accuracy study were below 100% for CoQ10H2 and above 100% for CoQ10. This could be due to ex vivo conversion of the reduced form to the oxidized form of Q10 during sample preparation and storage. To maintain the oxidation ratio, it is crucial to keep samples at a low temperature during work up. The stability of extracted samples was acceptable at 4 °C for up to 8 h. Claessens et al. [19] found 1-propanol extracted plasma to be stable in 4.5 h, but no antioxidant was added. The stability of extracted samples was only slightly increased using storage at −20 °C. Furthermore, some of the heart tissue extracted samples, which were clear before freezing, became cloudy after 24 h of freezing. Therefore, it is not advisable to store the Q10 extracted samples at −20 °C, and if doing so, at least have three samples available per time point for re-analysis.
Total Q10 concentration found in this study with the validated HPLC-ECD method using canine plasma was in a range from 0.65 to 1.24 µg/mL (n = 17). In agreement with our data, Svete et al. [31] measured a total coenzyme Q10 range in dogs affected by various types and stages of cardiac diseases, and found 25 and 75% percentiles to be between 0.3 and 2.5 µg/mL (n = 43) using LC-APCI-MS. A basal Q10 range of 0.1 to 0.5 ug/mL (n = 8) in healthy dogs was found by Yuan et al. with HPLC-UV detection [14]. Yeramilli-Rao et al. [32] found a basal plasma Q10 range in beagle dogs of 0.3 to 0.8 µg/mL (n = 8). The detection principle of the latter analysis is unclear. The obtained ranges from HPLC-ECD and LC-MS/MS analysis of canine plasma samples are similar to, or marginally higher than, those reported earlier for dogs. The oxidation ratio for canine plasma and heart tissue determined by the HPLC-ECD method was 1.9% (N = 17) and 65% oxidized (N = 2), respectively. The observed oxidation ratio in the heart is in good agreement with Niklowitz et al. finding 60% oxidized Q10 in swine heart [23]. Thus, although the observed oxidation ratio in the heart can only be regarded as an indication considering the low N-value, it is known that particularly the heart and lung display much higher than average tissue oxidation of antioxidants [33].
The correlation between the HPLC-ECD and LC-MS/MS method was found to be relatively good for both CoQ10H2 and CoQ10. However, actual results for CoQ10H2 were about 30% lower with the LC-MS/MS method, which is also reflected by the significantly different average oxidation ratio obtained in the two methods. The lower results in the LC-MS/MS method could to some extent be related to differences in calibration, since different calibration stock solutions were used. The variation, especially for CoQ10, appeared to be higher in the LC-MS/MS method (between 10.8 and 15.0% for LC-MS/MS in relation to less than 6.5% for HPLC-ECD). This could partly be explained by the fact that measurement of CoQ10 was near or at the LLOQ of the LC-MS/MS instrument making especially the CoQ10 comparison uncertain. Furthermore, the addition of a small volume of IS in hexane (2 µL) could have contributed to the increased variation observed in the LC-MS/MS method. The oxidation ratio obtained for plasma samples using LC-MS/MS was higher than for HPLC-ECD, which could indicate increased oxidation of CoQ10H2. The auto sampler connected to the MS instrument could only run with a temperature of around 8 °C, which may not have been sufficient to ensure stability of the analytes. Another explanation could be that oxidation of CoQ10H2 took place in the emitter, thereby increasing the concentration of CoQ10. However, the chromatographic trace from CoQ10 at the retention time of CoQ10H2 showed no visible peak for CoQ10 In conclusion, the data set used for comparing the two methods is quite small, making the method comparison preliminary and further investigations regarding the observed discrepancy is warranted.
The sensitivity of the HPLC-ECD method of 10 nM with an S/N ratio of 10 was found to be similar to or better than previous methods for Q10 quantification. An LOD based on a S/N ratio of 3 has been reported to 17 nM using HPLC-ECD [13]. Finckh et al. [15] found LOQs between 4 and 12 nM in plasma with an S/N ratio of 5. An LOQ of 17 nM for CoQ10H2 and 6 nM for CoQ10 was reported using UPLC-MS/MS [22]. The sensitivity of the developed LC-MS/MS method was comparable with the HPLC-ECD method regarding CoQ10H2, but, quite surprisingly, actually poorer regarding analysis of CoQ10. The utilization of 100% organic solvent in the mobile phase could potentially have led to lower responses, and poorer sensitivity of the mass spectrometer due to loss of protonation in the gas phase to the organic solvents as described in [34]. Only optimization of instrument parameters were performed in the MS analysis; the same column and mobile phase were used in both methods. Improved sensitivity could perhaps have been obtained by running the samples with negative APCI LC-MS as described by Hansen et al. [20].
5. Conclusions
A simple and rapid HPLC-ECD method with a good precision (<6.5%), accuracy (89–109%), and sensitivity (LLOQ of 10 nM) was developed for the quantification of CoQ10H2 and CoQ10 in canine plasma and heart tissue. Furthermore, the stability of CoQ10H2 and CoQ10 during sample preparation, analysis and storage was found to be acceptable. Our results indicate a difference between the results obtained by HPLC-ECD and LC-MS/MS, perhaps due to differences in calibration stock solutions, or increased oxidation during storage or analysis in the LC-MS/MS system. The two methods are therefore not considered to be interchangeable. The observed discrepancy could be investigated further by analyzing extracted heart tissue samples with the two developed methods.
Acknowledgments
Liselotte Bruun Christiansen & Lisbeth Høier Olsen, University of Copenhagen, are thanked for supplying canine samples. Ricki Thanning and Joan Frandsen are thanked for excellent technical assistance. This study was supported by the Lifepharm Centre for In Vivo Pharmacology.
Author Contributions
A.M.V.S.-P. and J.L. conceived the study. A.M.V.S.-P. and D.S. conducted the laboratory analyses. All authors contributed to interpretation of the data. A.M.V.S.-P. wrote the draft manuscript. All authors have critically edited and approved the final manuscript.
Funding
This research was funded by the LifePharm Centre for In Vivo Pharmacology.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Hargreaves I.P. Ubiquinone: cholesterol’s reclusive cousin. Ann. Clin. Biochem. 2003;40:207–218. doi: 10.1258/000456303321610493. [DOI] [PubMed] [Google Scholar]
- 2.Overvad K., Diamant B., Holm L., Holmer G., Mortensen S.A., Stender S. Coenzyme Q10 in health and disease. Eur. J. Clin. Nutr. 1999;53:764–770. doi: 10.1038/sj.ejcn.1600880. [DOI] [PubMed] [Google Scholar]
- 3.Overvad K., Diamant B., Holm L., Holmer G., Mortensen S.A., Stender S. Plasma ubiquinol-10 is decreased in patients with hyperlipidaemia. Atherosclerosis. 1997;129:119–126. doi: 10.1016/s0021-9150(96)06021-2. [DOI] [PubMed] [Google Scholar]
- 4.Bentinger M., Tekle M., Dallner G. Coenzyme Q-Biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Hekimi S. Understanding Ubiquinone. Trends Cell. Biol. 2016;26:367–378. doi: 10.1016/j.tcb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Sohmiya M., Tanaka M., Tak N.W., Yanagisawa M., Tanino Y., Suzuki Y., Okamoto K., Yamamoto Y. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J. Neurol. Sci. 2004;223:161–166. doi: 10.1016/j.jns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Aberg F., Appelkvist E.L., Dallner G., Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992;295:230–234. doi: 10.1016/0003-9861(92)90511-T. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A., Kaur H., Devi P., Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol. Thera. 2009;124:259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Madmani M.E., Solaiman A.Y., Agha K.T., Madmani Y., Shahrour Y., Essali A., Kadro W. Coenzyme Q10 for heart failure. Cochrane Database Syst. Rev. 2014;52:1435–1441. doi: 10.1002/14651858.CD008684.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen S.A., Rosenfeldt F., Kumar A., Dolliner P., Filipiak K.J., Pella D., Alehagen U., Steurer G., Littarru G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014;2:641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Dallner G., Sindelar P.J. Regulation of ubiquinone metabolism. Free Radic. Biol. Med. 2000;29:285–294. doi: 10.1016/S0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- 12.Franke A.A., Morrison C.M., Bakke J.L., Custer L.J., Li X., Cooney R.V. Coenzyme Q10 in human blood: Native levels and determinants of oxidation during processing and storage. Free Radic. Biol. Med. 2010;48:1610–1617. doi: 10.1016/j.freeradbiomed.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang P.H., Miles M.V., DeGrauw A., Hershey A., Pesce A. HPLC analysis of reduced and oxidized coenzyme Q(10) in human plasma. Clin. Chem. 2001;47:256–265. [PubMed] [Google Scholar]
- 14.Yuan B., Liu C., Xu P., Lin L., Pan C., Wang L., Validated H.X. Validated HPLC method for the quantitative determination of CoQ(10) in dog plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2011;25:1038–1044. doi: 10.1002/bmc.1567. [DOI] [PubMed] [Google Scholar]
- 15.Finckh B., Kontush A., Commentz J., Hubner C., Burdelski M., Kohlschutter A. High-performance liquid chromatography-coulometric electrochemical detection of ubiquinol 10, ubiquinone 10, carotenoids, and tocopherols in neonatal plasma. Methods Enzymol. 1999;299:341–348. doi: 10.1016/s0076-6879(99)99034-1. [DOI] [PubMed] [Google Scholar]
- 16.Lang J.K., Gohil K., Packer L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal. Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 17.Menke T., Niklowitz P., Adam S., Weber M., Schluter B., Andler W. Simultaneous detection of ubiquinol-10, ubiquinone-10, and tocopherols in human plasma microsamples and macrosamples as a marker of oxidative damage in neonates and infants. Anal. Biochem. 2000;282:209–217. doi: 10.1006/abio.2000.4579. [DOI] [PubMed] [Google Scholar]
- 18.Niklowitz P., Onur S., Fischer A., Laudes M., Palussen M., Menke T., Döring F. Coenzyme Q10 serum concentration and redox status in European adults: Influence of age, sex, and lipoprotein concentration. J. Clin. Biochem. Nutr. 2016;58:240–245. doi: 10.3164/jcbn.15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claessens A.J., Yeung C.K., Risler L.J., Phillips B.R., Himmelfarb J., Shen D.D. Rapid and sensitive analysis of reduced and oxidized coenzyme Q10 in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and application to studies in healthy human subjects. Ann. Clin. Biochem. 2016;53:265–273. doi: 10.1177/0004563215593097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen G., Christensen P., Tuchsen E., Lund T. Sensitive and selective analysis of coenzyme Q10 in human serum by negative APCI LC-MS. Analyst. 2004;129:45–50. doi: 10.1039/B308690A. [DOI] [PubMed] [Google Scholar]
- 21.Pandey R., Riley C.L., Mills E.M., Tiziani S. Highly sensitive and selective determination of redox states of coenzymes Q9 and Q10 in mice tissues: Application of orbitrap mass spectrometry. Anal. Chim. Acta. 2018;1011:68–76. doi: 10.1016/j.aca.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Z., Li S., Guan X., Schmitt-Kopplin P., Lin S., Cai Z. Rapid assessment of the coenzyme Q10 redox state using ultrahigh performance liquid chromatography tandem mass spectrometry. Analyst. 2014;139:5600–5604. doi: 10.1039/C4AN00760C. [DOI] [PubMed] [Google Scholar]
- 23.Niklowitz P., Doring F., Paulussen M., Menke T. Determination of coenzyme Q10 tissue status via high-performance liquid chromatography with electrochemical detection in swine tissues (Sus scrofa domestica) Anal. Biochem. 2013;437:88–94. doi: 10.1016/j.ab.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Tang P.H., Miles M.V., Miles L., Quinlan J., Wong B., Wenisch A., Bove K. Measurement of reduced and oxidized coenzyme Q9 and coenzyme Q10 levels in mouse tissues by HPLC with coulometric detection. Clin. Chim. Acta. 2004;341:173–184. doi: 10.1016/j.cccn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Vadhanavikit S., Morishita M., Duff G.A., Folkers K. Micro-analysis for coenzyme Q10 in endomyocardial biopsies of cardiac patients and data on bovine and canine hearts. Biochem. Biophys. Res. Commun. 1984;123:1165–1169. doi: 10.1016/S0006-291X(84)80255-7. [DOI] [PubMed] [Google Scholar]
- 26.Guidance for Industry. Bioanalytical Method Validation. FDA USA. Department of Health and Human Services, Food and Drug Administration; Silver Spring, MD, USA: [(accessed on 29 July 2019)]. Available online: https://www.fda.gov/media/70858/download. [Google Scholar]
- 27.Birkegard A.C., Reimann M.J., Martinussen T., Haggstrom J., Pedersen H.D., Olsen L.H. Breeding Restrictions Decrease the Prevalence of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels over an 8- to 10-Year Period. J. Vet. Intern. Med. 2016;30:63–68. doi: 10.1111/jvim.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podda M., Weber C., Traber M.G., Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J. Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 29.Littarru G.P., Mosca F., Fattorini D., Bompadre S., Battino M. Methods Enzymol. Academic Press; Cambridge, MA, USA: 2004. Assay of Coenzyme Q10 in Plasma by a Single Dilution Step; pp. 170–176. [DOI] [PubMed] [Google Scholar]
- 30.Acworth I.N., Ullucci P.A., Gamache P.H. Determination of Oxidized and Reduced CoQ10 and CoQ9 in Human Plasma/Serum Using HPLC-ECD. In: Armstrong D., editor. Advanced Protocols in Oxidative Stress I. Humana Press; Totowa, NJ, USA: 2008. pp. 245–258. [DOI] [PubMed] [Google Scholar]
- 31.Svete A.N., Verk B., Seliskar A., Tomsic K., Krizman P.J., Petric A.D. Plasma coenzyme Q10 concentration, antioxidant status, and serum N-terminal pro-brain natriuretic peptide concentration in dogs with various cardiovascular diseases and the effect of cardiac treatment on measured variables. Am. J. Vet. Res. 2017;78:447–457. doi: 10.2460/ajvr.78.4.447. [DOI] [PubMed] [Google Scholar]
- 32.Yerramilli-Rao P., Beal M.F., Watanabe D., Kieburtz K., Blieck E.A.d., Kitano M., Hosoe K., Funahashi I., Cudkowicz M.E. Oral repeated-dose toxicity studies of coenzyme Q10 in beagle dogs. Int. J. Toxicol. 2012;31:58–69. doi: 10.1177/1091581811425256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lykkesfeldt J., Moos T. Age-dependent change in Vitamin C status: A phenomenon of maturation rather than of ageing. Mech. Aging Dev. 2005;126:892–898. doi: 10.1016/j.mad.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S., Hamburger M. Effects of solvent composition on molecular ion response in electrospray mass spectrometry: Investigation of the ionization processes. Rapid Commun. Mass Spectrom. 1995;9:1516–1521. doi: 10.1002/rcm.1290091511. [DOI] [Google Scholar]