Abstract
HSP90 is a molecular chaperone that increases the stability of client proteins. Cancer cells show higher HSP90 expression than normal cells because many client proteins play an important role in the growth and survival of cancer cells. HSP90 inhibitors mainly bind to the ATP binding site of HSP90 and inhibit HSP90 activity, and these inhibitors can be distinguished as ansamycin and non-ansamycin depending on the structure. In addition, the histone deacetylase inhibitors inhibit the activity of HSP90 through acetylation of HSP90. These HSP90 inhibitors have undergone or are undergoing clinical trials for the treatment of cancer. On the other hand, recent studies have reported that various reagents induce cleavage of HSP90, resulting in reduced HSP90 client proteins and growth suppression in cancer cells. Cleavage of HSP90 can be divided into enzymatic cleavage and non-enzymatic cleavage. Therefore, reagents inducing cleavage of HSP90 can be classified as another class of HSP90 inhibitors. We discuss that the cleavage of HSP90 can be another mechanism in the cancer treatment by HSP90 inhibition.
Keywords: HSP90 inhibitors, Anti-cancer therapy, ATP binding, Acetylation, HSP90 cleavage
INTRODUCTION
Most living organisms commonly express heat shock proteins and their expression increases in response to a variety of stresses (Welch, 1993). Heat shock-induced expression of genes was first discovered in chromosomal puffing by heat shock in Drosophila busckii in 1962 (Ritossa, 1962). In 1974, it was first reported that the synthesis of a few proteins was enhanced by stresses such as heat shock in Drosophila cells (Tissieres et al., 1974). Heat shock protein 90 (HSP90) is a member of the heat shock protein family and functions as a molecular chaperone that supports the stability of client proteins. Typical examples of the client proteins are mutated p53, Bcr-Abl, Raf-1, Akt, human epidermal growth factor 2 (Her2/ErbB2), HIF-1α, etc. (Neckers and Workman, 2012).
HSP90 is evolutionarily conserved and has many isoforms, such as HSP90α, HSP90β, Grp94, and HSP75/TRAP1. Among these isoforms, HSP90α and HSP90β are localized in cytosol, HSP90α (major form) is constitutively expressed, and expression of HSP90β (minor form) is inducible (Sreedhar et al., 2004). HSP90 consists of three domains, N-terminal domain (N-domain), middle domain (M-domain), and C-terminal domain (C-domain). The N-domain has an ATP-binding pocket and ATPase activity (Prodromou et al., 1997). HSP90 forms a flexible dimer by interaction of C-domains. The formation and dissociation of compact dimers involving N-domains is important for the molecular chaperone activity (Rohl et al., 2013). Binding of ATP to the ATP-binding pocket of the N-domain promotes dimerization between the two N-domains, and the ATPase activity of the N-domain induces the hydrolysis of ATP to ADP, resulting in N-domain dissociation (Prodromou et al., 2000; Richter and Buchner, 2001). HSP90 requires co-chaperones, such as cdc37, Hop, p23, PP5, and Xap2, to function as a molecular chaperone. Co-chaperones interact with HSP90 and regulate ATPase activity for molecular chaperone activity of HSP90 and recruit client proteins to HSP90 (Zuehlke and Johnson, 2010; Rohl et al., 2013).
In most cancer cells, HSP90 and its client proteins are expressed at higher levels than normal cells. The client proteins, such as Her2/ErbB2, v-Src, Raf-1, Akt, hTERT, are important for cancer cell survival and growth (Ferrarini et al., 1992; Sharp and Workman, 2006; Miyata et al., 2013) (Table 1). HSP90 is also involved in the transition from benign to malignant cells (Boltze et al., 2004). Therefore, many researchers have investigated the potential of HSP90 as a target of anti-cancer drugs (Neckers et al., 1999; Sharp and Workman, 2006; Modi et al., 2011; Dickson et al., 2013). As a result, several HSP90 inhibitors have been studied for use as an anticancer agent, and some clinical trials are underway. In the first part of this review, we provide an overview on traditional HSP90 inhibitors and their effects on cancers. HSP90 inhibitors hamper HSP90 function by competitively binding to the ATP binding site of HSP90, blocking the interaction with co-chaperones, or modulating acetylation. In the second part, we present a novel group of HSP90 inhibitors inducing cleavage of HSP90 and suggest that cleavage of HSP90 can be another mechanism of HSP90 inhibitors to suppress the activity of HSP90.
Table 1.
Selected client proteins of HSP90 related with tumor growth and survival
| Class | Client protein of HSP90 | Function | References |
|---|---|---|---|
| Receptor tyrosine kinase | Her2/ErbB2 | Promotes cell proliferation and inhibits apoptosis | Moasser, 2007 |
| EGFR mutant | Promotes cell proliferation via activation of MAPK, AKT and JNK pathway | Voldborg et al., 1997 | |
| FLT3 | Regulates cell survival, proliferation and differentiation | Grafone et al., 2012 | |
| VEGFR | Promotes vasculogenesis and angiogenesis | Kliche and Waltenberger, 2001 | |
| Signaling molecule and Kinase | Akt | Plays a role in apoptosis, cell proliferation, transcription and cell migration | Yoeli-Lerner and Toker, 2006 |
| mTOR | Regulates cell proliferation, motility and survival | Guertin and Sabatini, 2007 | |
| p38 | Regulates cell proliferation, apoptosis and motility | Koul et al., 2013 | |
| v-Src | Promotes formation of cancer, cell movement and proliferation | Irby and Yeatman, 2000 | |
| Raf-1 b-Raf | Activates cell growth signaling, such as MEK1/2 and ERK1/2 | Leicht et al., 2007 | |
| JAK2 | Promotes cell proliferation and motility | Xu et al., 2017 | |
| Bcr-Abl | Promotes cell proliferation and reduces apoptosis | Quintas-Cardama and Cortes, 2009 | |
| Transcription factor | Twist1 | Promotes cancer metastasis and reduces apoptosis | Puisieux et al., 2006 |
| Hif-1α | Induce cell proliferation and angiogenesis | Tiburcio et al., 2014 | |
| NF-κB | Keeps cell proliferation and protects from apoptosis | Xia et al., 2014 | |
| p53 mutant | Transactivates growth-promoting and oncogenic genes | Ozaki and Nakagawara, 2011 | |
| Others | Cyclin D1 | Controls cell cycle | Qie and Diehl, 2016 |
| VDUP-1 | Inhibits cell growth and metastasis and contributes to apoptosis | Kaimul et al., 2007 | |
| MUC1 | Prevents cell death and promotes proliferation and invasion | Nath and Mukherjee, 2014 | |
| MMP2/9 | Plays a role in invasion and angiogenesis via breakdown of extracellular matrix | Gialeli et al., 2011 | |
| Survivin | Inhibits apoptosis and regulates mitosis | Mita et al., 2008 | |
| Vimentin | Increases migration and invasive capacity | Satelli and Li, 2011 |
HSP90 INHIBITORS BLOCKING ATP BINDING
HSP90 inhibitors generally interrupt ATP binding to HSP90 and can be classified into ansamycins and non-ansamycins depending on whether they have a benzoquinone structure (Table 2).
Table 2.
Selected HSP90 inhibitors
| Target | Inhibitor | References |
|---|---|---|
| ATP-binding site | Ansamycin | |
| Geldanamycin | Grenert et al., 1997 | |
| 17-AAG (Tanespimycin) | Krishnamoorthy et al., 2013 | |
| 17-DMAG (Alvespimycin) | Jez et al., 2003 | |
| IPI-504 (retaspimycin hydrochloride) | Sydor et al., 2006 | |
| Non-ansamycin | ||
| AUY922 (Luminespib) | Eccles et al., 2008 | |
| BIIB021 | Lundgren et al., 2009 | |
| HSP990 | Menezes et al., 2012 | |
| Debio0932 (CUDC-305) | Bao et al., 2009 | |
| STA-9090 (Ganetespib) | Ying et al., 2012 | |
| AT13387 (Onalespib) | Woodhead et al., 2010 | |
| SNX-5422 (PF-04929113) | Chandarlapaty et al., 2008 | |
| Deacetylation | LAQ824 | Chen et al., 2005 |
| Romidepsin | Yu et al., 2002 | |
| Vorinostat (SAHA) | Bali et al., 2005b |
Ansamycins
Ansamycins, including geldanamycin (GM), herbimycin A, and the macbecins, are antibiotics with anti-cancer activity and include the benzoquinone structure. These antibiotics induce death of tumor cells through HSP90 inhibition and degradation of the client proteins that are required for tumor cell survival and growth (Zhang and Zhang, 2011).
GM competitively binds to the ATP-binding pocket in the N-domain of HSP90 and inhibit chaperone activity of HSP90 via down-regulation of ATPase activity (Grenert et al., 1997). 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) is an analogue of GM with higher binding affinity and lower toxicity (Krishnamoorthy et al., 2013). 17-AAG inhibits cell proliferation and induces apoptosis via depleting HSP90 client proteins and downregulation of other downstream proteins in various types of cancer cells in vitro and in vivo (Hostein et al., 2001; Solit et al., 2002; Banerji et al., 2005b; Karkoulis et al., 2010). 17-AAG also induces cell cycle arrest in G1 phase (Solit et al., 2002; Karkoulis et al., 2010). In addition, these effects of 17-AAG in cancer cells are similar to those in glioma stem cells (Sauvageot et al., 2009).
17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG, alvespimycin), an analogue of 17-AAG, is a more potent and water-soluble derivative. 17-DMAG binds to ATP binding site of HSP90 and inhibits ATP binding and chaperone activity of HSP90. Nuclear factor-κB (NF-κB) regulates anti-apoptotic proteins and oncogenes, such as c-FLIP, Bcl2, Mcl1, and XIAP in chronic lymphocytic leukemia (CLL). 17-DMAG induces degradation of IκB kinase (IKK) and suppresses DNA binding of NF-κB. Consequently, 17-DMAG decreases transcription of NF-κB target genes and induces apoptosis (Hertlein et al., 2010). Her2/ErbB2 overexpression enhances cell growth and motility in breast and ovarian cancer. 17-DMAG-mediated HSP90 inactivation leads to degradation of Her2/ErbB2 (Niu et al., 2009). In non-small cell lung cancer (NSCLC), 17-DMAG treatment induces down-regulation of phospho-EGFR, phospho-Akt, and phospho-MAPK in EGFR-mutant cell lines than EGFR-WT cell line, and promotes cell apoptosis in EGFR-mutant cells (Kobayashi et al., 2012). In hepatocellular carcinoma cells, 17-DMAG induces apoptosis by degradation of survival-related proteins (Leng et al., 2012).
IPI-504 (retaspimycin hydrochloride) is a water-soluble analogue of 17-AAG and interacts with the ATP binding site of HSP90. IPI-504 inhibits myeloma tumor growth and has selective cytotoxicity to myeloma cancer cells compared with normal cells (Sydor et al., 2006). IPI-504 has anti-proliferation activity in several cancer cells through Her2/ErbB2 and Akt degradation. Tumor growth in vivo is also reduced by IPI-504 treatment through Her2/ErbB2 degradation (Leow et al., 2009). In diffuse large B-cell lymphoma (DLBCL) cell lines, IPI-504 inhibits cell growth and induces apoptosis. Among these cell lines, IPI-504-sensitive cell lines have high expression levels of phospho-Akt (Abramson et al., 2009).
Non-ansamycins
AUY992 (luminespib) binds to the ATP binding site in the N-domain of HSP90. AUY992 inhibits cell proliferation and induces G1/G2 cell cycle arrest and apoptosis in vitro. Furthermore, AUY992 inhibits growth and lung metastasis of b-Rafmutated human melanoma tumor in vivo (Eccles et al., 2008). p23 is a co-chaperone of HSP90 and plays an important role in the activity of HSP90 and stabilization of client proteins by association with HSP90, and HSP90-p23 interaction requires ATP binding in HSP90 (Sullivan et al., 2002). In a Her/ErbB-overexpressing estrogen receptor (ER)-positive breast cancer xenograft model, AUY992 reduces tumor growth by inducing dissociation of HSP90-p23 interaction and degradation of client proteins (Jensen et al., 2008). AUY992 induces apoptosis and depletion of Akt and IKK in acute myeloma leukemia (AML) cell lines and primary AML blasts (Walsby et al., 2013). AUY992 also inhibits cell growth and motility in hepatocellular carcinoma and pancreatic cancer cells and reduces growth factor-mediated and angiogenesis-related protein activation and vascularization in pancreatic cancer (Moser et al., 2012; Cheng et al., 2015).
BIIB021, an orally available inhibitor of HSP90, binds to the ATP binding pocket in the N-domain of HSP90. BIIB021 has anti-proliferation activity in various tumor cell lines in vitro and inhibits tumor growth in vivo. BIIB021 also induces degradation of HSP90 client proteins (Lundgren et al., 2009). In CLL cells, BIIB021 induces growth inhibition and apoptosis by the mitochondrial pathway and degradation of BCR-ABL protein. In addition, the BIIB021-induced apoptosis includes an autophagic response such as the formation of autophagosome by regulating the mTOR-Ulk1 pathway (He et al., 2016). 17-AAG and other ansamycin derivatives are inactive in cell lines expressing P-glycoprotein (P-gp) and/or multidrug resistance-associated protein 1 (MRP-1/ABCC1). In contrast, BIIB021 is active in these cell lines. Therefore, BIIB021 may be used for therapy of tumors protected by multidrug resistant (MDR) protein (Zhang et al., 2010a).
Debio0932 (CUDC-305) binds to the ATP binding site in the N-domain of HSP90. Debio0932 induces degradation of multiple oncoproteins, such as Akt, Raf-1, and Her2/ErbB2, and inhibits cell proliferation in various tissue-derived cancer cell lines. In glioblastoma, AML, breast cancer, colorectal cancer, and NSCLC mouse models, debio0932 also induces degradation of client proteins and inhibits tumor growth (Bao et al., 2009).
STA-9090 (Ganetespib), a synthetic small molecule inhibitor, binds to the ATP binding site in the N-domain of HSP90 (Ying et al., 2012). STA-9090 induces proteasome-mediated degradation of EGFR, JAK2, and FLT3 which are essential for growth and activation of STAT, MAPK, and Akt. In addition, STA-9090 induces cell cycle arrest in G1 and G2/M phase (Proia et al., 2011). In Her2/ErbB2-positive breast cancer cells, STA-9090 inhibits cell proliferation, cell cycle, survival, and activation/phosphorylation of Her2/ErbB2. Furthermore, the half-life of Her2/ErbB2 protein is decreased by STA-9090 treatment (Lee et al., 2018). In gastric cancer, STA9090 inhibits proliferation and induces G2/M arrest of cell cycle and apoptosis. The receptor tyrosine kinase (RTK) signaling pathway is suppressed by STA-9090 treatment (Lee et al., 2017). STA-9090 also inhibits cell growth and induces apoptosis in AML and NSCLC (Shimamura et al., 2012; Lazenby et al., 2015).
Heat shock protein 990 (HSP990) binds to the ATP binding site in the N-domain of HSP90. During in vitro experiments, HSP990 inhibits ATPase activity of TNF receptor associated protein 1 (TRAP1), a mitochondrial HSP90, by more than 90%. HSP990 inhibits the activity of HSP90 and growth of various types of cancer cell lines and has suppressive activity in most various cancer patient-derived tumors ex vivo. In gastric cancer, breast cancer, AML, and NSCLC mouse models, HSP990 treatment also suppresses tumor growth (Menezes et al., 2012).
KW-2478 binds to HSP90α with high affinity. In multiple myeloma, KW-2478 induces degradation of client proteins such as FGFR3, c-Raf, and cyclin D1, growth inhibition, and apoptosis in vitro and in vivo (Nakashima et al., 2010).
AT13387 (onalespib) has affinity for HSP90 by binding in the ATPase site of the N-domain (Woodhead et al., 2010). AT13387 suppresses proliferation and survival of cell lines from different types of tumors. In glioma cell lines, AT13387 depletes survival-related client proteins and suppresses their downstream signaling pathway. As a result, proliferation, motility, angiogenesis, and survival are decreased. In NSCLC, AT13387 shows long duration of effects in vitro and in vivo (Graham et al., 2012; Canella et al., 2017).
SNX-5422/2112 (PF-04928473) binds to the ATP binding site in the N-domain of HSP90. SNX-5422/2112 induces HSP90 client protein degradation, such as Her2/ErbB2, Akt, and cyclin D1, and inhibits cell proliferation in Her2/ErbB2-overespressing breast and ovarian cancers. In the body, SNX-5422 rapidly converts to SNX-2112, and SNX-2112 accumulates in tumors compared to normal tissues (Chandarlapaty et al., 2008). The growth inhibition activity of SNX-2112 is higher than 17-AAG in multiple myeloma and other hematologic tumors. In multiple myeloma cells, SNX-2112 induces apoptosis by caspase activation and suppresses Akt and ERK activation. SNX-2112 inhibits tube formation of HUVEC cells by suppression of the eNOS/Akt pathway and osteoclastogenesis of multiple myeloma cells by down-regulation of ERK/c-Fos and PU.1 (Okawa et al., 2009).
HSP90 INHIBITORS BLOCKING DEACETYLATION OF HSP90
The molecular chaperone activity of HSP90 is also controlled by acetylation and deacetylation of K294 and K287 in the M-domain of HSP90α and HSP90β, respectively (Bali et al., 2005a; Scroggins et al., 2007; Nishioka et al., 2008). Histone deacetylase 6 (HDAC6) deacetylates K294α/K287β of HSP90, and the deacetylated HSP90 acts as a molecular chaperone. Acetylation of K294α/K287β decreases the binding affinity of HSP90 to client proteins and co-chaperones (Bali et al., 2005a; Scroggins et al., 2007). In Her2/ErbB2-overexpressing breast cancer cell lines, vorinostat (also known as suberoylanilide hydroxamic acid (SAHA)) induces apoptosis via acetylation of HSP90, which dissociates Her2/ErbB2 from HSP90 and promotes polyubiquitinylation and degradation of Her2/ErbB2 (Bali et al., 2005b). LAQ824 induces acetylation of HSP90 in prostate cancer cells; the ATP binding activity of HSP90 is decreased as a result, and the androgen receptor is dissociated from HSP90 and degraded. Therefore, LAQ824 suppresses expression of androgen-induced prostate-specific antigen and induces anti-proliferation effects and apoptosis in prostate cancer cells. LAQ824 also reduces other HSP90 client protein levels (Chen et al., 2005). Romidepsin inhibits growth and induces apoptosis in wild type or mutant p53-expressing NSCLS cells. Romidepsin also reduces protein levels of ErbB1, ErbB2, Raf-1, and mutant p53, but not wild type p53. Romidepsin induces dissociation of mutant p53 and Raf-1 from HSP90, which is related with acetylation of HSP90 (Yu et al., 2002).
CLINICAL TRIALS OF TRADITIONAL HSP90 INHIBITORS FOR CANCER THERAPY
Since 17-AAG among HSP90 inhibitors first entered the clinical trial (Banerji et al., 2005a), many HSP90 inhibitors have entered clinical trials and are still underway. The HSP90 inhibitors that have been investigated in clinical trials are presented in Table 3. Clinical trials of HSP90 inhibitors were performed as HSP90 inhibitor monotherapy or combination therapy with other anti-cancer reagents for validation of safety, anti-cancer activity, and dosing schedule and dosage. While inhibitors of ansamycin and non-ansamycin classes have not been FDA approved, romidepsin and vorinostat among HDAC inhibitors have been FDA approved.
Table 3.
Clinical trials of anti-cancer therapy with HSP90 inhibitors
| HSP90 inhibitor | Phase | Tumor type | Reference |
|---|---|---|---|
| 17-AAG (Tanespimycin) | I | Relapsed or refractory acute myeloid leukemia | Walker et al., 2013 |
| II | Metastatic or locally advanced, unresectable breast cancer | Rajan et al., 2011 | |
| II | Metastatic melanoma | Solit et al., 2008 ; Pacey et al., 2012 | |
| I | Solid tumor | Tse et al., 2008 | |
| I | b-Raf or NRAS mutated melanoma | Banerji et al., 2008 | |
| I | Relapsed / refractory pediatric solid tumor | Weigel et al., 2007 | |
| I | Refractory advanced cancer | Ramanathan et al., 2005 | |
| I/II | Relapsed or relapsed and refractory multiple myeloma | Richardson et al., 2011 | |
| I | Relapsed multiple myeloma | Richardson et al., 2010 | |
| I | Her2/ErbB2-overexpressed breast cancer | Modi et al., 2007 | |
| 17-DMAG (Alvespimycin) | I | Chronic lymphocytic leukemia/small lymphatic lymphoma | Maddocks et al., 2016 |
| I | Advanced solid tumor | Pacey et al., 2011; Jhaveri et al., 2012 | |
| I | Acute myeloma leukemia | Lancet et al., 2010 | |
| I | Advanced malignancies | Kummar et al., 2010 | |
| AUY922 (Luminespib) | IB/II | Her2/ErbB2-positive metastatic breast cancer | Kong et al., 2016 |
| II | Advanced pancreatic cancer | Renouf et al., 2016 | |
| II | Gastrointestinal stromal cancer | Bendell et al., 2016 | |
| I | Advanced solid tumor | Sessa et al., 2013; Doi et al., 2014; Bendell et al., 2015 | |
| I/II | EGFR-mutant lung cancer | Johnson et al., 2015 | |
| I/IB | Multiple myeloma | Seggewiss-Bernhardt et al., 2015 | |
| BIIB021 | I | Advanced solid tumor | Saif et al., 2014 |
| I | Refractory metastatic or locally advanced solid tumor | Hong et al., 2013 | |
| II | Gastrointestinal stromal tumor | Dickson et al., 2013 | |
| Debio0932 (CUDC-305) | I | Advanced cancer | Isambert et al., 2015 |
| STA-9090 (Ganetespib) | I | Her2/ErbB2-positive metastatic breast cancer | Jhaveri et al., 2017 |
| II | Metastatic castrate-resistant prostate cancer | Thakur et al., 2016 | |
| II | Non-small cell lung cancer | Ramalingam et al., 2015 | |
| II | KRAS mutated and WT metastatic colorectal cancer | Cercek et al., 2014 | |
| I | Advanced hepatocellular carcinoma | Goyal et al., 2015 | |
| II | Metastatic breast cancer | Jhaveri et al., 2014 | |
| II | Advanced non-small lung cancer | Socinski et al., 2013 | |
| I | Solid malignancies | Goldman et al., 2013 | |
| HSP990 | I | Advanced solid malignancies | Spreafico et al., 2015 |
| KW-2478 | I/II | Multiple myeloma | Cavenagh et al., 2017 |
| I | B-cell malignancies | Yong et al., 2016 | |
| LAQ824 | I | Advanced solid tumor | de Bono et al., 2008 |
| AT13387 (Onalespib) | I | Advanced solid tumor | Do et al., 2015; Shapiro et al., 2015 |
| IPI-504 (Retaspimycin hydrochloride) | I | Gastrointestinal stromal cancer, soft-tissue sarcoma | Wagner et al., 2013 |
| II | Castrate-resistant prostate cancer | Oh et al., 2011 | |
| Romidepsin | II | Metastatic castrate-resistant prostate cancer | Molife et al., 2010 |
| SNX-5422 (PF-04929113) | I | Refractory solid tumor | Infante et al., 2014 |
| I | Refractory solid tumor malignancies and lymphomas | Rajan et al., 2011 | |
| Vorinostat (SAHA) | I/II | Locally advanced breast cancer | Tu et al., 2014 |
| I/II | Metastatic breast cancer | Ramaswamy et al., 2012 |
Another strategy that uses HSP90 in clinical trials is to suppress drug resistance using HSP90 inhibitor. Her2/ErbB2 and mutated EGFR are one of the proteins that cause drug resistance. Because Her2/ErbB2 and EGFR are clients of HSP90, clinical trials with HSP90 inhibitors were designed to overcome drug resistance by these proteins. In Her2/ErbB2-overexpressing breast cancer, 17-AAG, STA-9090, and AUY922 were used in Phase I and II clinical trials to overcome Her2/ErbB2-mediated resistance to trastuzumab (Herceptin, humanized anti-Her2/ErbB2 monoclonal antibody). The results proved that HSP90 inhibition may be used to overcome the trastuzumab resistance (Modi et al., 2007; Kong et al., 2016; Jhaveri et al., 2017). In addition, to overcome the resistance of EGFR mutation-positive lung cancer to erlotinib (Tarceva, receptor tyrosine kinase inhibitor), Phase I and II clinical trials using AUY922 have been performed (Johnson et al., 2015).
NOVEL CLASS OF HSP90 INHIBITORS INDUCING CLEAVAGE OF HSP90
Recently, it has been reported that HSP90 is cleaved by various stimuli. Cleavage of HSP90 is induced by various stimuli such as UVB irradiation (Chen et al., 2009), ascorbate/menadione (Beck et al., 2009, 2012), andrographolide (Liu et al., 2014), HDAC inhibitors (Park et al., 2015), proteasome inhibitors (Park et al., 2017), tumor necrosis factor (TNF) (Fritsch et al., 2016), a combination of gefitinib and vorinostat (Park et al., 2019), etc. (Table 4). Therefore, cleavage of HSP90 can be considered another mechanism of HSP90 regulation.
Table 4.
Novel class of HSP90 inhibitors inducing cleavage of HSP90
| Cleavage type | Inhibitor | References |
|---|---|---|
| Enzymatic cleavage | Histone deacetylase inhibitors | Park et al., 2015 |
| Proteasome inhibitors | Park et al., 2017 | |
| Ultra-violet irradiation | Chen et al., 2009 | |
| Non-enzymatic cleavage | Ascorbate/Menadione | Beck et al., 2009, 2012 |
| Oxidative stress (H2O2) | Castro et al., 2019 | |
| Others (undefined) | Tumor necrosis factor (TNF) | Fritsch et al., 2016 |
| Andrographolide | Liu et al., 2014 | |
| β-Lapachone | Wu et al., 2016 | |
| 17-AAG (Tanespimycin) | Karkoulis et al., 2010 | |
| As(III) and MMA(III) | Shen et al., 2008 |
HSP90 cleavage can be divided into enzymatic cleavage and non-enzymatic cleavage. The enzymatic cleavage generates an approximately 55 kDa fragment of HSP90 via caspase 10 activation, and the non-enzymatic cleavage generates an approximately 70 kDa fragment via chemical degradation by reactive oxygen species (ROS). There are some substances that have HSP90 cleavage activity, but it has not yet been determined whether enzymes are engaged in the process.
Chemicals and UV inducing enzymatic cleavage of HSP90
The enzymatic cleavage of HSP90 is induced by histone deacetylase inhibitors (including vorinostat), proteasome inhibitors (including MG132), and UVB irradiation. We found cleavage of HSP90 when treated with the histone deacetylase inhibitor vorinostat in leukemia cells (Park et al., 2015). HSP90 was also cleaved by other histone deacetylase inhibitors, sodium butyrate and valproic acid (Park et al., 2015). Vorinostat induces ROS generation in acute T cell leukemia cell line (Ruefli et al., 2001). On the other hand, generation of ROS by several stimuli leads to activation of caspase and triggers apoptosis in various types of cancer cells through an extrinsic or intrinsic pathway (Kim and Chung, 2007). According to our results, ROS-induced caspase 10 activation is responsible for enzymatic cleavage of HSP90 after treatment with vorinostat (Park et al., 2015). Furthermore, vorinostat-induced HSP90 cleavage needs newly synthesized protein(s), and Vitamin D up-regulating protein 1 (VDUP-1) may be one of the candidate substances (Park et al., 2015). Vorinostat was previously reported to increase the expression level of the VDUP-1 gene (Butler et al., 2002), and VDUP-1 negatively regulates thioredoxin, a cellular antioxidant (Junn et al., 2000).
We also found that MG132, a proteasome inhibitor, induces HSP90 cleavage through a mechanism similar to that of vorinostat treatment (Park et al., 2017). Although the mRNA level of VDUP-1 was not changed after treatment with MG132, the protein level of VDUP-1 was significantly increased. E3 ubiquitin ligase Itch-mediated ubiquitination and proteasomal degradation is involved in the regulation of VDUP-1 protein level (Zhang et al., 2010b). Therefore, MG132 may up-regulate the VDUP-1 protein level by inhibiting the proteasome activity and blocking the proteasomal degradation of VDUP-1. Another antioxidant glutathione was also decreased by MG132 treatment. In short, MG132 also induces HSP90 enzymatic cleavage via generation of ROS and subsequent activation of caspase 10.
UVB irradiation induces HSP90 cleavage by activating the Fas/Fas ligand axis (Chen et al., 2009). In this case, Fas ligand secretion and Fas expression were increased by UVB irradiation. Caspase 8 was activated by interaction with the FADD domain of active Fas, and HSP90 was cleaved by caspase 8-mediated active caspase 10. Importantly, apoptosis of cells increased when HSP90 was down-regulated and cells harboring mutation on the cleavage site of HSP90 showed better survival compared with control cells upon UVB irradiation. Therefore, it is likely that the HSP90 cleavage is not merely a side effect of caspase activation but an essential process for the regulation of apoptosis.
Chemicals inducing non-enzymatic cleavage of HSP90
In leukemia cells (K562 cell line), ascorbate/menadione (asc/men) treatment induces HSP90 cleavage with a molecular weight of approximately 70 kDa. The HSP90 cleavage is selectively induced in tumor cells, but not in normal cells. The asc/men-induced HSP90 cleavage triggers degradation of HSP90 client proteins, including Bcr-Abl, and the cleavage of HSP90 is induced by ROS generation and inhibited by antioxidant (Beck et al., 2009). The cleavage of HSP90 is induced by Fenton reaction in the presence of redox-active iron. The cleavage site in this reaction is between I131 and G132 in HSP90α and I126 and G127 in HSP90β (Beck et al., 2012).
In Jurkat cells, oxidative stress (H2O2) induces iron-dependent HSP90 cleavage with a molecular weight of approximately 70 kDa, and the HSP90 cleavage inversely correlates with cell proliferation. Cleaved HSP90 accumulates as aggregates in an insoluble form, and actin also aggregates in sequence. Using a cell-free in vitro model, it was proved that the cleaved HSP90 gains another function to directly induce aggregation of actins (Castro et al., 2019).
Others inducing cleavage of HSP90
TNF induces HSP90 cleavage depending on Cathepsin D (CtsD). TNF treatment in U937 (human myeloid leukemia) cells induced HSP90 cleavage in a time-dependent manner, which was blocked by treatment with pepstatin A (PepA), a CtsD inhibitor. In this case, HSP90 was observed to be cleaved into 60 kDa and 40 kDa fragments and it was concluded using the mutagenesis technique that the 465th tyrosine residue may be the target of HSP90 cleavage. Apoptosis of U937 cells expressing mutant HSP90 (Y465W) by TNF was decreased compared to control U937 cells (Fritsch et al., 2016).
Andrographolide (andro), a diterpenoid lactone isolated from Andrographis paniculata, has anti-inflammatory activity and inhibits cell transformation through v-Src degradation (Liang et al., 2008). When temperature-sensitive v-Src-expressing cell line (ts-v-Src; RK3E cell line) was treated with andro, 40 kDa fragments of HSP90 were also detected with v-Src degradation. Andro-induced HSP90 cleavage was related with a decrease of v-Src and cell apoptosis. In this phenomenon, andro-mediated ROS generation plays an important role in HSP90 cleavage and v-Src suppression. Furthermore, in leukemia cells, andro-induced HSP90 cleavage was correlated with BCR-ABL down-regulation and apoptosis (Liu et al., 2014).
The novel anti-cancer drug β-Lapachone (β-lap) induces HSP90 cleavage in NAD(P)H:quinone oxidoreductase-1 (NQO1)-expressing lung and prostate cancer cells and HUVEC cells. The cleavage of HSP90 is induced by β-lap-mediated ROS generation, and the cleavage of HSP90 and down-regulation of client proteins are restored by antioxidant treatment. NQO1-mediated activation of β-lap triggers a futile cycle, and NQO1-depedent quinones cannot cleave HSP90, unlike β-lap. Therefore, the futile redox cycle of β-lap may generate ROS, and the chemical structure of β-lap is a critical factor for HSP90 cleavage (Wu et al., 2016).
As described earlier, 17-AAG inhibits HSP90 by binding to the ATP-binding pocket of HSP90. In human urinary bladder cancer cells, 17-AAG induces cell apoptosis and down-regulation of client proteins, and the cleavage of HSP90 was also examined (Karkoulis et al., 2010). The detailed action mechanism involved in 17-AAG has not been defined yet.
Arsenic compounds arsenite (As(III)) and monomethylarsonous acid (MMA(III)) induce ROS-mediated apoptosis and HSP90 cleavage. It was reported that the NADPH inhibitor, diphenyleneiodonium chloride (DPI), inhibit As(III)-induced apoptosis and that HSP90 cleavage is also reduced by DPI treatment. In addition, JNK inhibitor, SP600125, blocks HSP90 cleavage, whereas the ERK inhibitor PD98059 does not. Therefore, As(III) and MMA(III) induce HSP90 cleavage via NADPH and JNK activation, thereby inducing cell apoptosis (Shen et al., 2008).
CONCLUSIONS
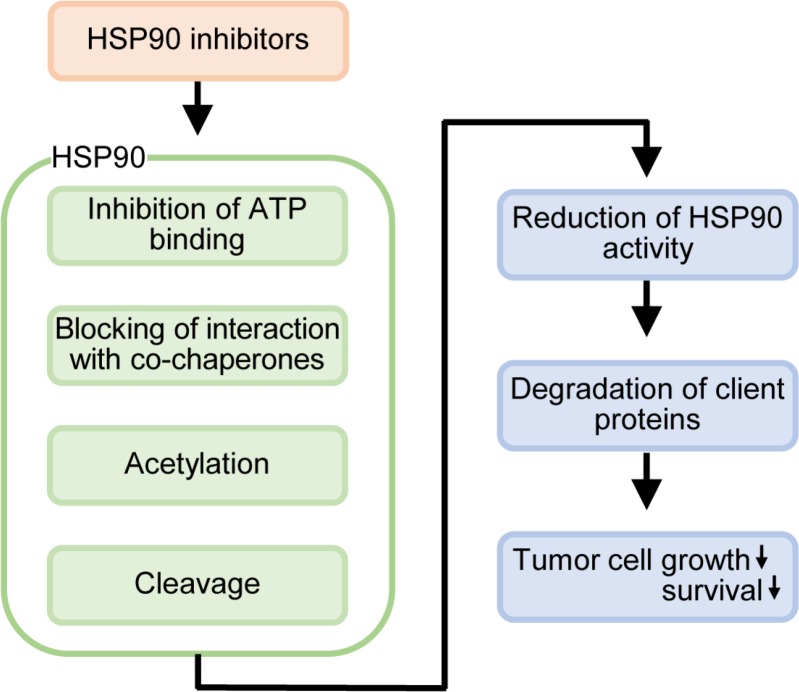
HSP90 is a molecular chaperone that supports folding and stabilization of the client proteins. Likely because many client proteins of HSP90 are required for cancer cell survival and growth, most cancer cells express HSP90 more highly than normal cells (Ferrarini et al., 1992; Sharp and Workman, 2006; Neckers and Workman, 2012; Miyata et al., 2013). Various inhibitors of HSP90 have been studied as anticancer drugs and clinical trials are underway. Most of the traditional inhibitors target ATP binding or deacetylation of HSP90, and thereby block the molecular chaperone activity of HSP90, resulting in degradation of the client proteins and increased cell death in cancer cells. Recently, several studies including ours have shown that the cleavage of HSP90 is correlated with degradation of client proteins and directly or indirectly affects the survival and growth of cancer cells (Shen et al., 2008; Chen et al., 2009; Liu et al., 2014; Fritsch et al., 2016; Wu et al., 2016). In addition, HSP90 cleavage inducing reagents, such as β-lap and vorinostat (Ramaswamy et al., 2012; Tu et al., 2014; Park et al., 2015; Wu et al., 2016), are also used for anti-cancer therapy. Therefore, it can be carefully postulated that chemicals inducing HSP90 cleavage may have anticancer activity. Taken together, the present results show that HSP90 cleavage may be another mechanism of HSP90 inhibitors and targeting of HSP90 cleavage is potentially another strategy for cancer chemotherapy (Fig. 1). We are presently screening chemicals that can induce HSP90 cleavage and are planning to verify whether they have anticancer activity when used alone or in combination. The results can be helpful to understand the mechanism of HSP90 inhibition in several aspects and may provide novel candidate drugs for cancer therapy.
Fig. 1.
Diagram of HSP90 inhibitor-mediated tumor cell suppression. HSP90 inhibitors are known to inhibit the binding of ATP, block binding to co-chaperones and regulate acetylation to inhibit the activity of HSP90. In addition, the cleavage of HSP90 discussed in this paper appears to suppress the activity of HSP90. Inhibition of HSP90 by HSP90 inhibitors reduces chaperone activity and inhibits growth and survival of tumor cells through degradation of the HSP90 client proteins.
Acknowledgments
This research was supported by grants from the National Research Foundation (NRF-2018R1A2B6002504) funded by the Ministry of Science and ICT in the Republic of Korea.
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Abramson JS, Chen W, Juszczynski P, Takahashi H, Neuberg D, Kutok JL, Takeyama K, Shipp MA. The heat shock protein 90 inhibitor IPI-504 induces apoptosis of AKT-dependent diffuse large B-cell lymphomas. Br J Haematol. 2009;144:358–366. doi: 10.1111/j.1365-2141.2008.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005a;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, Rocha K, Wang HG, Richon V, Bhalla K. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005b;11:6382–6389. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- Banerji U, Affolter A, Judson I, Marais R, Workman P. BRAF and NRAS mutations in melanoma: potential relationships to clinical response to HSP90 inhibitors. Mol Cancer Ther. 2008;7:737–739. doi: 10.1158/1535-7163.MCT-08-0145. [DOI] [PubMed] [Google Scholar]
- Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005a;23:4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res. 2005b;11:7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- Bao R, Lai CJ, Qu H, Wang D, Yin L, Zifcak B, Atoyan R, Wang J, Samson M, Forrester J, DellaRocca S, Xu GX, Tao X, Zhai HX, Cai X, Qian C. CUDC-305, a novel synthetic HSP90 inhibitor with unique pharmacologic properties for cancer therapy. Clin Cancer Res. 2009;15:4046–4057. doi: 10.1158/1078-0432.CCR-09-0152. [DOI] [PubMed] [Google Scholar]
- Beck R, Dejeans N, Glorieux C, Creton M, Delaive E, Dieu M, Raes M, Leveque P, Gallez B, Depuydt M, Collet JF, Calderon PB, Verrax J. Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS ONE. 2012;7:e40795. doi: 10.1371/journal.pone.0040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, Feron O, Calderon PB. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol. 2009;77:375–383. doi: 10.1016/j.bcp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Bauer TM, Lamar R, Joseph M, Penley W, Thompson DS, Spigel DR, Owera R, Lane CM, Earwood C, Burris HA., 3rd A phase 2 study of the Hsp90 inhibitor AUY922 as treatment for patients with refractory gastrointestinal stromal tumors. Cancer Invest. 2016;34:265–270. doi: 10.1080/07357907.2016.1193746. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Jones SF, Hart L, Pant S, Moyhuddin A, Lane CM, Earwood C, Murphy P, Patton J, Penley WC, Thompson D, Infante JR. A phase I study of the Hsp90 inhibitor AUY922 plus capecitabine for the treatment of patients with advanced solid tumors. Cancer Invest. 2015;33:477–482. doi: 10.3109/07357907.2015.1069834. [DOI] [PubMed] [Google Scholar]
- Boltze C, Lehnert H, Schneider-Stock R, Peters B, Hoang Vu C, Roessner A. Withdrawal. HSP90 is a key for telomerase activation and malignant transition in pheochromocytoma. Endocrine. 2004;23:229. doi: 10.1007/s12020-004-0002-4. [DOI] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci USA. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canella A, Welker AM, Yoo JY, Xu J, Abas FS, Kesanakurti D, Nagarajan P, Beattie CE, Sulman EP, Liu J, Gumin J, Lang FF, Gurcan MN, Kaur B, Sampath D, Puduvalli VK. Efficacy of onalespib, a long-acting second-generation HSP90 inhibitor, as a single agent and in combination with temozolomide against malignant gliomas. Clin Cancer Res. 2017;23:6215–6226. doi: 10.1158/1078-0432.CCR-16-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro JP, Fernando R, Reeg S, Meinl W, Almeida H, Grune T. Non-enzymatic cleavage of Hsp90 by oxidative stress leads to actin aggregate formation: A novel gain-of-function mechanism. Redox Biol. 2019;21:101108. doi: 10.1016/j.redox.2019.101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenagh J, Oakervee H, Baetiong-Caguioa P, Davies F, Gharibo M, Rabin N, Kurman M, Novak B, Shiraishi N, Nakashima D, Akinaga S, Yong K. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br. J. Cancer. 2017;117:1295–1302. doi: 10.1038/bjc.2017.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercek A, Shia J, Gollub M, Chou JF, Capanu M, Raasch P, Reidy-Lagunes D, Proia DA, Vakiani E, Solit DB, Saltz LB. Ganetespib, a novel Hsp90 inhibitor in patients with KRAS mutated and wild type, refractory metastatic colorectal cancer. Clin. Colorectal Cancer. 2014;13:207–212. doi: 10.1016/j.clcc.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Ye Q, Scott A, Silinski M, Huang K, Fadden P, Partdrige J, Hall S, Steed P, Norton L, Rosen N, Solit DB. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008;14:240–248. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xia Y, Fang D, Hawke D, Lu Z. Caspase-10-mediated heat shock protein 90 beta cleavage promotes UVB irradiation-induced cell apoptosis. Mol Cell Biol. 2009;29:3657–3664. doi: 10.1128/MCB.01640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Meng S, Wang H, Bali P, Bai W, Li B, Atadja P, Bhalla KN, Wu J. Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor LAQ824. Mol Cancer Ther. 2005;4:1311–1319. doi: 10.1158/1535-7163.MCT-04-0287. [DOI] [PubMed] [Google Scholar]
- Cheng W, Ainiwaer A, Xiao L, Cao Q, Wu G, Yang Y, Mao R, Bao Y. Role of the novel HSP90 inhibitor AUY922 in hepatocellular carcinoma: potential for therapy. Mol Med Rep. 2015;12:2451–2456. doi: 10.3892/mmr.2015.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, Mita M, Shaw H, Workman P, Kaye S, Rowinsky EK, Aherne W, Atadja P, Scott JW, Patnaik A. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res. 2008;14:6663–6673. doi: 10.1158/1078-0432.CCR-08-0376. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Okuno SH, Keohan ML, Maki RG, D’Adamo DR, Akhurst TJ, Antonescu CR, Schwartz GK. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann Oncol. 2013;24:252–257. doi: 10.1093/annonc/mds275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K, Speranza G, Chang LC, Polley EC, Bishop R, Zhu W, Trepel JB, Lee S, Lee MJ, Kinders RJ, Phillips L, Collins J, Lyons J, Jeong W, Antony R, Chen AP, Neckers L, Doroshow JH, Kummar S. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest New Drugs. 2015;33:921–930. doi: 10.1007/s10637-015-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Onozawa Y, Fuse N, Yoshino T, Yamazaki K, Watanabe J, Akimov M, Robson M, Boku N, Ohtsu A. Phase I dose-escalation study of the HSP90 inhibitor AUY922 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74:629–636. doi: 10.1007/s00280-014-2521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall F, Aherne W, Rowlands M, Hayes A, Martins V, Urban F, Boxall K, Prodromou C, Pearl L, James K, Matthews TP, Cheung KM, Kalusa A, Jones K, McDonald E, Barril X, Brough PA, Cansfield JE, Dymock B, Drysdale MJ, Finch H, Howes R, Hubbard RE, Surgenor A, Webb P, Wood M, Wright L, Workman P. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Fritsch J, Fickers R, Klawitter J, Sarchen V, Zingler P, Adam D, Janssen O, Krause E, Schutze S. TNF induced cleavage of HSP90 by cathepsin D potentiates apoptotic cell death. Oncotarget. 2016;7:75774–75789. doi: 10.18632/oncotarget.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FC, Guo R, Tian WL, Ge FF, Sun L, Jiang ZX. Proliferation and apoptosis of leukemia cell line K562 treated with HSP90 inhibitor 17-DMAG. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:998–1002. doi: 10.7534/j.issn.1009-2137.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Ge FF, Guo R, Tian WL, Gao FC, Sun L, Jiang ZX. Effect of heat shock protein 90 inhibitor 17-DMAG on proliferation and apoptosis of acute lymphocytic leukemia cell line Jurkat. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:1011–1015. doi: 10.7534/j.issn.1009-2137.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- Goldman JW, Raju RN, Gordon GA, El-Hariry I, Teofilivici F, Vukovic VM, Bradley R, Karol MD, Chen Y, Guo W, Inoue T, Rosen LS. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer. 2013;13 doi: 10.1186/1471-2407-13-152. 152-2407-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L, Wadlow RC, Blaszkowsky LS, Wolpin BM, Abrams TA, McCleary NJ, Sheehan S, Sundaram E, Karol MD, Chen J, Zhu AX. A phase I and pharmacokinetic study of ganetespib (STA-9090) in advanced hepatocellular carcinoma. Invest New Drugs. 2015;33:128–137. doi: 10.1007/s10637-014-0164-8. [DOI] [PubMed] [Google Scholar]
- Grafone T, Palmisano M, Nicci C, Storti S. An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: biology and treatment. Oncol Rev. 2012;6:e8. doi: 10.4081/oncol.2012.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B, Curry J, Smyth T, Fazal L, Feltell R, Harada I, Coyle J, Williams B, Reule M, Angove H, Cross DM, Lyons J, Wallis NG, Thompson NT. The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci. 2012;103:522–527. doi: 10.1111/j.1349-7006.2011.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- He W, Ye X, Huang X, Lel W, You L, Wang L, Chen X, Qian W. Hsp90 inhibitor, BIIB021, induces apoptosis and autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive and -resistant chronic myeloid leukemia cells. Int J Oncol. 2016;48:1710–1720. doi: 10.3892/ijo.2016.3382. [DOI] [PubMed] [Google Scholar]
- Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH, 3rd, Goettl V M, Zhang X, Jarjoura D, Raymond CA, West DA, Croce CM, Byrd JC, Johnson AJ. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood. 2010;116:45–53. doi: 10.1182/blood-2010-01-263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D, Said R, Falchook G, Naing A, Moulder S, Tsimberidou AM, Galluppi G, Dakappagari N, Storgard C, Kurzrock R, Rosen LS. Phase I study of BIIB028, a selective heat shock protein 90 inhibitor, in patients with refractory metastatic or locally advanced solid tumors. Clin Cancer Res. 2013;19:4824–4831. doi: 10.1158/1078-0432.CCR-13-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003–4009. [PubMed] [Google Scholar]
- Infante JR, Weiss GJ, Jones S, Tibes R, Bauer TM, Bendell JC, Hinson JM, Jr, Von Hoff DD, Burris HA, 3rd, Orlemans EO, Ramanathan RK. Phase I dose-escalation studies of SNX-5422, an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumours. Eur. J Cancer. 2014;50:2897–2904. doi: 10.1016/j.ejca.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- Isambert N, Delord JP, Soria JC, Hollebecque A, Gomez-Roca C, Purcea D, Rouits E, Belli R, Fumoleau P. Debio0932, a second-generation oral heat shock protein (HSP) inhibitor, in patients with advanced cancer-results of a first-in-man dose-escalation study with a fixed-dose extension phase. Ann Oncol. 2015;26:1005–1011. doi: 10.1093/annonc/mdv031. [DOI] [PubMed] [Google Scholar]
- Jensen MR, Schoepfer J, Radimerski T, Massey A, Guy CT, Brueggen J, Quadt C, Buckler A, Cozens R, Drysdale MJ, Garcia-Echeverria C, Chene P. NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008;10:R33. doi: 10.1186/bcr1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jez JM, Chen JC, Rastelli G, Stroud RM, Santi DV. Crystal structure and molecular modeling of 17-DMAG in complex with human Hsp90. Chem Biol. 2003;10:361–368. doi: 10.1016/S1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- Jhaveri K, Chandarlapaty S, Lake D, Gilewski T, Robson M, Goldfarb S, Drullinsky P, Sugarman S, Wasserheit-Leiblich C, Fasano J, Moynahan ME, D’Andrea G, Lim K, Reddington L, Haque S, Patil S, Bauman L, Vukovic V, El-Hariry I, Hudis C, Modi S. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014;14:154–160. doi: 10.1016/j.clbc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Jhaveri K, Miller K, Rosen L, Schneider B, Chap L, Hannah A, Zhong Z, Ma W, Hudis C, Modi S. A phase I dose-escalation trial of trastuzumab and alvespimycin hydrochloride (KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin Cancer Res. 2012;18:5090–5098. doi: 10.1158/1078-0432.CCR-11-3200. [DOI] [PubMed] [Google Scholar]
- Jhaveri K, Wang R, Teplinsky E, Chandarlapaty S, Solit D, Cadoo K, Speyer J, D’Andrea G, Adams S, Patil S, Haque S, O’Neill T, Friedman K, Esteva FJ, Hudis C, Modi S. A phase I trial of ganetespib in combination with paclitaxel and trastuzumab in patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer. Breast Cancer Res. 2017;19 doi: 10.1186/s13058-017-0879-5. 89-017-0879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Yu HA, Hart EM, Weitner BB, Rademaker AW, Patel JD, Kris MG, Riely GJ. Phase I/II study of HSP90 inhibitor AUY922 and erlotinib for EGFR-mutant lung cancer with acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol. 2015;33:1666–1673. doi: 10.1200/JCO.2014.59.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Radic Biol Med. 2007;43:861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Karkoulis PK, Stravopodis DJ, Margaritis LH, Voutsinas GE. 17-Allylamino-17-demethoxygeldanamycin induces downregulation of critical Hsp90 protein clients and results in cell cycle arrest and apoptosis of human urinary bladder cancer cells. BMC Cancer. 2010;10 doi: 10.1186/1471-2407-10-481. 481-2407-10-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem Biophys Res Commun. 2007;363:745–750. doi: 10.1016/j.bbrc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Kliche S, Waltenberger J. VEGF receptor signaling and endothelial function. IUBMB Life. 2001;52:61–66. doi: 10.1080/15216540252774784. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Toyooka S, Soh J, Yamamoto H, Dote H, Kawasaki K, Otani H, Kubo T, Jida M, Ueno T, Ando M, Ogino A, Kiura K, Miyoshi S. The anti-proliferative effect of heat shock protein 90 inhibitor, 17-DMAG, on non-small-cell lung cancers being resistant to EGFR tyrosine kinase inhibitor. Lung Cancer. 2012;75:161–166. doi: 10.1016/j.lungcan.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Kong A, Rea D, Ahmed S, Beck JT, Lopez Lopez R, Biganzoli L, Armstrong AC, Aglietta M, Alba E, Campone M, Hsu Schmitz SF, Lefebvre C, Akimov M, Lee SC. Phase 1B/2 study of the HSP90 inhibitor AUY922 plus trastuzumab in metastatic HER2-positive breast cancer patients who have progressed on trastuzumab-based regimen. Oncotarget. 2016;7:37680–37692. doi: 10.18632/oncotarget.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4:342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy GP, Guida T, Alfano L, Avilla E, Santoro M, Carlomagno F, Melillo RM. Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. J Biol Chem. 2013;288:17481–17494. doi: 10.1074/jbc.M112.439422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, Chen M, Steinberg SM, Muir CA, Yancey MA, Horneffer YR, Juwara L, Melillo G, Ivy SP, Merino M, Neckers L, Steeg PS, Conley BA, Giaccone G, Doroshow JH, Murgo AJ. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur. J Cancer. 2010;46:340–347. doi: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL, Baer MR. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- Lazenby M, Hills R, Burnett AK, Zabkiewicz J. The HSP90 inhibitor ganetespib: A potential effective agent for Acute Myeloid Leukemia in combination with cytarabine. Leuk Res. 2015;39:617–624. doi: 10.1016/j.leukres.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Saini N, Howard EW, Parris AB, Ma Z, Zhao Q, Zhao M, Liu B, Edgerton SM, Thor AD, Yang X. Ganetespib targets multiple levels of the receptor tyrosine kinase signaling cascade and preferentially inhibits ErbB2-overexpressing breast cancer cells. Sci Rep. 2018;8:6829. doi: 10.1038/s41598-018-25284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Saini N, Parris AB, Zhao M, Yang X. Ganetespib induces G2/M cell cycle arrest and apoptosis in gastric cancer cells through targeting of receptor tyrosine kinase signaling. Int J Oncol. 2017;51:967–974. doi: 10.3892/ijo.2017.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim. Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng AM, Liu T, Yang J, Cui JF, Li XH, Zhu YN, Xiong T, Zhang G, Chen Y. The apoptotic effect and associated signalling of HSP90 inhibitor 17-DMAG in hepatocellular carcinoma cells. Cell Biol Int. 2012;36:893–899. doi: 10.1042/CBI20110473. [DOI] [PubMed] [Google Scholar]
- Leow CC, Chesebrough J, Coffman KT, Fazenbaker CA, Gooya J, Weng D, Coats S, Jackson D, Jallal B, Chang Y. Antitumor efficacy of IPI-504, a selective heat shock protein 90 inhibitor against human epidermal growth factor receptor 2-positive human xenograft models as a single agent and in combination with trastuzumab or lapatinib. Mol Cancer Ther. 2009;8:2131–2141. doi: 10.1158/1535-7163.MCT-08-1038. [DOI] [PubMed] [Google Scholar]
- Liang FP, Lin CH, Kuo CD, Chao HP, Fu SL. Suppression of v-Src transformation by andrographolide via degradation of the v-Src protein and attenuation of the Erk signaling pathway. J Biol Chem. 2008;283:5023–5033. doi: 10.1074/jbc.M705877200. [DOI] [PubMed] [Google Scholar]
- Liu SH, Lin CH, Liang FP, Chen PF, Kuo CD, Alam MM, Maiti B, Hung SK, Chi CW, Sun CM, Fu SL. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem Pharmacol. 2014;87:229–242. doi: 10.1016/j.bcp.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Zhang H, Brekken J, Huser N, Powell RE, Timple N, Busch DJ, Neely L, Sensintaffar JL, Yang YC, McKenzie A, Friedman J, Scannevin R, Kamal A, Hong K, Kasibhatla SR, Boehm MF, Burrows FJ. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol Cancer Ther. 2009;8:921–929. doi: 10.1158/1535-7163.MCT-08-0758. [DOI] [PubMed] [Google Scholar]
- Maddocks K, Hertlein E, Chen TL, Wagner AJ, Ling Y, Flynn J, Phelps M, Johnson AJ, Byrd JC, Jones JA. A phase I trial of the intravenous Hsp90 inhibitor alvespimycin (17-DMAG) in patients with relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 2016;57:2212–2215. doi: 10.3109/10428194.2015.1129536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes DL, Taverna P, Jensen MR, Abrams T, Stuart D, Yu GK, Duhl D, Machajewski T, Sellers WR, Pryer NK, Gao Z. The novel oral Hsp90 inhibitor NVP-HSP990 exhibits potent and broad-spectrum antitumor activities in vitro and in vivo. Mol Cancer Ther. 2012;11:730–739. doi: 10.1158/1535-7163.MCT-11-0667. [DOI] [PubMed] [Google Scholar]
- Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19:347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D’Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, Cropp GF, Johnson RG, Hannah AL, Hudis CA. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- Molife LR, Attard G, Fong PC, Karavasilis V, Reid AH, Patterson S, Riggs CE, Jr, Higano C, Stadler WM, McCulloch W, Dearnaley D, Parker C, de Bono JS. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC) Ann Oncol. 2010;21:109–113. doi: 10.1093/annonc/mdp270. [DOI] [PubMed] [Google Scholar]
- Moser C, Lang SA, Hackl C, Wagner C, Scheiffert E, Schlitt HJ, Geissler EK, Stoeltzing O. Targeting HSP90 by the novel inhibitor NVP-AUY922 reduces growth and angiogenesis of pancreatic cancer. Anticancer Res. 2012;32:2551–2561. [PubMed] [Google Scholar]
- Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y, Akinaga S, Soga S, Shiotsu Y. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res. 2010;16:2792–2802. doi: 10.1158/1078-0432.CCR-09-3112. [DOI] [PubMed] [Google Scholar]
- Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L, Mimnaugh E, Schulte TW. Hsp90 as an anti-cancer target. Drug Resist Updat. 1999;2:165–172. doi: 10.1054/drup.1999.0082. [DOI] [PubMed] [Google Scholar]
- Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Takeuchi S, Koeffler HP, Yokoyama A. MS-275, a novel histone deacetylase inhibitor with selectivity against HDAC1, induces degradation of FLT3 via inhibition of chaperone function of heat shock protein 90 in AML cells. Leuk Res. 2008;32:1382–1392. doi: 10.1016/j.leukres.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Niu G, Li Z, Cao Q, Chen X. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with (64)Cu-DOTA-trastuzumab. Eur. J. Nucl. Med. Mol Imaging. 2009;36:1510–1519. doi: 10.1007/s00259-009-1158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WK, Galsky MD, Stadler WM, Srinivas S, Chu F, Bubley G, Goddard J, Dunbar J, Ross RW. Multicenter phase II trial of the heat shock protein 90 inhibitor, retaspimycin hydrochloride (IPI-504), in patients with castration-resistant prostate cancer. Urology. 2011;78:626–630. doi: 10.1016/j.urology.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa Y, Hideshima T, Steed P, Vallet S, Hall S, Huang K, Rice J, Barabasz A, Foley B, Ikeda H, Raje N, Kiziltepe T, Yasui H, Enatsu S, Anderson KC. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009;113:846–855. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Nakagawara A. Role of p53 in cell death and human cancers. Cancers (Basel) 2011;3:994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey S, Gore M, Chao D, Banerji U, Larkin J, Sarker S, Owen K, Asad Y, Raynaud F, Walton M, Judson I, Workman P, Eisen T. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs. 2012;30:341–349. doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U, Roels B, Peachey H, Aherne W, de Bono JS, Raynaud F, Workman P, Judson I. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JA, Kim YE, Song S, Kwon HJ, Lee Y. Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones. 2015;20:149–157. doi: 10.1007/s12192-014-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JA, Yoo H, Park HB, Lee Y. Proteasome inhibitor-induced cleavage of HSP90 is mediated by ROS generation and caspase 10-activation in human leukemic cells. Redox Biol. 2017;13:470–476. doi: 10.1016/j.redox.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SE, Kim DE, Kim MJ, Lee JS, Rho JK, Jeong SY, Choi EK, Kim CS, Hwang JJ. Vorinostat enhances gefitinibinduced cell death through reactive oxygen speciesdependent cleavage of HSP90 and its clients in nonsmall cell lung cancer with the EGFR mutation. Oncol Rep. 2019;41:525–533. doi: 10.3892/or.2018.6814. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Proia DA, Foley KP, Korbut T, Sang J, Smith D, Bates RC, Liu Y, Rosenberg AF, Zhou D, Koya K, Barsoum J, Blackman RK. Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling. PLoS ONE. 2011;6:e18552. doi: 10.1371/journal.pone.0018552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. Br. J Cancer. 2006;94:13–17. doi: 10.1038/sj.bjc.6602876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L, Mannargudi B, Figg WD, Houk BE, Shnaidman M, Brega N, Giaccone G. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res. 2011;17:6831–6839. doi: 10.1158/1078-0432.CCR-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S, Goss G, Rosell R, Schmid-Bindert G, Zaric B, Andric Z, Bondarenko I, Komov D, Ceric T, Khuri F, Samarzija M, Felip E, Ciuleanu T, Hirsh V, Wehler T, Spicer J, Salgia R, Shapiro G, Sheldon E, Teofilovici F, Vukovic V, Fennell D. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1) Ann Oncol. 2015;26:1741–1748. doi: 10.1093/annonc/mdv220. [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, Trump DL, Eiseman JL, Belani CP, Agarwala SS, Zuhowski EG, Lan J, Potter DM, Ivy SP, Ramalingam S, Brufsky AM, Wong MK, Tutchko S, Egorin MJ. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- Ramaswamy B, Fiskus W, Cohen B, Pellegrino C, Hershman DL, Chuang E, Luu T, Somlo G, Goetz M, Swaby R, Shapiro CL, Stearns V, Christos P, Espinoza-Delgado I, Bhalla K, Sparano JA. Phase I–II study of vorinostat plus paclitaxel and bevacizumab in metastatic breast cancer: evidence for vorinostat-induced tubulin acetylation and Hsp90 inhibition in vivo. Breast Cancer Res Treat. 2012;132:1063–1072. doi: 10.1007/s10549-011-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renouf DJ, Hedley D, Krzyzanowska MK, Schmuck M, Wang L, Moore MJ. A phase II study of the HSP90 inhibitor AUY922 in chemotherapy refractory advanced pancreatic cancer. Cancer Chemother Pharmacol. 2016;78:541–545. doi: 10.1007/s00280-016-3102-y. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Chanan-Khan AA, Alsina M, Albitar M, Berman D, Messina M, Mitsiades CS, Anderson KC. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose-escalation study. Br J Haematol. 2010;150:438–445. doi: 10.1111/j.1365-2141.2010.08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, Albitar M, Berman D, Messina M, Anderson KC. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153:729–740. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Rohl A, Rohrberg J, Buchner J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem Sci. 2013;38:253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif MW, Takimoto C, Mita M, Banerji U, Lamanna N, Castro J, O’Brien S, Stogard C, Von Hoff D. A phase 1, dose-escalation, pharmacokinetic and pharmacodynamic study of BIIB021 administered orally in patients with advanced solid tumors. Clin Cancer Res. 2014;20:445–455. doi: 10.1158/1078-0432.CCR-13-1257. [DOI] [PubMed] [Google Scholar]
- Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageot CM, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, Ramakrishna NR, Stiles CD, Kung AL, Kieran MW, Wen PY. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro-oncology. 2009;11:109–121. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggewiss-Bernhardt R, Bargou RC, Goh YT, Stewart AK, Spencer A, Alegre A, Blade J, Ottmann OG, Fernandez-Ibarra C, Lu H, Pain S, Akimov M, Iyer SP. Phase 1/1B trial of the heat shock protein 90 inhibitor NVP-AUY922 as monotherapy or in combination with bortezomib in patients with relapsed or refractory multiple myeloma. Cancer. 2015;121:2185–2192. doi: 10.1002/cncr.29339. [DOI] [PubMed] [Google Scholar]
- Sessa C, Shapiro GI, Bhalla KN, Britten C, Jacks KS, Mita M, Papadimitrakopoulou V, Pluard T, Samuel TA, Akimov M, Quadt C, Fernandez-Ibarra C, Lu H, Bailey S, Chica S, Banerji U. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res. 2013;19:3671–3680. doi: 10.1158/1078-0432.CCR-12-3404. [DOI] [PubMed] [Google Scholar]
- Shapiro GI, Kwak E, Dezube BJ, Yule M, Ayrton J, Lyons J, Mahadevan D. First-in-human phase I dose escalation study of a second-generation non-ansamycin HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:87–97. doi: 10.1158/1078-0432.CCR-14-0979. [DOI] [PubMed] [Google Scholar]
- Sharp S, Workman P. Inhibitors of the HSP90 molecular chaperone: current status. Adv Cancer Res. 2006;95:323–348. doi: 10.1016/S0065-230X(06)95009-X. [DOI] [PubMed] [Google Scholar]
- Shen SC, Yang LY, Lin HY, Wu CY, Su TH, Chen YC. Reactive oxygen species-dependent HSP90 protein cleavage participates in arsenical As(+3)- and MMA(+3)-induced apoptosis through inhibition of telomerase activity via JNK activation. Toxicol Appl Pharmacol. 2008;229:239–251. doi: 10.1016/j.taap.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, Chen L, Li D, Carretero J, Li YC, Sinha P, Carey CD, Borgman CL, Jimenez JP, Meyerson M, Ying W, Barsoum J, Wong KK, Shapiro GI. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18:4973–4985. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, Paschold E, Salgia R, West H, Sequist LV, Bonomi P, Brahmer J, Chen LC, Sandler A, Belani CP, Webb T, Harper H, Huberman M, Ramalingam S, Wong KK, Teofilovici F, Guo W, Shapiro GI. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19:3068–3077. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, Teitcher J, Wolchok JD, Germino FJ, Krown SE, Coit D, Rosen N, Chapman PB. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, Scher HI, Rosen N. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–993. [PubMed] [Google Scholar]
- Spreafico A, Delord JP, De Mattos-Arruda L, Berge Y, Rodon J, Cottura E, Bedard PL, Akimov M, Lu H, Pain S, Kaag A, Siu LL, Cortes J. A first-in-human phase I, dose-escalation, multicentre study of HSP990 administered orally in adult patients with advanced solid malignancies. Br. J Cancer. 2015;112:650–659. doi: 10.1038/bjc.2014.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/S0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- Sullivan WP, Owen BA, Toft DO. The influence of ATP and p23 on the conformation of hsp90. J Biol Chem. 2002;277:45942–45948. doi: 10.1074/jbc.M207754200. [DOI] [PubMed] [Google Scholar]
- Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J, Patterson J, Penders C, Pink M, Read MA, Sang J, Woodward C, Zhang Y, Grayzel DS, Wright J, Barrett JA, Palombella VJ, Adams J, Tong JK. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci USA. 2006;103:17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur MK, Heilbrun LK, Sheng S, Stein M, Liu G, Antonarakis ES, Vaishampayan U, Dzinic SH, Li X, Freeman S, Smith D, Heath EI. A phase II trial of ganetespib, a heat shock protein 90 Hsp90) inhibitor, in patients with docetaxel-pretreated metastatic castrate-resistant prostate cancer (CRPC)-a prostate cancer clinical trials consortium (PCCTC) study. Invest New Drugs. 2016;34:112–118. doi: 10.1007/s10637-015-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio PD, Choi H, Huang LE. Complex role of HIF in cancer: the known, the unknown, and the unexpected. Hypoxia (Auckl) 2014;2:59–70. doi: 10.2147/HP.S50651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Tse AN, Klimstra DS, Gonen M, Shah M, Sheikh T, Sikorski R, Carvajal R, Mui J, Tipian C, O’Reilly E, Chung K, Maki R, Lefkowitz R, Brown K, Manova-Todorova K, Wu N, Egorin MJ, Kelsen D, Schwartz GK. A phase 1 dose-escalation study of irinotecan in combination with 17-allylamino-17-demethoxygeldanamycin in patients with solid tumors. Clin Cancer Res. 2008;14:6704–6711. doi: 10.1158/1078-0432.CCR-08-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Hershman DL, Bhalla K, Fiskus W, Pellegrino CM, Andreopoulou E, Makower D, Kalinsky K, Fehn K, Fineberg S, Negassa A, Montgomery LL, Wiechmann LS, Alpaugh RK, Huang M, Sparano JA. A phase I–II study of the histone deacetylase inhibitor vorinostat plus sequential weekly paclitaxel and doxorubicin-cyclophosphamide in locally advanced breast cancer. Breast Cancer Res Treat. 2014;146:145–152. doi: 10.1007/s10549-014-3008-5. [DOI] [PubMed] [Google Scholar]
- Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8:1197–1206. doi: 10.1023/A:1008209720526. [DOI] [PubMed] [Google Scholar]
- Wagner AJ, Chugh R, Rosen LS, Morgan JA, George S, Gordon M, Dunbar J, Normant E, Grayzel D, Demetri GD. A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft-tissue sarcomas. Clin Cancer Res. 2013;19:6020–6029. doi: 10.1158/1078-0432.CCR-13-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Klisovic R, Johnston JS, Jiang Y, Geyer S, Kefauver C, Binkley P, Byrd JC, Grever MR, Garzon R, Phelps MA, Marcucci G, Blum KA, Blum W. Pharmacokinetics and dose escalation of the heat shock protein inhibitor 17-allyamino-17-demethoxygeldanamycin in combination with bortezomib in relapsed or refractory acute myeloid leukemia. Leuk Lymphoma. 2013;54:1996–2002. doi: 10.3109/10428194.2012.760733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby EJ, Lazenby M, Pepper CJ, Knapper S, Burnett AK. The HSP90 inhibitor NVP-AUY922-AG inhibits the PI3K and IKK signalling pathways and synergizes with cytarabine in acute myeloid leukaemia cells. Br J Haematol. 2013;161:57–67. doi: 10.1111/bjh.12215. [DOI] [PubMed] [Google Scholar]
- Weigel BJ, Blaney SM, Reid JM, Safgren SL, Bagatell R, Kersey J, Neglia JP, Ivy SP, Ingle AM, Whitesell L, Gilbertson RJ, Krailo M, Ames M, Adamson PC. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children’s Oncology Group study. Clin Cancer Res. 2007;13:1789–1793. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- Welch WJ. How cells respond to stress. Sci Am. 1993;268:56–64. doi: 10.1038/scientificamerican0593-56. [DOI] [PubMed] [Google Scholar]
- Woodhead AJ, Angove H, Carr MG, Chessari G, Congreve M, Coyle JE, Cosme J, Graham B, Day PJ, Downham R, Fazal L, Feltell R, Figueroa E, Frederickson M, Lewis J, McMenamin R, Murray CW, O’Brien MA, Parra L, Patel S, Phillips T, Rees DC, Rich S, Smith DM, Trewartha G, Vinkovic M, Williams B, Woolford AJ. Discovery of (2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-yl-methyl)-1,3-dihydrois oindol-2-yl]methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem. 2010;53:5956–5969. doi: 10.1021/jm100060b. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang X, Chang S, Lu W, Liu M, Pang X. beta-Lapachone induces NAD(P)H:quinone oxidoreductase-1- and oxidative stress-dependent heat shock protein 90 cleavage and inhibits tumor growth and angiogenesis. J Pharmacol Exp Ther. 2016;357:466–475. doi: 10.1124/jpet.116.232694. [DOI] [PubMed] [Google Scholar]
- Xia Y, Shen S, Verma IM. NF-kappaB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jin J, Xu J, Shao YW, Fan Y. JAK2 variations and functions in lung adenocarcinoma. Tumour Biol. 2017;39 doi: 10.1177/1010428317711140. 1010428317711140. [DOI] [PubMed] [Google Scholar]
- Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, Zhou D, Inoue T, Tatsuta N, Sang J, Ye S, Acquaviva J, Ogawa LS, Wada Y, Barsoum J, Koya K. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther. 2012;11:475–484. doi: 10.1158/1535-7163.MCT-11-0755. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Toker A. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle. 2006;5:603–605. doi: 10.4161/cc.5.6.2561. [DOI] [PubMed] [Google Scholar]
- Yong K, Cavet J, Johnson P, Morgan G, Williams C, Nakashima D, Akinaga S, Oakervee H, Cavenagh J. Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies. Br. J Cancer. 2016;114:7–13. doi: 10.1038/bjc.2015.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- Zhang H, Neely L, Lundgren K, Yang YC, Lough R, Timple N, Burrows F. BIIB021, a synthetic Hsp90 inhibitor, has broad application against tumors with acquired multidrug resistance. Int. J Cancer. 2010a;126:1226–1234. doi: 10.1002/ijc.24825. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang L. Ansamycin class of natural product Hsp90 inhibitors. In: Schwab M, editor. Encyclopedia of Cancer. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. pp. 198–201. [DOI] [Google Scholar]
- Zhang P, Wang C, Gao K, Wang D, Mao J, An J, Xu C, Wu D, Yu H, Liu JO, Yu L. The ubiquitin ligase itch regulates apoptosis by targeting thioredoxin-interacting protein for ubiquitin-dependent degradation. J Biol Chem. 2010b;285:8869–8879. doi: 10.1074/jbc.M109.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]