Abstract
Mycobacterium tuberculosisis highly endemic in the Philippines. The diagnosis is challenging with its non-specific presentation and the organism could extend to any of the organs. Interestingly, bacterial peritonitis arising spontaneously from gastrointestinal tuberculosis (TB) in an otherwise healthy, non-cirrhotic patient is quite unusual. In this paper, we discuss the case of a 27-year-old HIV-seronegative woman with massive intraperitoneal mixed bacterial and tuberculous abscess presenting 20 months after being diagnosed with bacteriologically confirmed gastrointestinal TB. Repeated large-volume paracentesis was done to drain out the infected ascites instead of inserting a percutaneously implanted catheter. Clinical improvement was noted and she was discharged after 12 days of intravenous antibiotics. She had completed 6 months of antituberculosis therapy and been well since then. The case has demonstrated that repeated paracentesis along with appropriate antibiotic regimen, may be a viable option for patients with TB and bacterial coinfected peritonitis. And possibly, peritoneal TB may increase the risk for (spontaneous) bacterial peritonitis.
Keywords: infections, ib and other respiratory infections, infection (gastroenterology)
Background
Philippines is one of the countries highly endemic to tuberculosis (TB), with 6% incidence rate in 2017.1 The diagnosis is challenging with its non-specific presentation and the organism could extend to any of the organs. Bacterial peritonitis arising spontaneously from gastrointestinal tuberculosis (GITB) in an otherwise healthy, non-cirrhotic patient is rare.
We demonstrate the efficacy of conservative management of repeated paracentesis versus percutaneously implanted indwelling catheter for drainage of infected peritoneal fluid in this case report. The case also highlighted the sufficiency of less than 2 weeks of culture-guided antibiotic treatment, even for massive intraperitoneal abscess.
Case presentation
A 27-year-old Filipina consulted the emergency department for increasing abdominal girth and abdominal pain for 1 month. This was associated with loose watery non-bloody stools, four to five times a day, intermittent fever (38°C–39°C), significant weight loss (~15 kg from her baseline 60 kg body weight) and anorexia. She denied smoking, alcohol beverage drinking or use of illicit drugs, nor any risky sexual behaviour. She was received with stable vital signs, with noted soft globular abdomen, prominent abdominal vessels and absence of any palpable abdominal mass. No oedema and no stigmata of liver disease were observed.
Twenty months prior to this consult, she was diagnosed with GITB at another institution after presenting with chronic diarrhoea, abdominal distention, abdominal pain and weight loss. At the time, a colonoscopy was performed which revealed a 6×6 mm ulcer at the terminal ileum and edematous mucosa. A biopsy of the ulcer was performed which showed chronic granulomatous inflammation consistent with TB—intestinal mucosal fragments with aggregates of epithelioid cells forming granulomas, some showing central caseation necrosis, and interspersed Langhan’s multinucleated giant cells surrounded by a lamina propria with lymphoplasmatic infiltrates. TB-PCR was positive for Mycobacterium tuberculosis (Mtb) complex further supported the diagnosis of GITB.
She was started on a combination of four drugs isoniazid, rifampicin, pyrazinamide and ethambutol (HRZE, 300, 600, 1600 and 1100 mg/day) but only for 3 weeks due to abdominal discomfort. During the interim, she reported resolution of her GI symptoms with persistence of intermittent cough.
Investigations
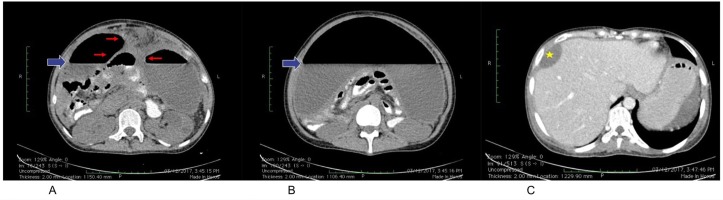
Paracentesis was done draining 3-litre of yellow, thick turbid, free-flowing, foul-smelling liquid (figure 1). Abdominal CT scan was performed showing loculated abscess (~3400 mL), perihepatic abscess (33 mL), and air-fluid levelling (figure 2). Hepatobiliary tree ultrasound was requested, which revealed a communicating perihepatic and intra-abdominal fluid collection, with absence of focal solid or cystic lesion at the liver. Rapid HIV test (HIV antibody, SD Brooke) was negative. Initial complete blood count showed hyperleukocytosis of 17×109/L with neutrophilic predominance of 75%. Ceftriaxone 2 g intravenous per day was started for spontaneous bacterial peritonitis and metronidazole 500 mg intravenous every 8 hours was added for anaerobic coverage. During her first week of hospital stay, she still complained of loose stools, intermittent low-grade fever, increasing abdominal girth and abdominal pain necessitating repeated therapeutic drainage of 2–3 L of ascitic fluid.
Figure 1.
A tan-coloured, thick, turbid, foul-smelling, 3 L peritoneal fluid drained on her first hospital day. The fluid has low glucose of <1.1 mmol/L, white blood cells (WBC) of 11 600×106/L, 94% distorted cells and bacteria 4+/hpf. Aerobic culture revealed Escherichia coli, ESBL+. Acid-fast bacilli (AFB) smear was negative, but TB culture yielded a pan-sensitive Mycobacterium tuberculosis. TB, tuberculosis.
Figure 2.
Abdominal CT scan with triple contrast, triphasic was done on her first hospital day. (A, B) Large, loculated, peripherally enhancing hypodense fluid collection (red arrow) exhibiting a layering air-fluid level with an approximate volume of 3400 mL (solid blue arrow). This collection displaces and compresses the contrast-filled, non-distended bowel loops posteriorly and the urinary bladder and uterus inferiorly, while maintaining clear planes of differentiation from these structures. (C) An ovoid, peripherally enhancing fluid collection measuring 7.2×4.5×2.0 cm with an approximate volume of 33 mL is seen in the right perihepatic space, causing focal marginal indentation of the adjacent portion of segment 8 (star).
Her peritoneal fluid yielded low glucose of <1.1 mmol/L, white blood cells (WBC) of 11 600×106/L, 94% distorted cells and bacteria 4+/hpf. Acid-fast bacilli (AFB) smear was negative. Aerobic culture of peritoneal fluid isolated Escherichia coli, ESBL+, which was sensitive to ertapenem, meropenem, cefipime and cefoxitin. Consequently, ceftriaxone was shifted to ertapenem 1 g intravenous per day. A category 1 TB treatment (4HRZE + 2 hour) was empirically started initially, along with ertapenem and metronidazole. Due to non-availability at our institution’s pharmacy later on, ertapenem was shifted to meropenem 1 g intravenous every 8 hours.
At this time, the service was managing her as a case of bacterial peritonitis with co-infected GI and possible peritoneal TB. Due to the foul-smelling nature of the ascitic fluid and in the absence of anaerobic culture, an anaerobic co-infection could not be totally ruled out and this warranted an anaerobic coverage.
Crepitus at the left lower quadrant extending to the left periumbilical area was observed on her 13th hospital day. But a ruptured viscus was readily ruled out after abdominal X-ray showed no signs of perforation. The observed subcutaneous emphysema was thought to be caused by the gas-forming organism.
On ertapenem/meropenem day 10, metronidazole day 9, HRZE day 7, her fever successfully lysed, she has formed stools, decreasing abdominal pain and consistently had decreasing abdominal girth. A total of four paracenteses were done throughout her hospital stay on as-needed-basis, on hospital days 1, 2, 6 and 12.
Her renal function tests during her hospital stay were within normal range—with initial blood urea nitrogen (BUN)of 14 mmol/L and serum creatinine of 63 µmol/L, which latter decreased to 36 µmol/L on discharge. However, a drop of serum albumin from 35 to 27 g/L after the second large-volume paracentesis then to 23 g/L after the third paracentesis was noted. This is likely due to the on-going inflammatory process. Liver enzymes were slightly elevated (Aspartate Aminotransferase, AST 2x upper-limit-of-normal and Alanine Aminotransferase, ALT 1.3x upper-limit-of-normal) but with normal bilirubin levels.
She was sent home after completing 12 days of meropenem/ertapenem, 10 days of metronidazole and 9 days of HRZE, with abdominal girth of 74 cm at umbilical level. She was enrolled to a local TB Directly Observed Treatment Short course for completion of TB category 1 regimen.
Peritoneal fluid which was sent for TB culture during her hospital confinement revealed Mtb after 6 weeks of incubation (BD BBL Mycobacteria Growth Indicator Tube), sensitive to streptomycin, isoniazid, rifampicin, ethambutol and pyrazinamide.
Differential diagnosis
Differential diagnosis showed ruptured viscus or complicated intra-abdominal infection (including abscess formation) that would warrant prompt surgical intervention. Clinical presentation along with the initial ascitic fluid studies may overlap with spontaneous bacterial peritonitis (SBP). But these causes of secondary peritonitis have been excluded on abdominal CT scan and the patient’s improvement even on antibiotics.
A neoplastic process is also a differential in this case—that is, a peritoneal carcinomatosis arising primarily from the gynecologic or gastrointestinal area, or a peritoneal lymphoma, although the latter is quite rare. The imaging and the cytology, however, were non-supportive of this differential.
Treatment
During her admission, she underwent four large-volume paracenteses and completed 12 days of culture-guided antibiotics (meropenem/ertapenem) and 10 days of metronidazole for empiric anaerobic coverage. She later finished 6 months of standard antituberculosis regimen for GITB—category 1 consisting of 2 months of HRZE (isoniazid/ rifampicin/ pyrazinimde/ ethambutol) and 4 months of HR (isoniazid/ rifampicin).
Outcome and follow-up
During her initial weekly follow-up visits, there was consistent decrease in abdominal girth to 63 cm (after 1 month). She denied any fever, and reported to have good appetite, regular bowel movement and weight gain. She completed her 6 months of TB category 1 regimen and was able to go back to work.
Discussion
GITB is generally managed with medical therapy with antituberculous drugs. Surgeries are usually conservative and are done only if absolutely indicated2—including free perforation, confined perforation with abscess or fistula, massive bleeding, complete obstruction or obstruction not responding to medical management.3
Nair et al proposed in their 2016 paper that Mtb could have an alternative route of entry, besides the classic theory involving ingestion of alveolar macrophage in the pulmonary system. This involves the microfold cells (M-cells), which are specialised epithelial cells that actively transport particles from the mucosal surface to the basal portion of the cell. Through the M-cells, Mtb could travel to various lymph nodes in the body through the lymphatics.4 This could also imply dissemination of Mtb anywhere in the body, including the peritoneum.
Peritoneal tuberculosis (or TB peritonitis) may result from either hematogenous spread from active pulmonary TB or spreading from adjacent intestinal foci or abdominal lymph nodes. TB peritonitis has been divided into three types, namely the more common wet ascitic type, the fixed fibrotic type and the dry plastic type. The wet ascitic type that was found in this case, has been associated with large amounts of free or loculated high-density fluid in abdomen.
The 2016 study by Hong et al reported variations in the commensal lung microbiota for patients with TB infection. This study had proposed the possible linkage of dysbiosis of airway flora for the pathophysiological process associated with TB disease.5 This dysbiosis may make the individual more susceptible to GI infection. An overpopulation of pathogenic microbes could disrupt the defense mechanism on the gut lining provided by the normal gut flora and may cause direct damage to the intestinal barrier. Mtb may also have direct effect to the gut lining. This will make the individual susceptible for bacterial gut translocation and consequently cause bacterial peritonitis.
Bacterial peritonitis can be either spontaneous or secondary. SBP is defined as the ‘infection of ascitic fluid in the absence of any intra-abdominal, surgically treatable source of infection’.6 A diagnosis of SBP is made if the polymorphonuclear cell count in the ascitic fluid is ≥250 cells/mm3, culture results are positive and secondary causes of peritonitis are excluded. SBP is largely seen in cirrhotic patients. This is in contrast with secondary bacterial peritonitis, which usually requires prompt surgical intervention, and antibiotics alone for this case is insufficient to control the infection.
A comparison of SBP and TB peritonitis was done in cirrhotic patients. The study revealed that TB peritonitis has more insidious onset (≥2 weeks), associated with extraperitoneal TB, seen more on Child-Pugh Class B, lower WBC count in ascites, and higher protein ascitic concentration.7
In contrast to this case, Mtb usually infects immunocompromised patients. Extrapulmonary TB has >50% prevalence in HIV-seropositive patients, as opposed to the 10%–15% among those HIV-seronegative patients.8 In 10-year study of TB peritonitis in Taiwan,9 36% of the 211 cases has pulmonary TB, 23.7% has liver cirrhosis, 20.9% has end-stage renal disease, 17.1% has diabetes mellitus, 10.4% has alcoholism, 7.1% were chronic steroid user and 1.4% has AIDS. The population were predominantly male (54.5%) with median age of 61 years (43–72).9
A concomitant infection of TB with another bacterial pathogen has warranted drainage for source control. This is done to lessen the bacterial burden, improve antibiotic efficacy and lessen antibiotic duration. In this case, the drainage of infected peritoneal fluid was done through repeated paracentesis. Percutaneous implanted catheter (or pigtail insertion) is another option. This method had been popularly used for recurrent malignant ascites but placing another foreign body in an infected compartment may cause persistence of the pathogen.
Repeated paracentesis was done on as-needed-basis, depending on patient’s clinical picture (ie, abdominal pain due to recurrence of ascites). This significantly improved the patient’s symptom, decreased the microbial burden and shortened the antibiotic duration. An appropriate antituberculosis regimen (2HRZE + 4 hour), culture-guided antibiotics and drainage were sufficient for the resolution of bacterial peritonitis.
The 2016 Philippine local TB guideline10 recommends 6 months treatment for new cases of GITB, consisting of 2 months of intensive phase followed by 4 months of maintenance phase. This category 1 treatment suggests a four-drug combination that includes isoniazid, rifampicin, pyrazinamide and ethambutol (HRZE) in the intensive phase, while a two-drug regimen consisting of isoniazid and rifampicin (HR) is advocated in the maintenance phase. Based on this local TB guideline,10 patients could still be started with the standard category 1 regimen (2HRZE + 4 hour) if patient took the medications for <4 weeks. Fortunately, the Mtb strain was pan-sensitive to the standard regimen.
Learning points.
Peritoneal tuberculosis (TB) may increase the risk for spontaneous bacterial peritonitis. Gastrointestinal TB in the ileocoecal region may extend to the peritoneum and increase the patient’s risk for bacterial gut translocation. And consequently, may cause spontaneous bacterial peritonitis.
Repeated paracentesis is a viable option for patients with tuberculous and bacterial peritonitis. It improves the patient’s symptoms, decreases microbial burden and possibly shortens antibiotic duration.
The frequency of paracentesis may be based on the patient’s clinical picture and could be done on as-needed-basis.
Directly observed therapy in TB management is a superior strategy to combat this endemic disease. Although this strategy is being executed in the Philippines for two decades, this case reflects the need for better implementation.
Footnotes
Contributors: CCS was the primary physician who managed the patient. She wrote the draft for this case report and consolidate inputs of all involved services. HC was the consultant-in-charge of this case and he directly supervise in the management and writing of this manuscript. IDVT and MLMMJ were actively engaged in the management of the patient. They both assisted in the editing of this manuscript. All authors are accountable in the integrity and accuracy of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Kanabus A. Information about tuberculosis. GHE 2018;1 www.tbfacts.org. [Google Scholar]
- 2. Debi U, Ravisankar V, Prasad KK, et al. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol 2014;20:14831–40. 10.3748/wjg.v20.i40.14831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weledji EP, Pokam BT. Abdominal tuberculosis: is there a role for surgery? World J Gastrointest Surg 2017;9:174–81. 10.4240/wjgs.v9.i8.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nair VR, Franco LH, Zacharia VM, et al. Microfold cells actively translocate Mycobacterium tuberculosis to initiate infection. Cell Rep 2016;16:1253–8. 10.1016/j.celrep.2016.06.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong BY, Maulén NP, Adami AJ, et al. Microbiome changes during tuberculosis and antituberculous therapy. Clin Microbiol Rev 2016;29:915–26. 10.1128/CMR.00096-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koulaouzidis A, Bhat S, Karagiannidis A, et al. Spontaneous bacterial peritonitis. Postgrad Med J 2007;83:379–83. 10.1136/pgmj.2006.056168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim NJ, Choo EJ, Kwak YG, et al. Tuberculous peritonitis in cirrhotic patients: comparison of spontaneous bacterial peritonitis caused by Escherichia coli with tuberculous peritonitis. Scand J Infect Dis 2009;41:852–6. 10.3109/00365540903214264 [DOI] [PubMed] [Google Scholar]
- 8. Chen HL, Wu MS, Chang WH, et al. Abdominal tuberculosis in southeastern Taiwan: 20 years of experience. J Formos Med Assoc 2009;108:195–201. 10.1016/S0929-6646(09)60052-8 [DOI] [PubMed] [Google Scholar]
- 9. Yeh HF, Chiu TF, Chen JC, et al. Tuberculous peritonitis: analysis of 211 cases in Taiwan. Dig Liver Dis 2012;44:111–7. 10.1016/j.dld.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 10. Philippine Coalition Against Tuberculosis. Clinical Practice Guidelines for the Diagnosis, Treatment, Prevention and Control of Tuberculosis in Adult Filipinos: 2016 Update, 2016. [Google Scholar]