Abstract
A 55-day-old boy was transferred to our unit with intestinal obstruction and obstructive jaundice after two neonatal operations for duodenal atresia and intestinal malrotation. Abdominal ultrasound showed dilated intrahepatic and extrahepatic ducts with cut-off at the distal common bile duct (CBD). He underwent emergency laparotomy for adhesive intestinal obstruction with a contained abscess from mid-jejunal perforation. Biliary dissection was not attempted due to poor preoperative nutritional status. Tube cholecystostomy was created for biliary decompression. Postoperative magnetic resonance cholangiopancreatography showed dilated CBD with cut-off at the ampulla but did not demonstrate pancreaticobiliary maljunction (PBMJ). The diagnostic dilemma was whether our patient had congenital PBMJ or had developed biliary stricture from perioperative ischaemic scarring. He underwent definitive surgery at 7 months: excision of dilated CBD with Roux-en-Y hepaticojejeunal reconstruction, excisional tapering duodenoplasty and jejunostomy creation. Intraoperative finding was type I choledochal cyst and subsequently confirmed on histology. Postoperative recovery was uneventful and bilirubin levels normalised.
Keywords: congenital disorders, paediatric surgery
Background
Congenital duodenal atresia is often associated with anomalies in the cardiac, urological, skeletal and central nervous systems. Associated hepaticopancreatic and gastrointestinal tract anomalies include oesophageal atresia, anorectal malformation, intestinal malrotation, pancreatic anomalies and rarely biliary tract anomalies.1 Early and late complications of duodenal atresia surgery include megaduodenum, gastro-oesophageal reflux disease, peptic ulcer, anastomotic leak or stricture, adhesive intestinal obstruction, motility disorder and biliary drainage problems.2 We summarise the literature and discuss the diagnostic challenge in managing a patient with duodenal atresia and malrotation who developed postoperative obstructive jaundice with subsequent diagnosis of choledochal cyst and megaduodenum.
Case presentation
A baby boy conceived via in vitro fertilisation, was delivered at 35 weeks and 5 days of gestation by normal vaginal delivery at a private hospital weighing 2195 g. He developed bilious vomiting and underwent laparotomy, duodenoduodenostomy and Ladd’s procedure for duodenal atresia and intestinal malrotation. Postoperatively, the patient developed intestinal obstruction and underwent laparotomy, adhesiolysis and revision of anastomosis on day 10 of life. He achieved oral feeding and was discharged home at the age of 28 days. Two weeks later, he developed vomiting associated with abdominal distension and hematochezia. He was readmitted to the private hospital and treated for enterocolitis with intravenous antibiotics. Subsequently, he continued to have high nasogastric aspirates with dilated bowel loops seen on abdominal X-ray and was then transferred to our institution for further management of intestinal obstruction.
On transfer at the age of 55 days, he was severely malnourished, weighing 3.00 kg. He had microtia with bilateral hearing loss but no other dysmorphism. Initial investigations showed conjugated hyperbilirubinaemia (bilirubin 147 μmol/L, alanine aminotransferase 26 U/L, aspartate aminotransferase 42 U/L, alkaline phosphatase 246 U/L, gamma-glutamyl transferase 145 U/L). Ultrasound scan of the abdomen showed dilatation of the intrahepatic ducts and common bile duct (CBD), with abrupt calibre change in the distal CBD adjacent to the pancreatic head and non-visualisation of the distal CBD within the pancreatic head. The patient was given total parenteral nutrition, intravenous antibiotics and underwent a trial of conservative management of presumed adhesive intestinal obstruction. At laparotomy, he was found to have extensive abdominal adhesions with mid-jejunal obstruction associated with perforation and contained abscess. Limited resection and primary anastomosis of the jejunum was performed. The previous duodenoduodenal anastomosis was intact without stenosis; however, there was massive megaduodenum with redundant duodenum folding on itself causing partial extrinsic obstruction. A naso-jejunal feeding tube was manipulated past the megaduodenum transition to normal distal bowel to stent the lumen. Previous Ladd’s procedure had been performed with wide base of small mesentery. There was a firm liver and a distended gallbladder. No attempt was made at biliary tree dissection because of the poor preoperative nutritional status. Tube cholecystostomy was created to decompress the biliary system.
The patient was discharged home on nasojejunal feeding a month after the operation with a weight of 3.41 kg with plan for definitive surgery at a later date. In the interim, he required close outpatient follow-up to manage enteral fluid calculations for high biliary drainage losses from the tube cholecystostomy and catch-up weight gain. He required admission for intravenous antibiotic treatment for an episode of cholangitis at the age of 5 months.
Investigations
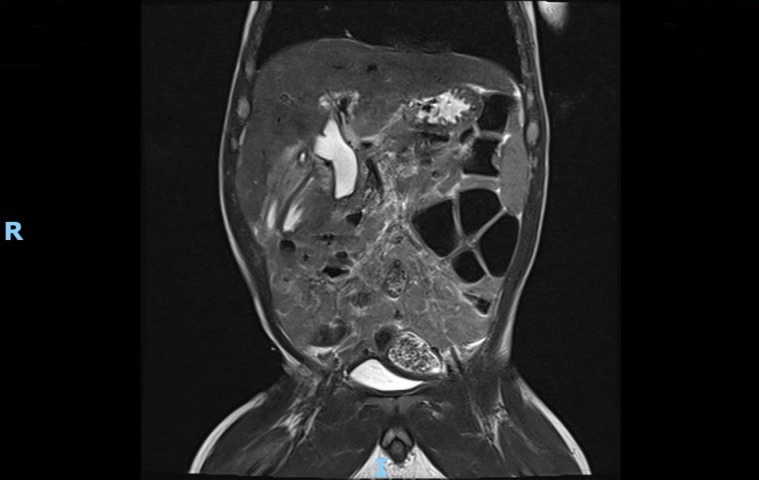
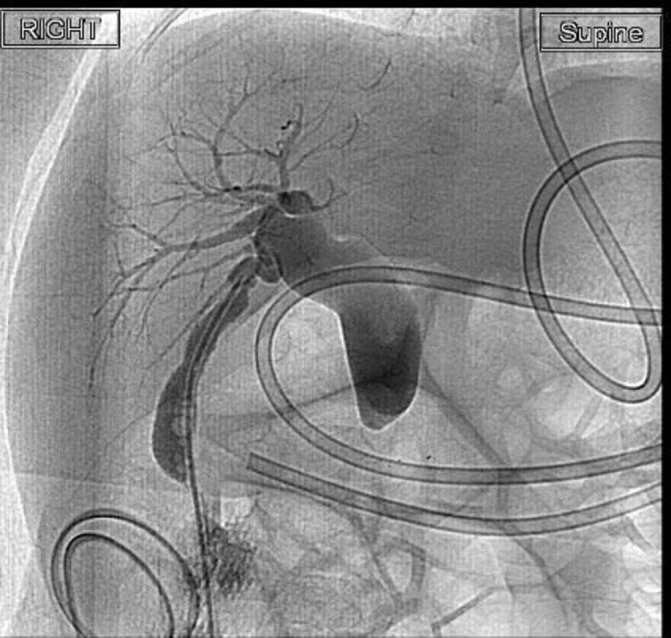
Tube cholecystogram that showed a dilated CBD with no passage of contrast into duodenum (figure 1). Magnetic resonance cholangiopancreatography showed dilated CBD up to 1.1 cm in diameter with abrupt narrowing of the distal CBD in the expected region of the ampulla but could not demonstrate a pancreaticobiliary maljunction (PBMJ). The pancreatic duct was not dilated (figure 2).
Figure 1.
Cholecystogram showing dilated CBD with no flow of contrast into duodenum. CBD, common bile duct.
Figure 2.
MRCP showing dilated CBD with abrupt cut-off in the expected region of the ampulla (coronal). CBD, common bile duct; MRCP, magnetic resonance cholangiopancreatography.
Treatment
He underwent definitive surgery at the age of 7 months: excision of the dilated CBD with Roux-en-Y hepaticojejeunal reconstruction, excisional tapering duodenoplasty and formal jejunostomy creation. Intraoperative findings were typical of fusiform type I choledochal cyst with insertion of the cystic duct just below the bifurcation into right and left hepatic ducts.
Outcome and follow-up
Postoperative recovery was uneventful and bilirubin levels normalised. Histology of the excised CBD was consistent with type I choledochal cyst.
Discussion
Embryological development of the biliary tract and the duodenum involve lumen formation through recanalisation and Boyden highlighted the possibility of combined developmental anomalies of duodenum and the pancreaticobiliary ductal system due to anatomical proximity.3 An early necropsy series by Reid described 9 neonatal patients with congenital biliary tract abnormalities out of a total of 167 patients with duodenal obstruction.4 In that series, there were five patients with distal biliary tract stenosis of which three had prior duodenal atresia operations, three patients with gallbladder agenesis and one patient with partial duplication of the CBD. Reid suggested that biliary obstruction resulted from luminal epithelial hyperplasia rather than failure to recanalise, since the patients had bile in the meconium and/or the gallbladder. Thus, he anticipated the possibility of choledochal cyst associated with duodenal atresia, although there were none in that series. Twenty years later, Bailey et al reported the first case of choledochal cyst operated in a patient 4 years after the initial duodenal atresia surgery.5 Another case report described a patient with choledochal cyst that developed 13 years after duodenojejunostomy which was treated by cyst excision, conversion to duodenoduodenostomy and hepaticojejunostomy reconstruction.1 Since then, nine cases of choledochal cyst associated with duodenal obstruction have been reported; the majority were associated with annular pancreas6–13 except for one with a normal pancreas.14 Most of these patients had an extrahepatic and/or extrapancreatic choledochal cyst, with two cases being intrapancreatic.
Choledochal cyst formation has been attributed to reflux of pancreatic enzymes into the biliary system due to congenital PBMJ, an anomaly in which the pancreatic and common bile duct fuse outside of the ampulla of Vater.15 Iwai et al speculated that the reason choledochal cysts were not recognised at the operation for duodenal atresia was because patients with pre-existing PBMJ initially had minimal CBD dilatation which subsequently grew more obvious with time.13 Shih et al highlighted the high prevalence of associated annular pancreas suggesting that embryological events around ventral pancreas rotation contributes to the risk of PBMJ and duodenal obstruction.12 Other authors have reported combinations of choledochal cyst with malrotation and anatomically proximal anomalies of annular pancreas16 or duodenojejunal atresia.17
The diagnostic concern for our patient was whether he had congenital PBMJ or had developed biliary stricture due to perioperative scarring and ischaemia. At the time of presentation with cholestatic jaundice, he had already undergone two operations in the region of the ampulla of Vater and did not have any prior hepatobiliary ultrasound imaging. A diagnosis of biliary stricture rather than choledochal cyst would allow the consideration of other management options like dilatation and stenting. There were also financial implications as medical insurance coverage for the biliary surgery would exclude congenital conditions. As preoperative imaging could not confirm the diagnosis, we opted for definitive surgery appropriate for choledochal cyst management.
At the age of 2 months, our patient is the youngest case report of choledochal cyst associated with duodenal atresia since prior paediatric patients ranged from 2 to 12 years of age.1 5–13 Hence, our case supports the assertion by previous authors3 12 13 that metachronous presentation of choledochal cyst and duodenal atresia represent delayed manifestation of a congenital PBMJ or distal biliary tract stenosis.
Megaduodenum is a known late complication after repair of duodenal obstruction and can occur up to 59 years postoperatively.18 Grosfeld and Rescorla reported two cases of megaduodenum which required tapering duodenoplasty to improve duodenal emptying.1 Escobar et al reported 4 cases of megaduodenum that required tapering duodenoplasty in the largest single institution series of 169 patients with duodenal obstruction.2 The cause of megaduodenum after duodenal atresia repair is unclear. Authors have suggested that it may result from chronic dysmotility of the duodenum that occurs after duodenal atresia repair19 since megaduodenum has been described resulting from chronic obstruction associated with malrotation.20 Operative management of megaduodenum, often tapering duodenoplasty, has the advantage of creating more effective coaptation of the duodenal wall to improve duodenal peristalsis and allowing earlier feeding but results in a relatively long suture line that predisposes to anastomotic leakage.21–28 It is difficult to predict which patients would benefit from duodenoplasty at the time of the initial surgery. Variable degree of proximal duodenal dilatation is always present in duodenal atresia and most patients have resolution of the duodenal dilatation after simple duodenoduodenostomy.2 29 Chen et al recommend routine tapering duodenoplasty when megaduodenum of >5 cm in diameter is present.21 We chose to perform elective tapering duodenoplasty at the same setting of the choledochal cyst surgery since our patient had already undergone prolonged nasojejunal stenting of the duodenoduodenal anastomosis with proximal nasogastric tube decompression without any improvement in the megaduodenum.
Learning points.
Duodenal atresia and/or malrotation may be associated with congenital biliary tract abnormalities.
Atypical presentations of duodenal atresia associated with malrotation or annular pancreas should raise the clinical suspicion of possible pancreaticobiliary maljunction.
Congenital biliary tract abnormalities may not be recognised during the initial surgery for duodenal atresia and may manifest later. Consider early evaluation of the biliary system to identify biliary tract abnormalities in atypical presentations of duodenal atresia.
Footnotes
Contributors: All the authors were involved in the operation and contributed to postoperative care. MFJG was involved in the write-up and preparation for submission. MHWM and YL were responsible for planning and editing the manuscript. CCPO was the lead consultant in charged of the patient’s care and was involved in obtaining patient consent and editing of the manuscript for submission. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Grosfeld JL, Rescorla FJ. Duodenal atresia and stenosis: reassessment of treatment and outcome based on antenatal diagnosis, pathologic variance, and long-term follow-up. World J Surg 1993;17:301–9. 10.1007/BF01658696 [DOI] [PubMed] [Google Scholar]
- 2. Escobar MA, Ladd AP, Grosfeld JL, et al. Duodenal atresia and stenosis: long-term follow-up over 30 years. J Pediatr Surg 2004;39:867–71. Discussion 867-871 10.1016/j.jpedsurg.2004.02.025 [DOI] [PubMed] [Google Scholar]
- 3. Boyden EA, Cope JG, Bill AH. Anatomy and embryology of congenital intrinsic obstruction of the duodenum. Am J Surg 1967;114:190–202. 10.1016/0002-9610(67)90372-8 [DOI] [PubMed] [Google Scholar]
- 4. Reid IS. Biliary tract abnormalities associated with duodenal atresia. Arch Dis Child 1973;48:952–7. 10.1136/adc.48.12.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey PV, Tracy TF, Connors RH, et al. Congenital duodenal obstruction: a 32-year review. J Pediatr Surg 1993;28:92–5. 10.1016/S0022-3468(05)80364-1 [DOI] [PubMed] [Google Scholar]
- 6. Komura J, Yano H, Tanaka Y, et al. A case of pancreaticobiliary maljunction associated with annular pancreas (in Japanese). In: Proceedings of 14th annual meeting of Japanese Study Group on Pancreaticobiliary Maljunction. 1991:82–3.
- 7. Okada K, Ohama K, Tsunezuka Y, et al. Two cases of congenital biliary dilatation associated with annular pancreas. J Jpn Soc Pediatr Surg 1993;4:302–8. [Google Scholar]
- 8. Nakamura T, Nagahara N, Kusafuka T, et al. Study of pathophysiology on congenital choledochal cyst—embryological consideration on choledochal cyst with annular pancreas (in Japanese). Annual report of 1994:135–8. [Google Scholar]
- 9. Komuro H, Makino S, Tahara K. Choledochal cyst associated with duodenal obstruction. J Pediatr Surg 2000;35:1259–62. 10.1053/jpsu.2000.8768 [DOI] [PubMed] [Google Scholar]
- 10. Sugimoto T, Yamagiwa I, Obata K, et al. Choledochal cyst and duodenal atresia: a rare combination of malformations. Pediatr Surg Int 2004;20:724–6. 10.1007/s00383-002-0783-6 [DOI] [PubMed] [Google Scholar]
- 11. Oowari M, Yonekura T, Kosumi T, et al. A case of pancreaticobiliary maljunction associated with annular pancreas (in Japanese). Jap J Pediatr Surg 2003;39:478. [Google Scholar]
- 12. Shih HS, Ko SF, Chaung JH. Is there an association between duodenal atresia and choledochal cyst? J Pediatr Gastroenterol Nutr 2005;40:378–81. 10.1097/01.MPG.0000148773.80213.03 [DOI] [PubMed] [Google Scholar]
- 13. Iwai A, Hamada Y, Takada K, et al. Choledochal cyst associated with duodenal atresia: case report and review of the literature. Pediatr Surg Int 2009;25:995–8. 10.1007/s00383-009-2462-3 [DOI] [PubMed] [Google Scholar]
- 14. Zoetsch S, Singer G, Sorantin E, et al. How to treat a neonate with duodenal atresia and intrapancreatic choledochocele causing persistent hyperbilirubinemia: A case report. Int J Surg Case Rep 2016;19:11–13. 10.1016/j.ijscr.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Todani T, Watanabe Y, Fujii T, et al. Anomalous arrangement of the pancreatobiliary ductal system in patients with a choledochal cyst. Am J Surg 1984;147:672–6. 10.1016/0002-9610(84)90139-9 [DOI] [PubMed] [Google Scholar]
- 16. Raman VS, Arora M, Khanna SK. Annular pancreas, type I choledochal cyst and malrotation in a low-birth weight newborn: A case report. J Indian Assoc Pediatr Surg 2015;20:155–6. 10.4103/0971-9261.154656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arbell D, Orkin B, Naveh Y, et al. Duodenojejunal atresia with absent dorsal mesentery, choledochal cyst, and malrotation in a premature newborn--a case report. J Pediatr Surg 2006;41:e11–3. 10.1016/j.jpedsurg.2006.02.032 [DOI] [PubMed] [Google Scholar]
- 18. Rueff J, Söllner O, Zuber M, et al. Megaduodenum in a 59-year-old man: a very late postoperative complication after duodenal atresia. BMJ Case Rep 2018;133:bcr-2017-221792 10.1136/bcr-2017-221792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalla Vecchia LK, Grosfeld JL, West KW, et al. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg 1998;133:490–6. Discussion 496-497 10.1001/archsurg.133.5.490 [DOI] [PubMed] [Google Scholar]
- 20. Russ A, Chin AC, Terry NE, et al. Presentation and management of late-onset duodenomegaly in a teenager with chronic obstruction from malrotation. J Pediatr Surg 2008;43:e21–4. 10.1016/j.jpedsurg.2008.03.070 [DOI] [PubMed] [Google Scholar]
- 21. Chen QJ, Gao ZG, Tou JF, et al. Congenital duodenal obstruction in neonates: a decade’s experience from one center. World J Pediatr 2014;10:238–44. 10.1007/s12519-014-0499-4 [DOI] [PubMed] [Google Scholar]
- 22. Adzick NS, Harrison MR, deLorimier AA. Tapering duodenoplasty for megaduodenum associated with duodenal atresia. J Pediatr Surg 1986;21:311–2. 10.1016/S0022-3468(86)80191-9 [DOI] [PubMed] [Google Scholar]
- 23. Alexander F, Difiore J, Stallion A. Triangular tapered duodenoplasty for the treatment of congenital duodenal obstruction. J Pediatr Surg 2002;37:862–4. 10.1053/jpsu.2002.32888 [DOI] [PubMed] [Google Scholar]
- 24. Dewan PA, Guiney EJ. Duodenoplasty in the management of duodenal atresia. Pediatr Surg Int 1990;5:253–4. 10.1007/BF00169664 [DOI] [Google Scholar]
- 25. Hutton KAR, Thomas DFM. Tapering duodenoplasty. Pediatr Surg Int 1988;3:132–4. [Google Scholar]
- 26. Kimura K, Perdzynski W, Soper RT. Elliptical seromuscular resection for tapering the proximal dilated bowel in duodenal or jejunal atresia. J Pediatr Surg 1996;31:1405–6. 10.1016/S0022-3468(96)90839-8 [DOI] [PubMed] [Google Scholar]
- 27. Sherman JO, Schulten M. Operative correction of duodenomegaly. J Pediatr Surg 1974;9:461–4. 10.1016/S0022-3468(74)80005-9 [DOI] [PubMed] [Google Scholar]
- 28. Young JS, Goco I, Pennell T. Duodenectomy and reimplantation of the ampulla of Vater for megaduodenum. Am Surg 1993;59:685–8. [PubMed] [Google Scholar]
- 29. Kimura K, Mukohara N, Nishijima E, et al. Diamond-shaped anastomosis for duodenal atresia: an experience with 44 patients over 15 years. J Pediatr Surg 1990;25:977–9. 10.1016/0022-3468(90)90241-Z [DOI] [PubMed] [Google Scholar]


