Abstract
Ectopic branches of the external carotid artery are rare but have critical diagnostic and therapeutic implications. We present a case involving a 70-year-old man who presented with recurrent left hemispheric strokes in the setting of a subocclusive left internal carotid stenosis. A left ascending pharyngeal artery with variant origin from the internal carotid artery helped maintain flow distal to the area of stenosis and allowed for safe and successful internal carotid artery stenting. Identification of this variant and recognition of the anastomotic network involving this connection were crucial to determine the safety of stenting. The patient had no further recurrent events and had sustained improvement on his 90-day follow-up.
Keywords: stroke, neuroimaging, neurosurgery, interventional radiology
Background
The cervical internal carotid artery (ICA) can usually be distinguished from the external carotid artery (ECA) by the lack of vascular branches immediately distal to the carotid bifurcation. Variants to this can cause difficulties in identifying the appropriate vasculature. This is particularly the case in steno-occlusive disease of the ICA, as the ECA, and to a lesser extent the vertebral artery, can provide collateral flow distal to the lesion. The ascending pharyngeal artery (APA) is one such variant. Often cited in cases of Le Fort I osteotomies, dural arteriovenous fistulas, tumours of the skull base, posterior fossa meningiomas and carotid endarterectomies, the APA has crucial vascular contributions.1–5 Given the APA’s anastomoses to other large vessels, it is important to be able to recognise its variations and clinical consequences.
Case presentation
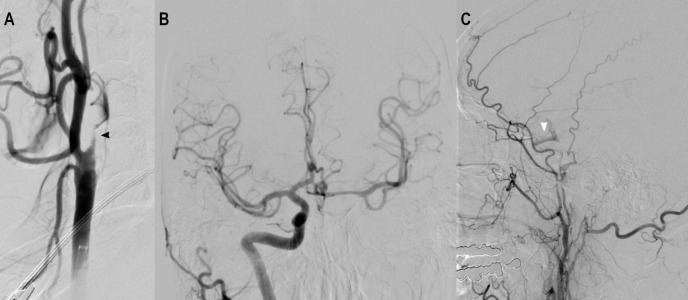
A 70-year-old man with history of transient ischaemic attacks presented with expressive aphasia and a right homonymous superior quadrantanopia on awakening. His National Institutes of Health Stroke Severity (NIHSS) score was 5. CT of the head revealed an old infarct in the left caudate and occipital lobe, with an Alberta Stroke Program Early CT Score (ASPECTS) of 10. His CT angiogram revealed a near-occlusive left cervical ICA atheromatous plaque (figure 1), and a fetal origin left posterior cerebral artery with a tandem P2 occlusion. Intravenous thrombolysis was not considered due to wake-up symptoms, but CT perfusion showed a core–penumbra mismatch with salvageable tissue encompassing the left thalamus and temporal lobe allowing for favourable intervention. The subsequent cerebral angiogram showed a subocclusive and flow-limiting left cervical ICA stenosis (figure 2A). The left hemisphere received excellent collateral flow from the right carotid circulation via the anterior communicating artery and from the left ECA via retrograde filling of the ophthalmic artery (figure 2B,C). Gentle manoeuvring of the guidewire was attempted but could not traverse the ICA lesion. Given the robust collaterals and low NIHSS score, the procedure was terminated. The patient was started on dual antiplatelet therapy and was discharged home with residual deficits of minor aphasia and partial vision loss (NIHSS score of 2).
Figure 1.
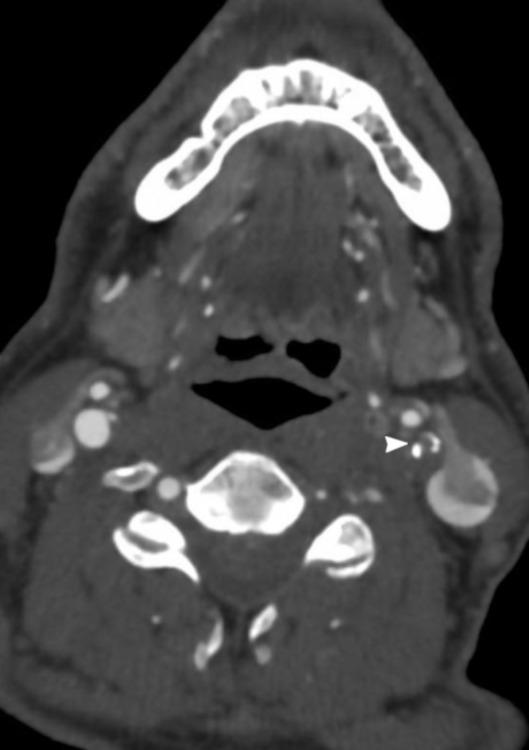
CT angiography showing subocclusive plaque of the left cervical internal carotid artery.
Figure 2.
(A) Angiogram of the left CCA showing a subocclusive left ICA stenosis. (B) Angiogram of the right ICA showing collateral flow into the left intracranial cerebral circulation through the anterior communicating artery. (C) Angiogram of the left external carotid artery showing retrograde flow through the ophthalmic artery to the left supraclinoid ICA. CCA, common carotid artery; ICA, internal carotid artery.
He returned 2 weeks later with worsening aphasia and a new haemodynamically dependent right hemiparesis (NIHSS score of 17). CT head (CTH) revealed new areas of acute infarction in the left insula and frontal lobe (ASPECTS of 8). CT angiogram (CTA) demonstrated lack of flow in the left petrous, cavernous and supraclinoid ICA with reconstitution at the level of the ICA terminus and a left middle cerebral artery distal (M3) branch occlusion.
Investigations
Angiogram of the left common carotid artery revealed a completely occluded cervical ICA (figure 3). Intracranially, flow was still maintained in the left hemisphere via the anterior communicating artery and retrograde through the left ophthalmic artery.
Figure 3.
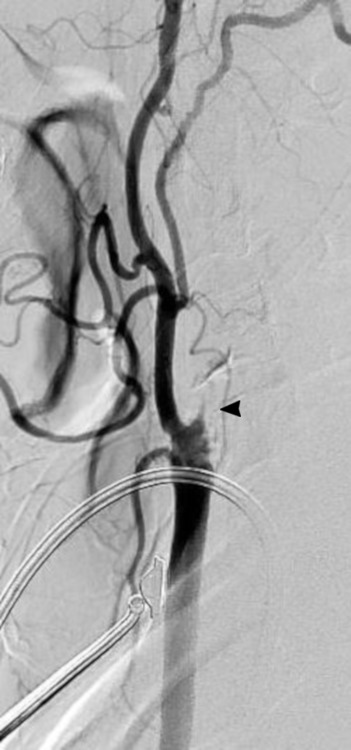
Angiogram of the left CCA on the patient’s second admission showing complete occlusion of the left internal carotid artery (black arrowhead).
During the right vertebral artery angiogram, there was anterograde flow in the left cervical ICA distal to the site of occlusion. This was supplied by a vessel identified to be the left APA, which had an aberrant origin off the left ICA distal to the occluded segment (figure 4A,B). The left APA was filling retrograde via collateral flow across the odontoid arcade from the right vertebral artery. Notably, there was no thrombus formation in the visualised segment of the distal left cervical ICA, so carotid stenting was deemed to be feasible.
Figure 4.
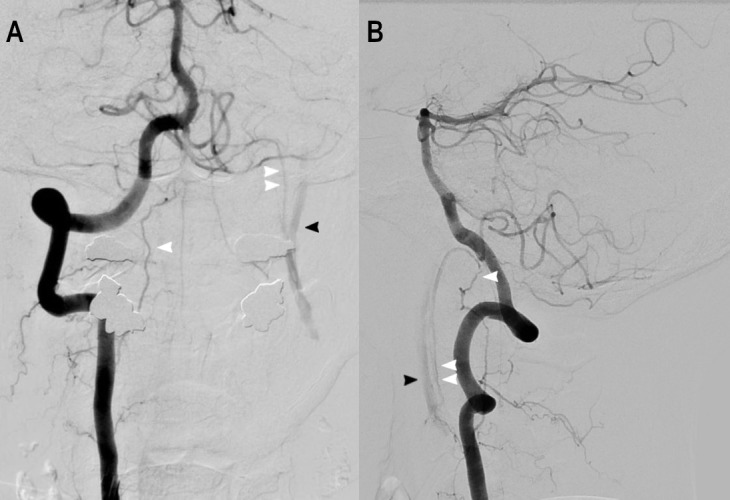
(A) Angiogram of the right vertebral artery revealing anterograde flow in the left cervical ICA (black arrowhead) via the odontoid arcade (single white arrowhead) and retrograde flow in the aberrant origin left ascending pharyngeal artery (double white arrowhead). (B) Lateral view of the right vertebral artery angiogram with simultaneous filling of the left cervical ICA via the network described above. ICA, internal carotid artery.
Treatment
The left common carotid artery was accessed with a FlowGate2 Balloon Guide Catheter (Stryker, Fremont, California), and the balloon was inflated to achieve flow arrest for embolic protection. A Synchro 2 standard microguidewire (Stryker) crossed the lesion and a SpiderFX distal embolic protection device (Medtronic, Minneapolis, Minnesota) was advanced over the microguidewire and deployed in the distal left cervical ICA. Predilation angioplasty was performed with a 3×20 mm Maverick balloon (Boston Scientific, Fremont, California). A 8×36 mm Carotid Wallstent (Boston Scientific) was successfully deployed. Angiographic runs from the left common carotid artery demonstrated restoration of full anterograde flow through the left ICA (figure 5).
Figure 5.
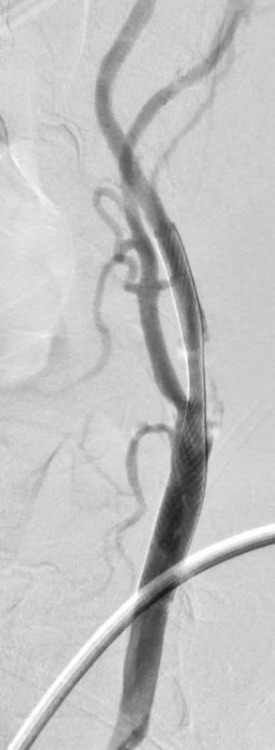
Angiogram of the left CCA after left internal carotid artery stent placement.
Outcome and follow-up
The patient was continued on dual antiplatelet therapy and discharged to rehabilitation with improved residual deficits of aphasia and right homonymous superior quadrantanopia but no motor deficits (NIHSS score of 4). He was seen at 90-day follow-up with mild but improving residual aphasia (NIHSS score of 3).
Discussion
The APA originates off the ECA from the posterior wall in 80% of cases.1 Variations include origination off the occipital artery, or rarely from a common trunk involving the lingual and facial artery.2 Cadaveric series demonstrated that the APA can also originate off the ICA in 0%–8% of cases.2 3 The APA typically ascends through the foramen magnum and divides into two distinct branches termed the pharyngeal and neuromeningeal trunks (NMT). For purposes of the report, only the NMT will be discussed.
The NMT is composed of two divisions termed the jugular branch and the hypoglossal branch. The jugular branch passes through the jugular foramen and supplies cranial nerves IX, X and XI, as well as the meninges of the internal auditory canal and dura of the inferior petrosal sinus.1 2 4 The hypoglossal branch travels through the hypoglossal canal and innervates the hypoglossal nerve. A subsequent posterior descending branch can anastomose with the arteries of the odontoid arch system (also termed odontoid arch, odontoid apical arcade).6 This arcade surrounds the odontoid process (dens) of C2 and has connections with the vertebral artery, occipital artery, and sometimes even the ICA.6–8
In our patient, brainstem vasculature involvement would typically be considered unlikely due to a lack of affected cranial nerves. However, complete evaluation of the cerebral angiogram helped to identify the causative lesion despite the presenting symptoms. The presence of an APA arising off the left ICA ensured safe intervention. Anterograde flow in the cervical ICA was accomplished through a complex anastomotic network involving the right vertebral artery, the odontoid arcade and the variant left APA. This was augmented by the increased demand after occlusion of the left cervical ICA. Visualisation of anterograde flow past the occlusion ensured absence of thrombi and provided reassurance that the necessary distal embolic protection device could be deployed. While the left APA may have also marginally contributed to the left hemispheric perfusion, it is important to note that perfusion was predominantly maintained via the collateral supply from the anterior communicating artery and the ophthalmic artery. This highlights the significance of neurovascular collateralisation and the importance of recognising this vast anastomotic network.
Patient’s perspective.
From the perspective of the wife: He’s improved so much since the strokes. It’s really remarkable how much he’s progressed. Physically, he’s perfect and even his comprehension is so that I can barely tell it’s been affected by the stroke. You can have a conversation and he’s understanding everything you are saying. That changed dramatically because he didn’t understand a lot at the beginning. Speech, unfortunately, I can’t put a percentage on because it’s still very affected. In the beginning, he could hardly say a few words. Now, he’s speaking in short 2–3 sentences but his naming is still very much affected. He does speech therapy 3x/week and uses a tablet to help him name and say things if he has trouble. But he is driving now; we’ve been living in this neighborhood for 47 years so he knows- drives locally and coordination is great so there’s no trouble. He goes to the grocery and hardware stores; he even told me when I made a wrong turn. Considering all that he went through…considering he was standing on his head before all this…I would say the progress has been remarkable.
Learning points.
Acute stroke cases should have thorough vessel imaging analysis to identify anomalies that can affect clinical symptoms.
Variant origins of external carotid artery (ECA) branches are critical to identify in both surgical and endovascular cases.
Anastomoses through ECA branches can provide collateralisation to an occluded or severely stenotic internal carotid artery (ICA).
The ascending pharyngeal artery should be considered for all skull base vascular and soft tissue pathologies with lower brainstem cranial nerve involvement.
Extracranial vertebral artery anastomosis with the ICA is not a common pathway of collateralisation in ICA stenosis, except in rare cases of variant origin vessels.
Footnotes
Contributors: BDK contributed to the design, writing, revision and submission of this manuscript. TJO and JTF contributed to data acquisition, analysis and interpretation, and revision of the manuscript. HS contributed to all of the above stated, including data acquisition, interpretation, and manuscript writing and revision. All authors have approved the final submission of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Hacein-Bey L, Daniels DL, Ulmer JL, et al. The ascending pharyngeal artery: branches, anastomoses, and clinical significance. AJNR Am J Neuroradiol 2002;23:1246–56. [PMC free article] [PubMed] [Google Scholar]
- 2. Cavalcanti DD, Reis CV, Hanel R, et al. The ascending pharyngeal artery and its relevance for neurosurgical and endovascular procedures. Neurosurgery 2009;65:ons114–20. 10.1227/01.NEU.0000339172.78949.5B [DOI] [PubMed] [Google Scholar]
- 3. De Freitas S, Malas MB. Ectopic origin of the ascending pharyngeal artery: implications for carotid surgery. Surg Radiol Anat 2018;40:1181–3. 10.1007/s00276-018-2088-z [DOI] [PubMed] [Google Scholar]
- 4. Cappabianca S, Somma F, Negro A, et al. Extracranial internal carotid artery: anatomical variations in asymptomatic patients. Surg Radiol Anat 2016;38:893–902. 10.1007/s00276-016-1652-7 [DOI] [PubMed] [Google Scholar]
- 5. Cortés-Franco S, Muñoz AL, Franco TC, et al. Anomalous ascending pharyngeal artery arising from the internal carotid artery: report of three cases. Ann Vasc Surg 2013;27:240.e1–240.e4. 10.1016/j.avsg.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 6. Schiff DC, Parke WW. The arterial supply of the odontoid process. J Bone Joint Surg Am 1973;55:1450–6. 10.2106/00004623-197355070-00012 [DOI] [PubMed] [Google Scholar]
- 7. Akobo S, Rizk E, Loukas M, et al. The odontoid process: a comprehensive review of its anatomy, embryology, and variations. Childs Nerv Syst 2015;31:2025–34. 10.1007/s00381-015-2866-4 [DOI] [PubMed] [Google Scholar]
- 8. Patel MC, Higgins JN, Kirkpatrick PJ. Endarterectomy of an Occluded ICA: short segment occlusion with distal patency maintained by an aberrant ascending pharyngeal artery. Interv Neuroradiol 1999;5:157–9. 10.1177/159101999900500208 [DOI] [PMC free article] [PubMed] [Google Scholar]