Abstract
Marchiafava-Bignami disease (MBD) is a rare complication of chronic alcoholism; however, MBD in a non-alcoholic diabetic patient has rarely been reported. The aetiology or pathophysiology of MBD is still unknown. A 50-year-old man with a history of untreated diabetes mellitus underwent on-pump beating coronary artery bypass graft surgery (CABG) surgery for three-vessel and left main coronary disease. 3 days after the surgery, he developed a fever over 40°C and entered a coma state. MRI revealed multiple lesions, including in the corpus callosum, globus pallidus, brain stem and upper cervical spinal cord, which suggested MBD. The patient did not respond to thiamine therapy, but partly responded to steroid therapy. He ultimately died of respiratory failure. The autopsy revealed MBD and haemophagocytic lymphohistiocytosis. It is rare, but systemic inflammatory response syndrome induced by on-pump beating CABG could develop these complication.
Keywords: neurology, cardiothoracic surgery
Background
Marchiafava-Bignami disease (MBD) is a rare complication of chronic alcoholism. MBD was first reported by Carducci in 1898 and named after the two Italian pathologists, Marchiafava and Bignami, who described in detail three patients who had been drinking cheap wine in 1903.1 MBD is common in 0.01% of patients with alcohol-related diseases.2 Over 300 cases have been reported in the literature, and most of these patients suffer from chronic alcoholism.
Some cases develop ‘non-alcoholic’, or diabetes mellitus (DM)-associated, MBD.3 To date, the pathogenesis of MBD is still unclear. As is described later, our case presented with the rare situation of developing in the postoperative period of coronary artery bypass graft surgery (CABG). CABG surgery is a well-known and popular procedure, and the postoperative complications of CABG are also well known. Complications of the central nervous system (CNS) are not rare, and the incidence of encephalopathy is 6.9%.4 Here, we describe a rare case of postoperative MBD early after on-pump beating CABG surgery.
Case presentation
A 50-year-old man with a history of untreated DM and history of alcohol abuse presented to our hospital and was diagnosed with congestive heart failure (CHF) due to an old myocardial infarction. Cardiac catheterisation and coronary angiography revealed three-vessel and left main coronary artery disease with a stenosis rate >90%–99%. Elective CABG was scheduled. Insulin therapy for DM and appropriate treatments for CHF, including nutritional therapy, were initiated.
A month and a half later, he underwent beating-heart CABG with intra-aortic balloon pumping. He developed a fever (>40°C) and lost consciousness on postoperative day (POD) 3. The patient’s fever, respiratory rate (>40 breathes/min), and heart rate (>100 beats/min) fulfilled the criteria for systemic inflammatory response syndrome (SIRS). Intraoperative or postoperative infection was suspected at first; however, apparent causative agents were not detected.On POD 6, a chest X-ray revealed possible signs of acute respiratory distress syndrome (ARDS). A head CT scan, performed to investigate the reason for the patient’s unconsciousness, revealed low density areas of the corpus callosum and bilateral globus pallidus.
Investigations
Patient’s general information on admission was as follows: height: 171 cm and weight: 62.2 kg (body mass index: 21). Level of consciousness was Glasgow Coma Scale 3 points on POD 3. Head MRI on POD 10 revealed multiple lesions, including in the corpus callosum (CC), which was possibly the cause of the patient’s unconsciousness (figure 1). The patient’s blood test results are presented; white cell count 5.32×109/L (reference values: 3.30–8.11×109/L), haemoglobin 70 g/L(reference values: 135–171/L), platelets 4.0×109/L (reference values: 14.0–32.4×109/L), fibrinogen 126 mg/dL (reference values: 170–410 mg/dL) on POD 2. Aspartate transaminase (AST) 465 U/L (reference values: 13–33 U/L), alanine aminotransferase (ALT) 280 U/L (reference values: 8–42 U/L), lactate dehydrogenase (LDH) 990 U/L (reference values: 119–229 U/L), C-reactive protein (CRP) 25.86 mg/dL (reference values: 0–0.3 mg/dL) on POD 10. Antinuclear antibody, syphilis, hepatitis B/C virus, and HIV tests and a PCR test for Epstein-Barr virus infection were all negative. Cerebrospinal fluid analysis on POD 10 showed the following: cell count <1/mm3; protein, 38 mg/dL; glucose, 93 mg/dL (CSF-blood glucose ratio, 0.49); myelin basic protein (MBP), 4120 pg/mL (reference values: <102 pg/mL); and negative findings for bacterium and malignancy. Although, intermittent or not simultaneous, continued fever over 40°C, clinical history of bicytopenia, significantly elevated AST, ALT and LDH, low natural killer cell activity, hyperferritinoaemia (3006.6 ng/mL) (reference values: 21.8–274.6 ng/mL), hypofibrinogenaemia and even ARDS potentially suggested haemophagocytic lymphohistiocytosis (HLH).5 We also performed a bone mallow aspiration on POD 65, although it was after steroid therapy. Bone marrow cellularity was low and immunostaining revealed large amounts of CD68 +cells or histiocytes, many of which contained haemosiderin, but only a few haemophagocytosis were detected.
Figure 1.
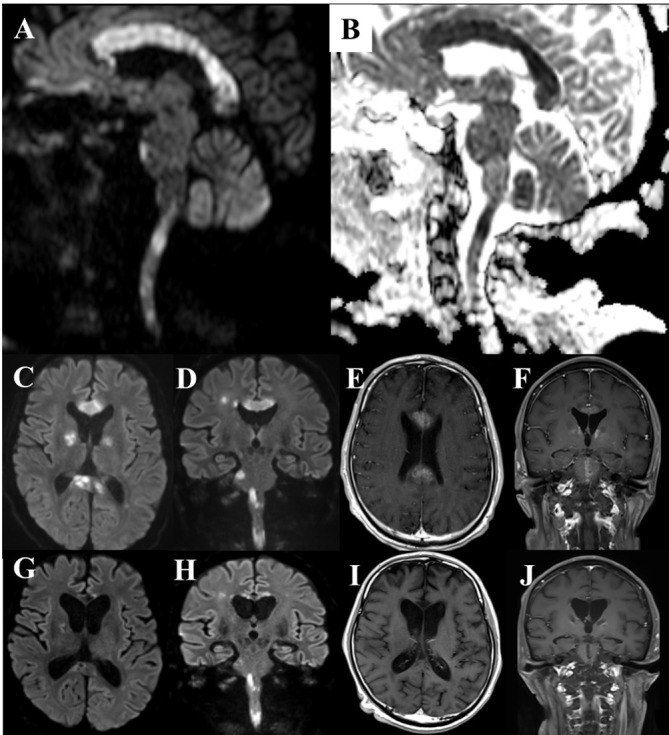
(A–B) DWI MRI on POD 10 showed an increased signal in all the areas of the corpus callosum (CC), globus pallidus, forefront ventral part of the pons, and the upper cervical spinal cord. ADC map showed cytotoxic oedema in these lesions. (C–D) Axial and coronal DWI on POD 30. (E–F) Gadolinium-enhanced T1-weighted image (Gd-T1WI) on POD 30 showed diffuse enhancement in the CC, globus pallidus and ventral part of the pons. Imaging in the pons differed from that of the central pontine myelinolysis (no imaging). The lesions did not seem to respond to thiamin therapy on imaging. (G–H) DWI on POD 78. The CC became thin and the previously seen increased signal tended to disappear. (I–J) Gd-T1WI on POD 78. Enhancement in the CC and brainstem disappeared after three sets of steroid pulse therapy. ADC, apparent diffusion coefficient; Gd-T1WI, Gadolinium-enhanced T1-weighted image; POD, postoperative day; DWI, diffusion-weighted.
Differential diagnosis
The entire callosal lesions on imaging suggested MBD first of all, MBD mimics are known as follows: ischaemic stroke, CNS lupus, CNS lymphoma, posterior reversible encephalopathysyndrome, multiple sclerosis, progressive multifocal leukoencephalopathy in HIV, acute disseminated encephalomyelitis, clinically mild encephalitis/encephalopathy with a reversible splenial lesion.
Treatment
Thiamine was administered for possible MBD beginning on POD 8, but the patient’s consciousness level did not improve. Steroid pulse therapy (methylprednisolone, 1000 mg/day) was administered for 5 days starting on POD 38. After the therapy, enhancement in the CC and brainstem disappeared and MBP was improved from 14 900 pg/mL on POD 24 to 900 pg/mL on POD 87. The patient’s consciousness level slightly improved, but he remained akinetic mutism, yet had sustained eye-opening, slow pursuit and escape response to pain stimulation. Two further rounds of steroid pulse therapy were administered. Cyclophosphamide was also administered, but it did not appear to yield any improvement.
Outcome and follow-up
Ultimately, the patient passed away on POD 99 due to respiratory failure. An autopsy revealed multiple lesions in the CNS were pathohistologically accumulated by macrophage, partly with cavity formation and the lesions in the CNS were from necrosis rather than demyelination (figure 2). Pathohistological findings in the CNS were consistent with MBD and did not support the other possibilities.6 The lesions in the cerebral peduncle, brain stem, spinal cord present with similar findings and all of the lesions were confined to white matter. Aside from the CNS, haemophagocytosis was detected in lymph nodes around the pancreas and oesophagus and a part of the lung, but was negative in the liver and spleen, although a lot of macrophages had accumulated.
Figure 2.
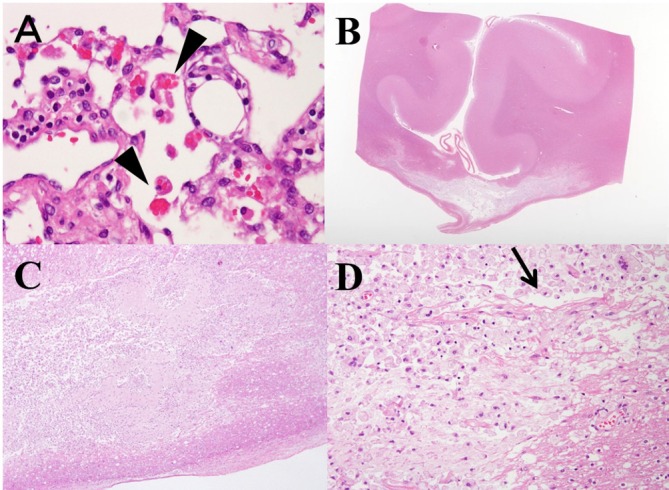
(A) Haemophagocytosis in the peripancreatic lymph node (arrow head). Haemophagocytosis is detected in the perioesophagus, peritrachial lymph node and right upper lung. (B) Histopathology of the corpus callosum (CC) through the magnifying glass revealed demyelination or necrosis in central part of the CC, while the dorsal and ventral layer was spared, which reflect characteristic ‘sandwich sign’ in MRI (H&E staining). (C) Necrotic lesions with cystic cavitations are seen (H&E staining, ×20 magnification). (D) At the central part of the lesion, most of the lesions are occupied by necrotic tissues with accumulated macrophages. There are no other inflammatory cells, like lymphocytes. Arrow head indicates cystic cavitation, which is sometimes identified with MBD patients. Myelin vacuolation seen at the lower right side suggests demyelination and few axons remains. (H&E staining, ×200 magnification). The lesions do not include old and new mixed and onset of the disease was suggested to be around the same time.
Discussion
The entire callosal lesion on imaging possibly suggested MBD. However, this case was atypical as MBD because the CNS lesions had spread beyond the corpus callosum. Nevertheless, characteristic callosal lesions were a key to understanding the patient’s condition.
The diagnosis of MBD mainly rests on the evidence of abnormality central demyelination or necrosis of the corpus callosum on images rather than on the variable clinical features.7 As a result, abnormality in imaging in the corpus callosum possibly contains MBD mimics. A past report suggests that abnormality of all parts of the corpus callosum and lesions that are enhanced by gadolinium are relatively specific to MBD.7 Our case fulfilled both of these conditions. In addition, histological examination of the CNS was consistent with MBD. Speaking about the spreading of lesions, Bellido et al reported unusual MRI findings in a patient who had onset of MBD in the early postoperative period of acute aortic dissection.8 They reported that the middle cerebellar peduncles and white matter tracts were included in MBD. Lesion in the corpus callosum is typical for MBD, but MBD could include a wider range of CNS areas.
On the other hand, the background of our case is somewhat atypical for MBD. It is true that our patient had a history of alcohol abuse, but he ceased drinking for several months before operation and was steadily in the hospital and received dietary management for one and a half months before the operation, so he was probably not malnourished or thiamine deficient. Summarising the above, our case presented with MBD on imaging and histological examination, but clinical background and response to treatment was somewhat atypical. Deficiency of vitamin B1 is known for the potential risk of MBD. It is true that many cases of MBD patients were improved by administration of vitamin B1, but detailed mechanism in MBD is still not clear. One possibility for postoperative MBD is, however hypothesis, is that reduction of the serum vitamin B1 during could occur intraoperating period, especially on-pump surgery.
It is also important to note the continued hyperinflammatory state of our patient. Our case meets the criteria for SIRS in the early stage of the postoperative period. Cardiac surgery, particularly on-pump, causes SIRS with a marked release of cytokines and SIRS after cardiac surgery is associated with postoperative multiorgan system dysfunction and major complications.9 HLH is sometimes less likely to be noticed, but should be suspected in cases of unexplained sudden onset SIRS.10 Our case fulfilled 6/8 of the HLH-2004 criteria during the duration of our patient’s stay. Considering the pathophysiology of our case, complication of HLH could be no coincidence. Inflammatory cytokine such as TNF-alpha causes elevation of extracellular glutamate and excitotoxic neuronal death.11 Moreover, white matters including the corpus callosum have high levels of glutamate and high activities of enzymes.12 These facts could be involved in the vulnerability of the corpus callosum to inflammation.13 Therefore, it is our hypothesis that hypercytokinaemia could have some involvement in the pathogenesis of MBD in our case.
Regrettably, our case has some limitations. First, although vitamin B1 deficiency is reported to be associated with MBD, serum vitamin B1 was not measured before the initiation of vitamin preparations. Second, based on imaging, we already assumed the patient’s unconsciousness was due to MBD on POD 10; however, we were hesitant to initiate steroid therapy because of the risk of potential infection, and it took a long time to notice the possibility of HLH. Moreover, the diagnostic difficulty for HLH was bycytopenia immediately after operation because anaemia and thrombocytopenia could be due to complications of cardiac surgery itself.
In conclusion, although we cannot rule out the possible influence of thiamine deficiency or alcoholism in this case, we suspect that hypercytokinaemia contributed to MBD in our patient. A clinical history of alcoholism or DM can increase the risk of MBD, but hypercytokinaemia could also do so.
Learning points.
Marchiafava-Bignami disease (MBD) is associated with malnutrition, vitamin B1 deficiency, or diabetes mellitus.
Lesion in the corpus callosum is typical for MBD, but MBD could include a wider range of central nervous system areas.
Postoperative systemic inflammatory response syndrome could be associated with Marhiafava-Bignami disease, especially on-pump beating coronary artery bypass graft surgery.
Footnotes
Contributors: NM performed an operation. KT took care of the patient, evaluated data and drafted the manuscript. KS and TK performed an autopsy and were in charge in pathological assessment.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Marchiafava E, Bignami A. Sopra un alterazione del corpo calloso osservata in soggetti alcoolisti. Rivistadi Patologia Nervosa e Mentale 1903;8:544–9. [Google Scholar]
- 2. Celik Y, Temizoz O, Genchellac H, et al. a Non-alcoholic patient with acute marchiafava-bignami disease associated with gynecologic malignancy: Paraneoplastic marchiafava-bignami disease? Clin Neurol Neurosurg 2007;109:505–8. 10.1016/j.clineuro.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 3. Suzuki Y, Oishi M, Ogawa K, et al. A patient with Marchiafava-Bignami disease as a complication of diabetes mellitus treated effectively with corticosteroid. J Clin Neurosci 2012;19:761–2. 10.1016/j.jocn.2011.07.040 [DOI] [PubMed] [Google Scholar]
- 4. McKhann GM, Grega MA, Borowicz LM, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol 2002;59:1422–8. [DOI] [PubMed] [Google Scholar]
- 5. Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: A case report. Medicine 2017;96:e9198 10.1097/MD.0000000000009198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuhlmann T, Lassmann H, Brück W. Diagnosis of inflammatory demyelination in biopsy specimens: a practical approach. Acta Neuropathol 2008;115:275–87. 10.1007/s00401-007-0320-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hillbom M, Saloheimo P, Fujioka S, et al. Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry 2014;85:168–73. 10.1136/jnnp-2013-305979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellido S, Navas I, Aranda MA, et al. Unusual MRI findings in a case of Marchiafava Bignami disease. Neurology 2012;78:1537 10.1212/WNL.0b013e3182553ced [DOI] [PubMed] [Google Scholar]
- 9. Baumann A, Buchwald D, Annecke T, et al. RECCAS - REmoval of Cytokines during CArdiac Surgery: study protocol for a randomised controlled trial. Trials 2016;17:137 10.1186/s13063-016-1265-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol 2013;139:713–27. 10.1309/AJCP4ZDKJ4ICOUAT [DOI] [PubMed] [Google Scholar]
- 11. Leonoudakis D, Braithwaite SP, Beattie MS, et al. TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol 2004;1:263–73. 10.1017/S1740925X05000608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassel B, Boldingh KA, Narvesen C, et al. Glutamate transport, glutamine synthetase and phosphate-activated glutaminase in rat CNS white matter. A quantitative study. J Neurochem 2003;87:230–7. 10.1046/j.1471-4159.2003.01984.x [DOI] [PubMed] [Google Scholar]
- 13. Starkey J, Kobayashi N, Numaguchi Y, et al. Cytotoxic lesions of the corpus callosum that show restricted diffusion: Mechanisms, causes, and manifestations. Radiographics 2017;37:562–76. 10.1148/rg.2017160085 [DOI] [PubMed] [Google Scholar]


