Abstract
Takotsubo cardiomyopathy (TC), otherwise known as stress cardiomyopathy, is characterised by acute, transient left ventricular systolic dysfunction with apical ballooning in the absence of obstructive epicardial coronary stenosis. The presentation of TC mimics that of acute myocardial infarction. More recently there has been a shift towards thinking of TC as a ‘microvascular acute coronary syndrome’. Our case is of an 82-year-old woman who presented with TC mimicking acute anterior ST elevation myocardial infarction in the context of sepsis. Slow flow noted in the left anterior descending artery prompted us to perform coronary physiology. Her fractional flow reserve was 0.91, with an index of myocardial resistance of 117 and a coronary flow reserve of 1.6. In combination these results are indicative of microvascular coronary dysfunction in the absence of significant epicardial stenosis.
Keywords: heart failure, ischaemic heart disease
Background
Takotsubo cardiomyopathy (TC), otherwise known as stress cardiomyopathy, is characterised by acute, transient left ventricular (LV) systolic dysfunction with apical ballooning in the absence of obstructive epicardial coronary stenosis.1 The name derives from the pathognomonic shape of the ballooned LV on ventriculogram resembling a Japanese octopus trap.2 Colloquially it is known as ‘broken heart syndrome’ due to its association with acute physical or emotional stress. TC is most prevalent in postmenopausal women, with this group accounting for up to 90% of cases.3
The presentation of TC mimics that of acute myocardial infarction, often presenting with chest pain, cardiac enzyme elevation and ischaemic ECG changes (ranging from mild T wave inversion to widespread ST elevation). It accounts for approximately 2% of patients undergoing emergency coronary angiography for acute coronary syndrome.4
The pathophysiology of TC is not yet fully understood. The association with emotional and physical stress naturally led investigators towards investigating the role of circulating catecholamines. Patients with TC have increased concentrations of plasma catecholamines that are counterintuitively even higher than those in patients suffering from ST elevation myocardial infarction (STEMI). These levels remain markedly elevated for days after symptom onset.5 There is ongoing debate regarding the mechanism of how this translates into acute LV dysfunction. One theory suggests direct toxicity of endogenous catecholamine causing myocardial necrosis similar to that observed in acute intracranial haemorrhage.6 Others suggest that the rapid stimulation of adrenoceptors enhances cardiac inotropy and chronotropy with a secondary oxygen supply–demand imbalance with resultant ischaemia.7
More recently there has been a shift towards thinking of TC as a ‘microvascular acute coronary syndrome’.8 Patients have angiographically slow coronary flow in the absence of epicardial coronary artery stenosis.9 The cause of this slow flow has been revealed to be acute microvascular impairment. We carried out a literature review using PubMed with the keywords ‘takotsubo’, ‘stress cardiomyopathy’, ‘microvascular’, ‘IMR’ and ‘CFR’. The identified case reports and papers are summarised in table 1.
Table 1.
Summary of the available published literature suggesting microvascular dysfunction in Takotsubo cardiomyopathy
| Authors | Journal | Year | Evidence of microvascular dysfunction |
| Kurisu et al 24 | JACC (Journal of the American College of Cardiology) | 2003 | Abnormal TIMI frame counts (TFC) in all major coronary vessels in 28 patients. |
| Bybee et al 25 | AJC (American Journal of Cardiology) | 2004 | Abnormal TFC in all major coronary vessels in 16 patients. |
| Fazio et al 26 | J Clin Monit Comput (Journal of clinical monitoring and computing) | 2010 | Abnormal TFC in all major coronary vessels in 23 of 24 patients. |
| Daniels and Fearon27 | CCI (Catheterization and Cardiovascular Interventions) | 2011 | Elevation of IMR reported in one case report. |
| Layland et al 28 | CRM (Cardiovascular revascularization medicine) | 2012 | Reversible elevation of IMR in one case report. |
| Khalid et al 9 | Int J Cardiol (International Journal of Cardiology) | 2015 | Corrected TFC in all major coronary vessels in 16 patients. |
| Warisawa et al 29 | J Circ (Circulation Journal) | 2016 | Reversible elevation of IMR in one case report. |
| Rivero et al 12 | JAMA Cardiology(Journal of the American Medical Association :Cardiology) | 2017 | Prospective demonstration of time-related improvement of IMR in 15 patients. |
| Loffi et al 30 | BioMed Res Int (Biomed research international) | 2018 | Retrospective analysis of TFC, blush grade and Quantitative myocardial Blush score (QuBE) score in 27 patients. |
IMR, index of myocardial resistance.
This is proposed to relate to primary microvascular coronary spasm mediated by the aforementioned increased concentrations of circulating catecholamines and increased levels of the potent vasoconstrictor endothelin-1 (which preferentially constricts the microcirculation).10 11 Intravenous adenosine has been shown to transiently improve myocardial perfusion, wall motion score index and LV ejection fraction in TC but not myocardial infarction, suggesting that microvascular dysfunction plays a major role in the pathogenesis. Microvascular function has been shown to gradually improve with time. This correlates with improving LV function, and both have been shown to normalise at 1-month follow-up.12 13
Additionally, Giusca et al 14 have published a case report of reversible epicardial coronary vasospasm in TC. Although epicardial vasospasm in TC is not commonly reported, this case may suggest the possibility of a spectrum of vasospastic responses, extending from microvascular to epicardial vasospasm in extreme cases.
A recent study has also highlighted the role of inflammation in the pathogenesis of TC.15 It is currently uncertain whether this inflammation is a primary or secondary process.
Case presentation
An 82-year-old woman was referred to her local district general hospital with 3 weeks of worsening left leg cellulitis and confusion. She had a background of hypertension, paroxysmal atrial fibrillation, corrected hypothyroidism, post-traumatic epilepsy, obesity and chronic bilateral lymphoedema with disseminated secondary eczema. She was clinically septic on arrival—febrile, tachycardic and hypotensive. She was treated with intravenous antibiotics. She was also noted to have an acute kidney injury and delirium. She responded well to initial treatment and avoided the need for inotropic support.
Over the coming week she had physical and biochemical improvement both in her cellulitis and acute kidney injury. Her delirium was slower to resolve and she remained intermittently confused. She was therefore transferred offsite to another district general hospital for a period of convalescence and rehabilitation prior to discharge. She was notably distressed and confused in the context of ongoing delirium and change of treatment team/surroundings.
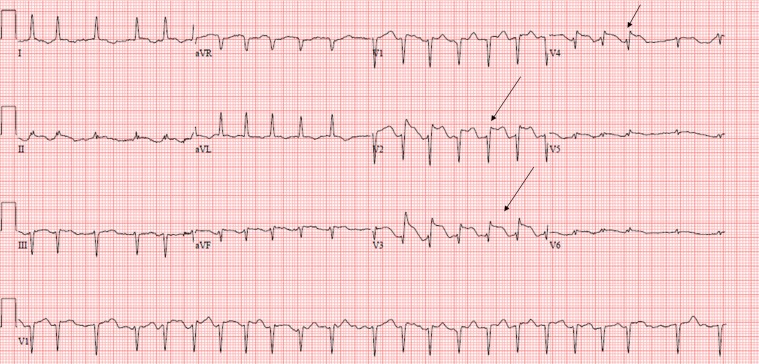
While in the rehabilitation ward, she developed acute severe chest pain. Her ECG (figure 1) was suggestive of an extensive anterior STEMI. She was discussed with and accepted by her nearest tertiary percutaneous intervention (PCI) centre for urgent coronary angiography.
Figure 1.
12-lead ECG showing ST marked anterior ST elevation (arrows) with Q waves. aVF and aVL, augmented vector F and L.
Her biochemistry and haematology results are summarised in table 2. These are consistent with a modest extent of myocardial necrosis. Of interest, we have bold-highlighted clinically relevant, abnormal results, including hypokalaemia, hypoalbuminaemia and systemic inflammation as revealed by C reactive protein (CRP). Interestingly, white cell and neutrophil counts were entirely normal.
Table 2.
Overview of the available laboratory data and blood testing
| Blood test (reference range, units) | t–5 hours | t+0 hour | t+1 hour | t+8 hours | t+17 hours |
| High sensitivity (Hs) troponin I (0–16, ng/L) | N/A | 228 | N/A | N/A | N/A |
| Troponin T (0–13 ng/L) | N/A | N/A | N/A | N/A | 150 |
| Sodium (133–146, mmol/L) | 139 | 138 | 136 | 136 | 140 |
| Potassium (3.5–5.3, mmol/L) | 3.5 | 3.2 | 2.7 | 3.5 | 4.1 |
| Urea (2.5–7.8, mmol/L) | 3.9 | 4 | 3.8 | 3.7 | 3.9 |
| Creatinine (40–130, μmol/L) | 71 | 69 | 77 | 83 | 94 |
| Bilirubin (<20, μmol/L) | 8 | 8 | 6 | 6 | 5 |
| Alanine Aminotransferase (ALT) (<50, U/L) | <6 | <6 | <6 | <6 | <6 |
| Aspartate Aminotransferase (AST) (<40, U/L) | 14 | 15 | 15 | 15 | 16 |
| Alkaline Phosphatase (ALP) (30–130, U/L) | 94 | 93 | 78 | 90 | 88 |
| Albumin (35–50, g/L) | 26 | 25 | 23 | 26 | 27 |
| C reactive protein (0–10, mg/L) | 61 | 59 | 42 | 46 | 45 |
| Haemoglobin (115–165, g/L) | 140 | 139 | 130 | N/A | 137 |
| White cell count (4.0–10.0, ×109/L) | 9.4 | 9.4 | 9.37 | N/A | 9.66 |
| Neutrophils (2.0–7.0, ×109/L) | 4.7 | 5 | 4.46 | N/A | 5.21 |
| Platelet count (150–410, ×109/L) | 321 | 317 | 300 | N/A | 306 |
‘T’ represents the time of onset of chest pain.
Highlighted in bold are clinically relevant, abnormal results.
N/A, not available.
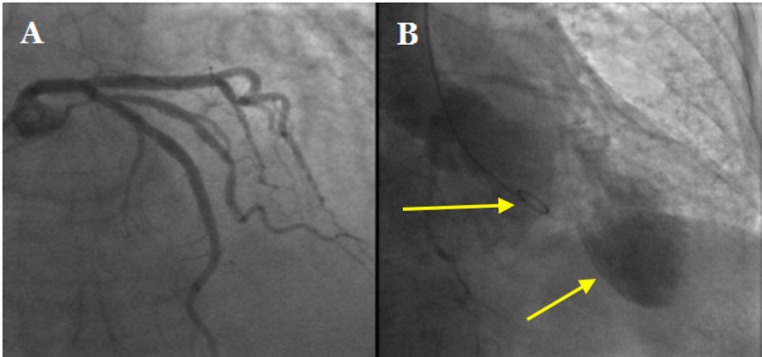
On arrival her coronary angiogram surprisingly revealed smooth epicardial coronary arteries (figure 2A). There was however slow flow in her left anterior descending artery (LAD).
Figure 2.
(A) Coronary angiogram showing smooth, obstructed left main stem, left anterior descending and circumflex arteries. (B) Left ventriculogram showing apical ballooning in keeping with Takotsubo cardiomyopathy. The arrows delineate the 5F pigtail catheter within the basal portion of the left ventricular cavity and the ballooning appearance in the left ventricular apex.
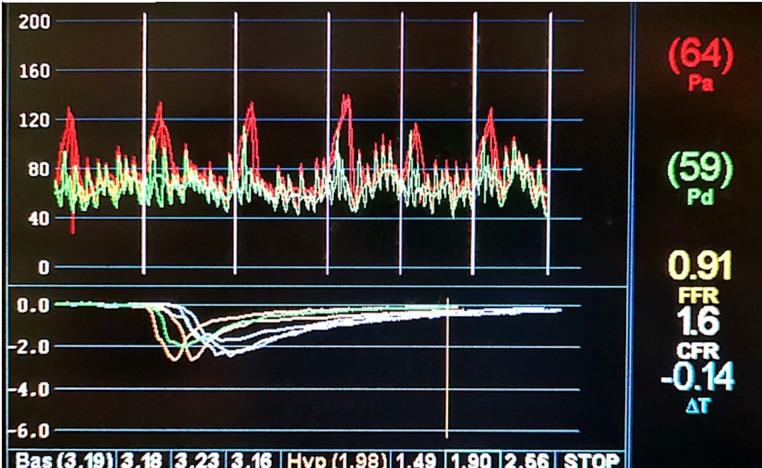
We proceeded to perform coronary physiology on her LAD (figure 3 and table 3).
Figure 3.
Coronary physiology testing aortic pressure (Pa), distal pressure (Pd), fractional flow reserve (FFR) and coronary flow reserve (CFR).
Table 3.
Coronary physiology using pressure wire in our patient’s left anterior descending artery
| Test | Value | Ischaemic reference range |
| Resting Pd/Pa (distal pressure/aortic pressure) | 0.93 | |
| Fractional flow reserve | 0.91 | <0.8 |
| Coronary flow reserve | 1.6 | <2.0 |
| Index of myocardial resistance | 117 | >25 |
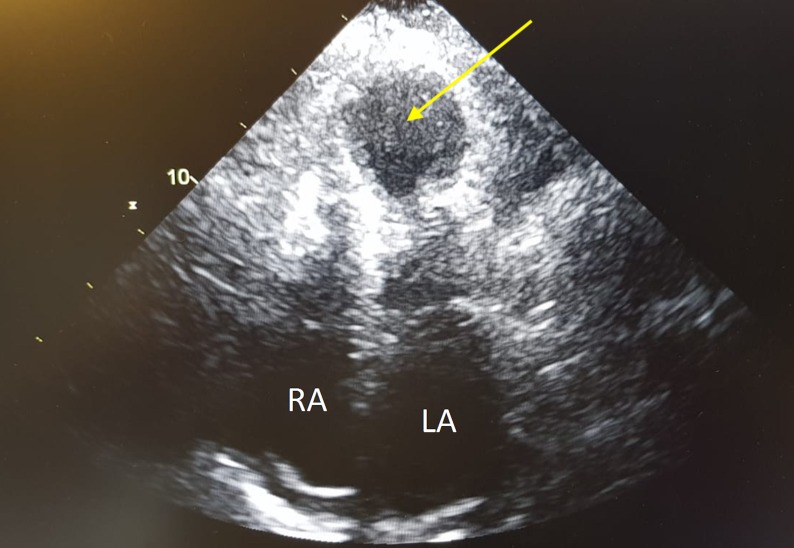
This suggested that, although there was no flow-limiting epicardial coronary stenosis, there was evidence of increased microvascular resistance. A left ventriculogram was then carried out, revealing LV apical ballooning consistent with TC (figure 2B). Her echocardiogram supported the diagnosis of TC, showing evidence of severe LV systolic dysfunction with apical ballooning and akinetic mid to apical regions with preservation of the basal segments (figure 4).
Figure 4.
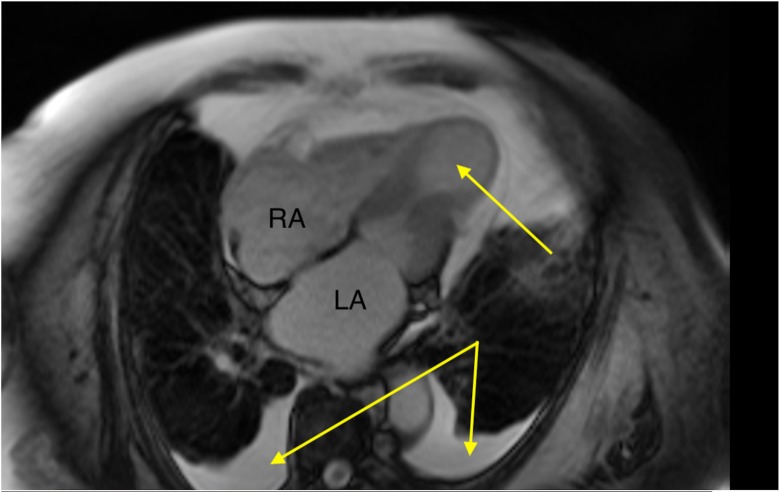
Transthoracic echocardiogram in the apical four-chamber view during systole. Theright (RA) and left (LA) atria are labelled to assist orientation. There is left ventricular apical ballooning (arrow) and akinesis with preservation of the basal segments.
A cardiac MRI (CMR) confirmed severely impaired LV function with akinetic mid to apical LV segments and apical ballooning (figure 5). There was evidence of myocardial oedema with elevated native T1 and T2 relaxation times, especially marked in the akinetic mid to apical segments. There was subtle, non-specific epicardial late gadolinium enhancement noted in the LV apex in a non-coronary distribution (figure 6). In combination this pattern is consistent with TC. Additionally, the CMR shows evidence of bilateral pleural effusions and a rim of pericardial fluid indicative of marked cardiac decompensation on presentation.
Figure 5.
Cardiac MRI during systole revealing apical ballooning (arrow) and akinesis with preservation of the basal segments. Bilateral pleural effusions (arrows) reveal the patient’s overloaded fluid status on presentation. LA, left atrium; RA, right atrium.
Figure 6.
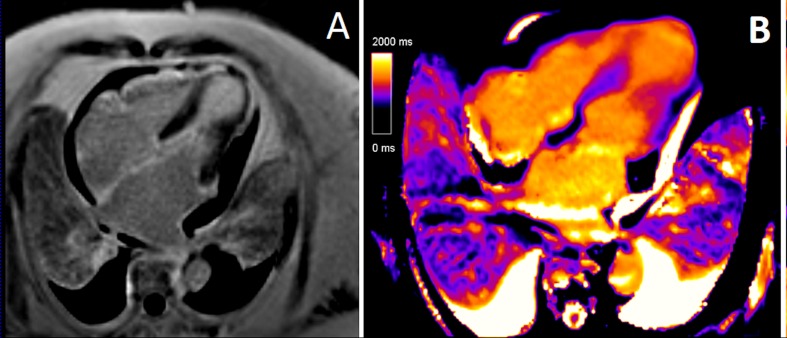
(A) Late gadolinium enhancement image of an apical four-chamber slice on cardiac MRI showing a subtle, non-specific epicardial late gadolinium enhancement noted in the left ventricular (LV) apex in a non-coronary distribution. (B) T1 map of an apical four-chamber slice on cardiac MRI showing elevated native T1, especially marked in the akinetic mid to apical segments. The native T1 relaxation time in the LV apex is markedly elevated at 1258 ms in comparison with 939 ms in the basal LV segments. This pattern is indicative of myocardial oedema.
Outcome and follow-up
Our patient returned to her district general hospital the day after her coronary angiogram. There she remained as an inpatient for a further 61 days while undertaking a prolonged programme of intensive rehabilitation due to frailty resulting from her critical illness with sepsis and TC. She ultimately returned to her functional baseline at time of discharge. Unfortunately, 36 days after discharge, she was readmitted with septic shock secondary to severe bilateral bronchopneumonia. She died following a short hospital admission.
She did not have any repeat cardiac imaging following her index admission as she died prior to her appointment for follow-up echocardiogram. We are therefore unable to comment on the degree of residual LV dysfunction at the time of her death.
Discussion
The combination of the above imaging modalities and coronary physiology confirmed the diagnosis of TC. Her trigger appears to have been a combination of physical stress resulting from her sepsis and emotional stress resulting from delirium.
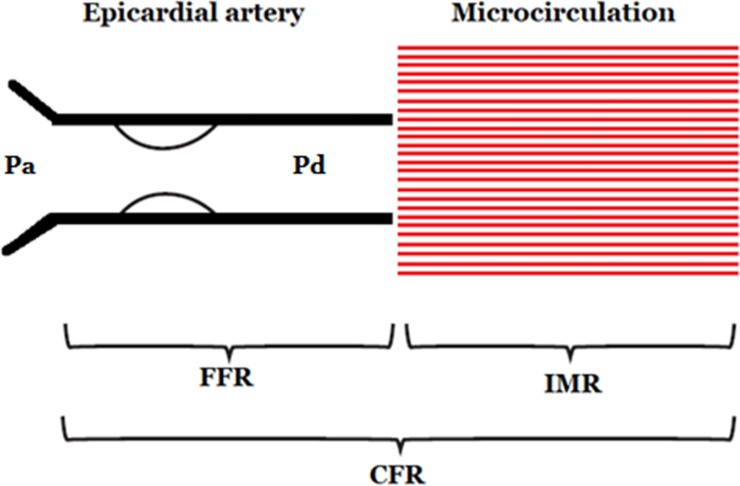
Fractional flow reserve (FFR) is the pressure distal to a coronary stenosis divided by the aortic pressure. It is calculated during hyperaemia (typically pharmacologically induced with adenosine) to minimise vascular resistance to ensure the observed pressure is directly proportional to coronary flow. It represents epicardial coronary flow only. The ischaemic threshold is ≤0.80.16
Coronary flow reserve (CFR) is a measure of coronary vasodilator capacity calculated by the ratio of maximum coronary blood flow to resting coronary blood flow. A pressure/thermistor-tipped guidewire is used to interpret multiple boluses of room temperature intracoronary saline to obtain coronary thermodilution curves and calculate the mean transit times. CFR is a well-established, prognostically validated measure of the vasodilator capacity of the coronary circulation, combining both epicardial and microvascular resistance. CFR may be estimated non-invasively and, as in this case, invasively. CFR may have limited test–retest repeatability and is strongly influenced by resting flow in addition to the response to hyperaemia. Notwithstanding these methodological considerations, the CFR value recorded in our patient (1.6) is severely depressed and very likely to fairly represent substantially depressed vasodilator reserve at that time. In the context of a normal FFR, a low CFR is indicative of microvascular dysfunction. The ischaemic threshold for CFR is ≤2.0.17–19
The index of myocardial resistance (IMR) is a measure of microvascular resistance. It is defined as the distal coronary pressure divided by the inverse of the hyperaemic mean transit time. It is the minimum microvascular resistance during steady-state, maximum hyperaemia. Unlike CFR, IMR is not influenced by resting conditions. IMR therefore specifically reflects microvascular resistance within a target coronary artery. The normal range of IMR is <25.20 21
All three measurements can be taken quickly in the catheter laboratory using a single pressure/thermistor-tipped guidewire. The results can then be interpreted together to comment on both epicardial and microvascular coronary function (figure 7).
Figure 7.
Summary of coronary physiology. CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of myocardial resistance; Pa, aortic pressure; Pd, distal pressure.
In this case the patient had impaired CFR and IMR with a normal FFR. This is indicative of microvascular dysfunction and is in keeping with the ‘microvascular acute coronary syndrome’ model of TC.
It is generally under-recognised in the medical community that TC is associated with considerable mortality. The International Takotsubo Registry, a consortium of 26 centres in Europe and the USA (n=1750), reported that 9.9% of patients with TC develop acute cardiogenic shock on presentation, with an overall inpatient mortality of 4.1%.22 This is comparable with the outcomes for STEMI in the primary PCI era.23
Current standard of care is the establishment of traditional heart failure medications (ACE inhibitors and beta blockers) without any major randomised control trial (RCT) data to support this. Invasive coronary physiology (FFR/CMR/IMR) may provide an easily assessable, quantitative test of treatment efficacy.
Further research is required to test whether the severity of microvascular dysfunction at diagnosis relates to disease severity or mortality in TC. However the observed simultaneous improvement of coronary flow and LV dysfunction suggests this is an avenue worth exploring.13
Learning points.
Takotsubo cardiomyopathy accounts for 2% of all acute coronary syndrome presentations and is associated with similar mortality to that of patients suffering from myocardial infarction in the era of primary percutaneous intervention.
Takotsubo cardiomyopathy is associated with an acute, reversible increase in microvascular resistance that gradually improves with time.
This temporal improvement in microvascular resistance correlates with improving left ventricular function.
Footnotes
Contributors: AJM was responsible for patient consent, appropriating data for publication and drafting the manuscript (both case report and discussion). PO’B, SN and CB jointly conceived the project, performed the reported diagnostic procedure and reviewed the manuscript, with each making critical changes prior to submission. CB was the senior consultant in charge of our patient’s care while in our centre and is the senior author of the paper. All authors have given final approval for the current version to be published.
Funding: This study was funded by the British Heart Foundation (grant RE/13/5/30177).
Competing interests: CB is employed by the University of Glasgow, which holds consultancy and research agreements with companies that have commercial interests in the diagnosis and treatment of angina. The companies include Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Menarini Pharmaceuticals and Siemens Healthcare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11–18. 10.1016/s0735-1097(01)01316-x [DOI] [PubMed] [Google Scholar]
- 2. Tofield A, Sato H. and Takotsubo cardiomyopathy. Eur Heart J 2016;37:2812. [DOI] [PubMed] [Google Scholar]
- 3. Dorfman TA, Iskandrian AE. Takotsubo cardiomyopathy: state-of-the-art review. J Nucl Cardiol 2009;16:122–34. 10.1007/s12350-008-9015-3 [DOI] [PubMed] [Google Scholar]
- 4. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. 10.1093/eurheartj/ehl032 [DOI] [PubMed] [Google Scholar]
- 5. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. 10.1056/NEJMoa043046 [DOI] [PubMed] [Google Scholar]
- 6. Pelliccia F, Kaski JC, Crea F, et al. Pathophysiology of Takotsubo Syndrome. Circulation 2017;135:2426–41. 10.1161/CIRCULATIONAHA.116.027121 [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Szeto C, Gao E, et al. Cardiotoxic and cardioprotective features of chronic β-adrenergic signaling. Circ Res 2013;112:498–509. 10.1161/CIRCRESAHA.112.273896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lüscher TF, Templin C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur Heart J 2016;37:2816–20. 10.1093/eurheartj/ehw057 [DOI] [PubMed] [Google Scholar]
- 9. Khalid N, Iqbal I, Coram R, et al. Thrombolysis In Myocardial Infarction Frame Count in Takotsubo Cardiomyopathy. Int J Cardiol 2015;191:107–8. 10.1016/j.ijcard.2015.04.192 [DOI] [PubMed] [Google Scholar]
- 10. Jaguszewski M, Osipova J, Ghadri JR, et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999–1006. 10.1093/eurheartj/eht392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiowski W, Lüscher TF, Linder L, et al. Endothelin-1-induced vasoconstriction in humans. Reversal by calcium channel blockade but not by nitrovasodilators or endothelium-derived relaxing factor. Circulation 1991;83:469–75. 10.1161/01.CIR.83.2.469 [DOI] [PubMed] [Google Scholar]
- 12. Rivero F, Cuesta J, García-Guimaraes M, et al. Time-Related Microcirculatory Dysfunction in Patients With Takotsubo Cardiomyopathy. JAMA Cardiol 2017;2:699–700. 10.1001/jamacardio.2016.5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J 2010;31:1319–27. 10.1093/eurheartj/ehq039 [DOI] [PubMed] [Google Scholar]
- 14. Giusca S, Eisele T, Nunninger P, et al. Aborted Sudden Cardiac Death in a Female Patient Presenting with Takotsubo-Like Cardiomyopathy due to Epicardial Coronary Vasospasm. Case Rep Cardiol 2017;2017:1–4. 10.1155/2017/7875240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scally C, Abbas H, Ahearn T, et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019;139:1581–92. 10.1161/CIRCULATIONAHA.118.037975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703–8. 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 17. Barbato E, Aarnoudse W, Aengevaeren WR, et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur Heart J 2004;25:219–23. 10.1016/j.ehj.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 18. Carrick D, Haig C, Ahmed N, et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation 2016;134:1833–47. 10.1161/CIRCULATIONAHA.116.022603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ford TJ, Corcoran D, Berry C. Coronary artery disease: physiology and prognosis. Eur Heart J 2017;38:1990–2. 10.1093/eurheartj/ehx226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Layland JJ, Whitbourn RJ, Burns AT, et al. The index of microvascular resistance identifies patients with periprocedural myocardial infarction in elective percutaneous coronary intervention. Heart 2012;98:1492–7. 10.1136/heartjnl-2012-302252 [DOI] [PubMed] [Google Scholar]
- 21. Melikian N, Vercauteren S, Fearon WF, et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention 2010;5:939–45. 10.4244/EIJV5I8A158 [DOI] [PubMed] [Google Scholar]
- 22. Templin C, Ghadri JR, Diekmann J, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015;373:929–38. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 23. Redfors B, Vedad R, Angerås O, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction - A report from the SWEDEHEART registry. Int J Cardiol 2015;185:282–9. 10.1016/j.ijcard.2015.03.162 [DOI] [PubMed] [Google Scholar]
- 24. Kurisu S, Inoue I, Kawagoe T, et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol 2003;41:743–8. 10.1016/S0735-1097(02)02924-8 [DOI] [PubMed] [Google Scholar]
- 25. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004;94:343–6. 10.1016/j.amjcard.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 26. Fazio G, Sarullo FM, Novo G, et al. Tako-tsubo cardiomyopathy and microcirculation. J Clin Monit Comput 2010;24:101–5. 10.1007/s10877-009-9217-5 [DOI] [PubMed] [Google Scholar]
- 27. Daniels DV, Fearon WF. The index of microcirculatory resistance (IMR) in takotsubo cardiomyopathy. Catheterization and Cardiovascular Interventions 2011;77:128–31. 10.1002/ccd.22599 [DOI] [PubMed] [Google Scholar]
- 28. Layland J, Whitbourn R, Macisaac A, et al. Takotsubo cardiomyopathy: reversible elevation in microcirculatory resistance. Cardiovasc Revasc Med 2012;13:66–8. 10.1016/j.carrev.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 29. Warisawa T, Naganuma T, Nakamura S. Reversible Microvascular Dysfunction in Takotsubo Syndrome Shown Using Index of Microcirculatory Resistance. Circ J 2016;80:750–2. 10.1253/circj.CJ-15-1283 [DOI] [PubMed] [Google Scholar]
- 30. Loffi M, Santangelo A, Kozel M, et al. Takotsubo Cardiomyopathy: One More Angiographic Evidence of Microvascular Dysfunction. Biomed Res Int 2018;2018:1–6. 10.1155/2018/5281485 [DOI] [PMC free article] [PubMed] [Google Scholar]