Abstract
A 52-year-old male patient with hyaluronic acid-based dermal fillers injected in his cheeks was diagnosed with glossotonsillary malignancy, and managed with concurrent cetuximab (epidermal growth factor receptor inhibitor) and radiation therapy. He developed significant inflammation around the dermal filler sites after first cycle of cetuximab which improved with dissolution of the dermal fillers with hyaluronidase. This suggests that cetuximab can lead to inflammation around the dermal filler sites, which can be treated with dissolution of the filler.
Keywords: head and neck cancer, chemotherapy, dermatology
Background
Monoclonal antibodies to the epidermal growth factor receptor (EGFR), such as cetuximab, are being used in the treatment of squamous cell carcinoma of the head and neck and K-ras wild type colorectal adenocarcinoma.1 2 A significant morbidity of this agent is the dermatological manifestations, primarily presenting as an acneiform rash.3 Dermal fillers are being increasingly used, indicated for wrinkles, folds and lines associated with ageing.
Case presentation
A 52-year-old male patient was diagnosed with a 3.1 cm p16-positive base-of-tongue primary with four left sided lymph nodes involved, staged as cT2N2bM0 (AJCC, 7th edition). He was treated with concurrent radiation (70 Gy/35 fractions) and cetuximab.
Significant background history included hyaluronic acid dermal fillers injected in both cheeks 6 months prior.
Two hours after first dose of cetuximab, the patient developed nodules and oedema around the dermal filler sites, the symptoms gradually progressed over the next 48 h as shown in figure 1. Dermal filer sites were reviewed by cosmetic specialist and a provisional diagnosis of cetuximab-related dermal filler reaction was made.
Figure 1.
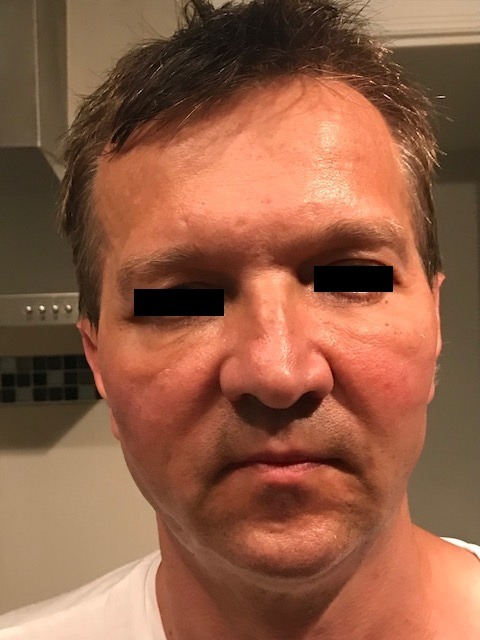
Nodules and oedema can be noted around the dermal filler site.
Treatment
After discussion with the oncology team, hyaluronidase was injected to dissolve the dermal fillers. Multiple injections (5000 IU in 5 mL) over 2 days were injected.
Outcome and follow-up
Within 24 h the nodules and oedema started to settle (figure 2). The patient continued cetuximab without recurrence of the dermal reaction around the filler sites. He developed typical cetuximab-related acneiform rash around nose and forehead, and later required dose reduction and ultimately final dose omission due to excessive oral mucositis and radiotherapy-induced in-field dermatitis.
Figure 2.
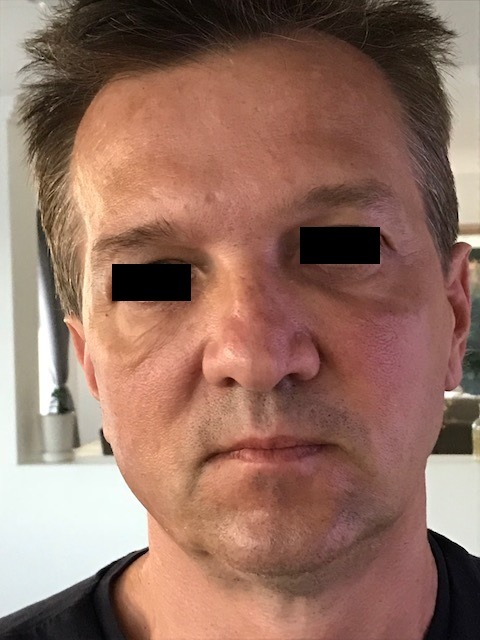
Resolution of the nodules and oedema postadministration of hyaluronidase to dissolve the dermal fillers.
Discussion
The diagnosis of cetuximab-induced skin reaction to the dermal filler is supported by both the timing (previously well-tolerated fillers become inflamed after the first dose of cetuximab) and the lack of reoccurrence of the reaction after the fillers had been dissolved, despite the continuation of cetuximab. The dermal filler reaction also occurred outside the radiation field. Typically, patients develop an acneiform rash to cetuximab which occurs in 60%–80% of patients and is the most common skin adverse effect. It primarily affects areas rich in sebaceous glands including scalp, face, and particularly the nose, cheeks, nasolabial folds and perioral region. It rarely affects the extremities, lower back, abdomen and buttocks. The rash is commonly treated with moisturising creams, topical corticosteroids and tetracycline antibiotics (minocycline and doxycycline), however, more severe forms may require treatment with systemic corticosteroids and/or cetuximab dose omission or reduction. Skin xerosis, nail toxicity and hair toxicity can also occur.3 Our patient’s reaction to cetuximab was atypical to the above presentation.
Pathogenesis of the skin rash has been related to EGFR expression in undifferentiated, proliferating basal and suprabasal keratinocytes. EGFR plays a critical role in epidermal homeostasis through regulation of keratinocyte proliferation, differentiation, migration and survival.4 Direct inhibition leads to diminished proliferation of basal keratinocyte, growth arrest and apoptosis with increased cell adhesivity and differentiation. Secondarily, there is increased synthesis of inflammatory chemokines leading to inflammation. The inflammatory cell infiltration leads to the cutaneous manifestations.5
Delayed reactions to hyaluronic acid dermal fillers are a recognised complication.6 Case series suggest presentations that include indurated papules or nodules with or without redness and tenderness, weeks to months after injection of fillers.7–9 The pathogenesis was unclear in the above reports, however, was attributed to an immune response, likely delayed type IV hypersensitivity reaction to the fillers.6–8 Chronic inflammation around the dermal filler has also been recognised, potentially occurring secondary to bacterial infiltration.9
A combination of the upregulation of inflammation secondary to the EGFR inhibition in the epidermis and the dermal fillers being more prone to both chronic inflammation or a hypersensitivity reaction may have led to the acute dermal filler inflammation observed in our patient. Although there have been independent articles on the dermatological complications of cetuximab therapy, and the delayed reactions associated with hyaluronic acid dermal fillers, this type of reaction has not been reported before. This observation is important since the treatment with cetuximab can be safely continued after dissolution of the filler.
Learning points.
Cetuximab can potentiate dermal filler inflammation.
Dissolution of the dermal filler can treat the inflammation and does not require dose reduction, or cessation of the cetuximab, as in the case of other associated skin adverse effects such as significant acneiform rash.
With the increasing use of dermal fillers and cetuximab, this reaction may become more common, and dissolution should remain a management option for optimal dosing of cetuximab.
Footnotes
Contributors: SP contributed via consenting the patient, data collection, literature view, analysis of literature and drafting the manuscript. MD (treating oncologist) contributed via consenting the patient, data collection, literature review, editing and finalising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. NCCN guidelines Version 1.2019 - March 6. 2019. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 2. NCCN guidelines Version 2.2019 - May 15. 2019. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 3. Pinto C, Barone CA, Girolomoni G, et al. Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist 2011;16:228–38. 10.1634/theoncologist.2010-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tran QT, Kennedy LH, Leon Carrion S, et al. EGFR regulation of epidermal barrier function. Physiol Genomics 2012;44:455–69. 10.1152/physiolgenomics.00176.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabbrocini G, Panariello L, Caro G, et al. Acneiform rash induced by EGFR inhibitors: review of the literature and new insights. Skin Appendage Disord 2015;1:31–7. 10.1159/000371821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beleznay K, Carruthers JD, Carruthers A, et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg 2015;41:929–39. 10.1097/DSS.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 7. Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Reports 2015;7:5851 10.4081/dr.2015.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open 2017;5:e1532 10.1097/GOX.0000000000001532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones D. Treatment of delayed reactions to dermal fillers. Dermatol Surg 2014;40:1180 10.1097/DSS.0000000000000158 [DOI] [PubMed] [Google Scholar]


