Abstract
A 65-year-old woman with long-standing rheumatoid arthritis (RA) experienced a recurrent tingling sensation in her left arm followed by aphasia and a tingling sensation in her right arm. A subsequent imaging study showed bilateral subdural fluid accumulation and we initially diagnosed her with a transient ischaemic attack and chronic subdural haematoma (CSDH). The cerebral spinal fluid study revealed an inflammatory response without any indications of infection or malignant tumours. After a meningeal biopsy, we redefined the diagnosis to rheumatoid meningitis (RM), and the patient showed remarkable improvement with prednisolone administration. RM should be considered as an alternative diagnosis when examining central nervous system diseases in patients with RA, as RM presents a highly variable clinical picture with image findings similar to those of CSDH.
Keywords: neuroimaging, immunology, pathology, rheumatoid arthritis
Background
Rheumatoid meningitis (RM) is a rare central neurological complication of rheumatoid arthritis (RA). We encountered a patient with long-standing RA with RM who was misdiagnosed with chronic subdural haematoma (CSDH) as a result of her brain MRI findings.
Case presentation
A 65-year-old woman with a 10-year history of RA was admitted to our neurology department, with a tingling sensation in her right arm and then developed mild aphasia.
Her RA had been well controlled by iguratimod and methotrexate for a long time, but her intake of methotrexate stopped 6 months before the onset of symptoms.
Approximately 2 months prior to admission, she experienced a transient tingling sensation in her left upper limb. The abnormal sensation occurred 3–4 times per day, with a duration of between 5 and 10 min. Light headaches and elementary hallucinations appeared concurrently. Two weeks prior to admission, she experienced a transient tingling sensation on the left side of her tongue and was diagnosed with a transient ischaemic attack (TIA) and began a regimen of aspirin (100 mg/day) and clopidogrel (75 mg/day). A fluid-attenuated inversion recovery (FLAIR) MRI sequence of the patient’s brain showed an area of hyperintensity in the bilateral subdural spaces. Therefore, in our opinion, her condition may have been complicated by CSDH (figure 1A). Her diffusion-weighted image (DWI) revealed that the right subdural lesion had restricted diffusion, while the left side appeared normal (figure 1B,C). Her abnormal sensations and headaches continued despite the use of dual antiplatelet drugs. Two weeks after the MRI study, she experienced a tingling sensation in her right upper limb and could not speak. Her family called for an ambulance and she was admitted to our department.
Figure 1.
FLAIR image showing areas of bright signal intensity under the bilateral dura mater 2 weeks prior to admission (A). Brain MRI also revealed restricted diffusion signals under the dura and the arachnoid on the right side (arrows) (B,C). FLAIR, fluid-attenuated inversion recovery.
Her medical history included no remarkable illnesses other than RA. She was a non-smoker and did not drink alcoholic beverages. On examination, she had no tingling sensations, but had mild aphasia; repetition and comprehension were partially impaired while naming was retained. There were no other marked systemic symptoms such as fever, arthralgia, respiratory manifestation and skin lesions. The mild aphasia disappeared 3 hours after admission and there were no other abnormal neurological findings. Subsequently, her transient aphasia and tingling sensations recurred on several occasions.
Investigations
Laboratory tests showed an increased platelet count 43.2×104/μL (normal<34.8×104 µL), C reactive protein (CRP) level 2.69 mg/dL (normal<0.14 mg/dL), and erythrocyte sedimentation rate of 83 mm/hour (2<normal<16 mm/hour) while her haemoglobin, white blood cell count, creatinine and liver enzyme levels were all within normal ranges. Inflammatory markers related to RA revealed elevated levels of rheumatoid factor (RF) (24.9 IU/mL (normal<15 IU/mL)) and antinuclear antibody (80 (normal<40)). Additional inflammatory markers, indicative of alternative autoimmune diseases such as double-stranded DNA antibodies, antineutrophil cytoplasmic antibodies, Sjögren’s syndrome-A/B antibody and myeloperoxidase/proteinase3 antineutrophil cytoplasmic antibody were all negative. Examination of the cerebrospinal fluid (CSF) showed an inflammatory profile: monocyte count of 65/μL (normal <3/μL), segmented cell count of 3/μL (normal <1/μL) and total protein of 75 mg/dL (15<normal< 40 mg/dL).
There were no evidence for tumour malignancy; major tumour markers such as carcinoembryonic antigen, alpha fetoprotein and carbohydrate antigen 125 were all negative. Whole-body contrast-enhanced CT revealed no abnormal findings. Cytology of CSF revealed many reactive inflammatory lymphocytes, but showed no evidence of malignancy. Moreover, flow cytometry analysis of CSF did not show monoclonality.
Workups of infectious diseases also revealed no abnormal findings: CSF cultures, CSF Cryptococcus antigens, herpes simplex PCR, CSF tuberculosis PCR and QuantiFERON- tuberculosis were all negative.
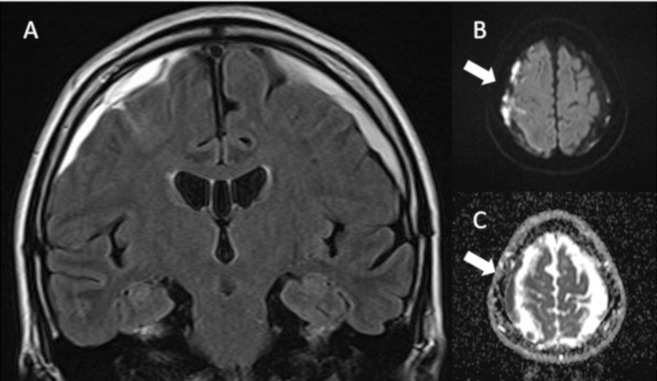
Her brain MRI did not show an area of clear abnormal intensity in the cerebral parenchyma. FLAIR images showed a decrease in the high intensity signal in the bilateral subdural space and gadolinium-enhanced T1-weighted images revealed an enhanced thick bilateral dura and enhanced right leptomeninges (figure 2A,B). A DWI revealed restricted diffusion in the bilateral subdural and subarachnoid spaces (figure 2C,D).
Figure 2.
The brain FLAIR MRI image on the day of admission showed decreased bilateral subdural fluid accumulation (A). Gadolinium-enhanced T1WI showed enhanced thick bilateral dura and enhanced right leptomeninges (arrows) (B). The DWI study revealed that a new area of restricted diffusion newly appeared in the left subdural space (arrows) (C,D). FLAIR, fluid-attenuated inversion recovery.
Differential diagnosis
We considered the possibility of a progressive subacute meningitis involving RM as a differential diagnosis. Infectious diseases were unlikely according to the laboratory results. Other than her long-standing RA, no additional autoimmune diseases were detected since she did not present with new systemic symptoms or autoantibodies. Major tumour markers were all negative and whole-body contrast-enhanced CT revealed no abnormal findings. Moreover, the findings from CSF cytology and flow cytometry did not indicate malignancy.
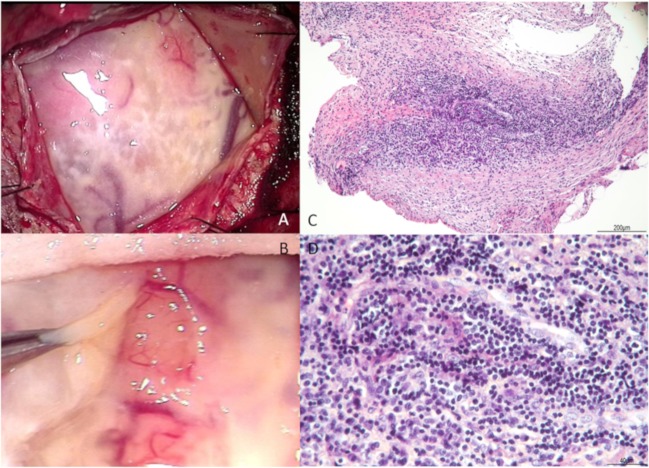
We performed a biopsy of her right frontal meninges to obtain a diagnosis. Intraoperative findings revealed viscous pale yellow effusions in the subdural and subarachnoid spaces (figure 3A,B). Histological sections showed a dense inflammatory infiltrate, predominantly consisting of lymphocytes and plasma cells (figure 3C,D). Meningeal biopsy also indicated no evidence of microorganism or dysplasia; therefore, we excluded infectious and neoplastic diseases. There was no remarkable fibrotic change and immunoglobulin G (IgG) 4 immunoreactive inflammatory cells were rarely observed, with lower counts than all other IgG immunoreactive inflammatory cells combined. New onset autoimmune diseases including IgG4-related disease were ruled out because of laboratory and biopsy results.
Figure 3.
During the meningeal biopsy, there were lots of viscous pale yellow effusions in the subdural (A) and the subarachnoid (B). Histopathology (H&E stain) of the meningeal biopsy: a low-power micrograph of the leptomeninges showed fibrous thickening changes (C, ×10) and a high-power micrograph of the area revealed infiltration with plasma cells and lymphocytes (D, ×40).
We diagnosed her with RM as other infectious, inflammatory and malignant diseases were excluded.
Treatment
Pulsed intravenous methylprednisolone therapy (1 g/day for 5 days) and subsequent oral prednisolone (55 mg/day) were administered with the oral prednisolone dosage tapering over several months.
Outcome and follow-up
Symptoms such as abnormal sensations and aphasia disappeared soon after the administration of intravenous methylprednisolone. Laboratory tests performed 1 month after treatment showed that her platelet count, CRP and RF returned to normal limits. There were no additional findings of interest other than increased neutrophils and decreased eosinophils due to prednisolone administration. Her CSF also indicated improvement with a monocyte count of 10/μL, a segmented cell count of 0/μL and a total protein of 41 mg/dL. MRIs indicated marked improvement 2 months after initiating treatment (figure 4A,B).
Figure 4.
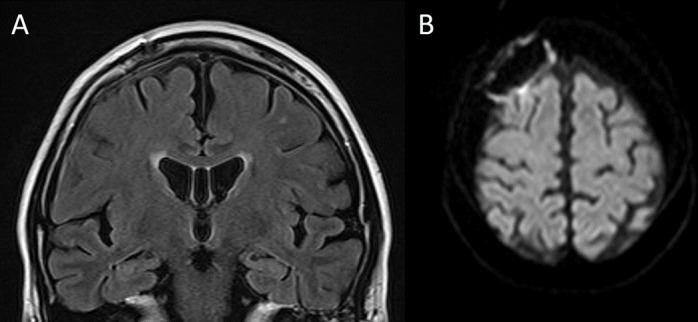
FLAIR images 2 months after treatment shoe a remarkable decrease in bilateral subdural fluid accumulation (A). DWI sequence also showed decline of hyperintensity area and vestige of craniotomy manipulation. DWI, diffusion-weighted image; FLAIR, fluid-attenuated inversion recovery.
Discussion
RM is a rare central nervous system complication of RA. Its clinical presentations are extremely variable, ranging from transient focal symptoms like TIAs to generalised seizure state.1–3 Since there are no definite diagnostic criteria, exclusion tests and meningeal biopsies must be performed.
A subset of patients with RM have very poor prognosis with late or no treatment.1 3
Several case reports have indicated that immunosuppressive therapy, including prednisolone treatment, can improve prognosis. Advancements in imaging modalities and resulting earlier diagnosis will be crucial to managing this condition.4–6
We misdiagnosed this case as a CSDH due to the radiological findings. Although CSDH typically causes subacute to chronic dementia or gait disturbances, some cases can present with transient neurological deficits like TIAs.7 8 The FLAIR MRI sequence findings showed a bright-intensity subdural lesion, while the lesions using a DWI sequence can show variable signals ranging from hypointensity to hyperintensity.9
There are different diseases that can mimic a CSDH, including metastasis, infection, lymphoma and autoimmune diseases such as granulomatosis with polyangiitis.10–12 Recently, Schuster and colleagues described a RM case in which brain CT showed right frontal subdural isodense areas mimicking haematoma.13 Our case, however, is characterised by its MRI findings resembling CSDH, which caused us to mistake RM for cerebrovascular disease.
Some case reports have suggested that DWI is useful for diagnosing RM by showing a bright signal in the subdural and subarachnoid spaces, which were related to viscous exudates.4 5 Our case showed a bright DWI signal area under the left pia mater when the patient experienced mild aphasia and tingling sensations in her right arm. Restricted diffusion may be caused by restriction of water molecule movement within dense inflammatory cells. Since there were many viscous components under the dura and arachnoid mater during biopsy, these viscous exudates most likely caused her symptoms.
Patient’s perspective.
I thought the tingling sensations in my left arm were my imagination at first. However, the tingling sensation recurred so many times that I visited a hospital. I was anxious that I really may have a stroke because my symptom had continued despite medications.
My memory about the day of admission is vague, but I was extremely afraid of my future.
As I had not suffered from arthralgias and stiffness for several years, I was really surprised when I received the explanation that my symptoms might be related to rheumatoid arthritis.
I was extremely anxious when they were explaining the procedure of meningeal biopsy, although I could understand it was necessary. My family and the medical staff encouraged me to have the surgery. As a result, I was diagnosed with rheumatoid meningitis and could receive appropriate treatment due to the biopsy.
My symptoms such as tingling sensation, headaches and aphasia disappeared soon after initiating treatment. The decision to undergo the biopsy was very difficult but the decision enabled me to return to my ordinary life. I am really grateful for my family and all of the medical staff.
Learning points.
Rheumatoid meningitis (RM) is a rare central neurological complication of rheumatoid arthritis and can present transient focal symptoms similar to transient ischaemic attacks.
The imaging findings from RM can mimic that of chronic subdural haematoma.
Early administration of prednisolone can result in a good outcome.
Footnotes
Contributors: KY is attending doctor and corresponding author of this article. AS greatly contributed to diagnosis and treatment according to her experience of rheumatoid meningitis. TS is the director of our department and provided us with excellent leadership.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Magaki S, Chang E, Hammond RR, et al. Two cases of rheumatoid meningitis. Neuropathology 2016;36:93–102. 10.1111/neup.12238 [DOI] [PubMed] [Google Scholar]
- 2. Bourgeois P, Rivest J, Bocti C. Rheumatoid meningitis presenting with stroke-like episodes. Neurology 2014;82:1564–5. 10.1212/WNL.0000000000000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bathon JM, Moreland LW, DiBartolomeo AG. Inflammatory central nervous system involvement in rheumatoid arthritis. Semin Arthritis Rheum 1989;18:258–66. 10.1016/0049-0172(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 4. Roques M, Tanchoux F, Calvière L, et al. MRI with DWI helps in depicting rheumatoid meningitis. J Neuroradiol 2014;41:275–7. 10.1016/j.neurad.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Hasiloglu ZI, Asik M, Erer B, et al. Magnetic resonance imaging of rheumatoid meningitis: a case report and literature review. Rheumatol Int 2012;32:3679–81. 10.1007/s00296-011-2105-6 [DOI] [PubMed] [Google Scholar]
- 6. Servioli MJ, Chugh C, Lee JM, et al. Rheumatoid meningitis. Front Neurol 2011;2:84 10.3389/fneur.2011.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilkinson CC, Multani J, Bailes JE. Chronic subdural hematoma presenting with symptoms of transient ischemic attack (TIA): a case report. W V Med J 2001;97:194. [PubMed] [Google Scholar]
- 8. Kaminski HJ, Hlavin ML, Likavec MJ, et al. Transient neurologic deficit caused by chronic subdural hematoma. Am J Med 1992;92:698–700. 10.1016/0002-9343(92)90790-I [DOI] [PubMed] [Google Scholar]
- 9. Lee SH, Choi JI, Lim DJ, et al. The Potential of Diffusion-Weighted Magnetic Resonance Imaging for Predicting the Outcomes of Chronic Subdural Hematomas. J Korean Neurosurg Soc 2018;61:97–104. 10.3340/jkns.2016.0606.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Catana D, Koziarz A, Cenic A, et al. Subdural Hematoma Mimickers: A Systematic Review. World Neurosurg 2016;93:73–80. 10.1016/j.wneu.2016.05.084 [DOI] [PubMed] [Google Scholar]
- 11. Shiotani A, Mukobayashi C, Oohata H, et al. Wegener’s granulomatosis with dural involvement as the initial clinical manifestation. Intern Med 1997;368:514. [DOI] [PubMed] [Google Scholar]
- 12. Kalra S, Yadav A, Agarwal S, et al. Wegener’s granulomatosis with subdural hematoma as the initial manifestation. Int J Crit Illn Inj Sci 2013;3:88 10.4103/2229-5151.109430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuster S, Braass H, Iking-Konert C, et al. Rheumatoid meningitis: A rare cause of aseptic meningitis with frequently stroke-like episodes. Neurol Clin Pract 2018;8:451–5. 10.1212/CPJ.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]