Abstract
The present work describes the chemical characterization and the phytotoxicity assessment of essential oils (EOs) obtained from spent materials or pruning waste of four plant species: Zingiber officinale Roscoe used in the juicing industry, Pistacia vera L. var. Bronte used in the food industry, discarded material of industrial hemp (Cannabis sativa L. var. Futura 75), and pruning waste from Cupressus sempervirens L. The phytochemical profile of the EOs was evaluated by gas chromatographic flame ionization detection (GC-FID) and GC-MS analyses, which highlighted the presence of several compounds with a wide range of biological activities. Among them, application possibilities in agriculture were evaluated by studying the phytotoxic activity in vitro against germination and initial radical growth of several seeds such as Raphanus sativus L., Lepidium sativum L., Lactuca sativa L., Solanum lycopersicum L., Lolium multiflorum Lam., and Portulaca oleracea L.
Keywords: Zingiber officinale Roscoe, Pistacia vera L., Cannabis sativa L., Cupressus sempervirens L., essential oil, phytotoxicity
1. Introduction
Modern agriculture uses many synthetic herbicides to manage weeds, and about 2.5 million tons of pesticides are used every year [1,2]. Over the years, the excessive use of synthetic herbicides and pesticides has harmed the environment and human health. Considering this, recently, much has been invested in the study of alternative strategies that can lead to the development of biodegradable and nontoxic products [3]. This is because many old herbicides have been withdrawn from the market for safety issues and there has been rapid evolution of resistance to new synthetic herbicides [4]. Moreover, the development of insecticide resistance has resulted in the loss of food [5].
Allelopathy is a biological phenomenon that affects the growth and development of plants using secondary metabolites produced in nature [6]. These latter compounds are also able to defend plants against phytopathogenic bacteria and/or fungi, plant-feeding insects, and herbivores [5,7].
Among the most investigated natural sources of secondary metabolites, essential oils (EOs) play a pivotal role [8].
In the last decade, many EOs have been studied for their phytotoxic and pesticidal properties, with terpenes being the class of bioactive compounds to which these biological activities are mainly attributable, although with generally higher concentrations needed to obtain phytotoxic effects to be used as insecticides or fungicides [9,10,11,12].
Indeed, it has been shown that many highly phytotoxic allelochemicals are derived from the terpenoid biosynthetic pathway [3].
Recently, considerable attention from the scientific community has been paid to the recovery of waste processing products from the agricultural and food industry. Indeed, it is well known that such waste products are an economic burden for processing companies due to the transport and disposal costs as special waste [13]. In other cases, biomass and byproducts are sources of compounds with technological and nutritional properties [14]. Moreover, the biomass of plant species subjected to pruning is rich in EOs.
In light of this, the aim of this study was to evaluate the phytochemical profile and the in vitro phytotoxic effects of the EOs isolated from Zingiber officinale Roscoe (Zingiberaceae, ginger), Pistacia vera L (Anacardiaceae, pistachio)., Cannabis sativa L. var. Futura 75 (Cannabaceae, hemp), and Cupressus sempervirens L. (Cupressaceae, cypress) byproducts on the germination and initial radical elongation of several seeds such as Raphanus sativus L. (radish), Lepidium sativum L. (garden cress), Lactuca sativa L. (lettuce), Solanum lycopersicum, L. (tomato), Lolium multiflorum Lam. (ryegrass), and Portulaca oleracea L. (purslane).
2. Results
2.1. Micromorphological Analysis
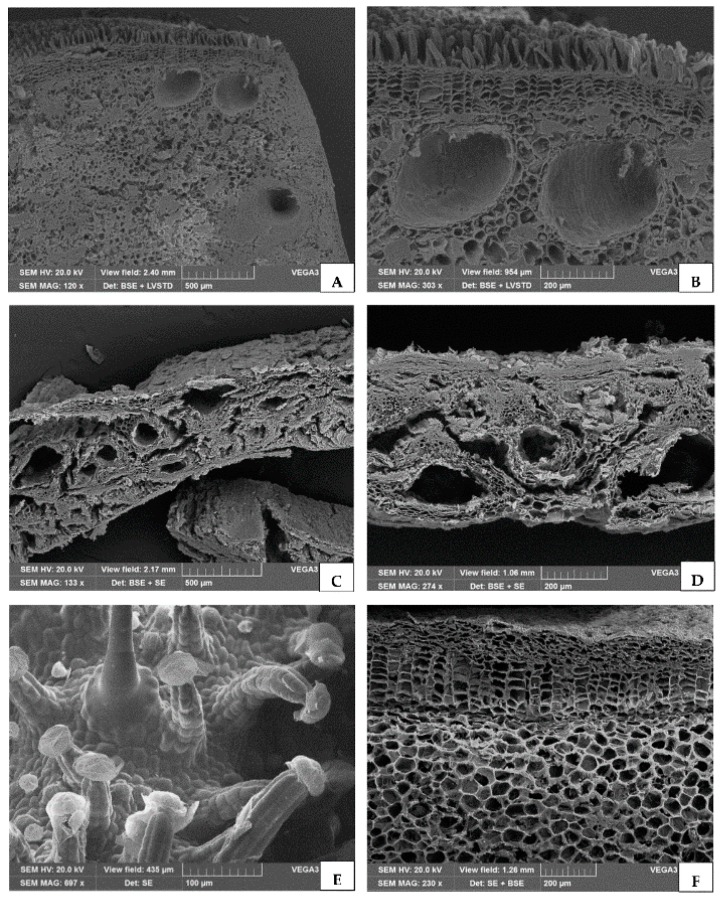
Samples from various species characterized by different kinds of secretory tissues, in which EO is synthesized and accumulated, were investigated. In female cones of C. sempervirens, the cross section of a cone scale showed several schizogenous resin ducts (Figure 1A,B). The hull of P. vera var. Bronte showed many resin ducts in the mesocarp, forming large schizogenous cavities where EO is synthesized (Figure 1C,D). Inflorescences of C. sativa var. Futura 75 showed long, multicellular, stalked glandular trichomes with large heads, where resin is produced and stored, together with a few small glandular trichomes with a bicellular head and a unicellular stalk (Figure 1E). In the rhizome of Z. officinale, a well-marked endodermis separates the cortex from vascular tissue, while oil globules are present in secretory cells scattered in the parenchyma (Figure 1F).
Figure 1.
SEM micrographs of different plant tissues used for EO isolation. (A,B) Cross section of a cone scale from Cupressus sempervirens L., showing several resin ducts at different magnifications. (C,D) Particular of the cross section of the mesocarp from the Pistacia vera L. var. Bronte hull, showing many resin ducts. (E) Inflorescences of Cannabis sativa L. var. Futura 75 showing long, multicellular, stalked glandular trichomes with large heads where resin is produced and stored. (F) Section of Zingiber officinale Roscoe rhizome containing oil globules in secretory cells scattered in the parenchyma.
2.2. Essential Oil Yields and Chemical Composition
Hydrodistillation of the aerial parts of C. sativa, hulls of P. vera, and cones of C. sempervirens furnished oils in 0.2%, 0.3%, and 0.6% yields on a dry mass basis, respectively, while hydrodistillation of the fresh rhizomes of Z. officinale furnished oil in a 0.7% yield.
The compositions of the EOs, with retention indices and area percentages for each compound, are given in Table 1, Table 2, Table 3 and Table 4.
Table 1.
Chemical composition of C. sativa L. var. Futura 75 EO.
| Compound Name | Area (%) | KI a | Identification b | |
|---|---|---|---|---|
| 1 | Heptanal | 0.02 | 217 | 1,2 |
| 2 | α-Thujene | 0.11 | 348 | 1,2 |
| 3 | α-(+)-Pinene | 7.82 | 401 | 1,2,3 |
| 4 | Camphene | 0.21 | 443 | 1,2,3 |
| 5 | (1S)-(−)-β-Pinene | 3.73 | 565 | 1,2,3 |
| 6 | β-Myrcene | 9.32 | 639 | 1,2,3 |
| 7 | α-Phellandrene | 0.44 | 662 | 1,2 |
| 8 | δ-3-Carene | 0.68 | 680 | 1,2,3 |
| 9 | (+)-4-Carene | 0.38 | 699 | 1,2 |
| 10 | p-Cymene | 0.09 | 722 | 1,2 |
| 11 | D-Limonene | 2.92 | 743 | 1,2,3 |
| 12 | cis-β-Ocimene | 0.54 | 769 | 1,2 |
| 13 | trans-β-Ocimene | 4.62 | 810 | 1,2 |
| 14 | γ-Terpinene | 0.30 | 827 | 1,2 |
| 15 | α-Terpinolen | 9.35 | 919 | 1,2 |
| 16 | β-Linalool | 0.10 | 934 | 1,2 |
| 17 | Fenchol | 0.05 | 961 | 1,2 |
| 18 | L-trans-Pinocarveol | 0.01 | 1015 | 1,2 |
| 19 | Borneol | 0.03 | 1070 | 1,2 |
| 20 | 4-Terpineol | 0.09 | 1093 | 1,2 |
| 21 | p-Cymen-8-ol | 0.09 | 1109 | 1,2 |
| 22 | α-Terpineol | 0.05 | 1118 | 1,2,3 |
| 23 | n-Hexyl butyrate | 0.04 | 1123 | 1,2 |
| 24 | (R)-(+)-β-Citronellol | 0.02 | 1184 | 1,2 |
| 25 | Bornyl acetate | 0.08 | 1270 | 1,2,3 |
| 26 | (+/−)-Lavandulol acetate | t | 1278 | 1,2 |
| 27 | Ylangene | 0.06 | 1379 | 1,2 |
| 28 | Copaene | 0.05 | 1384 | 1,2 |
| 29 | (+)-Sativene | 0.37 | 1403 | 1,2 |
| 30 | Isocaryophillene | 0.46 | 1419 | 1,2 |
| 31 | α-Caryophillene | 21.68 | 1447 | 1,2 |
| 32 | β-Gurjunene | 0.03 | 1451 | 1,2 |
| 33 | α-bergamotene | 3.22 | 1456 | 1,2 |
| 34 | γ-Gurjunene | 0.05 | 1459 | 1,2 |
| 35 | β-Caryophillene | 9.86 | 1478 | 1,2 |
| 36 | β-Farnesene | 0.80 | 1480 | 1,2 |
| 37 | allo-Aromadendrene | 1.07 | 1483 | 1,2 |
| 38 | α-Selinene | 0.02 | 1485 | 1,2 |
| 39 | γ-Cadinene | 0.42 | 1494 | 1,2 |
| 40 | β-Himachalene | 0.19 | 1497 | 1,2 |
| 41 | γ-Selinene | 0.49 | 1500 | 1,2 |
| 42 | α-Guaiene | 1.67 | 1506 | 1,2 |
| 43 | δ-Guaiene | 2.16 | 1514 | 1,2 |
| 44 | α-Muurolene | 0.08 | 1516 | 1,2 |
| 45 | β -Bisabolene | 1.23 | 1526 | 1,2 |
| 46 | γ-Muurolene | 0.31 | 1530 | 1,2 |
| 47 | (+)-Valencene | 0.12 | 1538 | 1,2 |
| 48 | β-Sesquiphellandrene | 0.80 | 1542 | 1,2 |
| 49 | β-Panasinsene | 1.72 | 1554 | 1,2 |
| 50 | δ-Selinene | 1.20 | 1558 | 1,2 |
| 51 | Selina-3,7(11)diene | 2.54 | 1561 | 1,2 |
| 52 | Elixene | 0.76 | 1575 | 1,2 |
| 53 | β-Maaliene | 0.41 | 1579 | 1,2 |
| 54 | trans-Nerolidol | 0.45 | 1582 | 1,2 |
| 55 | Caryophillene oxide | 3.83 | 1602 | 1,2 |
| 56 | Eremophilene | 0.09 | 1618 | 1,2 |
| 57 | Caryophylladienol II | 0.26 | 1653 | 1,2 |
| 58 | (+)-epi-Bicyclosesquiphellandrene | 0.30 | 1657 | 1,2 |
| 59 | α-Cadinol | 0.13 | 1670 | 1,2 |
| 60 | (+)-Ledene | 0.09 | 1673 | 1,2 |
| 61 | α-Bisabolol | 0.13 | 1697 | 1,2 |
| 62 | Guaia-3,9-diene | 0.01 | 1702 | 1,2 |
| 63 | Juniper camphor | 0.07 | 1710 | 1,2 |
| 64 | Z-9-Pentadecenol | 0.02 | 1742 | 1,2 |
| 65 | Hexahydrofarnesyl acetone | 0.04 | 1859 | 1,2 |
| 66 | Methyl palmitate | 0.02 | 1940 | 1,2 |
| 67 | Biformen | 0.08 | 2005 | 1,2 |
| 68 | Dehydroabietan | 0.04 | 2069 | 1,2 |
| 69 | Heneicosane | 0.02 | 2114 | 1,2 |
| 70 | Phytol | 0.06 | 2128 | 1,2 |
| 71 | Cryptopinone | 0.03 | 2178 | 1,2 |
| 72 | Dehydroabietal | 0.08 | 2282 | 1,2 |
| 73 | Methyl isopimarate | 0.02 | 2310 | 1,2 |
| 74 | Tricosane | 0.01 | 2315 | 1,2 |
| 75 | Methyl dehydroabietate | 0.03 | 2355 | 1,2 |
| 76 | Cannabidiol | 1.17 | 2445 | 1,2 |
| 77 | Cannabichromene | 0.07 | 2450 | 1,2 |
| 78 | Tetracosane | 0.03 | 2716 | 1,2 |
| 79 | Nonacosane | 0.06 | 2916 | 1,2 |
| Total | 100.00 | |||
| Monoterpene hydrocarbons | 40.51 | |||
| Oxygenated monoterpenes | 0.52 | |||
| Sesquiterpene hydrocarbons | 52.26 | |||
| Oxygenated sesquiterpenes | 4.87 | |||
| Cannabinoids | 1.24 | |||
| Others | 0.60 | |||
| Essential oil yield % (v/w) | 0.2 |
a Linear retention index on an HP-5MS column; b Identification method: 1 = linear retention index; 2 = identification based on the comparison of mass spectra; 3 = Co-injection with standard compounds; t = traces, less than 0.01%.
Table 2.
Chemical composition of EO from P. vera L. var. Bronte hull.
| Compound Name | Area (%) | KI a | Identification b | |
|---|---|---|---|---|
| 1 | Bornylene | 0.03 | 916 | 1,2 |
| 2 | Tricyclene | 0.72 | 923 | 1,2 |
| 3 | α-Pinene | 22.65 | 935 | 1,2,3 |
| 4 | Camphene | 3.88 | 950 | 1,2,3 |
| 5 | β-Pinene | 1.02 | 978 | 1,2,3 |
| 6 | β-Myrcene | 2.43 | 993 | 1,2,3 |
| 7 | 2-Carene | 1.05 | 995 | 1,2,3 |
| 8 | α-Phellandrene | 0.47 | 1006 | 1,2 |
| 9 | δ-3-Carene | 7.98 | 1011 | 1,2,3 |
| 10 | α-Terpinene | 2.33 | 1018 | 1,2,3 |
| 11 | p-Cymene | 1.42 | 1027 | 1,2 |
| 12 | D-Limonene | 8.50 | 1031 | 1,2,3 |
| 13 | trans-β-Ocimene | 0.48 | 1050 | 1,2 |
| 14 | cis-β-Ocimene | 0.35 | 1056 | 1,2 |
| 15 | γ-Terpinene | 0.58 | 1061 | 1,2 |
| 16 | 4-Carene | 32.03 | 1082 | 1,2 |
| 17 | α-Pinene oxide | 0.69 | 1096 | 1,2 |
| 18 | Linalool | 0.38 | 1101 | 1,2,3 |
| 19 | 2-Fenchanol | 0.44 | 1107 | 1,2 |
| 20 | 1,3,8-p-Menthatriene | 0.17 | 1130 | 1,2 |
| 21 | Camphor | 0.22 | 1148 | 1,2 |
| 22 | Menthone | 0.35 | 1150 | 1,2 |
| 23 | Borneol | 1.03 | 1169 | 1,2 |
| 24 | p-Cymen-8-ol | 0.74 | 1188 | 1,2 |
| 25 | α-Terpineol | 3.99 | 1194 | 1,2,3 |
| 26 | Myrtenal | 0.03 | 1197 | 1,2 |
| 27 | Myrtenol | 0.06 | 1202 | 1,2 |
| 28 | α-Methylcynnamaldehyde | 0.05 | 1210 | 1,2 |
| 29 | Piperitone | 0.53 | 1250 | 1,2 |
| 30 | Nerol | 0.34 | 1232 | 1,2 |
| 31 | Bornyl acetate | 2.37 | 1285 | 1,2,3 |
| 32 | Nerol acetate | 0.18 | 1365 | 1,2 |
| 33 | β-Bisabolene | 0.05 | 1513 | 1,2 |
| 34 | γ-Selinene | 0.09 | 1525 | 1,2 |
| 35 | δ-Cadinene | 0.07 | 1530 | 1,2 |
| 36 | cis-5-Dodecenoic acid | 0.13 | 1568 | 1,2 |
| 37 | 1,13-Tetradecadiene | 1.43 | 1810 | 1,2 |
| 38 | 1-Hexadecanol | 0.13 | 1880 | 1,2 |
| 39 | Palmitic acid | 0.05 | 1957 | 1,2 |
| 40 | 1,15-Hexadecadiene | 0.43 | 2549 | 1,2 |
| Total | 99.87 | |||
| Monoterpene hydrocarbons | 86.20 | |||
| Oxygenated monoterpenes | 11.37 | |||
| Sesquiterpene hydrocarbons | 0.21 | |||
| Oxygenated sesquiterpenes | 0.00 | |||
| Others | 2.22 | |||
| Essential oil yield % (v/w) | 0.3 |
a Linear retention index on an HP-5MS column; b Identification method: 1 = linear retention index; 2 = identification based on the comparison of mass spectra; 3 = Co-injection with standard compounds.
Table 3.
Chemical composition of Z. officinale L. rhizome EO.
| Compound Name | Area (%) | KI a | Identification b | |
|---|---|---|---|---|
| 1 | α-Pinene | 2.47 | 861 | 1,2 |
| 2 | Santolina triene | 6.29 | 873 | 1,2 |
| 3 | β-Pinene | 0.45 | 896 | 1,2 |
| 4 | m-Mentha-1(7),8-diene | 0.32 | 912 | 1,2 |
| 5 | Camphene | 1.28 | 914 | 1,2 |
| 6 | α-Phellandrene | 0.46 | 923 | 1,2 |
| 7 | iso-Sylvestrene | 0.06 | 927 | 1,2 |
| 8 | α-Terpinene | 0.08 | 934 | 1,2 |
| 9 | Eucalyptol | 10.32 | 949 | 1,2 |
| 10 | δ-3-Carene | t | 974 | 1,2 |
| 11 | (2E,4E)-Heptadienol | t | 975 | 1,2 |
| 12 | Sylvestrene | t | 983 | 1,2 |
| 13 | (Z)-β-Ocimene | 0.36 | 997 | 1,2 |
| 14 | m-Cymenene | t | 999 | 1,2 |
| 15 | 2-Nonanone | 0.22 | 1004 | 1,2 |
| 16 | 6-Camphenone | 0.24 | 1009 | 1,2 |
| 17 | Linalool | 0.55 | 1014 | 1,2 |
| 18 | 2-Nonanol | 0.22 | 1016 | 1,2 |
| 19 | 1,3,8-p-Menthatriene | t | 1019 | 1,2 |
| 20 | endo-Fenchol | t | 1024 | 1,2 |
| 21 | trans-p-Menth-2-en-1-ol | 0.07 | 1029 | 1,2 |
| 22 | allo-Ocimene | 0.12 | 1039 | 1,2 |
| 23 | 2-(1Z)-Propenyl-phenol | t | 1041 | 1,2 |
| 24 | (3E,6Z)-Nonadienol | t | 1044 | 1,2 |
| 25 | iso-Pulegol | 0.14 | 1054 | 1,2 |
| 26 | neo-iso-Pulegol | 0.71 | 1062 | 1,2 |
| 27 | Borneol | 1.52 | 1074 | 1,2 |
| 28 | Terpinen-4-ol | 0.13 | 1083 | 1,2 |
| 29 | (E)-Isocitral | 0.67 | 1091 | 1,2 |
| 30 | γ-Terpineol | 0.54 | 1098 | 1,2 |
| 31 | trans-Piperitol | 0.06 | 1100 | 1,2 |
| 32 | Citronellol | 0.94 | 1135 | 1,2 |
| 33 | Isobornyl formate | 4.89 | 1142 | 1,2 |
| 34 | Neral | 0.74 | 1161 | 1,2 |
| 35 | 2-Pentyl-ciclopent-2-en-1-one | 2.07 | 1172 | 1,2 |
| 36 | p-Menth-1-en-9-ol | 0.15 | 1182 | 1,2 |
| 37 | 2-Undecanone | 0.60 | 1193 | 1,2 |
| 38 | Undecen-10-en-1-al | 0.09 | 1196 | 1,2 |
| 39 | Cyclosativene | 0.21 | 1222 | 1,2 |
| 40 | α-Copaene | t | 1236 | 1,2 |
| 41 | 4aα,7α,7aβ-Nepetalactone | 0.12 | 1245 | 1,2 |
| 42 | iso-Longifolene | 0.39 | 1248 | 1,2 |
| 43 | α-Cubebene | 0.69 | 1260 | 1,2 |
| 44 | γ-Elemene | 0.27 | 1275 | 1,2 |
| 45 | α-Guaiene | 1.17 | 1278 | 1,2 |
| 46 | 6,9-Guaiadiene | 0.28 | 1293 | 1,2 |
| 47 | cis-Muurola-3,5-diene | 1.04 | 1295 | 1,2 |
| 48 | trans-Muurola-3,5-diene | 0.83 | 1305 | 1,2 |
| 49 | α-Humulene | 1.92 | 1312 | 1,2 |
| 50 | allo-Aromadendrene | 2.27 | 1336 | 1,2 |
| 51 | α-Acoradiene | 5.27 | 1360 | 1,2 |
| 52 | 9-epi-(E)-Caryophyllene | 1.56 | 1369 | 1,2 |
| 53 | β-Acoradiene | 5.72 | 1376 | 1,2 |
| 54 | α-Zingiberene | 15.92 | 1383 | 1,2 |
| 55 | γ-Amorphene | 11.55 | 1384 | 1,2 |
| 56 | Viridiflorene | 0.06 | 1395 | 1,2 |
| 57 | γ-Patchoulene | 8.77 | 1402 | 1,2 |
| 58 | (Z)-γ-Bisabolene | 0.37 | 1407 | 1,2 |
| 59 | cis-Cadinene-ether | 0.22 | 1411 | 1,2 |
| 60 | trans-Dauca-4(11),7-diene | 0.32 | 1424 | 1,2 |
| 61 | Germacrene B | 0.60 | 1428 | 1,2 |
| 62 | epi-Cedrol | 0.18 | 1463 | 1,2 |
| 63 | 1-epi-Cubenol | 0.47 | 1486 | 1,2 |
| 64 | allo-Aromadendrene epoxide | 0.38 | 1495 | 1,2 |
| 65 | β-Eudesmol | 0.30 | 1511 | 1,2 |
| 66 | α-Cadinol | 0.12 | 1517 | 1,2 |
| Total | 97.76 | |||
| Monoterpene hydrocarbons | 22.72 | |||
| Oxygenated monoterpenes | 11.73 | |||
| Sesquiterpene hydrocarbons | 60.57 | |||
| Oxygenated sesquiterpenes | 1.71 | |||
| Others | 3.27 | |||
| Essential oil yield % (v/w) | 0.7 |
a Linear retention index on an HP-5MS column; b Identification method: 1 = linear retention index; 2 = identification based on the comparison of mass spectra; 3 = Co-injection with standard compounds; t = traces, less than 0.01%.
Table 4.
Chemical composition of EO from C. sempervirens L. cones.
| Compound Name | Area (%) | KI a | Identification b | |
|---|---|---|---|---|
| 1 | α-Pinene | 43.25 | 820 | 1,2 |
| 2 | α-Fenchene | 0.96 | 873 | 1,2 |
| 3 | Verbenene | 0.06 | 893 | 1,2 |
| 4 | Sabinene | 3.24 | 897 | 1,2 |
| 5 | β-Pinene | 1.95 | 915 | 1,2 |
| 6 | α-Phellandrene | t | 924 | 1,2 |
| 7 | p-Mentha-1(7),8-diene | 16.47 | 932 | 1,2 |
| 8 | iso-Sylvestrene | 0.23 | 935 | 1,2 |
| 9 | p-Cimene | t | 938 | 1,2 |
| 10 | o-Cimene | 0.44 | 943 | 1,2 |
| 11 | (E)-β-Ocimene | 4.49 | 948 | 1,2 |
| 12 | γ-Terpinene | 1.37 | 958 | 1,2 |
| 13 | Terpinolene | t | 983 | 1,2 |
| 14 | p-Mentha-2-4(8)-diene | 3.76 | 999 | 1,2 |
| 15 | α-Pinene oxide | t | 1005 | 1,2 |
| 16 | cis-Limonene oxide | t | 1015 | 1,2 |
| 17 | 1,3,8-p-Menthatriene | t | 1020 | 1,2 |
| 18 | trans-p-Mentha-2,8-dien-1-ol | t | 1024 | 1,2 |
| 19 | allo-Ocimene | 2.61 | 1040 | 1,2 |
| 20 | cis-p-Mentha-2,8-dien-1-ol | t | 1046 | 1,2 |
| 21 | cis-Verbenol | 0.17 | 1048 | 1,2 |
| 22 | trans-Sabinol | t | 1059 | 1,2 |
| 23 | (E)-Tagetone | 0.06 | 1073 | 1,2 |
| 24 | neo-Isopulegol | 0.65 | 1083 | 1,2 |
| 25 | neo-iso-3-Thujanol | 0.10 | 1098 | 1,2 |
| 26 | Pentyl cyclohexa-1,3-diene | 0.06 | 1105 | 1,2 |
| 27 | (Z)-Isocitral | t | 1137 | 1,2 |
| 28 | Anisole | 0.39 | 1143 | 1,2 |
| 29 | Neo-iso-Isopulegol | 0.64 | 1180 | 1,2 |
| 30 | cis-Sabinene hydrate acetate | 0.66 | 1230 | 1,2 |
| 31 | α-Cubebene | 0.49 | 1236 | 1,2 |
| 32 | α-Terpinyl acetate | 2.25 | 1240 | 1,2 |
| 33 | α-Ylangene | 0.10 | 1260 | 1,2 |
| 34 | α-Copaene | 0.10 | 1278 | 1,2 |
| 35 | Longifolene | 0.58 | 1286 | 1,2 |
| 36 | α-Cedrene | 3.97 | 1294 | 1,2 |
| 37 | α-Gurjunene | 2.01 | 1305 | 1,2 |
| 38 | α-Santalene | 1.30 | 1329 | 1,2 |
| 39 | β-Cedrene | 0.62 | 1339 | 1,2 |
| 40 | β-Copaene | 1.67 | 1354 | 1,2 |
| 41 | Aromadendrene | 1.06 | 1372 | 1,2 |
| 42 | γ-Elemene | 0.48 | 1388 | 1,2 |
| 43 | Valencene | 0.62 | 1399 | 1,2 |
| 44 | Caryophyllene oxide | 0.17 | 1499 | 1,2 |
| 45 | Cedrol | 0.24 | 1470 | 1,2 |
| 46 | α-Acoradiene | 0.12 | 1477 | 1,2 |
| 47 | Himachalol | 0.15 | 1507 | 1,2 |
| 48 | α-Cadinol | 0.24 | 1517 | 1,2 |
| Total | 97.73 | |||
| Monoterpene hydrocarbons | 81.34 | |||
| Oxygenated monoterpenes | 3.96 | |||
| Sesquiterpene hydrocarbons | 13.30 | |||
| Oxygenated sesquiterpenes | 0.94 | |||
| Others | 0.46 | |||
| Essential oil yield % (v/w) | 0.6 |
a Linear retention index on an HP-5MS column; b Linear retention index on an HP-Innowax column; c Identification method: 1 = linear retention index; 2 = identification based on the comparison of mass spectra; 3 = Co-injection with standard compounds; t = traces, less than 0.01%.
Seventy-nine compounds were detected in the C. sativa var. Futura 75 EO (Table 1).
Sesquiterpenes represent the most abundant class (52.26%), followed by monoterpenes (40.51%), oxygenated sesquiterpenes (4.87%), cannabinoids (1.24%), and oxygenated monoterpenes (0.52%). Major sesquiterpenes include α-caryophillene (21.68%), β-caryophillene (9.86%), caryophillene oxide (3.83%), α-bergamotene (3.22%), selina-3,7(11)diene (2.54%), and δ-Guaiene (2.16%). The most abundant monoterpenes are α-terpinolene (9.35%), β-myrcene (9.32%), α-(+)-pinene (7.82%), trans-β-ocimene (4.62%), (1S)-(−)-β-pinene (3.73%), and D-limonene (2.92%). Among cannabinoids, cannabidiol is the most representative (1.17%).
Into the EO of P. vera L. var. Bronte hull, 40 compounds were detected (Table 2), belonging mainly to the class of monoterpene hydrocarbons (86.20%), and oxygenated monoterpenes (11.37%) and sequiterpenes (0.21%) are less represented.
Among monoterpenes, the most abundant compounds are 4-carene (32.03%), α-pinene (22.65%), D-limonene (8.50%), δ-3-carene (7.98%), α-terpineol (3.99%), camphene (3.88%), β-myrcene (2.43%), bornyl acetate (2.37%), and α-terpinene (2.33%).
Z. officinale EO showed a chemical composition similar to hemp EO regarding the relative abundance of terpene classes. In fact, among the 66 compounds identified, the most abundant ones belong to sesquiterpene hydrocarbons (60.57%), followed by monoterpenes (22.72%), oxygenated monoterpenes (11.73%), and oxygenated sesquiterpenes (1.71%). Major sesquiterpenes include α-zingiberene (15.92%), γ-amorphene (11.55%), γ-patchoulene (8.77%), β-acoradiene (5.72%), α-acoradiene (5.27%), and allo-aromadendrene (2.27%). Among monoterpenes, the most abundant are eucalyptol (10.32%), santolina triene (6.29%), isobornyl formate (4.89), and α-pinene (2.47%) (Table 3).
Finally, C. sempervirens EO showed the presence of 48 compounds belonging predominantly to monoterpene hydrocarbons (81.34%). Sesquiterpene hydrocarbons (13.30%), oxygenated monoterpenes (3.96%), and oxygenated sesquiterpenes (0.94%) were in less amounts (Table 4).
The most abundant monoterpenes are α-pinene (43.25%), p-mentha-1(7),8-diene (16.47%), (E)-β-ocimene (4.49%), p-mentha-2-4(8)-diene (3.76%), sabinene (3.24%), allo-ocimene (2.61%), and α-terpinyl acetate (2.25%). α-Cedrene (3.97%) and α-gurjunene (2.01%) predominated in sesquiterpenes.
The pistachio hull EO showed the highest content of monoterpenes, followed by cypress EO, while the ginger EO showed the highest content of sesquiterpenes, followed by hemp EO.
2.3. Phytotoxic Activity
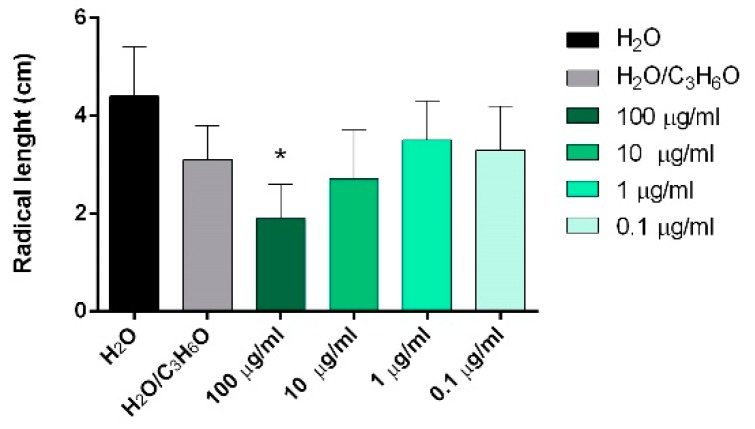
In order to evaluate the in vitro phytotoxic activity of the selected EOs, six seeds (radish, garden cress, lettuce, tomato, ryegrass, and purslane) were used, estimating their germination and radical elongation. Only P. vera EO showed activity against the germination of P. oleracea seeds after a treatment with 100, 10, and 1 μg/mL (Figure 2).
Figure 2.
Phytotoxic activity of P. vera EO against germination of Portulaca oleracea L., 120 h after sowing. Results are the mean ± standard deviation (n = 3) of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to control (ANOVA followed by Dunnett’s multiple comparison test).
C. sempervirens, Z. officinale, C. sativa, and P. vera EOs showed, in different ways, statistically significant activity against initial radical elongation on L. sativum, L. multiflorum, and R. sativus seeds (Figure 3, Figure 4 and Figure 5).
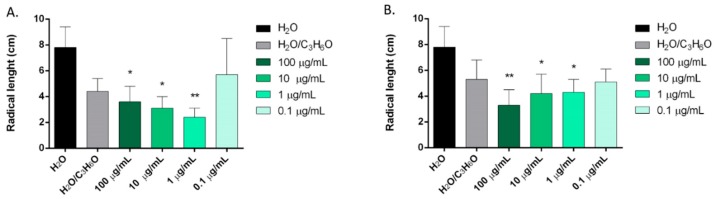
Figure 3.
Phytotoxic activity of C. sempervirens (A) and Z. officinale (B) EOs against radical elongation of Lepidium sativum L., 120 h after sowing. Results are the mean ± standard deviation (n = 3) of three independent experiments. * p < 0.05, ** p < 0.01 compared to control (ANOVA followed by Dunnett’s multiple comparison test).
Figure 4.
Phytotoxic activity of Z. officinale EO against radical elongation of Lolium multiflorum Lam.,120 h after sowing. Results are the mean ± standard deviation (n = 3) of three independent experiments. * p < 0.05, compared to control (ANOVA followed by Dunnett’s multiple comparison test).
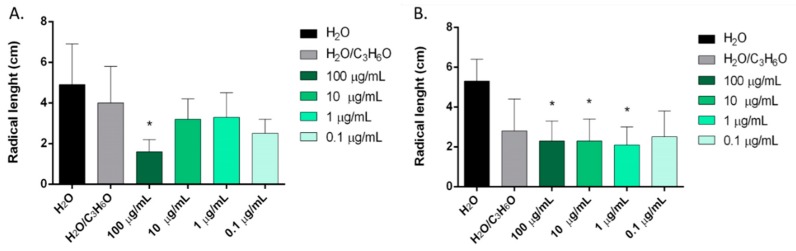
Figure 5.
Phytotoxic activity of P. vera (A) and C. sativa (B) EOs against radical elongation of Raphanus sativus L., 120 h after sowing. Results are the mean ± standard deviation (n = 3) of three independent experiments. * p < 0.05, compared to control (ANOVA followed by Dunnett’s multiple comparison test).
C. sempervirens and Z. officinale EOs were able to inhibit the radical elongation of L. sativum at concentrations of 100, 10, and 1 μg/mL (Figure 3).
Moreover, Z. officinale EO was the only one that also showed phytotoxic activity against the radical elongation of L. multiflorum seeds at the highest concentration tested (100 μg/mL) (Figure 4).
P. vera hull and C. sativa EOs were active against the radical elongation of R. sativus (Figure 5). P. vera EO inhibited the radical elongation at the highest concentration used (100 μg/mL), and C. sativa var. Futura 75 EO inhibited radical elongation at 100, 10, and 1 μg/mL.
3. Discussion
In this study, the chemical composition and possible phytotoxic activity of EOs obtained from different plant sources were investigated. In particular, EOs from the spent materials of Z. officinale, used in the juicing industry; discarded material of P. vera, used in in the food industry; discarded material of industrial hemp (C. sativa var. Futura 75); and pruning waste from the tree C. sempervirens were obtained. All these materials are generally considered to be byproducts or waste products; however, they can be a rich source of EOs with a wide range of applications.
Secretory tissues occurring in most vascular plants differ not only in structure and localization but also in terms of the secreted material. Different plant species synthesize lipophilic substances, such as EOs and resin, which have been used by humans throughout the ages for many purposes and are still of interest for environmental, agricultural, food, and medical applications. Secretory tissues may consist of single cells or hydathodes (e.g., in the Zingiber rhizome) or small to very large groups of cells. Glandular trichomes are located on the plant surface, such as in the female inflorescence of C. sativa, while secretory ducts are located inside the plant organs, as occurs in the resin ducts of the C. semprevirens cones and the P. vera hull.
There are few studies in the available literature on the chemical composition of C. sativa var. Futura 75. The EO analyzed in the present study showed a similar composition with respect to an EO isolated from hemp leaves by Benelli et al., with sesquiterpene hydrocarbons as the main constituents (52.5%) [15]. However, Nissen et al. showed a completely different phytochemical profile, with monoterpenes as the most abundant components [16].
The phytochemical profile of the EO from P. vera var. Bronte hull was superimposable with that which was previously analyzed; this is essentially due to the collection time and place, which were the same as previously reported [17]. However, the chemical composition of the EO reported in this study disagrees with results reported for P. vera EO belonging to other varieties and from different countries. P. vera EO var. Mateur from Tunisia is rich in α-pinene (42.5%) and terpinolene (32.2%), while the latter compound is totally absent in the sample here investigated [18]. Hashemi-Moghaddam et al. reported α-pinene (31.8%), α-terpinolene (20.3%), and myrcene (12.2%) as the main components of the EO obtained from P. vera var. Shahpasand hull cultivated in Iran [19].
Z. officinale EO was richest in sesquiterpenes, with a higher concentration of α-zingiberene than those reported in previous studies [20,21]. However, Lagha et al. already reported the most abundant presence of sesquiterpenes and α-zingiberene as the main compound in Z. officinale EO from France [22].
Few studies have analyzed the chemical composition of C. sempervirens cone EO. Our results are in accordance with Milos et al. [23] and Tumen et al. [24], who reported α-pinene as the main constituent of C. sempervirens cone EO, with percentages of 69.9% and 66.7%, respectively. Selim et al. also showed this as the main constituent in the EO from the aerial parts of C. sempervirens oil [25].
The possible phytotoxic effects of the EOs against germination and initial radical elongation of R. sativus L., L. sativum L., L. sativa L., S. lycopersicum, L., L. multiflorum Lam., and P. oleracea L. were evaluated. Few studies have been carried out to investigate the potential phytotoxicity of EOs against these six selected species.
In the present work, none of the EOs inhibited germination or radical elongation of S. lycopersicum. Nevertheless, Rolli et al. showed that C. sativa, Z. officinale, and C. sempervirens EOs are able to inhibit S. lycopersicum root length, with percentages of 47.9%, 73.8%, and 0.6%, respectively [26].
These differences are attributable to the different phytochemical profiles of the EOs investigated in the present work, which certainly influenced their phytotoxic activity [27]. Indeed, C. sativa EO showed a completely different phytochemical profile with respect to that reported in the previous work, with sesquiterpenes as the most abundant compounds with respect to monoterpenes and, in any case, a very low presence of oxygenated metabolites. C. sempervirens EO showed a similar phytochemical distribution, with monoterpenes as the most abundant compounds with respect to sesquiterpenes but, also in this case, with a low presence of oxygenated compounds. Finally, Z. officinale EO, even regarding the sesquiterpenes and monoterpenes ratios, showed a total inversion in the oxygenated sesquiterpenes content, which in the previous work represented about 99.70% of the total sesquiterpenes, compared with that investigated in the present work, in which they represent only 2.75%. In light of this, it is possible to hypothesize that the phytotoxic activity detected by Rolli et al. [26] against S. lycopersicum was mainly due to the presence of oxygenated metabolites and, in particular, to the oxygenated sesquiterpenes given the highlighted order of potency: Z. officinale > C. sativa > C. sempervirens.
The phytotoxic effect of an EO obtained by female inflorescences of another fiber hemp cultivar (Bialobrzeskie) was observed also against germination of other weeds and crops with redroot pigweed and rye brome, which resulted the most susceptible plant species. On the contrary, oilseed rape and oats have shown the most resistance [28].
Although in this study Z. officinale and C. sempevirens EOs showed radical elongation inhibition of L. sativum seeds, in literature, there are no other data on the phytotoxic activity of Z. officinale EO on this plant species and only one study showing a strong inhibitory effect of C. sempervirens aqueous extract on seed germination of lettuce, radish, and tomato [29,30].
C. sativa and P. vera EOs were active against radical elongation of R. sativus. Moreover, Z. officinale and P. vera EOs were active against the two weed species L. multiflorum and P. oleracea.
These results agree with Ismail et al., who showed that P. vera and P. terebinthus L. EOs strongly inhibited the germination and seedling growth of Sinapis arvensis L., Trifolium campestre Schreb., Lolium rigidium Gaudin, and Phalaris canariensis L. in a dose-dependent manner. No previous data were present on the possible phytotoxic activity of C. sativa EO on similar weed species [31].
However, recently, the allopathic effect of water extracts of fiber hemp on the germination energy and rate of monocot (spring wheat and winter rye) and dicot (yellow lupine and winter rape) crop species were investigated. Hemp extract decreased the germination rate in particular of monocot plants, although all four species investigated produced shorter roots at the highest concentration tested [32].
EOs are reported in literature for their phytotoxic activity, acting as inhibitors of both seed germination and radical elongation, with different potencies [3,33]. Monoterpenes and, in particular, oxygenated compounds seem to be responsible for such activity, above all when they are ketones, alcohols, aldehydes, and phenols [34,35].
However, it has been demonstrated that herbicidal activity as well as antimicrobial and insecticide activity is due to the synergy of major constituents of EOs with less phytotoxic components [36].
An advantage of the use of EOs as botanical pesticides with respect to synthetic ones, other than the already well-studied and endorsed toxic effects on the human health of the latter, is the development of growing resistance. The use of botanical pesticides and, in particular, of EOs could solve this problem because it has been demonstrated that complex mixtures rarely, or at least more slowly, cause resistance phenomena. The disadvantages of EOs are certainly the volatility and the high cost. It has been demonstrated that post-application temperature influences the insecticidal activity of EOs [37]. Also, if to date no data were available about the possible repercussions of temperature on the anti-germination activity of EOs, since they are very volatile compounds, an indirect correlation could be hypothesized.
In light of this, the results of the present research open new perspectives in the reutilization of byproducts of aromatic plants. In fact, this waste material can be used for some agricultural practices, with evident economic and environmental advantages. In particular, mulching with aromatic plant byproducts has been proposed as an effective method in organic agriculture [38,39].
4. Materials and Methods
4.1. Chemicals
C7–C40 saturated alkane standard mix and Na2SO4 were purchased from Sigma-Aldrich (Milan, Italy). Terpene standards (≥98%) were purchased from Extrasynthese (Genay, France). GC-grade dichloromethane was purchased from Merck (Darmstadt, Germany).
4.2. Plant Material and Isolation of Essential Oil
Spent materials or pruning waste from Z. officinale, C. sativa var. Futura 75, and C. sempervirens were obtained from FX Laboratorio Benessere s.r.l., Vicenza (Italy). P. vera var. Bronte hull was obtained from a local farmer in Bronte (Catania, Italy). Plant materials were subjected to steam distillation until no significant increase in the volume of the collected EO was observed (3 h). EOs were dried on Na2SO4 and stored in a dark-sealed vial with nitrogen headspace until analysis.
4.3. Micromorphological Analysis
Small pieces of each sample, approximately 1 cm2, were sectioned with a razor blade and fixed overnight at 4 °C in FineFIX working solution (Milestone s.r.l., Bergamo, Italy) with 70% ethanol, according to Chieco et al. [40]. The specimens were then dehydrated through a graded series of ethanol (80%, 90%, 95%, and 100%) and finally in CO2 by a critical point dryer apparatus (K850 CPD 2M Strumenti S.r.l., Roma, Italy). Dried samples were mounted on stubs, coated with 10 nm of gold, and observed with a Vega3 Tescan LMU scanning electron microscope (SEM) (Tescan USA Inc., Warrendale, PA, USA) at an accelerating voltage of 20 kV.
4.4. Gas Chromatographic Flame Ionization Detection (GC-FID) and GC-MS Analysis
GC analysis was performed on an Agilent gas chromatograph, model 7890A, equipped with a flame ionization detector (Agilent Technologies Santa Clara, CA, USA). An HP-5MS capillary column (30 mm, 0.25 mm coated with 5% phenyl methyl silicone, 95% dimethyl polysiloxane, 0.25 μm film thickness) and helium as the carrier gas (1 mL/min) were used. One microliter of 10% essential oil/CH2Cl2 v/v was injected by split mode (50:1). The injector and detector temperature were 250 °C and 280 °C, respectively. The following elution program was used: 60 °C for 6 min, increased to 270 °C at 3 °C/min, and held at 270 °C for 4 min. Percentages of compounds were determined from their peak areas in the GC-FID profiles. GC-MS analysis was carried out on the above instrument, coupled with an Agilent 5975C mass detector with the same column and the same operative conditions used for the analytical GC. We adjusted the ionization voltage to 70 eV, the electron multiplier to 900 V, and the ion source temperature to 230 °C.
4.5. Identification of the Essential Oil Components
Mass spectra data were acquired in scan mode (m/z range of 45–450 amu). Detected compounds were identified based on the following parameters: GC retention index (relative to C7–C40 n-alkanes on the HP-5MS column), values reported in the literature [41], matching of mass spectral data with those of the MS library (NIST 08) [42], comparison of MS fragmentation patterns with those reported in the literature, and co-injection with commercially available terpene standards.
4.6. Phytotoxic Activity
The phytotoxic activity was evaluated on the germination and radical elongation of six different plant species: R. sativus L. (radish), L. sativa L. (lettuce), L. sativum L. (garden cress), S. lycopersicum L. (tomato), L. multiflorum Lam. (ryegrass), and P. oleracea L. (purslane). These seeds are usually used in assays of phytotoxicity because they easily germinable and well known from the histological point of view. R. sativus, L. sativa, L. sativum, and S. lycopersicum seeds were purchased from the Blumen Group s.r.l. (Emilia Romagna); L. multiflorum seeds were purchased from Fratelli Ingegnoli Spa (Milano, Italy); and P. oleracea seeds from W. Legutko s.r.l. (Jutrosin, Polland). The seeds were surface sterilized in 95% ethanol for 15 s and sown in petri dishes (Ø = 90 mm) containing three layers of Whatman filter paper impregnated with distilled water (7 mL, control) or the tested solution of the essential oil (7 mL) at different doses. The germination conditions were 20 ± 1 °C with a natural photoperiod. The EOs, in a water–acetone mixture (99.5:0.5), were assayed at the doses of 100, 10, 1, and 0.1 µg/mL. Controls performed with the water–acetone mixture alone showed no appreciable differences in comparison to controls in water alone. Seed germination was observed directly in petri dishes every 24 h. A seed was considered germinated when the protrusion of the root became evident [43]. After 120 h (on the fifth day), the effects on radical elongation were measured in centimeters. Each determination was repeated three times, using petri dishes containing 10 seeds each. Data are expressed as the mean ± SD for both germination and radical elongation.
4.7. Statistical Analysis
All experiments were carried out in triplicate and the results are expressed as mean ± standard deviation (n = 3). Data of each experiment were statistically analyzed using GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA, USA), followed by comparison of means (one-way ANOVA) using Dunnett’s multiple comparisons test at the significance level of p < 0.05.
5. Conclusions
The EOs analyzed showed marked and selective phytotoxic properties, inhibiting the radical elongation of R. sativus, L. sativum, and L. multiflorum and the germination of P. oleracea. P. vera and Z. officinale EOs were more phytotoxic than C. sempervirens and C. sativa EOs; in fact, they were able to inhibit both weeds and food crops, so their use as a mulching material for crops or as a promising chemical herbicide alternative should be evaluated in depth.
This work opens new research perspectives on plant waste materials, in particular for aromatic plants. In fact, EOs are well known and studied for their antimicrobial and phytotoxic properties. However, they have a rather high cost due essentially to the value of the raw material. In this regard, the use of waste products or spent materials to obtain EOs useful in agriculture as phytotoxic and antimicrobial agents would be desirable. In light of this, further research is needed in order to support this thesis, investigating the phytotoxic activity of these EOs on other weed and food crop species and the mode of action by which EOs exert their allopathic activity.
Moreover, considering that EOs act and volatilize very quickly, further studies on alternative formulations, such as microencapsulation, are necessary to increase EO efficacy and reduce EO volatilization.
Author Contributions
Conceptualization, V.D.F., L.C. (Laura Cornara), and D.T.; methodology, A.S.; investigation, A.S. and L.C. (Lucia Caputo); formal analysis, M.V.; data curation, L.C. (Lucia Caputo); writing—original draft preparation, A.S., L.C. (Laura Cornara), and L.C. (Lucia Caputo); writing—review and editing, V.D.F.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the essential oils are available from the authors.
References
- 1.Dayan F.E., Cantrell C.L., Duke S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Koul O., Walia S., Dhaliwal G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008;4:63–84. [Google Scholar]
- 3.De Almeida L.F.R., Frei F., Mancini E., De Martino L., De Feo V. Phytotoxic activities of Mediterranean essential oils. Molecules. 2010;15:4309–4323. doi: 10.3390/molecules15064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heap I. The International Survey of Herbicide Resistant Weeds 2014. [(accessed on 27 February 2014)]; Available online: www/weedscience.org.
- 5.Said-Al Ahl H.A., Hikal W.M., Tkachenko K.G. Essential oils with potential as insecticidal agents: A review. Int. J. Environ. Plan. Manag. 2017;3:23–33. [Google Scholar]
- 6.Macías F.A., Molinillo J.M.G., Galindo J.C.G., Varela R.M., Simonet A.M., Castellano D. The Use of Allelopathic Studies in the Search for Natural Herbicides. J. Crop Prod. 2001;4:237–255. doi: 10.1300/J144v04n02_08. [DOI] [Google Scholar]
- 7.Della Pepa T., Elshafie H.S., Capasso R., De Feo V., Camele I., Nazzaro F., Scognamiglio M.R., Caputo L. Antimicrobial and Phytotoxic Activity of Origanum heracleoticum and O. majorana Essential Oils Growing in Cilento (Southern Italy) Molecules. 2019;24:2576. doi: 10.3390/molecules24142576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azirak S., Karaman S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. Sect. B-Soil Plant Sic. 2008;58:88–92. doi: 10.1080/09064710701228353. [DOI] [Google Scholar]
- 9.Mancini E., De Martino L., Marandino A., Scognamiglio M.R., De Feo V. Chemical composition and possible in vitro phytotoxic activity of Helichrsyum italicum (Roth) Don ssp. italicum. Molecules. 2011;16:7725–7735. doi: 10.3390/molecules16097725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkatullah I.M., Muhammad N., De Feo V. Chemical composition and biological activities of the essential oil of Skimmia laureola leaves. Molecules. 2015;20:4735–4745. doi: 10.3390/molecules20034735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apostolico I., Aliberti L., Caputo L., De Feo V., Fratianni F., Nazzaro F., Souza L.F., Khadhr M. Chemical Composition, Antibacterial and Phytotoxic Activities of Peganum harmala Seed Essential Oils from Five Different Localities in Northern Africa. Molecules. 2016;21:1235. doi: 10.3390/molecules21091235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamiri A., Lhaloui S., Benjilali B., Berrada M. Insecticidal effects of essential oils against Hessian fly, Mayetiola destructor (Say) Field Crop. Res. 2001;7:9–15. doi: 10.1016/S0378-4290(01)00139-3. [DOI] [Google Scholar]
- 13.Sharma K., Mahato N., Cho M.H., Lee Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Schieber A., Stintzing F.C., Carle R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001;12:401–413. doi: 10.1016/S0924-2244(02)00012-2. [DOI] [Google Scholar]
- 15.Benelli G., Pavela R., Lupidi G., Nabissi M., Petrelli R., Kamte S.L.N., Maggi F. The crop-residue of fiber hemp cv. Futura 75: From a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. 2018;25:10515–10525. doi: 10.1007/s11356-017-0635-5. [DOI] [PubMed] [Google Scholar]
- 16.Nissen L., Zatta A., Stefanini I., Grandi S., Sgorbati B., Biavati B., Monti A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.) Fitoterapia. 2010;81:413–419. doi: 10.1016/j.fitote.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Smeriglio A., Denaro M., Barreca D., Calderaro A., Bisignano C., Ginestra G., Bellocco E., Trombetta D. In Vitro Evaluation of the Antioxidant, Cytoprotective, and Antimicrobial Properties of Essential Oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017;18:1212. doi: 10.3390/ijms18061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chahed T., Dhifi W., Hosni K., Msaada K., Kchouk M.E., Marzouk B. Composition of Tunisian pistachio hull essential oil during fruit formation and ripening. J. Essent. Oil Res. 2008;20:122–125. doi: 10.1080/10412905.2008.9699971. [DOI] [Google Scholar]
- 19.Hashemi-Moghaddam H., Mohammdhosseini M., Salar M. Chemical composition of the essential oils from the hulls of Pistacia vera L. by using magnetic nanoparticle-assisted microwave (MW) distillation: Comparison with routine MW and conventional hydrodistillation. Anal. Methods. 2014;6:2572–2579. doi: 10.1039/C3AY42180E. [DOI] [Google Scholar]
- 20.Snuossi M., Trabelsi N., Ben Taleb S., Dehmeni A., Flamini G., De Feo V. Laurus nobilis, Zingiber officinale and Anethum graveolens Essential Oils: Composition, Antioxidant and Antibacterial Activities against Bacteria Isolated from Fish and Shellfish. Molecules. 2016;21:1414. doi: 10.3390/molecules21101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifi-Rad M., Varoni E.M., Salehi B., Sharifi-Rad J., Matthews K.R., Ayatollahi S.A., Kobarfard F., Ibrahim S.A., Mnayer D., Zakaria Z.A., et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules. 2017;22:2145. doi: 10.3390/molecules22122145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagha R., Ben Abdallah F., Al-Sarhan B.O., Al-Sodany Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules. 2019;24:1161. doi: 10.3390/molecules24061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miloš M., Mastelić J., Radonić A. Free and glycosidically bound volatile compounds from cypress cones (Cupressus sempervirens L.) Croat. Chem. Acta. 1998;71:139–145. [Google Scholar]
- 24.Tumen I., Hafizoglu H., Pranovich A., Reunanen M. Chemical constituents of cones and leaves of cypress (Cupressus sempervirens L.) grown in Turkey. Fresenius Environ. Bull. 2010;19:2268–2276. [Google Scholar]
- 25.Selim S.A., Adam M.E., Hassan S.M., Albalawi A.R. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the Mediterranean cypress (Cupressus sempervirens L.) BMC Complement. Altern. Med. 2014;14:179. doi: 10.1186/1472-6882-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolli E., Marieschi M., Maietti S., Sacchetti G., Bruni R. Comparative phytotoxicity of 25 essential oils on pre-and post-emergence development of Solanum lycopersicum L.: A multivariate approach. Ind. Crop. Prod. 2014;60:280–290. doi: 10.1016/j.indcrop.2014.06.021. [DOI] [Google Scholar]
- 27.Ismail A., Mancini E., De Martino L., Marandino A., Lamia H., Mohsen H., Bassem J., Scognamiglio M., Reverchon E., De Feo V. Chemical composition and biological activities of the essential oils from three Melaleuca species grown in Tunisia. Int. J. Mol. Sci. 2012;13:16580–16591. doi: 10.3390/ijms131216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agnieszka S., Magdalena R., Jan B., Katarzyna W., Malgorzata B., Krzysztof H., Danuta K. Phytotoxic Effect of Fiber Hemp Essential Oil on Germination of Some Weeds and Crops. J. Essent. Oil Bear. Plants. 2016;19:262–276. doi: 10.1080/0972060X.2015.1137236. [DOI] [Google Scholar]
- 29.M’barek K. Chemical composition and phytotoxicity of Cupressus sempervirens leaves against crops. J. Essent. Oil Bear. Plants. 2016;19:1582–1599. doi: 10.1080/0972060X.2016.1226964. [DOI] [Google Scholar]
- 30.M’barek K., Zribi I., Ullah M.J., Haouala R. The mode of action of allelochemicals aqueous leaf extracts of some Cupressaceae species on lettuce. Sci. Hortic. 2019;252:29–37. [Google Scholar]
- 31.Ismail A., Lamia H., Mohsen H., Bassem J. Herbicidal potential of essential oils from three mediterranean trees on different weeds. Curr. Bioact. Compd. 2012;8:3–12. doi: 10.2174/157340712799828197. [DOI] [Google Scholar]
- 32.Pudełko K., Majchrzak L., Narożna D. Allelopathic effect of fibre hemp (Cannabis sativa L.) on monocot and dicot plant species. Ind. Crop. Prod. 2014;56:191–199. doi: 10.1016/j.indcrop.2014.02.028. [DOI] [Google Scholar]
- 33.Dhima K.V., Vasilakoglou I.B., Gatsis T.D., Panou-Pholotheou E., Eleftherohorinos I.G. Effects of aromatic plants incorporated as green manure on weed and maize development. Filed Crop. Res. 2009;110:235–241. doi: 10.1016/j.fcr.2008.09.005. [DOI] [Google Scholar]
- 34.Vokou D., Douvli P., Blionis G.J., Halley J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003;29:2281–2301. doi: 10.1023/A:1026274430898. [DOI] [PubMed] [Google Scholar]
- 35.De Martino L., Mancini E., Rolim de Almeida L.F., De Feo V. The antigerminative activity of twenty-seven monoterpenes. Molecules. 2010;15:6630–6637. doi: 10.3390/molecules15096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasilakoglou I., Dhima K., Paschalidis K., Ritzoulis C. Herbicidal potential on Lolium rigidum of nineteen major essential oil components and their synergy. J. Essent. Oil Res. 2013;25:1–10. doi: 10.1080/10412905.2012.751054. [DOI] [Google Scholar]
- 37.Pavela R., Sedlák P. Post-application temperature as a factor influencing the insecticidal activity of essential oil from Thymus vulgaris. Ind. Crop. Prod. 2018;113:46–49. doi: 10.1016/j.indcrop.2018.01.021. [DOI] [Google Scholar]
- 38.De Falco E., De Feo V., Zaccardelli M., De Nicola M., Tarangelo M. Effects of different vegetal mulching on Rosmarinus officinalis L.—First resutls. Acta Hortic. 2006;723:447–452. doi: 10.17660/ActaHortic.2006.723.64. [DOI] [Google Scholar]
- 39.Kamariari I., Papastylianou P., Bilalis D., Travlos I.S., Kakabouki I. The role of mulching with residues of two medicinal plants on weed diversity in maize; Proceedings of the 4th ISOFAR Scientific Conference at the Organic World Congress on ‘Building Organic Bridges’; Istanbul, Turkey. 13–15 October 2014. [Google Scholar]
- 40.Chieco C., Rotondi A., Morrone L., Rapparini F., Baraldi R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013;88:109–119. doi: 10.3109/10520295.2012.746472. [DOI] [PubMed] [Google Scholar]
- 41.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 42.McLafferty F.W. The Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom. 7th ed. John Wiley & Sons; New York, NY, USA: 1998. [Google Scholar]
- 43.Bewley D., Black M. Seeds: Physiology of Development and Germination. Plenum Press; New York, NY, USA: 1985. [Google Scholar]