Abstract
Objectives
To evaluate the ability of glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase (UCH-L1) to detect concussion in children and adult trauma patients with a normal mental status and assess biomarker concentrations over time as gradients of injury in concussive and non-concussive head and body trauma.
Design
Large prospective cohort study.
Setting
Three level I trauma centres in the USA.
Participants
Paediatric and adult trauma patients of all ages, with and without head trauma, presenting with a normal mental status (Glasgow Coma Scale score of 15) within 4 hours of injury. Rigorous screening for concussive symptoms was conducted. Of 3462 trauma patients screened, 751 were enrolled and 712 had biomarker data. Repeated blood sampling was conducted at 4, 8, 12, 16, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, 168 and 180 hours postinjury in adults.
Main outcomes
Detection of concussion and gradients of injury in children versus adults by comparing three groups of patients: (1) those with concussion; (2) those with head trauma without overt signs of concussion (non-concussive head trauma controls) and (3) those with peripheral (body) trauma without head trauma or concussion (non-concussive body trauma controls).
Results
A total of 1904 samples from 712 trauma patients were analysed. Within 4 hours of injury, there were incremental increases in levels of both GFAP and UCH-L1 from non-concussive body trauma (lowest), to mild elevations in non-concussive head trauma, to highest levels in patients with concussion. In concussion patients, GFAP concentrations were significantly higher compared with body trauma controls (p<0.001) and with head trauma controls (p<0.001) in both children and adults, after controlling for multiple comparisons. However, for UCH-L1, there were no significant differences between concussion patients and head trauma controls (p=0.894) and between body trauma and head trauma controls in children. The AUC for initial GFAP levels to detect concussion was 0.80 (0.73–0.87) in children and 0.76 (0.71–0.80) in adults. This differed significantly from UCH-L1 with AUCs of 0.62 (0.53–0.72) in children and 0.69 (0.64–0.74) in adults.
Conclusions
In a cohort of trauma patients with normal mental status, GFAP outperformed UCH-L1 in detecting concussion in both children and adults. Blood levels of GFAP and UCH-L1 showed incremental elevations across three injury groups: from non-concussive body trauma, to non-concussive head trauma, to concussion. However, UCH-L1 was expressed at much higher levels than GFAP in those with non-concussive trauma, particularly in children. Elevations in both biomarkers in patients with non-concussive head trauma may be reflective of a subconcussive brain injury. This will require further study.
Keywords: biomarkers; concussion; mild traumatic brain injury; subconcussive, head trauma, trauma, children; paediatric; glial fibrillary acidic protein (GFAP); Ubiquitin C-terminal hydrolase (UCH-L1); blood test
Introduction
Glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase (UCH-L1) have been evaluated in several studies to determine the need for CT scan and neurosurgical intervention in mild to moderate traumatic brain injury patients (mmTBI) in adults1–7 and more recently in children with mmTBI.8–10 In early 2018, GFAP and UCH-L1 were Food and Drug Administration-approved for clinical use in adult patients with mmTBI to help determine the need for CT scan within 12 hours of injury.11 The approval was based on the ability to find lesions on CT scan but was not approved to diagnose a concussion or a mild TBI. Moreover, it was not approved for use in children.
Following trauma, patients often have a constellation of injuries and it is important that TBI biomarkers indicate brain-specific injury to be clinically useful. A number of articles have described how GFAP and UCH-L1 were able to distinguish mmTBI patients from orthopaedic controls and motor vehicle crash (MVC) controls as well as in those TBI patients with negative CTs.1 3 4 In these studies, many trauma control patients were exposed to significant trauma including the acceleration–deceleration vectors of MVCs and substantial falls and both GFAP and UCH-L1 showed a graded response to severity of injury from normal controls to trauma controls, to mmTBI. Moreover, GFAP has consistently shown very good specificity to brain injury in cases of polytrauma.3 12 GFAP has demonstrated the ability to detect intracranial lesions in victims of multiple trauma with mild TBI who had substantial extracranial injuries and fractures.3
Importantly, there is a group of individuals with head trauma who have been significantly understudied, and in whom biomarkers are rarely, if at all, examined. These are people who experience head trauma without symptoms of concussion. They may be classified as having ‘no injury’ or they may represent milder forms of concussion that do not elicit the typical signs or symptoms associated with concussion and are referred to as subconcussive injuries. To date, there is a paucity of studies addressing the effects of subconcussive head impacts following head trauma. The issue of subconcussive trauma has been a particular concern in military personnel13 and in athletes, as repetitive subconcussive impacts have the potential for long-term deleterious effects.14–16 Therefore, studying these biomarkers in patients with head trauma without symptoms could provide unique insights into how neuronal and glial biomarkers behave in subconcussive trauma.17
There are insufficient data on the diagnostic accuracy of GFAP and UCH-L1 in children and adults in determining which trauma patients with normal mental status have a concussion and how well they perform over time following different degrees of mild head trauma. This study evaluated the diagnostic accuracy of serum glial and neuronal serum biomarkers GFAP and UCH-L1, both individually and in combination, in detecting the presence of a concussion and grading potential subconcussive and non-concussive brain injury in paediatric and adult trauma patients presenting to the emergency department with a normal mental status (Glasgow Coma Scale (GCS) score of 15). Gradients of brain injury were defined by comparing serum biomarker concentrations in three groups of patients: (1) those with concussion, (2) those with blunt head trauma without overt signs or symptoms of concussion (head trauma controls) and (3) those with peripheral (body) trauma without head trauma or concussion (body trauma controls). In addition, the temporal profiles of GFAP and UCH-L1 were measured over 7 days in these three groups in adults.
Methods
Study population
This prospective cohort study enrolled a convenience sample of adult and paediatric trauma patients presenting to the emergency departments of three level I trauma centres: a paediatric level 1 trauma centre in Philadelphia, Pennsylvania; a paediatric level 1 trauma centre in Orlando, Florida; and an affiliated adult level 1 trauma centre in Orlando, Florida. This study was approved by the respective institutional review boards (IRBs) of each institution. Informed consent was obtained from patients and/or their legal authorised representatives prior to enrolment, and assent was obtained for children between the ages of 7 and 18 years.
Eligibility for concussion patients was determined by the treating physician based on the history of blunt head trauma followed by either loss of consciousness, amnesia or disorientation (or change in behaviour in children) and presenting to the emergency department within 4 hours of injury with a GCS score of 15. Eligibility was also prospectively verified by the research team prior to enrolment. Head CT scans were performed at the discretion of the treating physician. Patients were excluded if they: (1) had no history of trauma as their primary event (eg, syncope or seizure), (2) had known dementia, chronic psychosis or active central nervous system pathology, (3) were pregnant, (4) were incarcerated or (5) had haemodynamic instability.
Both non-concussive trauma groups, the body trauma control patients (no head trauma and no concussion) and the head trauma control patients (head trauma and no concussion) had a GCS score of 15 presenting to the emergency department with a traumatic mechanism of injury but without concussion. They experienced similar mechanisms of injury as the concussion group and all had a normal mental status since injury (as verified by the research team prospectively by at least two different sources) and had no evidence of acute brain injury or haemodynamic instability. Peripheral (body) trauma controls were primarily composed of orthopaedic and soft tissue injuries. These patients were carefully screened to ensure they had no loss of consciousness, no amnesia and no alteration in sensorium at any time after injury. The purpose of enrolling non-concussive body trauma controls and non-concussive head trauma controls was to have appropriate comparison groups to compare the accuracy of the biomarkers in detecting concussion and simulate the real-world challenges faced by clinicians. The head trauma controls provided an opportunity to assess biomarker release in the setting of head trauma without symptoms and the potential for subconcussive brain injury.
Study procedures
All initial patient assessments were made by board certified adult and paediatric emergency medicine physicians trained by a formal 1 hour session on evaluating patient eligibility for the study. Following the initial screening, a meticulous secondary assessment was conducted by the research team. All prehospital and emergency department records were reviewed; patients, families and witnesses (if available) were carefully questioned; and the final determination was made by the emergency physician together with the research team. Patient classification was performed prospectively. Blood samples were obtained within 4 hours of time of injury. Repeated blood sampling was conducted for as long as the patient remained in hospital at 4, 8, 12, 16, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, 168 and 180 hours after injury and discontinued when discharged. Patient management was not altered by the study. For each blood draw, a single vial of approximately 5 mL of blood was collected and placed in a serum separator tube and allowed to clot at room temperature. The blood was centrifuged within 30 min and the serum was placed in bar-coded aliquot containers and stored in a freezer at −70°C until it was transported to a central laboratory (Banyan Biomarkers, Alachua, Florida, USA). There, the samples were analysed in batches using sandwich ELISA to GFAP and UCH-L1. Laboratory personnel running the samples were blinded to the clinical data.
Outcome measures
Performance of GFAP and UCH-L1 was evaluated within 4 hours of injury in both adults and children and over a 7-day period in hospitalised adults. The main outcome measures included the performance of the biomarkers in: (1) detecting the presence of concussion compared with trauma patients without concussion in children and adults (separately and as a whole); (2) assessing gradients of injury defined by comparing three groups of patients: (A) those with concussion, (B) those with head trauma without overt signs of concussion (non-concussive head trauma controls) and (C) those with peripheral (body) trauma without head trauma or concussion (non-concussive body trauma controls); and (3) determining the time course of GFAP and UCH-L1 over 7 days after injury in these three groups in adults.
Statistical analysis
Descriptive statistics with means, medians, and proportions were used to describe the data. For statistical analysis, biomarker levels were treated as continuous data, measured in nanogram per millilitre and expressed as medians with IQR. Data were assessed for equality of variance and distribution. Logarithmic transformations were conducted on non-normally distributed data. Group comparisons for different trauma groups were performed using analysis of variance with multiple comparisons using Games-Howell post hoc test. Receiver operating characteristics (ROC) curves were created to explore the ability of the biomarkers to identify the presence of a concussion. Estimates of the area under these curves (AUC) were obtained (AUC=0.5 indicates no discrimination and an AUC=1.0 indicates a perfect diagnostic test).
A power analysis yielded a sample of 281 cases of concussion and 141 cases without concussion achieves an 80% power to detect a difference of 0.06 between the area under the ROC curve under the null hypothesis of 0.81 and an AUC under the alternative hypothesis of 0.75 using a two-sided z-test at a significance level of 0.05. All analyses were performed using the statistical software package SPSS V.22.0 (IBM, Somers, New York, USA).
Biomarker analysis
Serum GFAP and UCH-L1 levels were measured in duplicate for each sample using a validated ELISA platform (Banyan Biomarkers, Alachua, Florida, USA). For the GFAP assay, the lower limit of quantification (LLOQ) is 0.030 ng/mL and upper limit of quantification (ULOQ) is 50 ng/mL. The limit of detection (LoD) is 0.008 ng/mL. For the UCH-L1 assay, the LLOQ is 0.100 ng/mL and ULOQ is 9 ng/mL. The LoD is 0.045 ng/mL. Any samples yielding a signal over the quantification or calibrator range were diluted and reassayed.
Patient and public involvement
Patients and the public were not involved in the design, recruitment or conduct of the study.
Results
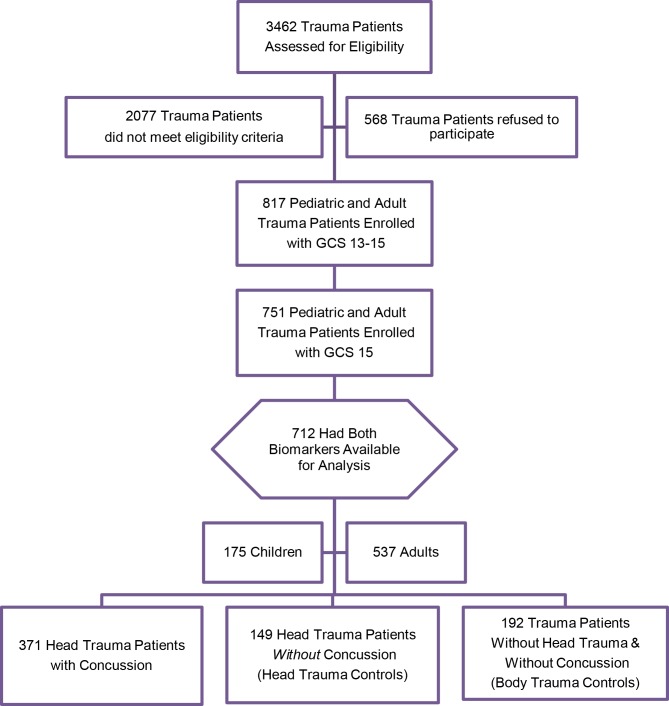
Over the study period, 3462 paediatric and adult trauma patients were screened, 1385 met eligibility criteria, 751 with a GCS score of 15 were enrolled and 712 had biomarker data available for analysis (figure 1). Of those enrolled, 371 (52%) had a concussion, 149 (21%) had non-concussive head trauma (head trauma controls) and 192 (27%) had non-concussive body trauma without head trauma (body trauma controls). The flow diagram in figure 1 describes the distribution of enrolled patients. There were 175 (25%) children and 537 (75%) adults. The distribution of clinical characteristics of all enrolled patients is presented in table 1. The overall injury severity score in children and adults was consistent with median scores of 4.
Figure 1.
Flow diagram of screened and enrolled patients. GCS, Glasgow Coma Scale.
Table 1.
Characteristics of enrolled patients
| Characteristics | Paediatric trauma patients without head trauma and without concussion (trauma controls), N=47 |
Paediatric head trauma patients without concussion (head trauma controls), N=34 |
Paediatric head trauma patients with concussion (concussion), N=94 |
Adult trauma patients without head trauma and without concussion (trauma controls), N=145 |
Adult head trauma patients without concussion (head trauma controls), N=115 |
Adult head trauma patients with concussion (concussion), N=277 |
| Mean age (years±SD), range | 9 (±4) (0–17) |
6 (±5) (1–16) |
9 (±6) (1–17) |
41 (±16) (18–83) |
40 (±16) (18–79) |
39 (±15) (18–78) |
| Gender, n (%) | ||||||
| Male | 33 (27) | 23 (19) | 66 (54) | 82 (25) | 68 (21) | 182 (55) |
| Female | 14 (26) | 11 (21) | 28 (53) | 63 (31) | 47 (23) | 95 (46) |
| Race, n (%) | ||||||
| Asian | 0 (0) | 1 (3) | 1 (1) | 2 (1) | 0 (0) | 6 (2) |
| Black | 19 (40) | 5 (15) | 31 (33) | 41 (28) | 32 (28) | 54 (20) |
| Hispanic | 7 (15) | 13 (38) | 17 (18) | 39 (27) | 20 (17) | 53 (19) |
| Native American | 0 (0) | 0 (0) | 0 (0) | 3 (2) | 0 (0) | 0 (0) |
| White | 19 (40) | 15 (44) | 42 (45) | 57 (39) | 60 (52) | 154 (56) |
| Other | 2 (4) | 0 (0) | 3 (3) | 3 (2) | 3 (3) | 10 (4) |
| Mechanism of injury, n (%) | ||||||
| Motor vehicle crash | 3 (6) | 1 (3) | 4 (4) | 94 (65) | 53 (46) | 134 (48) |
| Motorcycle crash | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 17 (15) | 29 (11) |
| Other motorised vehicle | 0 (0) | 3 (9) | 2 (2) | 0 (0) | 2 (2) | 3 (1) |
| Bicycle struck by vehicle | 0 (0) | 2 (6) | 3 (3) | 3 (2) | 7 (6) | 11 (4) |
| Fall off bicycle | 0 (0) | 1 (3) | 3 (3) | 1 (1) | 0 (0) | 5 (2) |
| Pedestrian struck | 1 (2) | 1 (3) | 6 (6) | 4 (3) | 3 (3) | 12 (4) |
| Fall | 33 (70) | 17 (50) | 42 (45) | 20 (14) | 12 (10) | 58 (21) |
| Sports injury | 9 (19) | 5 (15) | 23 (25) | 7 (5) | 2 (2) | 1 (<1) |
| Assault | 0 (0) | 0 (0) | 5 (5) | 0 (0) | 6 (5) | 10 (4) |
| Other | 1 (2) | 4 (12) | 6 (6) | 16 (11) | 13 (11) | 14 (5) |
| Loss of consciousness, n (%) | ||||||
| No | 47 (100) | 34 (100) | 34 (36) | 145 (100) | 115 (100) | 55 (20) |
| Yes | 0 (0) | 0 (0) | 37 (39) | 0 (0) | 0 (0) | 222 (80) |
| Unknown | 0 (0) | 0 (0) | 23 (24) | 0 (0) | 0 (0) | 0 (0) |
| Amnesia, n (%) | ||||||
| No | 47 (100) | 34 (100) | 36 (38) | 145 (100) | 115 (100) | 171 (62) |
| Yes | 0 (0) | 0 (0) | 17 (18) | 0 (0) | 0 (0) | 105 (20) |
| Unknown | 0 (0) | 0 (0) | 41 (44) | 0 (0) | 0 (0) | 1 (<1) |
| Injury Severity Score (n=629) | ||||||
| Median (IQR) | 4.0 (4.0–10) | 2.0 (1.0–4.0) | 4.0 (1.0–7.0) | 4.0 (1.0–4.0) | 4.0 (2.0–6.5) | 5.0 (2.0–8.8) |
| Means (95% CI) | 6.0 (3.0 to 9.1) | 3.3 (2.1 to 4.6) | 4.5 (3.6 to 5.4) | 4.2 (3.5 to 4.9) | 4.6 (4.0 to 5.3) | 6.1 (5.5 to 6.8) |
| Admitted to hospital, n (%) | 12 (26) | 8 (24) | 29 (31) | 42 (29) | 17 (15) | 101 (37) |
| CT head performed, n (%) | 0 (0) | 11 (32) | 74 (79) | 18 (12) | 80 (70) | 267 (96) |
| Intracranial lesions on CT, n (%) | 0 (0) | 0 (0) | 14 (16) | 0 (0) | 0 (0) | 22 (6) |
Due to rounding, percentages may not add up to 100.
There were a total of 1904 samples drawn in 712 patients. Patients had serum samples drawn within 4 hours of injury (16 children had samples drawn between 4 and 8 hours) with the average time from injury to serum sample collection of 3.1 hours (SD=0.9). Seven hundred and twelve patients had initial samples drawn at enrolment, 567 patients had samples at 4 hours postinjury, 109 at 8 hours postinjury, 80 at 12 hours postinjury, 73 at 16 hours postinjury, 70 at 20 hours postinjury, 67 at 24 hours postinjury, 46 at 36 hours postinjury, 40 at 48 hours postinjury, 33 at 60 hours postinjury, 32 at 72 hours postinjury, 20 at 84 hours postinjury, 17 at 96 hours postinjury, 8 at 108 hours postinjury, 8 at 120 hours postinjury, 4 at 132 hours postinjury, 6 at 144 hours postinjury, 6 at 156 hours postinjury and 4 at 168 hours postinjury.
Among body trauma control patients, 229 (60%) samples were below the LLOD and 90 (24%) below the LLOQ for GFAP. In head trauma control patients, 144 (38%) samples were below the LLOD and 88 (24%) below the LLOQ for GFAP. In concussion patients, 276 (24%) samples were below the LLOD and 172 (15%) below the LLOQ for GFAP. Among body trauma control patients, 61 (16%) samples were below the LLOD and 70 (19%) below the LLOQ for UCH-L1. In head trauma control patients, 34 (9%) samples were below the LLOD and 39 (10%) below the LLOQ for UCH-L1. In concussion patients, 128 (11%) samples were below the LLOD and 103 (11%) below the LLOQ for UCH-L1.
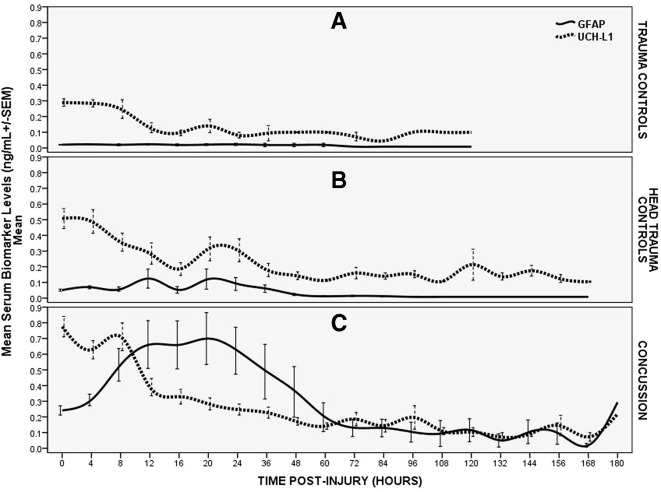
The time course of GFAP and UCH-L1 over a week post-trauma is depicted in figure 2 and contrasted in three groups of patients (concussion, head trauma controls and body trauma controls). In the concussion patients, the serum concentration of GFAP was detectable within 1 hour of injury and reached a peak at 20 hours postinjury and decreased over 72 hours. GFAP concentrations exhibited a slower decline thereafter but were still detectable at 168 hours (7 days) postinjury. In contrast, UCH-L1 rose very rapidly after injury, reached a peak at 8 hours, decreased quickly to 12 hours and was followed by a slower decline to 60 hours postinjury. Subsequently, UCH-L1, like GFAP, exhibited some smaller peaks and toughs over 7 days and was also detectable at 168 hours postinjury.
Figure 2.
Temporal profile of GFAP and UCH-L1 in three groups of trauma patients. (A) Temporal profile of GFAP and UCH-L1 in body trauma control patients. Means with error bars representing SEM. (B) Temporal profile of GFAP and UCH-L1 in head trauma control patients. Means with error bars representing SEM. (C) Temporal profile of GFAP and UCH-L1 in trauma patients with concussion. Means with error bars representing SEM. GFAP, glialfibrillary acidic protein; UCH-L1, ubiquitinC-terminal hydrolase.
In head trauma controls, GFAP levels were remarkably lower than in the concussion patients with very slight elevations until 48 hours. The peak appeared at 20 hours (as in the concussion patients) and after 48 hours, GFAP levels remained almost undetectable. Interestingly, in head trauma controls UCH-L1 levels peaked within 4 hours and remained lower than concussion patients over 12 hours. Thereafter, UCH-L1 concentrations become quite variable with levels either slightly lower, at par or slightly higher than concussion patients.
In body trauma control patients, concentrations of GFAP were negligible over time without any appreciable elevations. Initial UCH-L1 levels were slightly elevated but significantly lower than either head trauma or concussion patients. UCH-L1 levels decreased quickly over 12 hours (as it did in the head trauma controls) and levels remained noticeably elevated over the next several days.
The ability of GFAP and UCH-L1, individually and in combination, to distinguish concussion patients from body trauma controls (table 2) and head trauma controls (table 3) over time was assessed by calculating the area under the ROC curve at each timepoint postinjury. A comparison between concussion and both trauma and head trauma controls can be found in table 4. When comparing concussion patients to body trauma controls, GFAP demonstrated a range of AUCs between 0.75 (0.39–1.00) and 0.89 (0.69–1.00) and UCH-L1 demonstrated AUCs between 0.50 (0.05–0.23) and 0.78 (0.56–1.00). When GFAP and UCH-L1 were combined, the AUC ranged from 0.75 (0.49–1.00) to 0.90 (0.76–1.00) and closely mimicked the pattern of GFAP. GFAP outperformed UCH-L1 at all timepoints. The combination of GFAP and UCH-L1 marginally outperformed GFAP alone at some timepoints; however, the differences were not statistically significant.
Table 2.
Area under the curve for distinguishing between concussion and body trauma controls (no head trauma and no concussion symptoms)
| Hours postinjury | GFAP | UCH-L1 | Combination of GFAP and UCH-L1 |
| Enrolment (children) (n=141) | 0.80 (0.73–0.87) | 0.62 (0.53–0.72) | 0.80 (0.73–0.87) |
| Enrolment (adults) (n=422) | 0.76 (0.71–0.80) | 0.69 (0.64–0.74) | 0.78 (0.73–0.82) |
| Enrolment (both) (n=563) | 0.76 (0.72–0.80) | 0.67 (0.63–0.72) | 0.78 (0.74–0.81) |
| 4 hours (n=452) | 0.76 (0.72–0.80) | 0.65 (0.60–0.70) | 0.77 (0.73–0.81) |
| 8 hours (n=92) | 0.82 (0.72–0.91) | 0.72 (0.56–0.87) | 0.86 (0.75–0.96) |
| 12 hours (n=68) | 0.83 (0.73–0.93) | 0.74 (0.58–0.90) | 0.85 (0.74–0.96) |
| 16 hours (n=66) | 0.84 (0.73–0.95) | 0.77 (0.62–0.91) | 0.86 (0.75–0.97) |
| 20 hours (n=61) | 0.82 (0.71–0.94) | 0.63 (0.43–0.84) | 0.82 (0.70–0.94) |
| 24 hours (n=56) | 0.87 (0.76–0.98) | 0.74 (0.58–0.90) | 0.87 (0.74–1.00) |
| 36 hours (n=35) | 0.86 (0.72–1.00) | 0.76 (0.51–1.00) | 0.90 (0.76–1.00) |
| 48 hours (n=30) | 0.85 (0.68–1.00) | 0.71 (0.53–0.90) | 0.85 (0.65–1.00) |
| 60 hours (n=27) | 0.79 (0.57–1.00) | 0.70 (0.50–0.90) | 0.83 (0.61–1.00) |
| 72 hours (n=25) | 0.87 (0.71–1.00) | 0.78 (0.56–1.00) | 0.94 (0.82–1.00) |
| 84 hours (n=15) | 0.89 (0.69–1.00) | 0.75 (0.40–1.00) | 0.89 (0.69–1.00) |
| 96 hours (n=13) | 0.75 (0.39–1.00) | 0.54 (0.18–0.90) | 0.75 (0.49–1.00) |
| 108 hours (n=6) | n/a | n/a | n/a |
| 120 hours (n=6) | 0.80 (0.40–1.00) | 0.50 (0.05–0.23) | 0.90 (0.62–1.00) |
| 132 hours (n=2) | n/a | n/a | n/a |
| 144 hours (n=4) | n/a | n/a | n/a |
| 156 hours (n=4) | n/a | n/a | n/a |
| 168 hours (n=2) | n/a | n/a | n/a |
In this table, performance of GFAP and UCH-L1 alone and in combination is shown.
GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase; n/a, data not available.
Table 3.
Area under the curve for distinguishing between concussion and head trauma controls (no concussion symptoms) in patients with head trauma
| Hours postinjury | GFAP | UCH-L1 | Combination of GFAP and UCH-L1 |
| Enrolment (children) (n=128) | 0.64 (0.54–0.73) | 0.54 (0.43–0.66) | 0.63 (0.53–0.72) |
| Enrolment (adults) (n=392) | 0.66 (0.61–0.71) | 0.58 (0.51–0.64) | 0.66 (0.61–0.72) |
| Enrolment (both) (n=520) | 0.65 (0.60–0.70) | 0.56 (0.51–0.62) | 0.65 (0.60–0.70) |
| 4 hours (n=421) | 0.62 (0.57–0.68) | 0.55 (0.49–0.61) | 0.62 (0.57–0.88) |
| 8 hours (n=99) | 0.72 (0.61–0.82) | 0.61 (0.49–0.73) | 0.72 (0.61–0.82) |
| 12 hours (n=74) | 0.69 (0.55–0.82) | 0.51 (0.37–0.66) | 0.67 (0.54–0.80) |
| 16 hours (n=69) | 0.76 (0.64–0.88) | 0.58 (0.39–0.76) | 0.74 (0.62–0.85) |
| 20 hours (n=65) | 0.68 (0.53–0.83) | 0.41 (0.23–0.59) | 0.66 (0.52–0.81) |
| 24 hours (n=64) | 0.74 (0.60–0.88) | 0.40 (0.24–0.56) | 0.72 (0.59–0.86) |
| 36 hours (n=44) | 0.76 (0.61–0.91) | 0.56 (0.37–0.75) | 0.76 (0.61–0.92) |
| 48 hours (n=38) | 0.81 (0.67–0.95) | 0.52 (0.32–0.73) | 0.79 (0.64–0.93) |
| 60 hours (n=31) | 0.86 (0.73–1.00) | 0.54 (0.34–0.74) | 0.86 (0.73–1.00) |
| 72 hours (n=30) | 0.78 (0.60–0.95) | 0.43 (0.20–0.66) | 0.71 (0.51–0.91) |
| 84 hours (n=19) | 0.84 (0.65–1.00) | 0.37 (0.13–0.62) | 0.74 (0.52–0.97) |
| 96 hours (n=16) | 0.75 (0.51–0.99) | 0.28 (0.02–0.55) | 0.65 (0.38–0.91) |
| 108 hours (n=8) | 0.67 (0.26–1.00) | 0.17 (0–0.47) | 0.33 (0–0.71) |
| 120 hours (n=7) | 0.80 (0.46–1.00) | 0.20 (0–0.58) | 0.70 (0.30–1.00) |
| 132 hours (n=4) | 0.75 (0.20–1.00) | 0 (0–0) | 0.50 (0–1.00) |
| 144 hours (n=6) | 0.75 (0.20–1.00) | 0 (0–0) | 0.50 (0.01–0.99) |
| 156 hours (n=6) | 0.75 (0.34–1.00) | 0 (0–0) | 0.50 (0.01–0.99) |
| 168 hours (n=4) | 0.75 (0.20–1.00) | 0.13 (0–1.00) | 0.63 (0–1.00) |
In this table, performance of GFAP and UCH-L1 alone and in combination is shown.
GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase.
Table 4.
Area under the curve for distinguishing between concussion and all trauma controls (head and body controls with no concussion)
| Hours postinjury trauma | GFAP | UCH-L1 | Combination of GFAP and UCH-L1 |
| Enrolment (children) (n=175) | 0.73 (0.66–0.81) | 0.59 (0.51–0.67) | 0.73 (0.65–0.80) |
| Enrolment (adults) (n=537) | 0.71 (0.67–0.76) | 0.64 (0.59–0.69) | 0.73 (0.68–0.77) |
| Enrolment (both) (n=712) | 0.71 (0.67–0.75) | 0.63 (0.59–0.67) | 0.72 (0.68–0.76) |
| 4 hours (n=567) | 0.70 (0.66–0.74) | 0.61 (0.56–0.65) | 0.70 (0.66–0.75) |
| 8 hours (n=109) | 0.75 (0.66–0.84) | 0.65 (0.54–0.75) | 0.77 (0.68–0.86) |
| 12 hours (n=80) | 0.73 (0.62–0.85) | 0.59 (0.46–0.72) | 0.73 (0.62–0.85) |
| 16 hours (n=73) | 0.79 (0.69–0.90) | 0.64 (0.49–0.80) | 0.78 (0.68–0.89) |
| 20 hours (n=70) | 0.73 (0.61–0.86) | 0.49 (0.33–0.65) | 0.72 (0.59–0.84) |
| 24 hours (n=67) | 0.77 (0.64–0.89) | 0.47 (0.31–0.63) | 0.75 (0.63–0.88) |
| 36 hours (n=46) | 0.77 (0.63–0.91) | 0.59 (0.41–0.77) | 0.78 (0.64–0.92) |
| 48 hours (n=40) | 0.81 (0.68–0.95) | 0.55 (0.37–0.74) | 0.80 (0.66–0.93) |
| 60 hours (n=33) | 0.85 (0.71–0.98) | 0.58 (0.39–0.77) | 0.86 (0.73–0.98) |
| 72 hours (n=32) | 0.80 (0.64–0.95) | 0.51 (0.29–0.73) | 0.76 (0.59–0.94) |
| 84 hours (n=20) | 0.85 (0.67–1.00) | 0.44 (0.18–0.69) | 0.77 (0.56–0.98) |
| 96 hours (n=17) | 0.75 (0.51–0.98) | 0.33 (0.07–0.60) | 0.67 (0.41–0.92) |
| 108 hours (n=8) | 0.67 (0.26–1.00) | 0.17 (0–0.47) | 0.33 (0–0.71) |
| 120 hours (n=8) | 0.80 (0.48–1.00) | 0.30 (0–0.69) | 0.77 (0.42–1.00) |
| 132 hours (n=4) | 0.75 (0.20–1.00) | 0 (0–0) | 0.50 (0–1.00) |
| 144 hours (n=6) | 0.75 (0.34–1.00) | 0 (0–0) | 0.50 (0.01–0.99) |
| 156 hours (n=6) | 0.75 (0.34–1.00) | 0.38 (0–0.85) | 0.63 (0.15–1.00) |
| 168 hours (n=4) | 0.75 (0.20–1.00) | 0.13 (0–1.00) | 0.63 (0–1.00) |
In this table, performance of GFAP and UCH-L1 alone and in combination is shown.
GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase.
When comparing concussion patients to head trauma controls, GFAP demonstrated a range of AUCs between 0.62 (0.57–0.68) and 0.86 (0.73–1.00) and UCH-L1 demonstrated AUCs between 0.13 (0–1.00) and 0.61 (0.49–0.73). When GFAP and UCH-L1 were combined, the AUC ranged from 0.33 (0–0.71) to 0.86 (0.73–1.00). GFAP outperformed UCH-L1 at all timepoints and outperformed the combination of the two biomarkers at all timepoints.
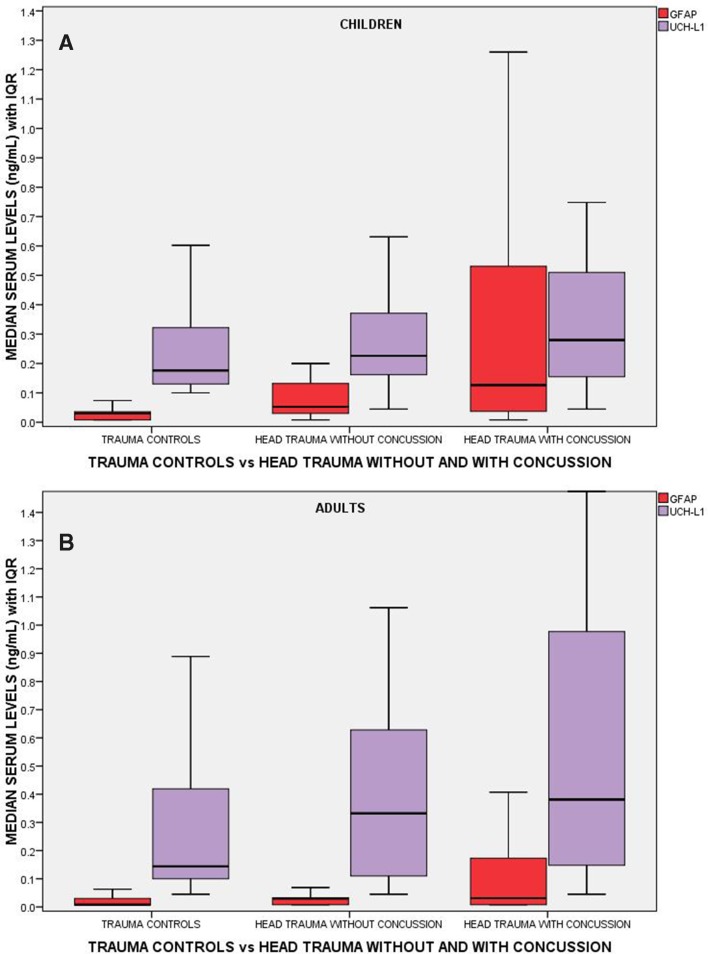
A comparison of the performance of GFAP and UCH-L1 measured within 4 hours of injury in children and adults is shown in figure 3. There are incremental increases in levels of GFAP and UCH-L1 from non-concussive body trauma controls to non-concussive head trauma controls to patients with concussion. In concussion patients, GFAP concentrations were significantly higher compared with body trauma controls (p<0.001) and to head trauma controls (p<0.001) in both children and adults, after controlling for multiple comparisons. There were also significantly higher levels of GFAP in head trauma controls compared with body trauma controls in children (p<0.001) and adults (p<0.001). In adults, concentrations of UCH-L1 measured within 4 hours of injury were also significantly higher in concussion patients than body trauma controls (p<0.001) and head trauma controls (p=0.002). There were also significantly higher levels of UCH-L1 in head trauma controls compared with body trauma controls (p=0.017). Similarly, in children, concentrations of UCH-L1 were significantly higher in concussion patients than that in body trauma controls (p=0.045). However, there were no significant differences between concussion patients and head trauma controls (p=0.894) and between body trauma and head trauma controls in children.
Figure 3.
Boxplot comparing initial levels of GFAP and UCH-L1 in three groups of trauma patients: (1) trauma controls without head trauma (controls), (2) head trauma controls without concussion (no concussion) and (3) head trauma with concussion (concussion). There were 47, 34 and 94 children in each group, respectively, and 145, 115 and 277 adult patients, respectively. Medians with bars representing IQR. GFAP, glialfibrillary acidic protein; UCH-L1, ubiquitinC-terminal hydrolase.
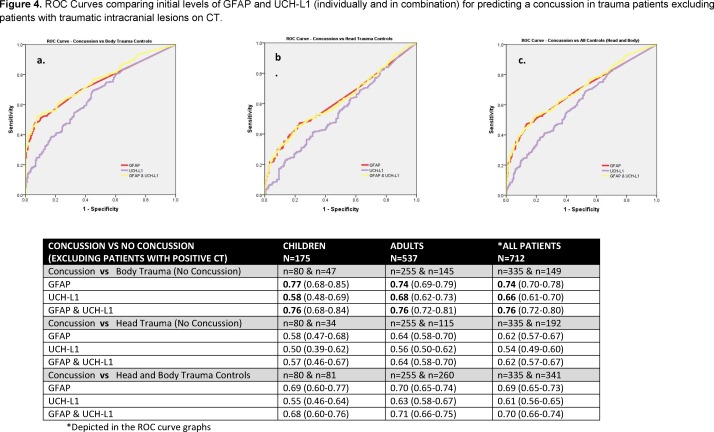
When ROC curves were compared in children and adults, the AUCs demonstrated that initial GFAP levels were able to distinguish concussion patients from body trauma controls with an AUC of 0.80 (0.73–0.87) in children and 0.76 (0.71–0.80) in adults (table 2). In contrast, initial UCH-L1 levels distinguished concussion from body trauma controls with a significantly lower AUC of 0.62 (0.53–0.72) in children (p=0.003) and 0.69 (0.64–0.74) in adults (p=0.04) (table 2). The AUCs for GFAP for distinguishing concussion patients from head trauma controls were 0.64 (0.54–0.73) in children and 0.66 (0.61–0.71) in adults. AUCs were lower for UCH-L1 with an AUC of 0.54 (0.43–0.66) in children (p=0.23) and 0.58 (0.51–0.64) in adults (p=0.04) (table 3). Overall, when concussion was compared with all (head and body) trauma control cases without concussion, the AUC for GFAP was 0.73 (0.66–0.81) in children and 0.71 (0.67–0.76) in adults. AUCs were significantly lower for UCH-L1 with an AUC of 0.59 (0.51–0.67) in children (p=0.013) and 0.64 (0.59–0.69) in adults (p=0.030) (table 4). There were significantly higher levels of GFAP and UCH-L1 in those with intracranial lesions on CT; therefore, we excluded the 36 (8%) patients with CT lesions and found similar results (figure 4).
Figure 4.
ROC curves comparing initial levels of GFAP and UCH-L1 (individually and in combination) for predicting a concussion in trauma patients excluding patients with traumatic intracranial lesions on CT. GFAP, glialfibrillary acidic protein; ROC, receiver operating characteristics; UCH-L1, ubiquitinC-terminal hydrolase.
Discussion
This prospective study assessed the diagnostic accuracy of glial and neuronal biomarkers, GFAP and UCH-L1, for detecting concussion in a very large cohort of children and adult trauma patients presenting to three level I trauma centres. The study investigated the pattern of biomarker release in trauma patients with and without concussion at 20 distinct timepoints, making it among the first and largest studies to assess the temporal profile of these two biomarkers in three groups of trauma patients with a normal mental status. In both children and adults, GFAP and UCH-L1 concentrations increased incrementally from those with non-concussive body trauma to those with non-concussive head trauma with highest levels in patients with concussion. GFAP showed very distinct patterns of release in all three groups, whereas UCH-L1 demonstrated similar patterns of release in all three groups but at much higher concentrations in both non-concussive trauma groups. There were significant differences between the three groups controlling for multiple comparisons for both biomarkers in adults; however, UCH-L1 could not distinguish concussive from non-concussive head trauma in children.
When the time course of GFAP and UCH-L1 was contrasted in three groups of patients (concussion, head trauma controls and body trauma controls), GFAP showed a clear increase over the first 20 hours postinjury and decline from 20 to 72 hours in concussion patients and was still detectable at 7 days postinjury making it potentially useful over a week from injury. Although GFAP was mildly elevated in head trauma without concussion, the expression was very low and very early compared with concussion. In body trauma control patients, concentrations of GFAP were negligible over all timepoints suggesting very good specificity for concussion. These results are consistent with previous studies showing how robust it is in multiple trauma.3 4 In contrast, UCH-L1 rose more rapidly after concussion than GFAP, peaking within 8 hours and steadily decreasing from 12 to 60 hours. Unexpectedly, UCH-L1 was much higher in the head trauma control group compared with GFAP at all timepoints from injury over 7 days. Even more surprising, was that UCH-L1 was elevated in body trauma too and showed a similar pattern of release as head trauma control patients. Possible explanations for UCH-L1 elevations in control patients include that (1) UCH-L1 may not be completely brain specific and is released from other organ or tissue trauma or (2) UCH-L1 is an ultrasensitive marker of any neuronal disruption that may occur from impacts to the body that jostle the brain.
Given that GFAP appears to be so brain specific and that it also showed low level elevations in the first 48 hours following head trauma without concussion symptoms (head trauma controls), these elevations may represent milder forms of concussion that do not elicit typical signs or symptoms associated with concussion. These injuries may be irrelevant, or they may represent important trauma that is just below the level of clinical detection and referred to as subconcussive trauma. Emerging data have demonstrated that significant alterations in brain function can occur in the absence of clinically obvious symptoms following even a single head trauma.15 18 19 Given the lack of concussive symptoms acutely, biomarkers (such as GFAP and UCH-L1) could provide a more objective measure of injury and potentially identify those at risk for neurocognitive problems. Studies in athletes have documented that both clinically diagnosed concussion and subconcussive traumas can induce similar changes in brain structure and functions on brain imaging.15 19–21 These changes include alterations in white matter and cerebrovascular integrity, blood flow, neuroinflammation, brain activation during working memory tasks, resting-state functional connectivity and brain chemistry as measured by various forms of MRI.20 22 23 The effect of thousands of subconcussive impacts has the potential for long-term deleterious effects on brain function and neurodegeneration in select individuals.14 15 19 In athletes, UCH-L1 has shown elevations in both concussive24 and subconcussive trauma.25
To date, there is a lack of research addressing the effects of subconcussive head impacts following head trauma in an emergency department population. Acute biomarkers may have a role in assessing these patients if the markers can be shown to correlate with long-term neurocognitive dysfunction. Most recently, microRNA biomarkers measured pre-season and post-season in collegiate football players were associated with worsening neurocognitive functioning over the course of a season in those with no concussions.26
Concussion is a clinical diagnosis that could benefit from a relatively non-invasive complementary tool such as a blood test.8 9 27–29 Based on these results, the potential utility of GFAP to distinguish concussion from body trauma controls over 7 days postinjury was fair to excellent with AUCs of 0.75 to 0.89, and UCH-L1’s ability was guarded and variable with AUCs from poor to good depending on timing of samples (AUCs of 0.54 to 0.78) with earlier samples being better. The combination of the both biomarkers did not significantly improve concussion detection among trauma control patients. The distinction between patients with head trauma with and without concussion was not as robust as with body trauma controls. GFAP performed with fair to very good AUCs (0.62 to 0.86) over the week postinjury, with optimal performance between 24 and 96 hours. The ability of UCH-L1 to distinguish concussion from head trauma control patients was very poor with AUCs of 0.13–0.61 and did not contribute to or improve the performance of GFAP alone.
Since it is not uncommon for patients who have suffered a concussion or mild TBI not to seek immediate medical attention, understanding when to use the biomarkers for detection of injury is critical. In the context of developing a point-of-care test, UCH-L1’s early and rapid rise could be useful in the early postinjury setting such as in the ambulance, on the playing field or on the battlefield. The longer half-life of GFAP makes it a very favourable marker to use in both the acute and subacute phases of injury as it can detect concussion for up to 7 days after injury.
The authors recognise that there are limitations to this study. This study addressed diagnosis of concussion in the acute care setting and did not describe long-term outcome in these patients. The main outcomes used in this study reflect current standards of practice and accepted definitions of concussion. However, future studies to better define the severity of concussion and mild TBI need to be pursued, particularly when neuroimaging is negative. Accordingly, we performed an analysis of patients with negative neuroimaging acutely and found no significant differences in the results whether we included those with positive scans or not. The number of samples available for analysis decreased over the course of the study. This reflects the challenge of obtaining samples over time in patients with less severe injuries because they are not hospitalised as long. However, there were many patients without TBI and patients with mild TBI who were captured in our longitudinal sample because they were admitted for other injuries. Important next steps will be to capture samples within minutes of injury. Uninjured controls were not included in this analysis as the concentrations of these two biomarkers have already been well characterised in uninjured normal control patients.1 2
Conclusions
In a cohort of trauma patients with normal mental status, GFAP outperformed UCH-L1 in detecting concussion in both children and adults. Blood levels of GFAP and UCH-L1 showed incremental elevations across three injury groups: from non-concussive body trauma, to non-concussive head trauma, to concussion. However, UCH-L1 was expressed at much higher levels than GFAP in those with non-concussive trauma, particularly in children, suggesting that UCH-L1 is either not completely brain specific or ultrasensitive to subtle impacts. Each biomarker exhibited a distinct temporal profile in each trauma group over 7 days with earlier elevations in UCH-L1 and more consistent and sustained elevations in GFAP. Furthermore, elevations in both biomarkers in patients with non-concussive head trauma may be reflective of a subconcussive brain injury. The stage is set for future studies to verify these findings.
What is known about the subject?
In 2018, serum biomarkers GFAP and UCH-L1 were Food and Drug Administration-approved in adults to guide CT scan ordering in mild to moderate traumatic brain injury. However, their ability to detect concussion in either children or adults has not been determined and there currently exists no objective measure to diagnose concussion acutely after injury. The challenge for clinicians is to detect concussion in the setting of head and/or peripheral trauma when patients have a normal mental status.
What this study adds?
GFAP outperformed UCH-L1 in detecting concussion in both children and adults within 4 hours of injury. Blood levels of GFAP and UCH-L1 showed incremental elevations across three injury groups: from non-concussive body trauma, to non-concussive head trauma, to concussion. UCH-L1 was expressed at much higher levels than GFAP in those with non-concussive trauma, particularly in children. Elevations of these biomarkers in non-concussive head trauma suggests possible subconcussive brain injury. GFAP could be potentially useful to detect concussion for up to a week postinjury.
Supplementary Material
Footnotes
Contributors: LP had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. LP conceived and designed the study. Data were acquired by LP, JR, PAG, CFB, CNT, NJA, MAL, CAH, DMG, MRZ and MKM. All authors were involved in the analysis and interpretation of the data. LP drafted the manuscript and all authors were involved in critical revision of the manuscript for important intellectual content. Statistical analysis was conducted by LP. Funding was obtained by LP. Administrative, technical or material support was provided by LP, PAG, MRZ and MKM. The study was supervised by LP, JR, PAG, CFB, CNT, NJA, MAL, CAH, DMG, MRZ and MKM. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by Award Number R01NS057676 (LP, Principal Investigator) from the National Institute of Neurological Disorders and Stroke.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Dissemination of results to participants is not possible/applicable.
Competing interests: LP is an unpaid scientific consultant for Banyan Biomarkers, but receives no stocks or royalties from the company and will not benefit financially from this publication. RDW and LML receive contract research funding from Banyan Biomarkers. They do not receive stocks or royalties from the company and will not benefit financially from this publication.
Ethics approval: This study was approved by the respective IRBs of each institution (Orlando Regional Medical Center IRB, Arnold Palmer Hospital for Children IRB and Children’s Hospital of Philadelphia IRB).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are not available for sharing at this time.
References
- 1.Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med 2012;59:471–83. 10.1016/j.annemergmed.2011.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papa L, Lewis LM, Silvestri S, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg 2012;72:1335–44. 10.1097/TA.0b013e3182491e3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papa L, Silvestri S, Brophy GM, et al. Gfap out-performs S100β in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J Neurotrauma 2014;31:1815–22. 10.1089/neu.2013.3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol 2016;73:551–60. 10.1001/jamaneurol.2016.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch RD, Ellis M, Lewis LM, et al. Modeling the kinetics of serum glial fibrillary acidic protein, ubiquitin carboxyl-terminal hydrolase-L1, and S100B concentrations in patients with traumatic brain injury. J Neurotrauma 2017;34:1957–71. 10.1089/neu.2016.4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis LM, Schloemann DT, Papa L, et al. Utility of serum biomarkers in the diagnosis and stratification of mild traumatic brain injury. Acad Emerg Med 2017;24:710–20. 10.1111/acem.13174 [DOI] [PubMed] [Google Scholar]
- 7.Bazarian JJ, Biberthaler P, Welch RD, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol 2018;17:782–9. 10.1016/S1474-4422(18)30231-X [DOI] [PubMed] [Google Scholar]
- 8.Papa L, Zonfrillo MR, Ramirez J, et al. Performance of glial fibrillary acidic protein in detecting traumatic intracranial lesions on computed tomography in children and youth with mild head trauma. Acad Emerg Med 2015;22:1274–82. 10.1111/acem.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papa L, Mittal MK, Ramirez J, et al. In children and youth with mild and moderate traumatic brain injury, glial fibrillary acidic protein Out-Performs S100β in detecting traumatic intracranial lesions on computed tomography. J Neurotrauma 2016;33:58–64. 10.1089/neu.2015.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papa L, Mittal MK, Ramirez J, et al. Neuronal biomarker ubiquitin C-terminal hydrolase detects traumatic intracranial lesions on computed tomography in children and youth with mild traumatic brain injury. J Neurotrauma 2017;34:2132–40. 10.1089/neu.2016.4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA authorizes marketing of first blood test to aid in the evaluation of concussion in adults. Silver Springs: US Food & Drug Administration;, 2018. Available: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm596531.htm
- 12.Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma 2004;57:1006–12. 10.1097/01.TA.0000108998.48026.C3 [DOI] [PubMed] [Google Scholar]
- 13.Tate CM, Wang KKW, Eonta S, et al. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. J Neurotrauma 2013;30:1620–30. 10.1089/neu.2012.2683 [DOI] [PubMed] [Google Scholar]
- 14.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011;30:179–88. xi 10.1016/j.csm.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailes JE, Dashnaw ML, Petraglia AL, et al. Cumulative effects of repetitive mild traumatic brain injury. Prog Neurol Surg 2014;28:50–62. 10.1159/000358765 [DOI] [PubMed] [Google Scholar]
- 16.Huber BR, Alosco ML, Stein TD, et al. Potential long-term consequences of concussive and Subconcussive injury. Phys Med Rehabil Clin N Am 2016;27:503–11. 10.1016/j.pmr.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papa L, Wang KKW. Raising the bar for traumatic brain injury biomarker research: methods make a difference. J Neurotrauma 2017;34:2187–9. 10.1089/neu.2017.5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Kierans A, Kenul D, et al. Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology 2013;267:880–90. 10.1148/radiol.13122542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailes JE, Petraglia AL, Omalu BI, et al. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg 2013;119:1235–45. 10.3171/2013.7.JNS121822 [DOI] [PubMed] [Google Scholar]
- 20.Slobounov SM, Walter A, Breiter HC, et al. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: a multi-modal neuroimaging study. Neuroimage Clin 2017;14:708–18. 10.1016/j.nicl.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds BB, Stanton AN, Soldozy S, et al. Investigating the effects of subconcussion on functional connectivity using mass-univariate and multivariate approaches. Brain Imaging Behav 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson B, Neuberger T, Gay M, et al. Effects of subconcussive head trauma on the default mode network of the brain. J Neurotrauma 2014;31:1907–13. 10.1089/neu.2014.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahrami N, Sharma D, Rosenthal S, et al. Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology 2016;281:919–26. 10.1148/radiol.2016160564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier TB, Nelson LD, Huber DL, et al. Prospective assessment of acute blood markers of brain injury in sport-related concussion. J Neurotrauma 2017;34:3134–42. 10.1089/neu.2017.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph JR, Swallow JS, Willsey K, et al. Elevated markers of brain injury as a result of clinically asymptomatic high-acceleration head impacts in high-school football athletes. J Neurosurg 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 26.Papa L, Slobounov SM, Breiter HC, et al. Elevations in microRNA biomarkers in serum are associated with measures of concussion, neurocognitive function, and Subconcussive trauma over a single national collegiate athletic association division I season in collegiate football players. J Neurotrauma 2019;36:1343–51. 10.1089/neu.2018.6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papa L. Potential blood-based biomarkers for concussion. Sports Med Arthrosc Rev 2016;24:108–15. 10.1097/JSA.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papa L, Ramia MM, Kelly JM, et al. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma 2013;30:324–38. 10.1089/neu.2012.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papa L, Ramia MM, Edwards D, et al. Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J Neurotrauma 2015;32:661–73. 10.1089/neu.2014.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.