Abstract
A 62-year-old man was admitted to the emergency department due to fever and acute heart failure. A transthoracic echocardiogram revealed severe aortic valve obstruction. He was an hepatic transplant recipient and was medicated with everolimus. He underwent mitral and aortic valve replacement with prosthetic valves 4 years ago. Due to his medical background, therapy and clinical presentation, empirical therapy for infective endocarditis was started. Transoesophageal echocardiogram showed severe aortic valve regurgitation but no other findings suggestive of endocarditis. Computed tomography (CT) revealed pulmonary infiltrates compatible with infection and no evidence of septic embolisation. Multiple sets of blood cultures were negative. Proteus mirabilis was isolated in bronchial lavage and antibiotic therapy was adjusted. The patient underwent aortic valve replacement, with no macroscopic findings suggestive of endocarditis. P. mirabilis was isolated in the surgically removed valve. Dual antibiotic therapy was successfully administered for 6 weeks.
Keywords: valvar diseases, infections
Background
Infective endocarditis is an uncommon and serious condition. Its precise incidence is difficult to ascertain due to the use of different definitions over time1 and distinct epidemiology depending on geographical/economic regions.2 A population-based observational study in France documented 33.8 cases per million inhabitants in 1 year.3 The most common microorganisms implicated are staphylococci, streptococci and enterococci; gram-negative agents are rarely implicated—the HACEK (Haemophilus aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae) group accounts for 2% of cases and other gram-negative agents (among which Proteus mirabilis) encompass another 2% of cases.4
Immunocompromised patients are at increased risk for systemic infection but reports regarding the incidence of infective endocarditis in transplant recipients are lacking. In a retrospective analysis of solid organ transplant recipients in an Austrian Hospital, the incidence was 1% and the reported mortality was 44%.5
Case presentation
The patient was a 62-year-old obese, hypertensive, diabetic man. Five years ago, he had been submitted to an hepatic transplant due to hepatocolangiocarcinoma and was on everolimus. A year later, he was hospitalised due to infective endocarditis and, as part of treatment, he underwent valvular replacement surgery, in which the mitral and aortic valves were substituted by biological prosthesis St Jude Epic and Trifecta, respectively.
The patient sought the emergency room for dyspnoea on exertion for the previous 2 weeks. He also complained of orthopnoea but denied chest pain, cough or sputum. His blood pressure was 90/50 mm Hg; pulse rate was 150 beats/min and auricular temperature was 38.2°C. At cardiac auscultation, an aortic systolic murmur was noted. There were no clinical signs of vascular phenomena.
Blood chemistry showed evidence of inflammation and acute renal failure. An ECG showed atrial fibrillation with a rapid ventricular rate and there were no consolidations in the radiography of the chest. While still in the emergency room, a transthoracic echocardiogram was performed, which showed severe stenotic aortic valve dysfunction (mean gradient 43 mm Hg) and moderate to severe left ventricular systolic dysfunction. There were segmental motility alterations but no vegetations, abscesses or pseudoaneurysms. Digoxin was administered; the patient was admitted to an intermediate care unit; blood samples were drawn for microbiology and empirical therapy for late prosthetic valve infective endocarditis with ampicillin, flucloxacillin and gentamicin was started.
Investigations
Initial laboratory workup showed elevation of C reactive protein (CRP) (149.2 mg/dL), no leukocytosis, elevation of high-sensitivity troponin I (791.5 ng/L), B-type natriuretic peptide (3824.1 pg/mL) and plasma creatinine (2.15 mg/dL).
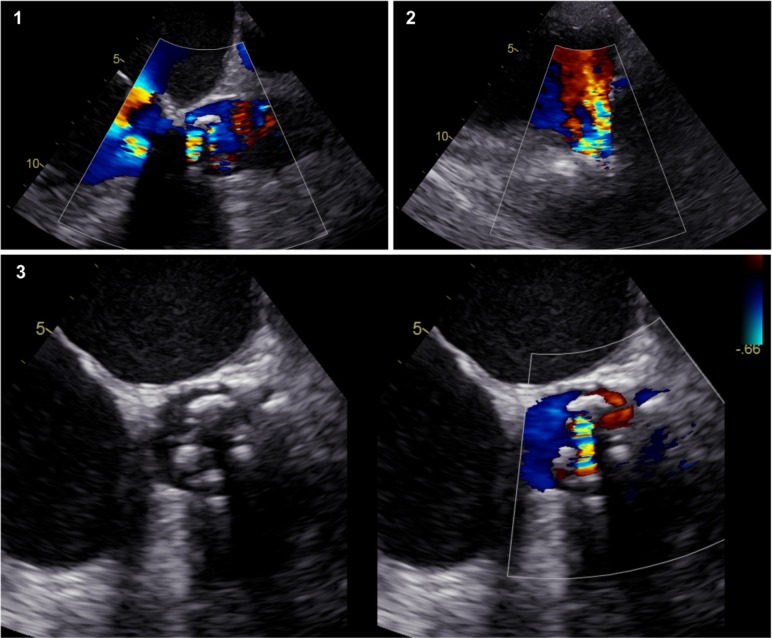
The transoesophageal echocardiogram that was performed in the first day of hospitalisation documented severe aortic regurgitation, as shown in figure 1. No vegetations or abscesses were observed, and the obstructive component of valvular dysfunction appeared to be degenerative with thickened leaflets. A CT of the thorax and abdomen showed no signs of systemic embolisation, which could substantiate the diagnosis of endocarditis. Instead, it revealed pulmonary infiltrates suggesting pneumonia. A bronchoscopy was then performed, and bronchial and bronchoalveolar lavage were sent for microbiological examination. Multiple sets of blood cultures were drawn consecutively after admission, which were negative and lead to testing for indolent and unusual infective agents. Given the state of immunosuppression, opportunistic infections namely by fungi (eg, Pneumocystis carinii, Aspergillus spp, Candida spp) and virus (eg, cytomegalovirus) were investigated and discarded. Serologies for Bartonella, Coxiella and Brucella spp were also negative.
Figure 1.
Transoesophageal echocardiogram of the aortic prosthetic valve. 1- Transoesophageal view of the aortic valve with colour Doppler showing severe regurgitation. 2- Transgrastric view. 3- Aortic valve with thickened cusps. Colour Doppler showing intravalvular and paravalvular regurgitant jets.
After extensive serological and microbiological testing, the only identified infective agent was P. mirabilis and was isolated in the bronchial lavage.
Differential diagnosis
In case of fever, elevated CRP, acute heart failure and newly diagnosed prosthetic heart valve dysfunction, the main diagnostic hypothesis was infective endocarditis.
Although this diagnosis was a strong possibility, evidence of involvement of the heart valves by an infectious process was lacking, and with persistently negative blood cultures, other sites and fungal, viral and indolent infective agents were considered. The valvular and myocardial dysfunction also had to be explained otherwise, if infective endocarditis was not the diagnosis. Degenerative valvular dysfunction seemed to be obvious by echocardiographic imaging, but whether it was enough to cause myocardial dysfunction was unclear. Myocarditis could explain both myocardial dysfunction and elevated markers of myocardial necrosis, but there was no history of recent viral illness. The alterations in left ventricle segmental motility suggested myocardial infarction instead, although there was no chest pain or electrocardiographic alterations consistent with the diagnosis.
Treatment
After 6 days of hospitalisation and under empirical therapy for infective endocarditis, the only identified infective agent was P. mirabilis and was isolated in a bronchial lavage. Although the sample was of questionable quality (with <10 epithelial cells/power field and <10 neutrophils/power field), the evidence of pneumonia, at this time, outweighed that of infective endocarditis. As such, antimicrobial therapy was adjusted, initiating piperacillin-tazobactam. Fever subsided, but CRP remained elevated.
Unfortunately, due to clinical deterioration with refractory pulmonary oedema, urgent aortic valve replacement surgery had to be performed. There was no indication that an uncontrolled infectious process was the reason why the patient was worsening. Intraoperatively, there were no macroscopic findings suggestive of endocarditis and the patient underwent surgery without any complications (the aortic valve was replaced by another biological prosthesis Magna Ease).
Three days after surgery, in the absence of fever, positive blood cultures and imaging or macroscopic observation suggestive of endocarditis, infective endocarditis was deemed unlikely and it was decided to stop antimicrobial therapy. One week after surgery, fever recurred and P. mirabilis was identified in the prosthetic valve that had been removed, confirming infective endocarditis.
According to the sensitivity report, dual antimicrobial therapy was started with cefepime and levofloxacin. Gentamicin was also an option as a synergic agent with cefepime, but concerns about renal function led us to decide for levofloxacin instead. Antimicrobial therapy was maintained for 6 weeks.
Outcome and follow-up
After the first week of treatment, the patient was apiretic, the analytical parameters measuring inflammation/infection were declining steadily and echocardiographic reexamination showed a normally functioning aortic valve without vegetations and mild myocardial dysfunction. The patient was discharged after 6 weeks of therapy in good clinical condition.
Discussion
P. mirabilis is a rarely implicated causative agent of infective endocarditis6 but, when present, often causes severe disease.7 Only 11 cases came to our knowledge so far and of those, only 6 patients survived. Only two patients had prosthetic valves. All patients had positive blood cultures.
The absence of positive blood cultures in our case clearly hindered our approach. In the absence of bacteremia or echocardiographic evidence of endocarditis and in face of clues to an alternative source of infection, we became less suspicious of endocarditis.
Only one other patient who we know of had P. mirabilis pneumonia simultaneously.8 One patient’s bacteremia and endocarditis stemmed from an infected pressure ulcer9 and four from urinary tract infections.7
None of the patients reported so far were immunocompromised, which was an additional difficulty in our case, since it significantly broadens the span of infectious diseases that had to be considered.
After definitive diagnosis, the choice of antimicrobial therapy posed another challenge. There are no guidelines concerning this topic as there are also insufficient data to elaborate international recommendations from.10 However, it is generally accepted that therapy should be prolonged and that dual antimicrobial therapy with synergic and bactericidal agents should be administered. In this particular patient, the obvious synergy between beta lactam antibiotics and aminoglycosides raised concerns about renal function. Therefore, therapy with cefepime and levofloxacin was chosen and continued for 6 weeks. Despite this rationale, it should be noted that there are reports of two patients who were treated solely with ceftriaxone for 3 or 4 weeks and were cured.7
Learning points.
Blood culture negative infective endocarditis poses an important diagnostic challenge and requires extensive workup, more so in patients under immunosuppressive medication.
Proteus mirabilis is a rarely implicated agent in infective endocarditis.
Treatment of infective endocarditis caused by gram-negative agents is challenging and there are no international guidelines regarding this topic.
Footnotes
Contributors: IA, ARS and MSC contributed by reviewing literature on infective endocarditis and similar cases to ours and writing the paper. FF assisted in reviewing the drafting of the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025–35. 10.1378/chest.06-2048 [DOI] [PubMed] [Google Scholar]
- 2. Ambrosioni J, Hernandez-Meneses M, Téllez A, et al. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep 2017;19 10.1007/s11908-017-0574-9 [DOI] [PubMed] [Google Scholar]
- 3. Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012;54:1230–9. 10.1093/cid/cis199 [DOI] [PubMed] [Google Scholar]
- 4. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruttmann E, Bonatti H, Legit C, et al. Severe endocarditis in transplant recipients—an epidemiologic study. Transplant International 2005;18:690–6. 10.1111/j.1432-2277.2005.00120.x [DOI] [PubMed] [Google Scholar]
- 6. Brotzki CR, Mergenhagen KA, Bulman ZP, et al. Native valve Proteus mirabilis endocarditis: successful treatment of a rare entity formulated by in vitro synergy antibiotic testing. BMJ Case Rep 2016:bcr2016215956 10.1136/bcr-2016-215956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalra A, Cooley C, Tsigrelis C. Treatment of endocarditis due to Proteus species: a literature review. Int J Infect Dis 2011;15:e222–5. 10.1016/j.ijid.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 8. Salsano A, Sportelli E, Borile S, et al. Proteus mirabilis bioprosthetic tricuspid valve endocarditis with massive right ventricular vegetation: a new entity in the prosthetic valve endocarditis aetiology. Eur J Cardiothorac Surg 2016;50:581–2. 10.1093/ejcts/ezw126 [DOI] [PubMed] [Google Scholar]
- 9. Liu C-H, Chang W-J, Chin C. An unusual cause of infective endocarditis: Proteus mirabilis bacteremia from an infected pressure ulcer. International Journal of Gerontology 2015;9:243–5. 10.1016/j.ijge.2014.09.003 [DOI] [Google Scholar]
- 10. Habib G. ESC Guidelines for the management of infective endocarditis. European Heart Journal 2015. [DOI] [PubMed] [Google Scholar]