Abstract
Liver transplant recipients are immunocompromised by the virtue of being on immunosuppressive agents which put them at risk of having infections from unusual and even multiple concomitant pathogens. We present a case of a 39-year-old man who developed septicaemia with Enterococcus casseliflavus, Streptococcus equinus and Klebsiella oxytoca in the setting of perinephric haematoma which resulted following a kidney biopsy performed to evaluate his nephrotic range proteinuria. E. casseliflavus has been known to cause infections in patients with liver disease/cirrhosis; however, simultaneous infection with S. equinus and K. oxytoca along with E. casseliflavus has never been reported earlier in post-transplant state.
Keywords: malignant disease and immunosuppression, cirrhosis, gastroenterology, infectious diseases
Background
Enterococcus casseliflavus is a rare Gram-positive vancomycin-resistant enterococcus that resides in human gastrointestinal and hepatobiliary tract. It has been implicated in causing a multitude of conditions in immunocompromised hosts. Streptococcus equinus is part of human gut flora and can act as an opportunistic pathogen. No previous reports of this bacterium causing infection in liver transplant patients were found on our detailed literature search. Klebsiella oxytoca is a member of family Enterobacteriaceae and is usually pathogenic though it can be found in healthy individuals as well.1 This case helps reader recognise the importance of multiple co-infections in an immunocompromised host and its implications and challenges in formulating treatment plans in such situations.
Case presentation
A 39-year-old Caucasian man presented to the emergency department with a fever of 40.6°C, profuse watery diarrhoea (15–20 bowel movements per day) of 2-day duration. There was no associated abdominal cramping, weight loss or intake of exotic/unusual food items. Other accompanying symptoms included headache and mild chest pain.
His medical history was significant for type 1 diabetes mellitus, hypertension, chronic kidney disease stage 5 (CKD 5), nephrotic range proteinuria, liver transplantation in 1993 and 2001 for hepatitis C virus cirrhosis (acquired by blood transfusion in 1979), biopsy-proven allograft cirrhosis and small oesophageal varices. His hepatitis C had been treated and cured with simeprevir/sofosbuvir and ribavirin 4 years ago. Past surgeries included liver transplantation (twice), Roux-en-Y surgery for biliary duct stricture and incisional hernia repairs. Family history was significant for diabetes mellitus, coronary artery disease and hypertension in mother and hypertension in father. Personal and social history revealed that he was a factory worker who was a lifelong non-alcoholic, non-smoker and did not use illicit drugs. He had no exposure to farm animals and horses, and he did not travel within or outside the USA.
His prescription medications included buspirone, biphasic-type insulin mixed with both 70% of intermediate insulin and 30% of regular insulin, calcium acetate, calcium/vitamin D, multivitamin, melatonin, oxycodone, tramadol, nadolol, erythropoietin alpha, potassium chloride, simethicone, rifaximin, pantoprazole and tacrolimus.
Four months ago, he was admitted to the hospital for shock liver, acute kidney injury on top of CKD 5 after developing haemorrhagic and septic shock with infected retroperitoneal bleed/haematoma after an attempted kidney biopsy which required coil embolisation to stop bleeding. Patient underwent kidney biopsy to ascertain the cause of his rapidly worsening proteinuria (progressed from 5.5 g/day to 9.2 g/day in 4 months) in the setting of sirolimus use and the background of diabetes mellitus. He had been switched from sirolimus to tacrolimus owing to worsening proteinuria. Biopsy resulted consistent with diabetic nephrosclerosis. As a result of this biopsy, the patient developed a perinephric haematoma which then became infected. This nidus of infection caused fever with septicaemia from K. oxytoca and E. casseliflavus both of which were sensitive to ampicillin-sulbactam. Septic/haemorrhagic shock required need for intravenous norepinephrine and temporary dependence on haemodialysis. He was treated with ampicillin-sulbactam for 2 weeks and then was discharged home on a prescription of amoxicillin-clavulanate for additional 7 days. A transthoracic echocardiogram was done which did not show any evidence of infective endocarditis.
His vital signs in the emergency department (ED) showed a blood pressure of 154/104 mm Hg, temperature of 40.6°C, heart rate 97 beats/min, respiratory rate 20 breaths/min, oxygen saturation 96% on room air. Physical examination was unremarkable except for fever and previous upper abdominal scar from liver transplant surgery.
Investigations
His laboratory test results suggested an ongoing infection as evident by elevated white blood cell count and serum procalcitonin (see table 1). Serum tacrolimus level (trough) was 2.0 ng/mL. Urinalysis showed 3+ albumin and moderate amount of blood.
Table 1.
Summary of laboratory results
| Test result | Value | Reference range |
| Hb | 151 | 128–164 g/L |
| WBC | 10.3×109 | 4.2–10.2×109/L |
| PMN | 9.0×109 | 1.8–7.1×109/L |
| Plt | 102×109 | 150–400×109/L |
| INR | 1.7 | 0.8–1.0 |
| PT | 19.4 | 11.7–14.5 s |
| Na+ | 135 | 136–145 mEq/L |
| Cr | 3.1 | 0.7–1.3 mg/dL |
| Alb | 3.1 | 3.4–5.0 g/dL |
| T-Bil | 1.1 | 0.2–1.0 mg/dL |
| Alk Phos | 199 | 45–117 U/L |
| AST | 44 | 15–37 U/L |
| ALT | 31 | 16–61 U/L |
| PCT | 3.17 | <2 ng/mL |
Alk Phos, alkaline phosphatase; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD, Chronic Kidney Disease; Cr, creatinine; CT, Computed Tomography; ED, Emergency Department; ESBL, Extended Spectrum Beta Lactamase; Hb, haemoglobin; INR, International Normalised Ratio; IV, intravenous; MALDI-TOF, Matrix-Assisted Laser Desorption Ionisation Time-Of-Flight; Na +, sodium ion; NDM-1, New Delhi Metallo-beta-lactamases; PCT, procalcitonin; PICC, Peripherally Inserted Central Catheter; Plt, platelets; PMN, polymorphonuclear neutrophils; PT, Prothrombin time; SBSEC, S. bovis/S. equinus complex; T-Bil, Total bilirubin; WBC, white blood cells.
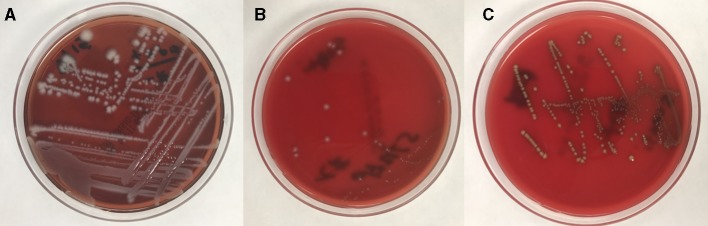
A CT abdomen was done which showed a decrease in the size of left perinephric haematoma from 23×13 cm to 10.6×8.1 cm. Splenomegaly was present (figure 1). He was empirically started on vancomycin and piperacillin-tazobactam in the ED after obtaining blood cultures. Perinephric haematoma was drained by interventional radiology. Infectious disease service was consulted and his antibiotics were changed to daptomycin and meropenem to avoid nephrotoxicity associated with the previous combination. Blood cultures drawn in emergency department grew K. oxytoca (extended-spectrum beta-lactamase (ESBL) producer though susceptible to ertapenem), S. equinus and E. casseliflavus susceptible to ampicillin (figure 2) in 2/2 blood culture bottles (BACTEC, Becton Dickinson, New Jersey, USA). Bacterial identification was done using matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) mass spectrometry technique (Microflex, Bruker, Massachussets, USA) and antibiotic sensitivities using DxM MicroScan WalkAway System (Beckman Coulter, California, USA). A transthoracic echocardiogram was done which did not show any evidence of vegetations, so infective endocarditis was ruled out. Stool tested for diarrhoea came back positive for Clostridium difficile toxin.
Figure 1.

Coronal plane CT scan image with side-by-side panels showing left perinephric haematoma during previous admission (A) measuring 24 cm in length and 12.5 cm in width which on current admission (B) had reduced to 10.7 cm in length (see yellow arrows).
Figure 2.
(A) Blood agar plate showing growth of Klebsiella oxytoca as large, mucoid, whitish colonies. (B) Blood agar plate showing growth of Streptococcus equinus as small, whitish colonies with peripheral halo. (C) Blood agar plate showing growth of Enterococcus casseliflavus as round, yellowish pigment producing colonies of various sizes.
Treatment
Patient was started on fidaxomicin for C. difficile infection and his diarrhoea improved. Given concern for ongoing infection, his immunosuppression was held. A peripherally inserted central catheter line was inserted to allow for intravenous antibiotic infusion as an outpatient. He was switched to intravenous ertapenem for 6 weeks and amoxicillin-clavulanate and was discharged home.
Outcome and follow-up
The patient was seen in clinic 6 weeks later and was afebrile and was doing well.
Discussion
Immunosuppressed patients (post-transplant on immunosuppression) can develop infections that are typically not seen in the general population. This case illustrates unusual combination of bacteria causing septicaemia in a patient who had been on sirolimus following liver transplant.
Enterococcus casseliflavus
E. casseliflavus is a Gram-positive, catalase-negative, facultatively anaerobic, non-spore forming vancomycin-resistant enterococcus (non-faecium, non-faecalis type). It is part of normal intestinal flora and is responsible for 1%–2% of all enterococcal infections.2 It forms yellow colonies on trypticase soy agar and has VanC-2/3 gene which confers it resistance against vancomycin.2 3 Colonisation by E. casseliflavus has been seen in intensive care unit patients with renal failure who have had prior exposure to antibiotics, specifically carbapenems.4
In addition to causing septicaemia, E. casseliflavus has been reported to cause various other infections such as meningitis, peritonitis, endocarditis, cholangiolitis and empyema.3 A large retrospective study found that patients with biliary disease, biliary drainage catheters, cancer, hypertension, diabetes and prior hospitalisation were more susceptible to bacteraemia from E. casseliflavus.5
Based on susceptibility, E. casseliflavus is usually treated with beta-lactams such as amoxicillin/ampicillin, penicillin G, ceftriaxone, imipenem/cisplatin.3 When resistant to beta-lactams, antibiotics used to treat vancomycin-resistant enterococci (VRE) (ie, linezolid, daptomycin, tigecycline, dalfopristin/quinupristin, telavancin and others) may be used.3 However, treatment of E. casseliflavus should be focused on local susceptibilities given increasing resistance to various antibiotics.
Streptococcus equinus
S. equinus, which was first isolated from horse dung in 1906,6 belongs to the S. bovis/S. equinus complex (SBSEC) group of bacteria which are a member of Lancefield group D streptococci, a taxonomic revision which was created in 2003.7 It is a commensal and is found in human gastrointestinal tract, however it can behave as an opportunistic pathogen. It can be acquired following close contact with horses and cows. S. equinus is a catalase-negative, Gram-positive coccus which forms small, non-beta-haemolytic colonies on blood agar. It can be distinguished from enterococcus based on its inabilities to grow in 6.5% salt broth and to hydrolyse arginine.
SBSEC bacteraemia, especially S. gallolyticus (previously known as S. bovis) bacteraemia, is a well-known cause of infective endocarditis. Comorbidities such as chronic liver disease, especially cirrhosis, and diabetes are more significant risk factors when infective endocarditis is caused by SBSEC than intravenous drug use.8 9 SBSEC septicaemia has been associated with a higher rate of colorectal cancer.10 11 S. equinus has been implicated in causing infective endocarditis12 and peritonitis13 in adults, urinary tract infections in children14 and mastitis in cows.15
Treatment of S. equinus should be based on local susceptibility pattern. Treatment is dependent on several coexisting factors such as the presence of prosthetic valves, distant septic foci, cardiovascular comorbidities and individual patient circumstances.16 Generally, treatment with penicillin G (with or without gentamicin synergy) or ceftriaxone is highly effective. For patients with beta-lactam intolerance, vancomycin may be used. Treatment duration varies between 2 and 4 weeks of antibiotics depending on coexisting factors.17 Other agents highly active against SBSEC are daptomycin, linezolid and tigecycline. Macrolides, tetracyclines, trimethoprim-sulfamethoxazole and clindamycin have weak activity against SBSEC and should not be used. Resistance to aztreonam has previously been reported.17 Given the high rate of infective endocarditis and colorectal cancer, an echocardiogram and colonoscopy should be performed in all patients with SBSEC bacteraemia.
Klebsiella oxytoca
K. oxytoca is a Gram-negative, lactose-fermenting, non-motile, aerobic rod-shaped bacterium belonging to the family of Enterobacteriaceae. K. oxytoca is indole-positive, as opposed to the more widely known pathogen K. pneumoniae, which is indole-negative. It is found on the surface of housefly eggs. K. oxytoca has been reported as a cause of antibiotic-associated haemorrhagic colitis,18 pneumonia,19 tricuspid valve endocarditis,20 ascending cholangitis,21 ileitis and portal venous gas formation,22 purpura fulminans,23 pseudomembranous enterocolitis,24 pneumatosis intestinalis,25 meningitis,26 endophthalmitis,27 liver abscess,28 septic arthritis,29 neck30 and other infections.
Treatment of K. oxytoca should be based on local susceptibilities. K. oxytoca commonly produces ESBL and is resistant to carbapenems. Strains producing New Delhi Metallo-beta-lactamases and imipenemase have been reported as well.31 32 Given variable resistance pattern, it is prudent to tailor choice of antibiotics based on local susceptibility results. In general, Infectious Diseases Society of America recommends use of piperacillin/tazobactam, cefepime, ceftazidime, laevofloxacin, ciprofloxacin, meropenem, imipenem/cilastatin or aztreonam for treatment of K. oxytoca infection.33
To the best of our knowledge, this is the first reported case of co-infection with E. casseliflavus, S. equinus and K. oxytoca septicaemia in a post-liver transplant patient. This patient had several comorbidities that predisposed him to infection with each microorganism, including chronic immunosuppression, nephropathy and previous antibiotic therapy. Patients with cirrhosis are at increased risk of developing infections. Readers need to be aware that multiple concurrent infections can occur in immunosuppressed individuals (such as those on immunosuppressive medications or those with cirrhosis) and that infected haematoma must be promptly drained to avoid providing a lingering nidus of ongoing infection which can lead to prolonged septicaemia as in this case.
Learning points.
Transplant recipients on immunosuppressive agents or patients with cirrhosis are immunocompromised individuals and can have infections caused by unusual and rare bacteria.
Transthoracic (or if required transoesophageal) echocardiogram should be performed when faced with septicaemia with Enterococcus or Streptococcus bacteria to rule out infective endocarditis.
An infected haematoma should be promptly drained, otherwise it can act as a nidus and persistent source of infection.
Although empirical antibiotic administration at the outset is acceptable, local susceptibility results should be followed when deciding on treatment of unusual bacterial infections.
Footnotes
Contributors: RV and SPN took care of the patient. YS worked on microbiology results of the patient cultures. RPH and RV drafted the initial version of the manuscript. All authors (RV, RPH, YS, SPN) contributed to the manuscript, made edits and approved the final version of manuscript for submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Högenauer C, Langner C, Beubler E, et al. . Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. New England Journal of Medicine 2006;355:2418–26. 10.1056/NEJMoa054765 [DOI] [PubMed] [Google Scholar]
- 2. Toye B, Shymanski J, Bobrowska M, et al. . Clinical and epidemiological significance of enterococci intrinsically resistant to vancomycin (possessing the vanC genotype). J Clin Microbiol 1997;35:3166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verma R, Baroco AL. Enterococcus casseliflavus septicaemia associated with hepatobiliary infection in a 75-year-old man. BMJ Case Rep 2017;34:bcr-2017-219636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batistão DWdaF, Gontijo-Filho PP, Conceição N, et al. . Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63. 10.1590/S0074-02762012000100008 [DOI] [PubMed] [Google Scholar]
- 5. Choi SH, Lee SO, Kim TH, et al. . Clinical features and outcomes of bacteremia caused by Enterococcus casseliflavus and Enterococcus gallinarum: analysis of 56 cases. Clin Infect Dis 2004;38:53–61. 10.1086/380452 [DOI] [PubMed] [Google Scholar]
- 6. Hodge HM, Sherman JM. Streptococcus equinus. J Bacteriol 1937;33:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jans C, Meile L, Lacroix C, et al. . Genomics, evolution, and molecular epidemiology of the Streptococcus bovis/Streptococcus equinus complex (SBSEC). Infect Genet Evol 2015;33:419–36. 10.1016/j.meegid.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 8. Corredoira J, Alonso MP, Coira A, et al. . Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis 2008;27:285–91. 10.1007/s10096-007-0441-y [DOI] [PubMed] [Google Scholar]
- 9. Tripodi MF, Adinolfi LE, Ragone E, et al. . Streptococcus bovis endocarditis and its association with chronic liver disease: an underestimated risk factor. Clin Infect Dis 2004;38:1394–400. 10.1086/392503 [DOI] [PubMed] [Google Scholar]
- 10. Klein RS, Recco RA, Catalano MT, et al. . Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med 1977;297:800–2. 10.1056/NEJM197710132971503 [DOI] [PubMed] [Google Scholar]
- 11. Gilon D, Moses A. Carcinoma of the colon presenting as Streptococcus equinus bacteremia. Am J Med 1989;86:135–6. 10.1016/0002-9343(89)90249-0 [DOI] [PubMed] [Google Scholar]
- 12. Sechi LA, De Carli S, Ciani R. Streptococcus equinus endocarditis in a patient with pulmonary histiocytosis X. Am J Med 2000;108:522–3. 10.1016/S0002-9343(99)00331-9 [DOI] [PubMed] [Google Scholar]
- 13. Tuncer M, Ozcan S, Vural T, et al. . Streptococcus equinus peritonitis in a CAPD patient. Perit Dial Int 1998;18:654. [PubMed] [Google Scholar]
- 14. Bump CM. Isolation of Streptococcus equinus from non-respiratory sources in children. J Clin Microbiol 1977;6:433–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broadbent JR, Chou YC, Gillies K, et al. . Nisin inhibits several Gram-positive, mastitis-causing pathogens. J Dairy Sci 1989;72:3342–5. 10.3168/jds.S0022-0302(89)79496-0 [DOI] [PubMed] [Google Scholar]
- 16. Baddour LM, Wilson WR, Bayer AS, et al. . American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- 17. Ariza J, Gudiol F, Dolz C, et al. . Evaluation of aztreonam in the treatment of spontaneous bacterial peritonitis in patients with cirrhosis. Hepatology 1986;6:906–10. 10.1002/hep.1840060516 [DOI] [PubMed] [Google Scholar]
- 18. Fisher A, Halalau A. A case report and literature review of Clostridium difficile negative antibiotic associated hemorrhagic colitis caused by Klebsiella oxytoca . Case Rep Gastrointest Med 2018;2018:1–4. 10.1155/2018/7264613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee KM, Morris-Love J, Cabral DJ, et al. . Coinfection with influenza A virus and Klebsiella oxytoca: an underrecognized impact on host resistance and tolerance to pulmonary infections. Front Immunol 2018;9:2377 10.3389/fimmu.2018.02377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Memon W, Miller M, Shabbir Z. Klebsiella oxytoca tricuspid valve endocarditis in an elderly patient without known predisposing factors. BMJ Case Rep 2018:2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venkatanarasimha N, Yong YR, Gogna A, et al. . Case 265. Radiology 2019;290:262–3. 10.1148/radiol.2018162374 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka H, Watanabe T, Nagai T, et al. . Hepatic portal venous gas associated with Klebsiella oxytoca infection in the absence of preceding antibiotic treatment. Clin J Gastroenterol 2019;12:316–9. 10.1007/s12328-019-00947-1 [DOI] [PubMed] [Google Scholar]
- 23. Tsubouchi N, Tsurukiri J, Numata J, et al. . Acute infectious purpura fulminans caused by Klebsiella oxytoca . Intern Med 2019;58:1801–2. 10.2169/internalmedicine.2350-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagamura T, Tanaka Y, Terayama T, et al. . Fulminant pseudomembranous enterocolitis caused by Klebsiella oxytoca: an autopsy case report. Acute Med Surg 2019;6:78–82. 10.1002/ams2.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tahara S, Sakai Y, Katsuno H, et al. . Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus. Medicine 2019;98:e14079 10.1097/MD.0000000000014079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carrie C, Walewski V, Levy C, et al. . Klebsiella pneumoniae and Klebsiella oxytoca meningitis in infants. Epidemiological and clinical features. Arch Pediatr 2019;26:12–15. 10.1016/j.arcped.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 27. Joseph J, Sharma S, Dave VP. Filamentous gram-negative bacteria masquerading as actinomycetes in infectious endophthalmitis: a review of three cases. J Ophthalmic Inflamm Infect 2018;8:15 10.1186/s12348-018-0157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paasch C, Wilczek S, Strik MW. Liver abscess and sepsis caused by Clostridium perfringens and Klebsiella oxytoca . Int J Surg Case Rep 2017;41:180–3. 10.1016/j.ijscr.2017.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hertting O, Gremark O, Källman O, et al. . An infant with Klebsiella oxytoca septic arthritis. J Microbiol Immunol Infect 2018;51:153–4. 10.1016/j.jmii.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 30. Cordesmeyer R, Kauffmann P, Markus T, et al. . Bacterial and histopathological findings in deep head and neck infections: a retrospective analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;124:11–15. 10.1016/j.oooo.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 31. Wang J, Yuan M, Chen H, et al. . First report of Klebsiella oxytoca strain simultaneously producing NDM-1, IMP-4, and KPC-2 carbapenemases. Antimicrob Agents Chemother 2017;61:e00877–17. 10.1128/AAC.00877-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang S, Hemarajata P, Shevy L, et al. . Unusual carbapenem resistant but ceftriaxone and cefepime susceptible Klebsiella oxytoca isolated from a blood culture: case report and whole-genome sequencing investigation. IDCases 2018;11:9–11. 10.1016/j.idcr.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalil AC, Metersky ML, Klompas M, et al. . Management of adults With hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61–e111. 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]