Abstract
This report discusses a case of superior mesenteric artery (SMA) syndrome in a previously healthy 15-year-old boy with no weight loss or other common risk factors. The patient presented to the emergency department with acute bilious vomiting and epigastric pain after acute consumption of a meal and excessive quantities of water. The patient was diagnosed with SMA syndrome based on the findings of contrasted CT of the abdomen. In early puberty, boys have a significant increase in lean body mass and a concomitant loss of adipose tissues. These pubertal changes lead to a narrowing of the aortomesenteric space. The acute consumption of food and water caused a transient obstruction at the already-narrowed space, which resulted in the manifestation of SMA syndrome. This case demonstrates that pubertal growth spurt is a risk factor for SMA syndrome, and acute excessive ingestion can trigger SMA syndrome among those in puberty.
Keywords: paediatrics, stomach and duodenum, primary care
Background
Superior mesenteric artery (SMA) syndrome is thought to be a rare entity in which the third portion of the duodenum is compressed by the narrow angle between the SMA and the aorta. The usual factors in the acute angulation between the SMA and the aorta can be categorised as follows: (1) significant weight loss leading to the depletion or loss of retroperitoneal fatty tissues; (2) external compression by belts or spica casts; and (3) anatomical defects and congenital anomalies, including corrective spinal surgery for scoliosis, a high insertion of the duodenum at the ligament of Treitz and intestinal malrotation.1 The symptoms are postprandial epigastric pain, bilious vomiting, nausea and early satiety. Typically, patients with SMA syndrome present after rapid weight loss or spinal surgery.2 3 Therefore, SMA syndrome is generally unexpected in young populations who lack classic risk factors such as severe weight loss, psychological disorders or spinal surgery.
Case presentation
A 15-year-old boy presented to the emergency department with acute bilious vomiting and epigastric pain. The patient appeared to suffer from considerable pain which occurred while participating in a basketball game, and gradually worsened after taking a meal and more than 2 L of water during the sports game. The patient reported that he received several forceful blows to his abdomen from other players. In addition, for the last one and a half years, the patient had early satiety and postprandial epigastric pain which was relieved when he was in a knee-chest position. One year prior to this admission, the patient had been brought to a different hospital for similar abdominal symptoms that had resolved without treatment within several hours.
His initial vital signs were normal. The patient had a strong, muscular and athletic build, a height of 185 cm and a weight of 66.0 kg. He reported a growth in height of 9 cm and no weight change for the past year. Palpation of his abdomen revealed generalised tenderness, but no guarding, and no abdominal masses were detected. Blood tests, including blood count (haemoglobin 154 g/L, white cell count 7.60×109/L and platelet count 22.9×109/L), chemistries (sodium 142 mmol/L, potassium 4.6 mmol/L, chloride 105 mmol/L, blood urea nitrogen 6.57 mmol/L and serum creatinine 59.3 μmol/L), liver function tests (albumin 56 g/L, bilirubin 21.2 μmol/L, aspartate aminotransferase 28 IU/L and alanine aminotransferase 18 IU/L) and inflammatory marker (C reactive protein 0.1 mg/L), were normal, with the exception of a slight elevation of amylase (163 U/L). The urinalysis was within normal limits. The abdominal ultrasound (US) showed no abnormalities other than a dilated stomach.
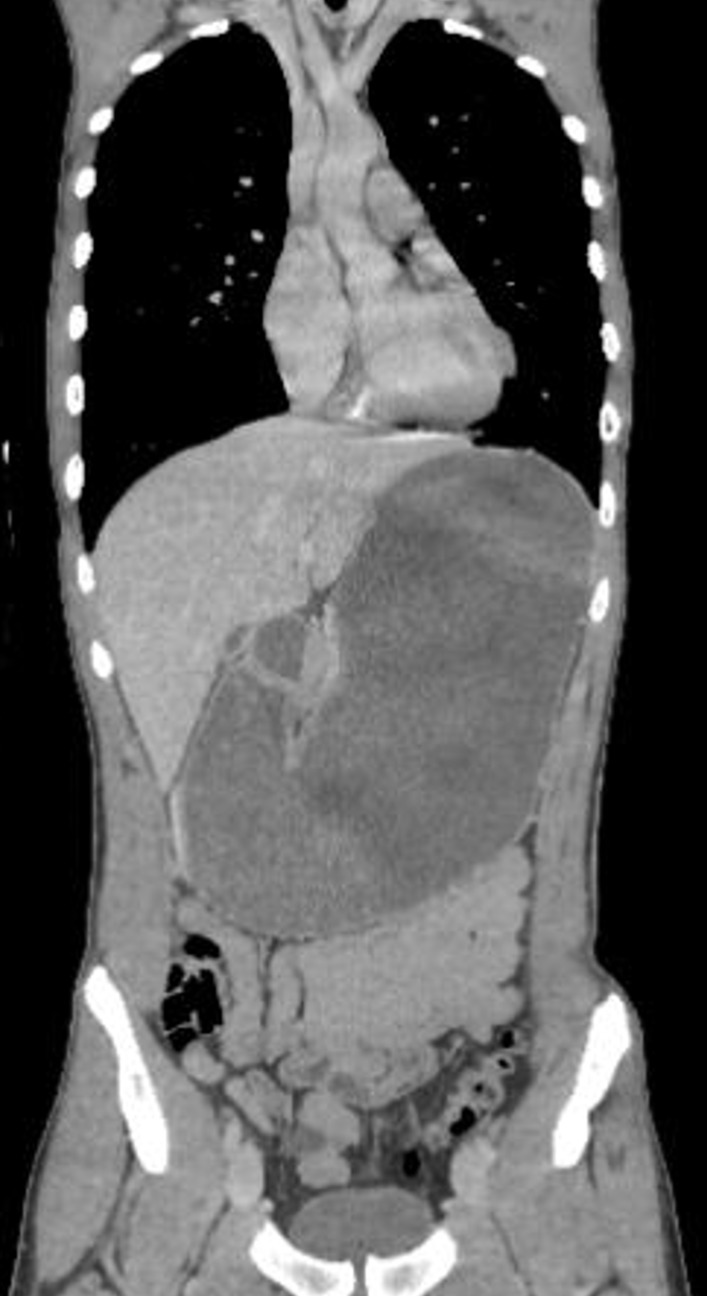
Despite the patient’s abdominal findings, his severe abdominal pain and history of forceful blows to the abdomen suggested the possibility of abdominal injuries, including intestinal perforation and abdominal haemorrhage. As a result, a contrasted CT of the abdomen and pelvis was performed. This CT was initially interpreted by a doctor on night duty as normal apart from a massively distended stomach (figure 1). After the CT scan, the patient lay in prone and knee-chest position for about an hour and then his symptoms resolved spontaneously. Therefore, gastric decompression with a nasogastric tube was not performed. The patient only underwent observation with intravenous hydration. No vomiting was found during the observation period. The next morning, the patient made a swift recovery. He had no abdominal symptoms, including postprandial pain. The follow-up abdominal US showed the volume of the stomach significantly decreased as normal and no other abnormalities were found. These suggested that there was a transient intestinal obstruction that was thought not to require emergency interventions. The patient was discharged, and a follow-up visit was arranged.
Figure 1.
A massively distended stomach.
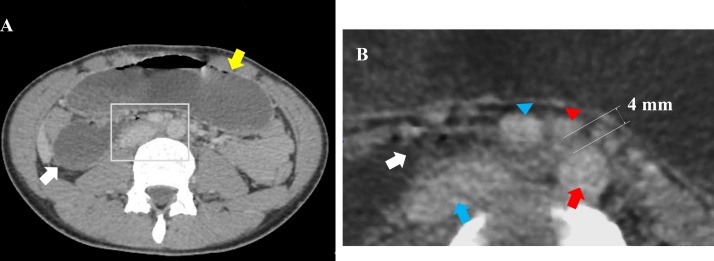
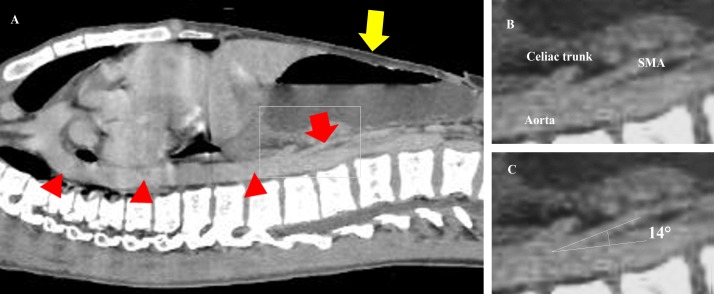
After his discharge, the CT review by an experienced radiologist revealed, however, a dilated proximal duodenum and an abrupt calibre change in the third portion of the duodenum (figure 2). The aortomesenteric angle and distance were 14° (normal range: 38°–65°4) and 4 mm (normal range: 10–28 mm5), respectively (figures 2 and 3). In addition, the abdominal fat was significantly low (figure 4). Furthermore, the third part of the duodenum passed anterior to the aorta in the retroperitoneal space. The position of the SMA and superior mesenteric vein was normal (figure 2). These suggested that there was no intestinal malrotation or volvulus in this patient. Based on these radiological findings and his clinical history, the patient was diagnosed with SMA syndrome.
Figure 2.
(A) CT of the abdomen showing an abrupt calibre change in the third portion of the duodenum (white arrow) and a dilated stomach (yellow arrow). (B) An enlarged image of the aorta (red arrow), the inferior vena cava (blue arrow), the SMA (red arrowhead) and the SMV (blue arrowhead), and an abrupt calibre change in the third portion of the duodenum (white arrow). The aortomesenteric distance was 4 mm (normal range: 10–28 mm). In addition, the CT revealed that the course of the duodenum, the position of the SMA and the SMV (the SMV was located to the right of the SMA), and the location of the caecum (not shown here) were normal. SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Figure 3.
(A) CT sagittal section showing the SMA (red arrow) and the aorta (red arrowheads) and a dilated stomach (yellow arrow). (B) An enlarged image of the SMA arising from the abdominal aorta immediately below the coeliac trunk. (C) The angle between the SMA and the aorta was 14° (normal range: 38°–65°). SMA, superior mesenteric artery.
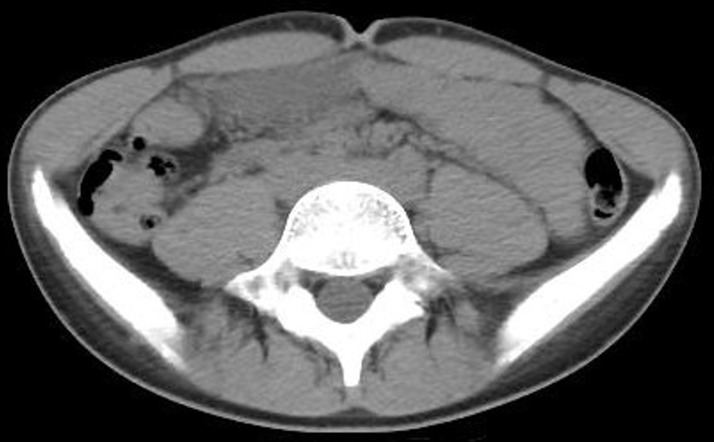
Figure 4.
A significant decrease in the patient’s adipose tissue.
Outcome and follow-up
At outpatient follow-up a week after discharge, the patient and guardians were informed of the diagnosis and advised to avoid excessive intake of fluid and food, to increase body weight by eating several small meals throughout the day, and to take a knee-chest position after eating. At 6-month follow-up, the patient was asymptomatic and had gained 3.9 kg in weight. Thus, the clinical course confirmed the diagnosis of SMA syndrome.
Discussion
In early puberty, boys experience a significant increase in lean body mass and a concomitant loss of adipose tissues.6 These pubertal changes in body composition often lead to a narrowing of the aortomesenteric space due to a depletion of the mesenteric fat pad. Therefore, as previous studies indicate, a growth spurt is a risk factor for SMA syndrome,3 7–9 and any event that further exacerbates the narrowing of the aortomesenteric space is likely to result in a manifestation of SMA syndrome. Potential triggers identified thus far for such exacerbation include extra weight loss due to infection10 and overeating after dieting.11 In the present case, considering his linear growth without weight gain, the patient’s mesenteric fat tissue had already been depleted. The abdominal CT also reveals the significant decrease in his adipose tissue (figure 4). Therefore, the retarded passage of the ingested food at the duodenum and the acute consumption of a large volume of water caused a transient obstruction that spontaneously resolved with postural changes.
This case supports the previous findings that weight loss is not necessary for the development of SMA syndrome in the paediatric population.7 8 For example, Biank and Werlin3 reported no weight loss was observed in 50% of the 22 paediatric cases of SMA syndrome. The reason for the development of SMA syndrome in children without weight loss is currently unexplained. However, the present case speculates that insufficient weight gain relative to height growth causes a decrease in visceral fat and predisposes children to SMA syndrome.
SMA syndrome can be managed initially with conservative therapy such as gastric decompression, electrolyte correction, and nutritional support which is an important component of conservative treatment. Psychiatric assessment is necessary for those with eating disorders. When oral feeding is not tolerated, enteral feeding can be performed through a nasojejunal tube placed distal to the obstruction.1 3 12 Total parenteral nutrition may be required in order to provide sufficient nutrition. Paediatric patients with acute presentation are likely to benefit from conservative management alone.3 However, if conservative management fails, surgical options, including Strong’s procedure, gastrojejunostomy and duodenojejunostomy, are indicated.
A paediatric patient presenting with bilious vomiting should be presumed to have an intestinal obstruction that is a surgical emergency. An upper gastrointestinal series should be performed when intestinal malrotation is suspected. In the present case, the patient should have been kept hospitalised until malrotation was ruled out and the underlying causes were identified.
Recurrent abdominal pain that resolves spontaneously within several hours, as observed in this patient, is frequently encountered in paediatric emergency care. Our case provides insight into the clinical observation. As stated above, a growth spurt is a predisposing condition for SMA syndrome, and acute consumption of food and water, which is often observed among adolescents, is identified as a trigger for the manifestation of the syndrome. These suggest that SMA syndrome might occur more frequently in such population than previously thought. In addition, the present case describes a case of SMA syndrome with a history of recurrent abdominal pain and spontaneous resolution of the symptoms, and draws attention to the fact that SMA syndrome with such presentation may be missed or misdiagnosed as functional disorders. These imply that SMA syndrome may be responsible for some of the unexplained abdominal symptoms among the pubertal group. Therefore, SMA syndrome should be considered in adolescents with recurrent abdominal symptoms.
Learning points.
A pubertal growth spurt is a risk factor for superior mesenteric artery (SMA) syndrome, and weight loss is not necessary for the development of the syndrome in the paediatric population.
Acute and excessive ingestion of water and food can trigger a manifestation of SMA syndrome in adolescents whose visceral adipose tissue has already been depleted.
A paediatric patient presenting with bilious vomiting should be presumed to have an intestinal obstruction.
Patients should not be sent home without ruling out surgical emergencies.
It is important to consider SMA syndrome in the differential diagnosis of adolescents with recurrent abdominal symptoms.
Footnotes
Contributors: TO is the principal author of the case report and contributed to the management of the patient. TS has been responsible for critically revising the manuscript and supervising the management of the patient by TO. YS has aided in providing the radiological interpretations.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Welsch T, Büchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg 2007;24:149–56. 10.1159/000102097 [DOI] [PubMed] [Google Scholar]
- 2. Anderson CM, Dalrymple MA, Podberesky DJ, et al. . Superior mesenteric artery syndrome in a basic military trainee. Mil Med 2007;172:24–6. 10.7205/milmed.172.1.24 [DOI] [PubMed] [Google Scholar]
- 3. Biank V, Werlin S. Superior mesenteric artery syndrome in children: a 20-year experience. J Pediatr Gastroenterol Nutr 2006;42:522–5. 10.1097/01.mpg.0000221888.36501.f2 [DOI] [PubMed] [Google Scholar]
- 4. Derrick JR, Fadhli HA. Surgical anatomy of the superior mesenteric artery. Am Surg 1965;31:545–7. [PubMed] [Google Scholar]
- 5. Sapkas G, O’Brien JP. Vascular compression of the duodenum (cast syndrome) associated with the treatment of spinal deformities. A report of six cases. Arch Orthop Trauma Surg 1981;98:7–11. 10.1007/BF00389703 [DOI] [PubMed] [Google Scholar]
- 6. Holland-Hall C, Burstein GR, et al. . Adolescent physical and social development : Kliegman RM, Stanton BF, St Geme JW, Schor NF, Nelson textbook of pediatrics. 20th edn Philadelphia: Elsevier, 2016. [Google Scholar]
- 7. Shin MS, Kim JY. Optimal duration of medical treatment in superior mesenteric artery syndrome in children. J Korean Med Sci 2013;28:1220–5. 10.3346/jkms.2013.28.8.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee TH, Lee JS, Jo Y, et al. . Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg 2012;16:2203–11. 10.1007/s11605-012-2049-5 [DOI] [PubMed] [Google Scholar]
- 9. Kogawa K, Kusama Y. Superior mesenteric artery syndrome in a healthy adolescent. BMJ Case Rep 2017;2017:bcr-2017-220609 10.1136/bcr-2017-220609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakker ME, van Delft R, Vaessens NA, et al. . Superior mesenteric artery syndrome in a 15-year-old boy during Ramadan. Eur J Pediatr 2014;173:1619–21. 10.1007/s00431-013-2190-5 [DOI] [PubMed] [Google Scholar]
- 11. Nishiguchi S, Shirobe T. Acute abdominal pain caused by superior mesenteric artery syndrome in a healthy young boy. BMJ Case Rep 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forsyth JM, Muhammad K, Mahmood K. Superior mesenteric artery syndrome as a cause for recurrent abdominal pain and vomiting in a 9-year-old girl. BMJ Case Rep 2015;2015 10.1136/bcr-2014-209270 [DOI] [PMC free article] [PubMed] [Google Scholar]