Abstract
Background:
Evidence-based treatments for posttraumatic stress disorder (PTSD) have poor uptake and remission rates, suggesting that alternative treatments are needed. Morning bright light may be an effective treatment for PTSD given its established effects on mood and sleep; however, there are no published trials.
Methods:
We conducted a placebo-controlled pilot trial of a wearable light device, the Re-timer®, for individuals with probable PTSD. Individuals were randomly assigned to the active Re-timer® (n = 9) or a placebo Re-timer® dimmed with neutral density filters (n = 6). Participants self-administered the treatment at home 1 hour each morning over 4 weeks. PTSD and depression symptoms were assessed at pre and post treatment.
Results:
The Re-timer® was well tolerated and perceived benefit was high, though treatment adherence was only moderate. Those in the active group were more likely to achieve a minimal clinically important change in PTSD and depression symptoms and had larger symptom reductions than those in the placebo group.
Conclusions:
A wearable morning light treatment was acceptable and feasible for patients with probable PTSD. This study provides initial proof-of-concept that light treatment can improve PTSD. A larger trial is warranted to establish treatment efficacy.
Keywords: Posttraumatic stress disorder, trauma, bright light, treatment, device, randomized controlled trial
Introduction
The mental health burden of posttraumatic stress disorder (PTSD) is costly for individuals and society (Kessler, 2000). Trauma-focused psychotherapies are considered to be first-line treatments for PTSD (Lee et al., 2016); however, evidence suggests that many individuals fail to receive these treatments let alone a therapeutic dose (Hoge et al., 2014). Treatment uptake is poor for several reasons including avoidance and lack of availability (Kantor, Knefel, & Lueger-Schuster, 2017). Moreover, many individuals remain symptomatic despite receiving treatment (Bradley, Greene, Russ, Dutra, & Westen, 2005). Alternative treatments are needed that are effective, acceptable, and accessible to patients.
Morning light treatment may be an effective treatment for PTSD. Although there is limited research examining circadian disturbances in PTSD, evidence suggests that an evening chronotype is associated with worse PTSD symptoms (Hasler, Insana, James, & Germain, 2013; Yun, Ahn, Jeong, Joo, & Choi, 2015). Later circadian timing (phase delay) is also associated with worse mood and sleep quantity / quality (Emens, Lewy, Kinzie, Arntz, & Rough 2009; Hasler, Buysee, Kupfer, & Germain, 2010), which are core symptoms of PTSD (Friedman, 2013; Spoormaker & Montgomery, 2008). Morning bright light treatment effectively advances circadian timing (i.e., shift toward morningness; St. Hilaire et al., 2012), and meta-analyses have shown morning light treatment is effective for non-seasonal depression (Al-Karawi & Jubair, 2016), and can meaningfully improve subjective and objective sleep (van Maanen, Meijer, van der Heijden, & Oort, 2016). However, to our knowledge, there are no published studies examining the effects of morning bright light treatment for PTSD.
In addition to light’s potential therapeutic effects, light treatment is safe and non-invasive with minimal side effects. Although some side effects have been reported (headache, eyestrain, nausea, agitation), these often spontaneously remit and patients rarely discontinue due to side effects (Pail et al., 2011; Terman & Terman, 2005). Light treatments are also typically self-administered, which makes them easily disseminable and scalable. Early research on light treatment used light boxes; however, newer wearable devices make it more feasible for patients to receive therapeutic doses as individuals can be ambulatory while receiving light treatment. The goal of this randomized controlled pilot trial was to evaluate the acceptability and feasibility of a wearable morning light treatment for probable PTSD, and make a preliminary assessment of the treatment’s effectiveness in improving PTSD symptoms.
Methods
Participants and Procedures
Fifteen participants with probable PTSD (i.e., PTSD symptoms were established using a self-report measure rather than a gold-standard clinical assessment) were randomly assigned to active (n = 9) and placebo (n = 6) conditions. A CONSORT diagram of participant flow is presented in Figure 1. Participant demographics are reported in Table 1.
Figure 1.
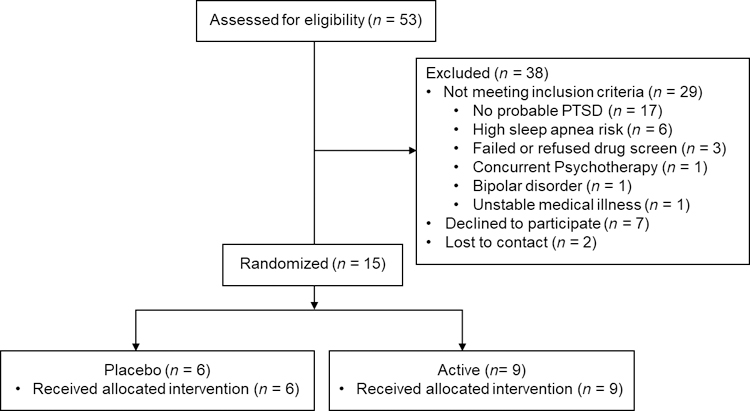
CONSORT diagram of participant flow
Table 1.
Sample Characteristics
| Total sample | Active | Placebo | |
|---|---|---|---|
| Variable | M (SD) | M (SD) | M (SD) |
| Age | 44.93 (11.83) | 40.89 (9.36) | 51.00 (13.33) |
| Adherence: days initiated treatment | 21.5 (6.1) | 19.67 (5.81) | 24.17 (6.01) |
| Adherence: minutes per treatment day in prescribed window | 35.2 (6.3) | 32.45 (5.30) | 39.22 (5.81) |
| Variable | n (%) | n (%) | n (%) |
| Male | 7 (46.7) | 5 (55.6) | 2 (33.3) |
| Ethnicity | |||
| Non-Hispanic | 11 (73.3) | 6 (66.7) | 5 (83.3) |
| Hispanic / Latino | 4 (26.7) | 3 (33.3) | 1 (16.7) |
| Race | |||
| White | 4 (26.7) | 4 (44.4) | 0 (0.0) |
| Black / African American | 8 (53.3) | 3 (33.3) | 5 (83.3) |
| Mixed | 2 (13.3) | 1 (11.1) | 1 (16.7) |
| Decline to answer | 1 (6.7) | 1 (11.1) | 0 (0.0) |
| Marital Status | |||
| Single | 12 (80.0) | 7 (77.8) | 5 (83.3) |
| Married / domestic partnership / engaged | 3 (20.0) | 2 (22.2) | 1 (16.7) |
| Highest Degree | |||
| Less than high school | 2 (13.3) | 2 (22.2) | 0 (0.0) |
| High school / GED | 4 (26.7) | 1 (11.1) | 3 (50.0) |
| Vocational / Associates Degree / Some college | 6 (40.0) | 5 (55.5) | 1 (16.7) |
| Baccalaureate or Master’s degree | 3 (20.0) | 1 (11.1) | 2 (33.3) |
| Index trauma type | |||
| Physical / Sexual assault | 5 (33.3) | 2 (22.2) | 3 (50.0) |
| Being shot / Shot at | 4 (26.7) | 4 (44.4) | 0 (0.0) |
| Witnessing serious harm or death of others | 3 (20.0) | 2 (22.2) | 1 (16.7) |
| Combat | 2 (13.3) | 1 (11.1) | 1 (16.7) |
| Motor vehicle accident | 1 (6.7) | 0 (0.0) | 1 (16.7) |
Note. Total sample N = 14. Active group n = 9. Placebo group n = 6. “Adherence: days initiated treatment” refers to the number of days that participants completed part or all of the morning light treatment out of the 28 treatment days. “Adherence: minutes per treatment day in prescribed window” refers to the minutes of light that participants received in the treatment window on days when they initiated treatment.
Participants had to meet the following inclusion criteria: PTSD Checklist for DSM-5 score > 33 (Bovin et al., 2016) rated based on an index trauma that met DSM-5 criterion A for PTSD; 18–70 years old; and fluent in English. Exclusion criteria are reported in Table 2. When scheduling participants, study staff ensured that participants would not be engaged in the protocol during the bi-annual time change for daylight saving time or any special events that might disrupt sleep.
Table 2.
Exclusion criteria
| Exclusion Criteria |
|---|
| Past 6 month substance use disorder |
| Lifetime psychotic or bipolar disorder |
| Lifetime diagnosis of winter depression |
| Significant suicidal ideation or behaviors in past 6 months |
| Severe hearing and memory problems |
| Cognitive impairment that would interfere with consent |
| Pending legal cases or litigation |
| Initiation of psychotherapy in the past 30 days |
| Engaged in an evidence based psychotherapy for PTSD |
| Serious unstable medical condition likely to result in hospitalization in the next year |
| Chronic migraine triggered by bright light |
| Vision problems, retinal disease, history of eye surgery, or history of light treatment |
| Photosensitizing medication use |
| Unstable dose of psychiatric medication (hypnotics, sleep aids, and antidepressants must be stable for 30 days before and during the study) |
| High risk for sleep apnea (Netzer, Stoohs, Netzer, Clark & Strohl, 1999) or restless leg syndrome (Hening & Allen, 2003) |
| Worked night shift in the past month |
| Pregnant, trying to get pregnant, or breastfeeding |
| Travel outside the study time zone in the past month |
Procedures
Participants were recruited from local advertisements. An initial phone screen was conducted to establish preliminary inclusion criteria; those who appeared eligible were invited for an in-person screening visit. All participants provided written informed consent prior to any study procedures. During the screening visit, participants completed a series of questionnaires and were given detailed instructions regarding the study procedures. Eligible participants who chose to enroll in the study completed a pre-treatment visit (day 1; visit 1), which included a urine drug test, a breathalyzer test, and questionnaires. Those who failed the drug or breathalyzer tests were excluded from the study. Those who passed received a wrist activity monitor, daily sleep logs and event logs at the day 1 visit and were told to sleep ad lib at home, following their usual sleep schedule. On day 8 (visit 2), participants completed a baseline session in which they completed questionnaires and were randomized 1:1 to the bright (active) or dim (placebo) Re-timer® using a simple randomization scheme. Participants were instructed on how to use the Re-timer® and only saw the Re-timer® device they were assigned (single blinded).
On day 9, subjects began 4 weeks of self-administered bright light treatment at home. Participants were told to use the Re-timer® for 1 hour each morning starting at their usual wake time or up to 1 hour earlier if required to fit into their daily schedule (determined from baseline week of wrist actigraphy) and to maintain their habitual sleep duration. Participants returned to the lab weekly for 4 weeks (visits 3 – 6) for repeat questionnaires and review of their treatment adherence (including immediate feedback based on their data to encourage adherence). Participants were compensated $75 for each for visits 2 through 5 as well as $150 for visit 6 in the form of a check or gift cards. This study was approved by the Institutional Review Board at Rush University Medical Center.
Re-timer® and Placebo Devices
The Re-timer® is commercially available and permits ambulation while receiving light from LEDs positioned below the eyes. The Re-timer® can be worn over glasses and does not substantially interfere with vision. The LEDs emit green light (~500nm, 230 µW/m2, 500 lux), close to the peak sensitivity of circadian photoreceptors (~480 nm; LeGates, Fernandez, & Hattar, 2014). The Re-timer® has previously been shown to shift circadian timing (Lovato & Lack, 2016).
We created a placebo device using neutral density filters to reduce the light intensity to a level that will not shift circadian timing (irradiance 3 μW/m2, 7 lux, Zeitzer, Dijk, Kronauer, Brown, & Czeisler, 2000). The placebo (dim) Re-timer® appears identical to those on the Re-timer® website.
Measures
All self-report measures were collected at pretreatment (visit 2) and posttreatment (visit 6). Objective sleep and treatment adherence were assessed throughout the study.
Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5; Weathers et al., 2013).
This 20-item self-report measure asks participants to rate how much they are bothered by DSM-5 PTSD symptoms based on their index trauma. Signal detection analyses have shown that a score of 31–33 had the highest efficiency for diagnosing PTSD (Bovin et al., 2016). At the screening visit, participants were asked to rate their symptoms for the past month to establish study eligibility. At visits 2 and 6, participants were asked to rate their symptoms for the past week to detect changes that occurred during the intervention. A 10-point improvement is considered to be a minimal clinically important difference (MCID) for the DSM-IV version of the scale (PTSD: National Center for PTSD, 2017). Given that there are not updated standards for the PCL-5, we used that as our threshold. Cronbach’s alpha was .90 at both pretreatment and posttreatment.
Patient Health Questionnaire-9 (PHQ-9; Kroenke, Spitzer, & Williams, 2001).
The PHQ-9 assesses severity of depression symptoms over the past two weeks. A 5-point improvement on the PHQ-9 has been established as a MCID (Löwe, Unützer, Callahan, Perkins, & Kroenke, 2004). Cronbach’s alpha was .72 at pretreatment and .83 at posttreatment.
Pittsburgh Sleep Quality Index (PSQI; Buysee, Reynolds, Monk, Berman, & Kupfer, 1989).
The PSQI is a 19-item self-rated instrument that measures seven domains of sleep complications over the past month. Items from each domain are rated on a 0–3 scale and summed to create a global score. Higher composite scores signify poorer sleep quality.
Objective sleep measures.
Between visits 1 and 6, participants were asked to wear a wrist actigraphy monitor (30 second epochs, Actiwatch Spectrum, Respironics, Bend, OR) on their non-dominant wrist, complete sleep logs, and press the event marker on the monitor before and after sleep each day. Data was analyzed with the Actiware 6.0.9 program (Respironics, Bend, OR). The setting of nightly rest intervals for analysis was guided by the event markers, sleep logs, light data and activity levels (Patel et al., 2015). Objective actigraphic estimates of sleep onset time (clock time of the first epoch scored as sleep in each rest interval), wake time (clock time of the last epoch scored as sleep in each rest interval), total sleep time (TST, number of minutes scored as sleep in each rest interval) and wake after sleep onset (WASO, number of minutes scored as wake between sleep onset and wake time) were extracted for each study day, then averaged for the baseline week and last treatment week.
Treatment adherence.
Adherence was evaluated using light and activity readings from a monitor (30 second epochs, Actiwatch Spectrum, Respironics, Bend, OR) attached to the inside of the Re-timer®. These readings allowed us to evaluate green light to determine Re-timer® on/off times and activity to confirm that the Re-timers® were worn at the assigned times.
Treatment expectancy, perceived benefit, and blinding.
Treatment expectancy was evaluated at the baseline visit after participants were introduced to their assigned Re-timer®. Participants were asked to report, “How much do you expect to benefit from the 4-week light treatment” on a 10-point Likert-type scale from 0 (not at all) to 10 (a lot). Perceived benefit was evaluated at the endpoint session. Participants were asked to report, “How much did you benefit from the 4-week light treatment” on a 10-point Likert-type scale from 0 (not at all) to 10 (a lot). At the end of treatment, participants were asked to return an anonymous survey via mail that asked about their experience in the trial including whether they thought they were in the active or placebo group.
Statistical Approach
Given the small sample size for this pilot study, we did not conduct significance testing using inferential statistics. For expectancy, perceived benefit, and adherence, we reported the distribution of these variables in each arm, including the median response. We also evaluated the degree to which our acceptability / feasibility measures (expectancy, side effects, attrition, adherence) met specific success criteria (Thabane et al., 2010): (a) overall median treatment expectancy would be greater than the midpoint of the scale, (b) less than 38% of the sample would report any side effects (based on Cascade, Kalali, & Kennedy, 2009), (c) less than 21.2% of the sample would drop out after initiating treatment (based on Bradley, Greene, Russ, Dutra, & Westen, 2005), and (d) participants would receive a minimum of 12 minutes of light therapy per day for the first 2 weeks (based on Al-Karawi & Jubair, 2016 and Chang et al., 2012). For sleep variables, we reported descriptive statistics at pre and post treatment and calculated the magnitude of the difference in change from pre to post treatment for the active v. placebo (calculated as a change score) group using Cohen’s d. We calculated similar effect sizes for our clinical outcome variables (PCL-5, PHQ-9) and also evaluated what percentage of participants in each treatment arm achieved a MCID.
Results
Expectancy, perceived benefit, adherence, and tolerance
The overall median treatment expectancy was 8, which exceeded our success criteria of 5. All participants in the active group reported treatment expectancy at or above the midpoint of the scale (range 5 – 10, median = 8). Five of six participants in the placebo group (83.3%) reported treatment expectancy at or above the midpoint of the scale (median = 7); one participant reported a treatment expectancy of 0. At the end of treatment, all participants reported perceived benefit of the treatment at or above the midpoint of the scale (median = 7 for both active and placebo groups). Nine of the fifteen participants completed the anonymous feedback survey; all nine thought they were in the active treatment group.
All participants that were randomized completed the 4-week intervention, which met our attrition success criteria. On average, participants initiated morning light treatment on 21.5 of the 28 treatment days; on days when participants initiated treatment, they received an average of 35.2 minutes per day within the prescribed treatment window (see Table 1). Adherence was similar in the active and placebo groups. Only 3 individuals (all in the placebo group) met our adherence success criteria.
Only one person in the active group (11.1% of active group participants) reported a mild headache on day 7 of light treatment, which met our success criteria. The participant chose to continue treatment and reported no other headaches throughout the trial. No participants in the placebo group reported side effects.
Changes in sleep
Descriptive statistics for subjective and objective sleep measures are reported by treatment group in Table 3. Active group participants reported greater improvements in subjective sleep quality from pre to posttreatment than placebo participants; however, this difference was small (d = .29). There was little change in sleep start times in both groups; however, the placebo group saw a slightly greater advance in sleep start time relative to the active group (d = .29). Active participants saw a greater advance in wake time relative to placebo participants (d = .68). Correspondingly, active participants revealed a decrease in TST from pre to posttreatment of approximately 36 minutes, whereas placebo participants revealed no change in TST, resulting in a large difference in TST change from pre to posttreatment between the groups (d = .77). Active participants also showed a decrease in WASO from pre to posttreatment, indicating greater sleep continuity, whereas placebo participants showed a slight increase in WASO from pre to posttreatment. This resulted in a moderate difference in WASO change between the groups (d = .48).
Table 3.
Changes in Subjective and Objective Sleep by Treatment Group
| Active (n = 9) | Placebo (n = 6) | ||||
|---|---|---|---|---|---|
| Pre M(SD) |
Post M(SD) |
Pre M(SD) |
Post M(SD) |
| d | | |
| PSQI | 8.67 (3.64) | 6.67 (3.04) | 7.67 (1.37) | 6.50 (3.99) | .29 |
| Start time | 24.23 (1.13) | 24.10 (1.48) | 23.31 (1.11) | 22.95 (1.58) | .29 |
| Wake time | 7.72 (1.02) | 6.89 (0.94) | 7.35 (1.50) | 7.00 (1.20) | .68 |
| WASO | 66.34 (34.21) | 60.08 (33.71) | 60.82 (26.57) | 61.40 (27.48) | .48 |
| Sleep time (min) | 383.19 (68.49) | 347.21 (62.48) | 421.35 (46.76) | 421.49 (35.71) | .77 |
Note. For sleep variables, pre reflects the average baseline week of sleep prior to the initiation of light treatment and post reflects the average of week of sleep during week 4 of light treatment. PSQI = Pittsburgh Sleep Quality Index; higher scores indicate worse sleep quality. Start time = time subjects fell asleep in military decimal time. End time = time subjects woke up in military decimal time. WASO = wake after sleep onset. Sleep time = total sleep time. d = effect size of the difference between the active and placebo group in change from pre to posttreatment (calculated as a change score).
Clinical outcomes
Table 4 reports pre and posttreatment means and standard deviations for the outcome measures by treatment group, the percentage of participants in each group that achieved a MCID, and the effect size of the difference in pre-post change for the active versus placebo group. A higher proportion of those in the active group achieved a MCID in PTSD and depression symptoms. On average, participants in the active group also had larger reductions in PTSD and depression symptoms from pre to posttreatment than those in the placebo group (d = .94 and .74, respectively).
Table 4.
Changes in PTSD and Depression by Treatment Group
| Active | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Pre M(SD) |
Post M(SD) |
MCID n(%) |
n | Pre M(SD) |
Post M(SD) |
MCID n(%) |
d | |
| PCL-5 | 9 | 43.11 (12.77) | 28.00 (14.41) | 6 (66.7) | 6 | 34.17 (15.33) | 31.67 (17.26) | 2 (33.3) | .94 |
| PCL-5 sensitivity analysisa | 8 | 46.00 (10.03) | 30.75 (11.30) | 5 (62.5) | 5 | 38.80 (11.52) | 36.60 (13.78) | 2 (40.0) | .91 |
| PHQ-9 | 9 | 10.44 (4.33) | 5.67 (3.94) | 5 (55.6) | 6 | 10.00 (4.43) | 8.83 (5.81) | 1 (16.7) | .74 |
Note. Pre = pretreatment visit 2. Post = posttreatment visit 6. MCID = minimal clinically important difference. PCL-5 = PTSD Checklist for DSM-5; MCID = 10 points. PHQ-9 = Patient Health Questionnaire-9; MCID = 5 points. d = effect size of the difference between the active and placebo group in change from pre to posttreatment (calculated as a change score); positive effect sizes indicate greater improvement in the active compared to the placebo group.
A sensitivity analysis was conducted examining only those who scored a minimum of 25 points on the PCL-5 at visit 2.
An issue that emerged is that some participants experienced a decrease in PTSD symptoms between the screening and the initiation of morning light treatment. Therefore, not all participants had a PCL-5 score > 33 when treatment was initiated. Wortmann and colleagues (2016) conducted a psychometric analysis of the PCL-5 and evaluated optimally efficient cut scores for the PCL-5 relative to various scoring rules established for DSM-IV PTSD measures. They reported a score of 25 as the lowest PCL-5 score that corresponded to a previously established cut score. Therefore, we conducted a sensitivity analysis examining change in PTSD symptoms for those who scored a minimum of 25 points on the PCL-5 at visit 2 (8 active, 5 placebo). The findings were similar to the original analyses (see Table 4).
Discussion
This is the first published study evaluating a morning bright light treatment for individuals with probable PTSD. Our findings indicated that a 4-week wearable bright light treatment was acceptable and feasible for participants. Only 18% (7 / 38) of individuals who expressed interest in the study declined to participate. It is unclear whether these individuals were not interested in the treatment or did not want to undergo the study procedures. Thus, the treatment appeared to be acceptable to the majority of potential participants. Treatment expectancy was high prior to treatment initiation, suggesting that participants believed that the treatment was plausible and had the potential to reduce their symptoms. The treatment was also well tolerated by participants; all those who initiated the treatment completed the treatment and only one participant in the active group reported transient side effects.
Our findings do indicate that adherence to a 1-hour treatment within a prescribed time window may be a challenge for patients. Patients initiated light therapy on 77% of the treatment days, indicating that the 4-week timeframe was feasible. However, they only received an average of 35 minutes per treatment day in the prescribed window and very few individuals received what could be considered a minimally active daily course of treatment. It is important to note that participants may have continued to use the light treatment outside of the prescribed window and therefore may have gotten a larger dose of treatment than is indicated by our conservative metric. Moreover, our objective sleep measures suggested that active group participants experienced a circadian shift with the amount of light received, evidenced by earlier morning wake times. Thus, future research examining the minimal dose needed for therapeutic effects is much needed.
With respect to changes in sleep, the active group showed expected advances in wake time and decreases in WASO relative to the placebo group. The decrease in TST was not anticipated, but appeared to result largely from the earlier awakenings in the active group. The fact that TST was reduced to less than 6 hours in the active group at posttreatment suggests that greater emphasis should be placed on earlier bedtimes to avoid sleep deprivation when introducing light treatment. The greater sleep deprivation in the active group may have contributed to the greater reduction in WASO (improved sleep continuity) and improvement in subjective sleep quality in the active group. Nonetheless, our findings suggest that providers should continue to monitor sleep duration and possible negative side effects of sleep loss when recommending morning light treatment.
Clinical outcomes showed that a higher proportion of individuals in the active group demonstrated a clinically meaningful improvement in PTSD symptoms relative to the placebo group and the magnitude of difference in PTSD symptom reduction between the active and placebo group was large. These findings should be taken with caution given the small sample size, unequal pretreatment symptoms in the two groups, and differences in sample characteristics across the two groups. However, our results provide initial proof-of-concept and suggest that a larger efficacy trial is warranted.
A key innovation of this study was to develop a credible placebo device that would isolate the effects of the light treatment from the stabilization of wake time, which also occurred as part of the treatment. The use of neutral density filters dimmed the light to a non-therapeutic level while maintaining a similar appearance to the active Re-timer®, including lighting up of the LEDs when the placebo Re-timer® was turned on. Although there was one participant in the placebo group who reported low treatment expectancy prior to treatment initiation, all participants reported high perceived benefit at the end of treatment, suggesting that the placebo was credible for participants. Moreover, all participants who completed the anonymous feedback questionnaire thought they were in the active group; this likely included participants who had been in the placebo group.
There are several limitations to consider when interpreting the results of this study. Because this was a pilot study, we evaluated PTSD symptoms using a self-report measure and did not conduct a follow-up assessment to establish whether the effects we found were maintained over time. Clinician-rated measures are considered the gold standard for diagnosing and evaluating PTSD. The lack of a clinician-rated measure may have contributed to the fact that some participants’ PTSD scores decreased prior to the initiation of light treatment. However, this could also be due to the measurement strategy used (i.e., use of a past month PCL-5 score for the screening visit and a past week PCL-5 score for pre and posttreatment visits) or positive expectancy about the receipt of treatment. Because of the small sample size, our two groups were not balanced with respect to pretreatment PTSD symptoms. Participants in the active group were on average 8.9 points more severe at pretreatment than participants in the placebo group. It is possible that higher baseline scores in the active group could have contributed to larger symptoms reductions. However, we did see objective sleep changes in the active group relative to the placebo group, suggesting there was a differential biological impact of light in the two treatment groups. We excluded participants with specific sleep disorders (sleep apnea, restless leg syndrome) and substance use disorders because these symptoms might interfere with our ability to detect treatment effects. Given that these are common comorbid conditions for individuals with PTSD (Chilcoat & Menard, 2003; Krakow, Ulibarri, Moore, & McIver, 2015), this limits the generalizability of our findings. Finally, only 60% of participants responded to our anonymous feedback survey.
Conclusion
With the poor uptake and limited efficacy of existing evidence-based treatments for PTSD, novel interventions are needed that are acceptable and feasible for patients. Wearable bright light treatments that are self-administered have the potential to be easily disseminable and scalable and might align well with patients’ priorities for treatment given that sleep disturbance is one of the most common and pressing concerns among patients with PTSD (McLay, Klam, & Volkert, 2010; Rosen, Adler, & Tiet, 2013). Previous studies show that there is clear theoretical plausibility that morning bright light could have therapeutic effects in patients with PTSD. Our findings provide initial proof-of-concept regarding the acceptability, feasibility, and efficacy of this treatment and suggest that the efficacy of morning bright light for PTSD should be explored in a larger randomized controlled trial.
Acknowledgment
We would like to thank Denise Zou, Muneer Rizvydeen, and Thomas Molina for their assistance with data collection. Alyson Zalta is supported by a career development award from the National Institute of Mental Health (K23 MH103394). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Mark Pollack receives grant funding from the National Institute of Health, Janssen Pharmaceuticals, and Wounded Warrior Project; he serves on advisory boards for Aptinyx, Atlas Venture, Bracket Global, Lundbeck, and Palo Alto Health Sciences; he has equity from Argus, Doyen Medical, Mensante Corporation, Mindsite, and Targia Pharmaceuticals; and he receives royalties from the SIGH-A and the SAFER interviews. Dr. Burgess serves on the scientific advisory board for Natrol, LLC.
Footnotes
Conflicts of Interest
All other authors report no conflicts of interest.
Data Accessibility
The datasets generated and analyzed during the current study are not publicly available because they contain more than two indirect identifiers of human research participants that cannot be sufficiently anonymized for a public repository. The datasets are available from the corresponding author on reasonable request.
References
- Al-Karawi D, & Jubair L (2016). Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. Journal of Affective Disorders, 198, 64–71. doi: 10.1016/j.jad.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Bovin MH, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, & Keane TM (2016). Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual Disorder – Fifth Edition (PCL-5) in veterans. Psychological Assessment, 28, 1379–1391. doi: 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162, 214–27. [DOI] [PubMed] [Google Scholar]
- Buysee DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cascade E, Kalali AH, & Kennedy SH (2009). Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont), 6(2), 16–18. [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Santhi N, Hilaire M St., Gronfier C, Bradstreet DS, Duffy JF, … & Czeisler CA (2012). Human responses to bright light of different durations. The Journal of Physiology, 590, 3103–3112. doi: 10.1113/jphysiol.2011.226555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, & Menard C (2003). Epidemiological investigations: Comorbidity of posttraumatic stress disorder and substance use disorder. In Ouimette P & Brown PJ (Eds.), Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders (pp. 9–28). Washington, DC, US: American Psychological Association. [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, & Rough J (2009). Circadian misalignment in major depressive disorder. Psychiatry Research, 168, 259–61. doi: 10.1016/j.psychres.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Friedman MJ (2013). Finalizing PTSD in DSM‐5: Getting here from there and where to go next. Journal of Traumatic Stress, 26, 548–56. doi: 10.1002/jts.21840 [DOI] [PubMed] [Google Scholar]
- Harrison Y (2013). The impact of daylight saving time on sleep and related behaviours. Sleep Medicine Review, 17, 285–92. doi: 10.1016/j.smrv.2012.10.001 [DOI] [PubMed] [Google Scholar]
- Hasler BP, Buysse DJ, Kupfer DJ, & Germain A (2010). Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: Further evidence for circadian misalignment in non-seasonal depression. Psychiatry Research, 178, 205–207. doi: 10.1016/j.psychres.2010.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Insana SP, James JA, & Germain A (2013). Evening-type military veterans report worse lifetime posttraumatic stress symptoms and greater brainstem activity across wakefulness and REM sleep. Biological Psychology, 94, 255–262. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hening WA, & Allen RP (2003). Restless legs syndrome (RLS): the continuing development of diagnostic standards and severity measures. Sleep Medicine, 4, 95–97. doi: 10.1016/S1389-9457(03)00009-1 [DOI] [PubMed] [Google Scholar]
- Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, Wilk JE (2014). PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatric Services, 65, 997–1004. doi: 10.1176/appi.ps.201300307 [DOI] [PubMed] [Google Scholar]
- Kantor V, Knefel M, & Lueger-Schuster B (2017). Perceived barriers and facilitators of mental health service utilization in adult trauma survivors: A systematic review. Clinical Psychology Review, 52, 52–68. doi: 10.1016/j.cpr.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Kessler RC (2000). Posttraumatic stress disorder: The burden to the individual and to society. The Journal of Clinical Psychiatry, 61 (Suppl 5), 4–12. [PubMed] [Google Scholar]
- Krakow BJ, Ulibarri VA, Moore BA, & McIver ND (2015). Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Medicine Reviews, 24, 37–45. doi: 10.1016/j.smrv.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General and Internal Medicine, 16, 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, & Hoge CW (2016). Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: Systemic review and meta‐analyses to determine first‐line treatments. Depression and Anxiety, 33, 792–806. doi: 10.1002/da.22511 [DOI] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, & Hattar S (2014). Light as a central modulator of circadian rhythms, sleep and affect. Nature Reviews Neuroscience, 15, 443–54. doi: 10.1038/nrn3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato N, & Lack L (2016). Circadian phase delay using the newly developed re-timer portable light device. Sleep and Biological Rhythms, 14, 157–164. doi: 10.1007/s41105-015-0034-6 [DOI] [Google Scholar]
- Löwe B, Unützer J, Callahan CM, Perkins AJ, & Kroenke K (2004). Monitoring depression treatment outcomes with the patient health questionnaire-9. Medical care, 42, 1194–1201. doi: 10.1097/00005650-200412000-00006 [DOI] [PubMed] [Google Scholar]
- McLay RN, Klam WP, & Volkert SL (2010). Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Military Medicine, 175, 759–62. doi: 10.7205/MILMED-D-10-00193 [DOI] [PubMed] [Google Scholar]
- National Center for PTSD. (2017). PTSD Checklist for DSM-5 (PCL-5) Retrieved August 26, 2018, from https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- Netzer NC, Stoohs RA, Netzer CM, Clark K, & Strohl KP (1999). Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine, 131, 485–491. doi: 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N, & Kasper S (2011). Bright-light therapy in the treatment of mood disorders. Neuropsychobiology, 64, 152–62. doi: 10.1159/000328950 [DOI] [PubMed] [Google Scholar]
- Patel SR, Weng J, Rueschman M, Dudley KA, Loredo JS, Mossavar-Rahmani Y, … & Sotres-Alvarez D (2015). Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep, 38, 1497–1503. doi: 10.5665/sleep.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C, Adler E, & Tiet Q (2013). Presenting concerns of veterans entering treatment for posttraumatic stress disorder. Journal of Traumatic Stress, 26, 640–643. doi: 10.1002/jts.21841 [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, & Montgomery P (2008). Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12, 169–184. doi: 10.1016/j.smrv.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Hilaire MA St., Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, & Lockley SW (2012). Human phase response curve to a 1h pulse of bright white light. Journal of Physiology, 590, 3035–3045. doi: 10.1113/jphysiol.2012.227892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M, & Terman JS (2005). Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectrums, 10, 647–63. doi: 10.1017/S1092852900019611 [DOI] [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, … & Goldsmith CH (2010). A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology, 10: 1. doi: 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen A, Meijer AM, van der Heijden KB, & Oort FJ (2016). The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Medicine Review, 29, 52–62. doi: 10.1016/j.smrv.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri P, Marx B, & Schnurr P (2013). The PTSD checklist for DSM-5 (PCL-5). National Center for PTSD doi: 10.1037/t02622-000 [DOI]
- Wortmann JH, Jordan AH, Weathers FW, Resick PA, Dondanville KA, Hall-Clark B, Foa EB, Young-McCaughan S, Yarvis JS, Hembree EA, Mintz J, Peterson AL, & Litz BT (2016). Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychological Assessment, 28, 1392–1403. doi: 10.1037/pas0000260 [DOI] [PubMed] [Google Scholar]
- Yun JA, Ahn YS, Jeong KS, Joo EJ, & Choi KS (2015). The relationship between chronotype and sleep quality in Korean firefighters. Clinical Psychopharmacology and Neuroscience, 13, 201–208. doi: 10.9758/cpn.2015.13.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, & Czeisler CA (2000). Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. The Journal of Physiology, 526, 695–702. doi: 10.1111/j.1469-7793.2000.00695.x [DOI] [PMC free article] [PubMed] [Google Scholar]


