Abstract
Purpose:
Radiation therapy has direct cytotoxic effects on tumor-infiltrating lymphocytes, but it also has immune stimulatory effects that increase immune cell infiltration. The dynamics of these competing effects on immune cells at the site of the tumor are poorly characterized during chemoradiation treatment (CRT) because of the difficulty of obtaining consecutive tumor biopsies. We used a minimally invasive cervical cytobrushing method to analyze the kinetics of intratumoral immune cell changes in patients with cervical cancer during CRT.
Methods and Materials:
Cervical brushings were obtained from 20 patients with cervical cancer at baseline and during fractionated radiation thera†py and cisplatin (weeks 1, 3, and 5). Matching peripheral blood mononuclear cells were obtained from 9 patients at the same time points. Cells were analyzed using multispectral flow cytometry to identify T cell and myeloid cell subsets and their activation status. Changes in immune cell subsets throughout treatment were calculated using matched-pair analysis with Wilcoxon rank sum test.
Results:
We observed a significant decline in CD3+ total T cells, as well as CD8+ and CD4+ T-cell subsets in the first week of treatment from baseline, followed by variable expansion at weeks 3 and 5. This coincided with higher levels of proliferating CD8 T cells expressing Ki67 at week 3 of treatment. The percentages of activated CD8 T cells expressing CD69 continuously increased over the course of treatment, whereas the percentage of activated CD11c CD11be dendritic cells was highest during the first week. Many of these changes were not observed in the blood.
Conclusions:
Our results identified immune dynamic changes during CRT, indicating that CRT may be immune activating at the site of the tumor. This study also suggests the importance of sequential analyses of the local tumor microenvironment in addition to peripheral blood.
Summary
Cytobrush methodology identified dynamic changes in intratumoral T cell and myeloid cell populations and their activation status in patients with cervical cancer undergoing chemoradiation treatment. These changes were not evident from the analyses of peripheral blood.
Introduction
Cervical cancer is among the most common malignancies among female patients worldwide, with an annual incidence of more than 500,000 women and an annual death rate of more than 250,000 women. (1) Locally advanced cervical cancer can be treated effectively with chemoradiation therapy (CRT) over a 7-week course of treatment requiring daily external beam radiation followed by brachytherapy (2); however, the rate of response to treatment is highly variable. (3, 4) Rapid responders to CRT are more likely to remain free of disease in the long term, but the mechanisms that underlie this heterogeneity in response rates are not well understood. Historically, radiation therapy (RT) was considered to have immunosuppressive effects; lymphocytes are one of the most radiosensitive cell types, (5) and peripheral lymphocyte counts generally decrease through the course of pelvic radiation. (6) More recently, radiation has been shown to uncover tumor antigens through tumor cell death, which enhances antigen presentation and induces antitumor T-cell responses. (7) Furthermore, radiation increases the release of damage associated molecular patterns, which attract and activate cells of the innate and adaptive immune system. (8)
Because of the challenges of obtaining frequent biopsies during RT, studies of immune activation are based on animal models using hypofractionated regimens, clinical studies with evaluation of tumors at very limited time points, or a focus on peripheral immune responses. As a result, the kinetics of intratumoral immune activation during RT are poorly understood. To discern the sequential changes within the cervix tumor microenvironment during CRT, we adapted the cytobrush methodology, demonstrated to be a reliable and minimally invasive technique to characterize immune cells within the female genital tract of HIV-positive patients in a multicenter clinical trial. (9) We recently reported the utility of this methodology for monitoring CD4 T cells at multiple mucosal tissues after intranasal vaccination in rhesus macaques. (10) By using this minimally invasive method, we characterized the immune infiltrate with multispectral flow cytometry at baseline and at weeks 1, 3, and 5 of CRT. Because T-cell infiltration has been associated with better prognosis in patients with cervical cancer and myeloid cells are known to modulate this infiltration, we focused our analysis on these populations. (11)
Methods and Materials
Patient population
Between January 2016 and January 2018, 20 patients were enrolled in a prospective observational clinical trial at MD Anderson Cancer Center and Lyndon B. Johnson Hospital designed to evaluate immunologic and metagenomic changes in the cervical and intestinal microbiota during CRT. Institutional review board approval was obtained, and all participants provided informed consent before enrolling in the study. Inclusion criteria and treatment are available in Methods E1 (available online at 10. 1016/j.ijrobp.2018.06.404).
Sample collection and processing
An attending radiation or gynecologic oncologist took the cervical brushing by directly sampling the visible exophytic cervical tumor. The brush was rotated against the visible tumor several times to collect enough cells from the tumor surface. Patients underwent additional tumor brushings after week 1 (5 fractions), after week 3 (10–15 fractions), and at the time of brachytherapy (week 5) for a total of 3 time points during radiation. In cases in which the tumor had resolved at later time points, the site of the original tumor on the cervix was sampled with the cytobrush.
Peripheral blood was also collected at these 4 time points (Methods E1, available online at https://doi.org/10.1016/j.ijrobp.2018.06.404). To analyze lymphocytes present in the tumor by using cytobrush, we followed a similar previously published method. (10, 12) Briefly, a sterile disposable cytobrush Plus GT (Cooper Surgical, Trumbull, CT) was used to collect cells from the cervix by rotating the brush 360° with 3 to 4 full rotations against the tumor. After collection of the sample, the brush is placed in complete RPMI 1640 media, placed on ice, and processed within 3 hours of sample collection. The brush was vortexed to collect all possible cells, and cells were then washed with complete RPMI 1640 media. Cells were treated with 2 mM dithiothreitol (Sigma, St Louis, MO) in 1X Hanks balanced salt solution (Invitrogen, Grand Island, NY) with 4% bovine serum albumin (Sigma, St Louis, MO) for 10 minutes at room temperature to remove any mucus present in the cells. Cells were then washed in complete media and passed through a 70-mm nylon cell strainer to remove any debris or mucus. Cells were finally plated in a 96-well rounded bottom plate. To detect cytokine production, cells were stimulated with phorbol myristate acetate (PMA)/ionomycin cocktail (final concentration for PMA 50 ng/mL and ionomycin 250 ng/mL) for 4 to 6 hours at 37°C after addition of Golgi plug to be further used for flow cytometry analysis.
Flow cytometry
Cells from the cytobrush and blood were washed with fluorescence-activated cell sorting (FACS) buffer (1X phosphate-buffered saline with 2% fetal bovine serum and 2mM EDTA) to be used for immune cell phenotyping. For the first 5 patients, we used a 7-color antibody panel to assess the feasibility of the study. The panel was composed of CD3, CD4, CD8, PD-1, FoxP3, CTLA-4, and a live/ dead fixable dead cell stain kit. For the rest of the patients, a 16-color panel was used. The panel used for the analysis includes antibodies against surface and intracellular markers; this and the MiFlowCyt standard are given in Methods E1 (available online at https://doi.org/10.1016/j.ijrobp.2018.06.404).
The cells were incubated with the antibodies for surface markers at 4°C in dark for 30 minutes. They were then washed twice with FACS buffer and fixed and permeabilized with FOXP3 Fix/perm Kit (ThermoFisher Scientific, Waltham, MA). Next, intracellular staining was performed by preparing the antibodies in permeabilization buffer and incubating the cells for 30 minutes at 4°C in the dark. Cells were washed with FACS buffer twice and prepared for acquisition on an LSR Fortessa X-20 analyzer at the Flow Cytometry Core at MD Anderson Cancer Center and were analyzed using FlowJo software (FlowJo, LLC, Ashland, OR). Compensation controls were prepared using OneComp ebeads (eBioscience, Waltham, MA) and fluorescence minus one controls were used. Figure E1 (available online at https://doi.org/10.1016/j.ijrobp.2018.06.404) shows the gating strategy used to analyze T cells and myeloid cells from cervical tumor and the fluorescence minus one controls used to specifically detect different activation markers on T cells.
T-cell receptor sequencing
T-cell receptor (TCR) sequencing was performed on samples from 11 patients (13). DNA extraction was performed using Isohelix protocol DSK-50. Multiplex polymerase chain reaction ebased deep sequencing of the CDR3 region of the TCR b was performed using the ImmunoSEQ immune profiling system13 (Adaptive Biotechnologies). This technique uses a library of known forward primers, each specific to a TCR Vb segment, and reverse primers specific to a TCR Jb segment. Both productive and nonproductive (CDR3 regions predicting out-of-frame receptor gene or premature stop) templates were analyzed, but absolute productive templates were used for the purposes of this analysis.
Statistical methods
Descriptive statistics were generated for patient, treatment, and outcome characteristics. Linear regression was used to determine associations between productive templates using TCR data and flow analysis. Changes in immune cell subsets at different time points were evaluated and calculated using matched-pair analysis with Wilcoxon rank sum test.
Results
Patient and tumor characteristics
Twenty patients with locally advanced cervical cancer (stage IBI-IIIB) were enrolled (Table E1; available online at https://doi.org/10.1016/j.ijrobp.2018.06.404). All patients completed treatment with external beam radiation with concurrent weekly cisplatin followed by pulsed-dose-rate or high-dose-rate brachytherapy.
T cells are transiently decreased after CRT followed by expansion in the cervical tumor
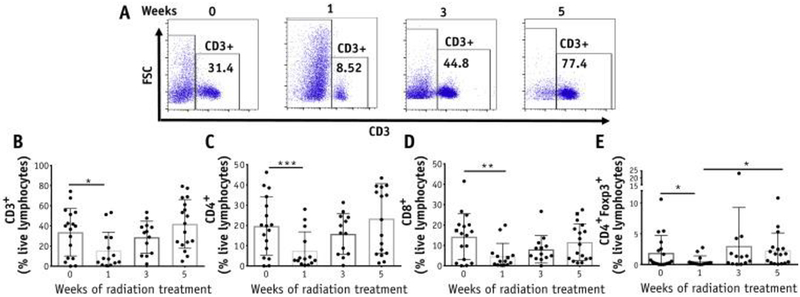
Cervical brushes were collected from visible tumors in patients; for patients with tumors that were not visible at week 5, the treated tumor site was brushed. On average, the yield of CD3 T cells from the cervical brushings varied (24–107,535, median 7809). We set an arbitrary cutoff of 400 CD3 T cells as the minimum required for flow cytometry analysis. Samples with fewer cells than this amount (8 out of 69 from different patients and time points) were excluded from the analysis. The number of T cells quantified by flow cytometry correlated with the number of TCRs present (representative of the total T cells) in the genomic DNA from the same samples (Fig. E2; available online at https://doi.org/10.1016/j.ijrobp.2018.06.404), validating the cytobrush methodology. Furthermore, the CD4 and CD8 T cells collected from the cervix are functional based on the response to stimulation with PMA and ionomycin resulting in the production of interferon-g (Fig. E3; available online at https://doi.org/10.1016/j.ijrobp.2018.06.404). Analyses of the frequencies of total CD3 T cells from the cytobrush samples showed a significant decrease between pretreatment (33.7%) and week 1 (15.7%) of the treatment, with a similar decline in CD4 and CD8 T-cell subsets. The number of CD3 T cells subsequently increased at week 3 (28.8%) and further increased at week 5 (42%), exceeding the frequencies found before treatment (Fig. 1A and 1B). These changes between baseline and through 5 weeks of treatment were consistent among the CD4 and CD8 T-cell subsets (Fig. 1C and 1D). The CD4 FoxP3 immunosuppressive regulatory T cells also decreased in the first week of treatment (1.9% and 0.6%, respectively) and subsequently expanded (Fig. 1E). Therefore, the ratio of CD8 T cells to regulatory T cells remained constant over time (Fig. E4; available online at https://doi.org/10.1016/j.ijrobp.2018.06.404). These data suggested that CRT induces an initial decline in intratumoral T-cell frequency followed by a variable expansion.
Fig. 1.
Changes in T-cell frequencies in the cervix throughout chemoradiation therapy (CRT). (A) Representative plots for CD3+ T cells in the cervix from 1 patient before and after 1, 3, and 5 weeks of treatment. (B) Percentages of CD3+, CD4+, CD8+ and CD4+FoxP3+ T-cell subsets among live lymphocytes were quantified (n = 20).
Intratumoral T-cell activation during CRT
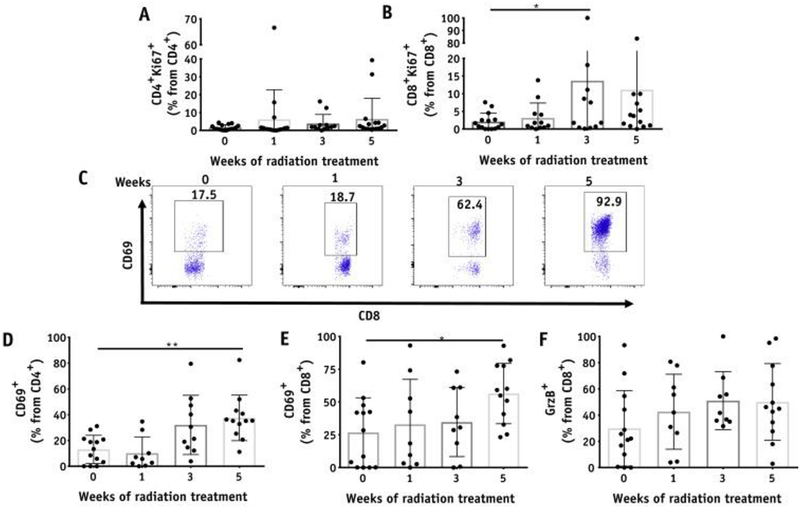
To determine whether the observed expansion of T cells after the initial decrease was accompanied by proliferation of the T cells, we analyzed the expression of Ki67, a surrogate for proliferation. We observed that Ki67 populations of CD8 T cells were higher at week 3 relative to pretreatment levels, accounting for the higher numbers of T cells detected at later time points (Fig. 2A and 2B). We also investigated whether the T cells underwent activation during CRT by analyzing for changes in the populations expressing the activation marker CD69 or the cytotoxic molecule granzyme B. Strikingly, we observed that both CD4 and CD8 T cells expressing CD69 significantly increased over time, from 13% and 26.8% at baseline to 37.5% and 56.4% at week 5, respectively (Fig. 2C–2E). Frequencies of granzyme B CD8 T cells also showed an increasing trend throughout the different time points (Fig. 2F). On the other hand, the percentages of CD4 and CD8 T cells expressing the inhibitory receptors CTLA-4 and PD-1 in the cervix remained unchanged during CRT (Fig. E5; available online at https://doi.org/10.1016/j.ijrobp.2018.06.404).
Fig. 2.
T-cell activation in the cervix throughout chemoradiation therapy. Kinetics of Ki67 expression on (A) CD4+ and (B) CD8+ T cells in the cervix (n = 20). (C) Representative plots for CD69+CD8+ T cells in the cervix from 1 patient before and after 1, 3, and 5 weeks of treatment. (D-F) Percentages of CD69+CD4+ (D), CD69+CD8+ (E), and granzyme B+CD8+ T cells (F) (n = 15).
Infiltration and activation of myeloid cells in the cervix during CRT
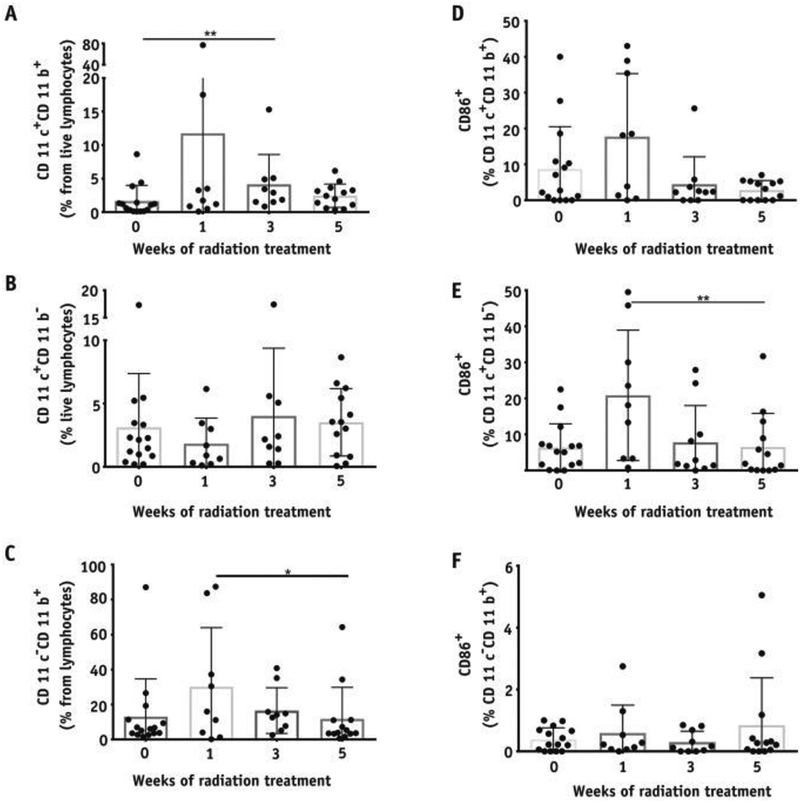
Different subsets of myeloid cell populations are known to be present in mucosal tissues and within tumors. (14) To determine the kinetics of changes in myeloid cells and their activation status in the cervical tumors during CRT, we analyzed for different subsets that included CD11c CD11b (monocytes and dendritic cells), CD11c CD11be (mostly plasmacytoid dendritic cells and monocytes), and CD11ceCD11b cells (mostly macrophages and neutrophils) using the gating strategy shown in Figure E1C (available online at https://doi.org/10.1016/j.ijrobp.2018.06.404). We observed increases in CD11c CD11b myeloid subsets between baseline (1.7%) and week 3 (4.1%) of treatment, with no change in the levels of CD11c CD11be cells (Fig. 3A–3C). The highest proportion of cells expressing the costimulatory molecule CD86 on CD11c CD11be cells was at week 1 (20.8%) with a decrease to baseline levels at week 5 (6.5%) (Fig. 3D–3F), suggesting an enrichment of activated dendritic cells in the first week of CRT.
Fig. 3.
Changes in myeloid cell frequencies and their activation status in the cervix throughout chemoradiation therapy. Percentages of (A) CD11c+CD11b+, (B) CD11c+CD11b–, and (C) CD11b+CD11c– cells among live lymphocytes in the cervix. Percentages of CD86+ cells among (D) CD11b+CD11c–, (E) CD11c+CD11b–, and (F) CD11b+CD11c– cells (n = 15).
Comparative analyses of immune cell changes in peripheral blood versus cervix during CRT
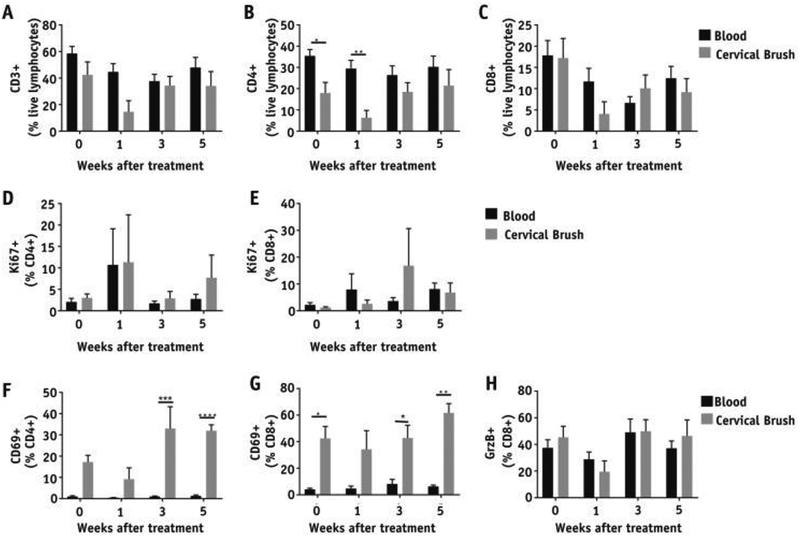
Because peripheral blood is more commonly assessed for the manifestation of treatment-induced immune changes, we compared changes in intratumoral immune activation with peripheral immune subsets. For this, we obtained blood samples from a subset of patients (n Z 9) at time points matching the cervical brushings during CRT. Although total CD3 T-cell frequencies did not significantly differ between blood and cervix during the treatment (Fig. 4A), the CD4 T-cell frequencies were significantly lower in the cervix relative to blood at baseline and week 1 (Fig. 4B). Furthermore, CRT induced a transient decrease in CD8 T-cell frequencies followed by an increase at week 5 in both blood and cervix, but these changes were significant only in the cervix (Fig. 4C). Absolute lymphocyte counts from complete blood count (CBC) demonstrate peripheral lymphodepletion resulting from CRT (Fig. E6; available online at https://doi.org/10.1016/j.ijrobp.2018.06.404). Analysis of the frequencies of T cells expressing Ki67 showed no significant differences between blood and cervix (Fig. 4D–4E). Interestingly, the percentage of CD4 and CD8 T cells expressing the activation marker CD69 was higher in intratumoral T cells compared with that in blood (Fig. 4F–4G), especially after 3 weeks of treatment. We also analyzed the expression of the coinhibitory receptors CTLA-4 and PD-1 on T cells and observed no differences in blood and cervix (data not shown). Overall, these data suggest that CRT-mediated changes in the populations and/ or activation status of T cells and myeloid cells are more pronounced in the local tumor microenvironment relative to the periphery.
Fig. 4.
Comparison of T-cell frequencies and their activation status in the cervix and blood from patients with cervical cancer throughout chemoradiation therapy. Percentages of (A) CD3+, (B) CD4+, and (C) CD8+ T cells among live lymphocytes. Percentages of Ki67+ cells among (D) CD4+ and (E) CD8+ T cells. Percentages of CD69+ cells among (F) CD4+ and (G) CD8+ T cells. Percentages of granzyme B+ cells among CD8+ T cells (H) in cervical brushes and blood (n = 9).
Discussion
The kinetics of immune activation within the local tumor microenvironment during CRT has not been well investigated because of challenges in obtaining serial biopsies during treatment. To our knowledge, this is the first study to evaluate the kinetics of changes in intratumoral immune cell subsets and their activation status during CRT.
We found that CRT induces an early and transient decrease of T cells during the first week of treatment. The percentage of activated CD69 T cells increased continuously during treatment, and the CD8 T cells expressing the proliferation marker Ki67 peaked at week 3. In contrast, the percentage of activated dendritic cells was highest at week 1, suggesting the first week of CRT is a potentially critical time for enhanced antigen presentation to subsequently promote activated effector T-cell populations important for generating antitumor immunity. Interestingly, these changes appeared to be specific to the intratumoral microenvironment and were not detected in peripheral immune subsets. Although the specific mechanism involved in the decline and further expansion of T cells in the cervix is not understood, radiation has been reported to promote dendritic cell migration to the tumor-draining lymph node to enhance antigen presentation and further induce T-cell activation and trafficking to the tumor in the context of dendritic cell vaccination. (15) Therefore, analysis of regional lymph nodes along with cervical cytobrushes could provide more insight regarding the potential induction and trafficking of immune cells within both of these areas during CRT.
Human papillomavirus (HPV)-associated cancers are known to be highly radiation sensitive, a feature generally attributed to cell cycle changes induced by the HPV E6 and E7 oncogenes. (16, 17) Because these viral oncogene products also serve as unique tumor antigens, antitumor immunity specific to these viral targets may also contribute to the radiation sensitivity of HPV cancers. (18) In preclinical models, RT has been shown to alter the T-cell repertoire, potentially inducing HPV-specific T cells. (19, 20) Although we cannot separate the immune changes resulting from chemotherapy versus radiation, it would be relevant to determine whether the expanded T cells observed at week 5 in our study are specific to HPV antigens. By using the cytobrush methodology, it may be feasible to identify HPV-specific T cells because they respond to the strong T-cell stimulator, PMA, and ionomycin. Optimizations to detect HPV-specific T cells are currently ongoing in our laboratory.
The transient decline of T cells in the periphery after CRT has been previously observed. (21, 22) In our study, we observed that this decline is more profound in the cervix, specifically after 1 week of treatment, but these cells are recovered after 5 weeks of treatment, reaching levels similar to baseline. Although the underlying mechanism for this increase is not fully understood, we observed increased T-cell proliferation at the site of the tumor. Another possibility would include enhanced trafficking of T cells to this location after CRT. The transient T-cell decrease was accompanied by an increase in activated CD11c CD11be, constituted mostly by dendritic cells and monocytes at week 1 after CRT, which may allow for increased antigen presentation and contribute to the expansion of activated T cells observed later during radiation. Additionally, the higher frequency of CD11b CD11ce at week 1, mostly constituted by macrophages and neutrophils, suggests that CRT shifts the balance of T cells to myeloid cells during early treatment. Because subsets of myeloid cells, such as myeloid-suppressor cells, exert highly immunosuppressive functions towards T cells, (23) it is also important to evaluate for any changes, such immunosuppressive populations, throughout treatment to discern their influence on CRT responsiveness in terms of clinical responsiveness and cancer recurrence.
Although data from the analyses of blood provide some insights about the systemic effects of CRT, it is thought that local immune changes provide information more pertinent to antitumor immunity and treatment outcomes. (24) In agreement with this, we noticed that the changes in T-cell proliferation and activation markers observed in the cervix were not evident in peripheral T cells. These results suggest that sequential analyses of the local tumor microenvironment, using the minimally invasive cytobrush methodology, provide valuable information regarding the immune changes that may be predictive of the treatment outcome. Our study allowed the prospective monitoring of immune cells in the cervix at 4 different time points throughout treatment. Although comparison of the cells obtained from cytobrush with biopsies would provide insights regarding the differences between the immune changes at different regions of the tumor, these analyses allowed us to identify the kinetic of changes in T-cell populations, along with their proliferation and activation status, and suggest that optimal integration of immunotherapy with CRT should take this chronology into account. Further studies with a larger cohort, biopsy analyses for comparison, and more detailed immune analyses may provide insights into the effects of CRT at the site of the tumor and identify immune phenotypes that correlate with CRT responsiveness and efficacy of combined immunotherapy with CRT.
Supplementary Material
Acknowledgments
This study was funded by The University of Texas MD Anderson Cancer Center HPV-related Cancers Moonshot. The Flow Cytometry Core at MD Anderson Cancer Center is supported by the Cancer Center Support Grant NCI# P30 CA16672.
Footnotes
Conflict of interest: J.W. reports being a Healios cofounder, a MolecularMatch cofounder, a MolecularMatch officer, an OncoResponse cofounder/SAB, on the Reflexion Medical Scientific Advisor Board, on the Checkmate Pharmaceuticals Scientific Advisor Board, and on the Mavu Scientific Advisory Board. J.W. also reports BMS Clinical research support, Merck Clinical research support, Varian Laboratory research support, Incyte Laboratory research support, Merck Laboratory research support, Calithera Laboratory research support, Checkmate Pharmaceuticals Laboratory research support, and OncoResponse Laboratory research support. L.L.L reports an investigator-initiated study that is supported by AstraZeneca.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2018.06.404.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLO-BOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Stern PL, van der Burg SH, Hampson IN, et al. Therapy of human papillomavirus-related disease. Vaccine 2012;30(Suppl 5):F71–F82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Mayr NA, Yuh WT, et al. Predicting outcomes in cervical cancer: A kinetic model of tumor regression during radiation therapy. Cancer Res 2010;70:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayr NA, Taoka T, Yuh WT, et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2002;52: 14–22. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe RT, Fuks ZY, Strober S, et al. The long term effects of radiation of T and B lymphocytes in the peripheral blood after regional irradiation. Cancer 1977;40:2071–2078. [DOI] [PubMed] [Google Scholar]
- 6.Spary LK, Al-Taei S, Salimu J, et al. Enhancement of T cell responses as a result of synergy between lower doses of radiation and T cell stimulation. J Immunol 2014;192:3101–3110. [DOI] [PubMed] [Google Scholar]
- 7.Demaria S, Formenti SC. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front Oncol 2012;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz LE, Peter C, Herrmann M, et al. Scent of dying cells: The role of attraction signals in the clearance of apoptotic cells and its immunological consequences. Autoimmun Rev 2010;9:425–430. [DOI] [PubMed] [Google Scholar]
- 9.McKinnon LR, Nyanga B, Chege D, et al. Characterization of a human cervical CD4þ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol 2011;187:6032–6042. [DOI] [PubMed] [Google Scholar]
- 10.Dorta-Estremera S, Nehete PN, Yang G, et al. Minimally invasive monitoring of CD4 T cells at multiple mucosal tissues after intranasal vaccination in rhesus macaques. PLoS One 2017;12:e0188807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piersma SJ, Jordanova ES, van Poelgeest MI, et al. High number of intraepithelial CD8 tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res 2007;67:354–361. [DOI] [PubMed] [Google Scholar]
- 12.McKinnon LR, Hughes SM, De Rosa SC, et al. Optimizing viable leukocyte sampling from the female genital tract for clinical trials: An international multi-site study. PLoS One 2014;9:e85675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robins HS, Campregher PV, Srivastava SK, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 2009;114:4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheldon IM, Owens SE, Turner ML. Innate immunity and the sensing of infection, damage and danger in the female genital tract. J Reprod Immunol 2017;119:67–73. [DOI] [PubMed] [Google Scholar]
- 15.Teitz-Tennenbaum S, Li Q, Okuyama R, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother 2008;31:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arenz A, Ziemann F, Mayer C, et al. Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther Onkol 2014;190: 839–846. [DOI] [PubMed] [Google Scholar]
- 17.Awwad M, North RJ. Sublethal, whole-body ionizing irradiation can be tumor promotive or tumor destructive depending on the stage of development of underlying antitumor immunity. Cancer Immunol Immunother 1988;26:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung CF, Wu TC, Monie A, et al. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol Rev 2008;222:43–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudqvist NP, Pilones KA, Lhuillier C, et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol Res 2018;6:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res 2017;23:5514–5526. [DOI] [PubMed] [Google Scholar]
- 21.Rey J, Fauriat C, Kochbati E, et al. Kinetics of cytotoxic lymphocytes reconstitution after induction chemotherapy in elderly AML patients reveals progressive recovery of normal phenotypic and functional features in NK cells. Front Immunol 2017;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Meir H, Nout RA, Welters MJ, et al. Impact of (chemo) radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2016;6:e1267095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol 2009;182:4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heusinkveld M, Goedemans R, Briet RJP, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer 2012;131:E74–E85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.