Abstract
Patient-derived xenograft (PDX) models are created by engraftment of patient tumor tissues into immunocompetent mice. Since a PDX model retains the characteristics of the primary patient tumor including gene expression profiles and drug responses, it has become the most reliable in vivo human cancer model. The engraftment rate increases with the introduction of Non-obese diabetic Severe combined immunodeficiency (NOD/SCID)-based immunocompromised mice, especially the NK-deficient NOD strains NOD/SCID/interleukin-2 receptor gamma chain(IL2Rγ)null (NOG/NSG) and NOD/SCID/Jak3(Janus kinase 3)null (NOJ). Success rates differ with tumor origin: gastrointestinal tumors acquire a higher engraftment rate, while the rate is lower for breast cancers. Subcutaneous transplantation is the most popular method to establish PDX, but some tumors require specific environments, e.g., orthotropic or renal capsule transplantation. Human hormone treatment is necessary to establish hormone-dependent cancers such as prostate and breast cancers. PDX mice with human hematopoietic and immune systems (humanized PDX) are powerful tools for the analysis of tumor–immune system interaction and evaluation of immunotherapy response. A PDX biobank equipped with patients’ clinical data, gene-expression patterns, mutational statuses, tumor tissue architects, and drug responsiveness will be an authoritative resource for developing specific tumor biomarkers for chemotherapeutic predictions, creating individualized therapy, and establishing precise cancer medicine.
Keywords: patient-derived xenograft, immunocompromised mice, precision medicine, drug screening, cancer, cell line, cancer immunotherapy, humanized mice
1. Introduction
Preclinical studies using animal models are essential for drug development. Fewer than 10% of candidate drugs are approved for the market, even if preclinical trials are successful [1]. This figure is lower for oncology drugs, at approximately 5% [2]. One possible reason is the lack of appropriate human cancer models. Mouse tumors and human-cell-line-transplanted animal models are not always representative of human cancer pathologies, contributing to distinct drug responses [3]. Mice and humans are considerably different [4], and human cancer cell lines somehow lose their original tumor characteristics when transplanted [5]. Accordingly, the National Cancer Institute (NCI, MD, USA) recently decided to replace the NCI-60, a panel of 60 human cell lines, with patient-derived xenografts (PDXs) for drug screening [3]. The PDXs are established by direct engraftment of a patient tumor into an immunocompromised mouse, maintaining the tumor growth in vivo. This has become an essential tool for preclinical and translational research, particularly for investigations of tumor pathology and for chemotherapeutic drug development. With the introduction of highly immunocompromised mice as recipients, PDX use is now widespread and is becoming a standard “Avatar” model for human cancer research.
2. Establishment of Immunocompromised Mice
2.1. Nude Mice
In 1962, the first known immunocompromised mice were discovered by Grist (Ruchill Hospital, Glasgow, UK). The “nude” nickname was given because they lacked body fur. Flanagan [6] showed that nude mice also lacked thymus and T lymphocytes; as a result, the adaptive immune responses, including T cell-mediated immune responses and antibody formation that require helper T cells, are defective in nude mice. Since then, nude mice have been used as recipients for human tumor xenografts. However, the intact (or rather activated) innate immunity in nude mice limits the options for human cancer transplantation [7]. In addition, nude mice show leakage of T cells with age [8].
2.2. Severe Combined Immunodeficient Mice
In 1983, Bosma [9] (Fox Chase Cancer Institute, PA, USA) first described the severe combined immunodeficient (SCID) mice that lack both functional T and B lymphocytes. The maturation deficiencies of B and T lymphocytes in SCID mice are due to the deletion of Prkdc (protein kinase, DNA activated, catalytic polypeptide: DNA-PKCs) and the absence of variable (V)-diveresity (D)- joining (J) recombination (V(D)J recombination). The SCID mice were first used as recipients of human hematopoietic stem cells (HSCs) and peripheral blood mononuclear cell (PBMC) transplantation [10,11]. The engraftment efficiency of human tumors is higher in SCID mice compared to nude mice [12]. However, the transplantation efficiencies of human blood cells and tumor cells are not as high as expected, as the remnant natural killer (NK) cells prevent homing and maintenance of human cells. To overcome the effects of NK cells, SCID/Beige mice were established by crossbreeding SCID mice and Beige mice [13]. In addition to the T and B deficiency of SCID mice, the SCID/Beige mice displayed severely reduced NK cell functioning along with the phagocytosis of Beige mice [14]. The uptake rate of human tumor cells increased in SCID/Beige mice compared with SCID mice, as expected [14], but the engraftment rate of human HSCs was not noticeably increased [15].
2.3. Non-Obese Diabetic/SCID Mice and NOD/SCID-Based Immunocompromised Mice
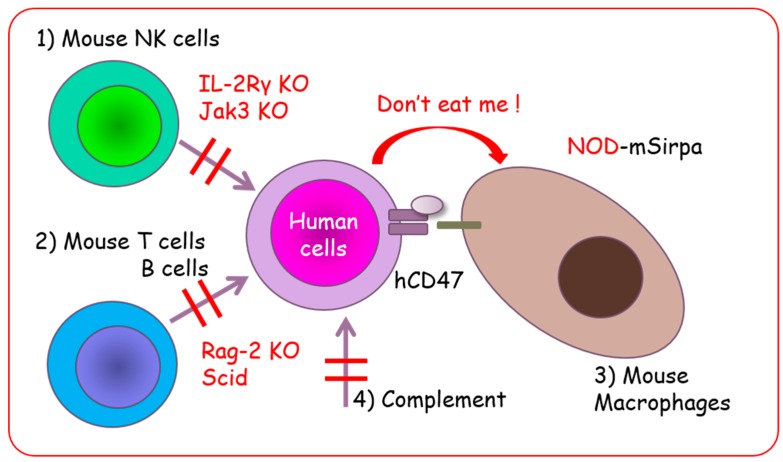
Non-obese diabetic (NOD) mice, discovered in 1980 by Makino (Shionogi Co. Osaka, Japan), have diabetes mellitus caused by infiltration of and pancreatic islet destruction by T lymphocytes [16]. Later, it was discovered that NOD mice acquire multiple immune abnormalities including loss of complement and impaired NK, macrophage, and dendritic cell functions [17]. The NOD/SCID mice were established by crossbreeding NOD and SCID mice. The NOD/SCID mice do not develop diabetes because they lose functional T lymphocytes. Moreover, multiple defects in innate and adaptive immunity are seen in these mice, making them better recipients for human hematopoietic stem cell and human solid tumor transplantation [18]. However, there are some residual NK activities in NOD/SCID mice, and several attempts were made to eliminate or suppress these and to improve transplantation efficiency. These attempts included the use of anti-interleukin (IL)-2 receptor antibody or anti-ganglio-N-tetraosylceramide (asialoGM1) antibody and crossbreeding with β2 macroglobulin- or perforin-deficient mice. The common γ chain (γc, CD132), also known as IL-2 receptor subunit gamma (IL2Rγ), is a cytokine receptor sub-unit. It is common to the receptor complexes for six different interleukin receptors, IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, which are critical for lymphocyte and NK cell development [19]. The IL2Rγ interacts with the Janus kinase 3 (Jak3) non-receptor-type tyrosine kinase for signal transduction. Therefore, IL2Rγ- and Jak3-deficient mice show common phenotypes such as NK deficiency and reduction of T and B lymphocytes [20]. Thus, NOD/SCID mice with complete loss of NK cells were established by crossbreeding with IL-2 receptor γ-deficient mice (NOD/SCID/IL2Rγnul: NOG [21], NOD/SCID/IL2Rγnul: NSG [22]) or Jak3-deficient mice (NOD/SCID/Jak3null: NOJ [23]) (Table 1). NOG mice have a NOD/ShiJic-Prkdcscid background with partial deficiency of IL2Rγ [21], whereas NSG mice have a NOD/ShiSzJ-Prkdcscid background with complete deficiency of IL2Rγ [10]. The NSG mice acquire higher engraftment capacity of cord-blood-derived CD34+ cells [24] and higher body weights [25], but these differences do not appear to affect the PDX transplantation efficiency [25]. Moreover, signal regulatory protein alpha (Sirpα) polymorphism in NOD strains provides superior opportunities for human cell engraftment because SIRPα interacts with human CD47 [26] and suppresses macrophage-mediated phagocytosis, or contributes to the so-called “don’t eat me” signal (Figure 1) [27,28].
Table 1.
NOD/SCID-based immunocompromised mice.
| Mice | NOD/SCID | NOG | NSG | NOJ |
|---|---|---|---|---|
| Strain | NOD.Cg-Prkdcscid | NOD.Cg-PrkdcscidIl2rgtm1Sug/Jic | NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ | NOD.Cg-PrkdcscidJak3tm1card |
| Genetic defects | SCID | SCID, IL-2γ Partial deficiency | SCID, IL-2Rγ Complete deficiency | SCID, Jak3 deficiency |
| Developer | CIEA 1, Jackson Laboratory |
CIEA 1 | Jackson Laboratory | Kumamoto University |
| Supplier | Japan Clea Charles River |
Japan Clea | Charles River | Kumamoto University |
| NK activities | NK cell dysfunction | Complete loss of NK cells | Complete loss of NK cells | Complete loss of NK cells |
| Reference | [18] | [21] | [22] | [23] |
1 Central Institute for Experimental Animals (CIEA). NOD = non-obese diabetic; SCID = severe combined immunodeficient; NOG = NOD/SCID/IL-2 receptor γ-deficient (IL2Rγnul); NSG = NOD/SCID/IL2Rγnul; NOJ = NOD/SCID/Janus kinase 3 deficient (Jak3); Prkdc = protein kinase, DNA activated, catalytic polypeptide; NK = natural killer.
Figure 1.
NOG, NSG, and NOJ mice with multiple immune deficiencies are excellent recipients for human cell engraftment. (1) Loss of NK cells; (2) loss of acquired immunity by T and B lymphocyte deficiency; (3) “Don’t eat me” signal by NOD-signal regulatory protein alpha (Sirpα); and (4) loss of complement.
2.4. BALB/c Background Immunocompromised Mice
The BALB/c mice also have Sirpα polymorphisms that acquire binding affinity to human CD47. Hence, BALB/c strain immunocompromised mice, such as BALB/c Rag-2null/IL2Rγnull (BRG) [29] and Rag-2null/Jak3null (BRJ) mice [30] have lower macrophage-mediated phagocytosis of human cells, and might be useful recipients for human cell and tissue transplantations [31,32]. Other mice such as C57/BL6 have lower affinity for engrafting human normal and malignant cells [30,33]. Even though the mutations in SCID mice are useful for T and B cell elimination, there are several disadvantages to SCID mice, such as high susceptibility to irradiation or drugs, and leakage of T lymphocytes. (Recombination activating gene-1/2 (Rag-1/Rag-2) knockout mice are generated to 1) solve these problems and 2) eliminate mature lymphocytes (Table 2) [29,34]. The BRG mice with human Sirpα (SRG) were also generated to improve the engraftment efficiency of PDX (Figure 1) [35].
Table 2.
Comparison of SCID and Rag-1/Rag-2 mutation.
| Mice | SCID | Rag-1/Rag-2 Knock Out Mice |
|---|---|---|
| Chromosome | Chr.16 | Chr.11 p13 |
| Mutated gene | Prkdc | Recombination-activation gene-1/-2 |
| Mutation | Natural mutant | Homologous recombination |
| Immunological phenotype | Deficiency of mature B and T lymphocytes NK cells are normal |
Deficiency of mature B and T lymphocytes NK cells were normal |
| Radiation sensitivity | Sensitive (Lethal dose < 3 Gy) |
Normal (Lethal dose 9 Gy) |
| Leakiness | Leaky | None |
Rag = recombination activating gene.
3. Establishment and Application of Nude/Hairless Immunocompromised Mice
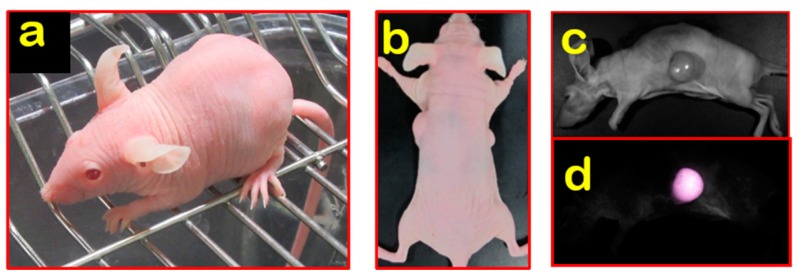
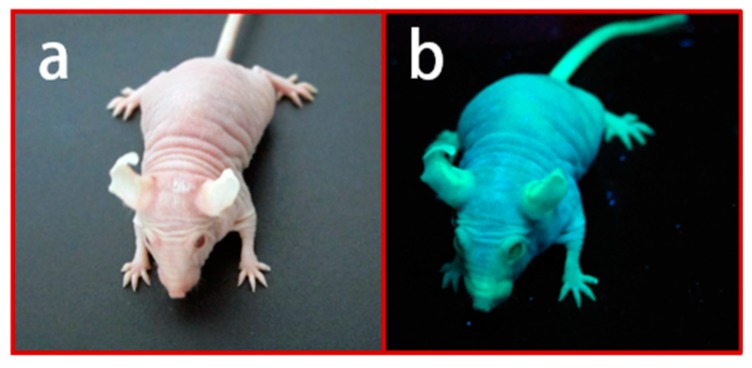
Although more combined immunocompromised mice have been developed, nude mice remained valuable for human tumor engraftment due to the fact of their advantages for tumor monitoring. Tumor visualization by direct observation and imaging in nude mice provides an excellent opportunity for tumor observation in vivo. For this reason, BALB/c Nude Rag-2/Jak3 double-deficient (Nude R/J) mice were established in our lab by mating nude mice with Rag-2null and Jak3null mice with a BALB/c background [36,37]. Nude R/J mice have no B and T lymphocytes with Rag-2 deficiency, no NK cells with Jak3 deficiency, and have the “don’t eat me signal” with a BALB/c background. Nude R/J mice retained the advantages of no fur and a higher immunocompromisation level than Nude mice, and were, consequently, optimal for in vivo imaging (Figure 2). Another type of furless mouse, namely, the hairless mouse, is also available. Hairless mice have no noticeable immunocompromised phenotypes [38,39]. The SCID Hairless (SHO) mice (Charles River, MA, USA) and Hairless NOD/SCID mice (Envigo, Huntingdon, UK) were established by crossbreeding with hairless mice and used for in vivo imaging (Table 3) [40,41]; however, the engraftment efficiencies were lower than for NK-deficient strains [42]. Mice expressing fluorescence proteins are a powerful tool in cancer research, particularly for visualization of the tumor–host interaction [43]. Several fluorescence-expressing immunocompromised mice have been established and utilized (Figure 3) [44,45,46,47]. The relationships between human tumors and the host microenvironment, including vessels, tumor-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs), can potentially be studied using these models [48].
Figure 2.
Nude R/J mice: (a) BALB/c Nude Rag-2/Jak3 double-deficient (Nude R/J) hairless phenotype; (b) direct visualization of subcutaneous tumor nodules in Nude R/J; (c–d) fluorescent signals observed in Nude R/J.
Table 3.
Comparison of hairless mice.
| Mice | Hairless | Nude | SCID Hairless | Nude R/J | |
|---|---|---|---|---|---|
| Strain | BALB/c | BALB/c | CB17.Cg/ICR | BALB/c | |
| Gene abnormality | Hairless | Foxn1 | Hairless, SCID |
Foxn1, Rag-2, Jak3 | |
| Immune system | T cells | + | − | − | − |
| B cells | + | + | − | − | |
| NK cells | + | + | + | − | |
| Hair coat | None | None | None | None | |
+: intact certain immune cells, −: lack of certain cells. Foxn1 = forkhead box N1.
Figure 3.
Green fluorescence protein (GFP)-expressing Nude R/J mice: (a) GFP Nude R/J phenotype; (b) strong GFP expression under β-actin promoter yields a very bright green signal under UV light [46].
4. Establishment of PDX Models Using Various Immunocompromised Mice
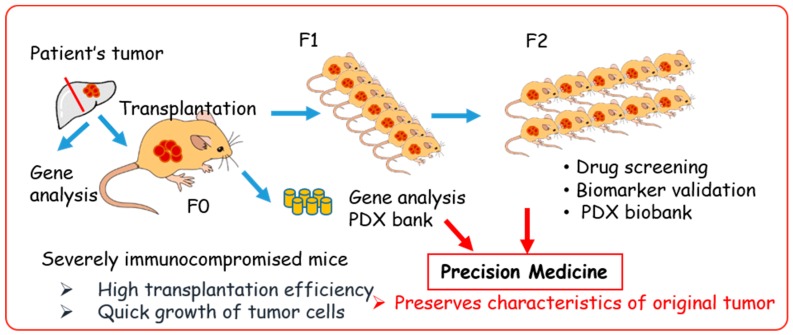
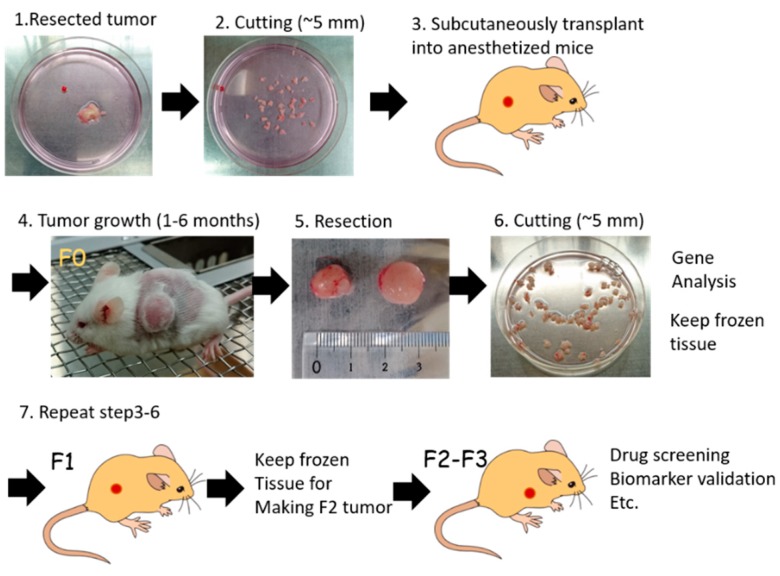
The PDX models were generated by direct transplantation of patient tumor samples into immunocompromised mice (Figure 4). A significant advantage of PDX models is that they retain key characteristics of the patient’s tumor, such as the gene expression profile and heterogeneity of tumor cells [49,50,51]. Currently, PDX models are the most clinically relevant in vivo cancer models and the most concordant drug response model to human cancer [31,49,52,53,54,55,56]. Thus, the US National Cancer Institute (NCI) decided to substitute a panel of 60 human cell lines (NCI-60) with PDX models for drug screening [3].
Figure 4.
Patient-derived xenograft (PDX) model in precision medicine.
The duration of tumor establishment in mice differs among tumors, taking from a few days to a few months for the tumor nodule to be observed (first generation; F0). After serial transplantation, the duration of tumor growth becomes stable, with approximately 40–50 days required to obtain certain sized tumors [53,57]. The PDX samples should be stored together with the patient’s clinical data, gene-expression patterns, mutational statuses, drug responsiveness, and pathological analysis to generate a PDX library.
Nude mice have been used to generate PDX models with reasonable efficacy and are used as a standard recipient (Table 4). With this model, the engraftment efficiencies of gastrointestinal tumors are relatively high, while the establishment of hematological malignancy PDXs are almost impossible in nude mice. The introduction of SCID and NOD/SCID mice increased PDX success rates [58]. As NOD/SCID mice have relatively short lifespans and spontaneously develop thymic lymphoma [18], more immunocompromised mice, such as NOG/NSG mice [59,60,61], are a more appropriate model. The NOG/NSG mice are the most immunocompromised mice available and show the highest engraftment efficiencies for both normal and malignant human tissues [53]. However, NOG/NSG mice must be kept under especially clean specific pathogen-free (SPF) conditions; hence, culture prices are relatively high (100 US$ per mouse for academic use in Japan). Moreover, breeding of these mice is not possible by the users. Since nude mice have benefits such as a relatively high engraftment ratios of gastrointestinal tumors, easy observation of subcutaneous tumors, and relatively low price, they remain an important resource for PDX establishment [31,53,62]. The BRJ mice have been used as alternative recipients of cholangiocarcinoma PDX, with a high engraftment ratio (75%) [57,63]. Other solid tumors, such as head and neck tumors, gastric cancers, and bladder cancers, are now under investigation. From our preliminary study, BRJ successfully acquired human solid cancers with relatively high engraftment ratios (compared to currently available models, data not shown) (Figure 5). Since BRJ mice are easy to breed and maintain by users, they are good candidates for PDX, and since Nude R/J mice have the benefits of both BRJ and nude mice, they may be the ideal model for passaging and drug evaluation [36].
Table 4.
Immunocompromised mouse strains for PDX.
| Mouse Strain | Phenotype | Advantage | Disadvantage/Consideration | Success Rate of PDX |
|---|---|---|---|---|
| Nude | No thymus, no coat of hair |
Well characterized, easy to detect s.c. tumor |
Functional B and NK cells, increased T cell leakage with age |
Low |
| SCID | No mature T and B cells | Better engraftment compared with nude | Functional NK cell, leakage of T cells, radiosensitive |
Low |
| SCID/Beige | No mature T and B cells, impaired Mφ and NK function |
Better engraftment compared with SCID | Leakage of T cells, radiosensitive |
Moderate |
| NOD/SCID | No mature T and B cells Impaired NK function Impaired Mφ & DC |
Better engraftment | Spontaneous lymphoma Short life span (av. 36wks) Radiosensitive |
Moderate |
| NOG/NSG/NOJ | No mature T and B cells, no NK cells, impaired Mφ and DC |
Excellent engraftment of PDX including hematopoietic malignancies | Need strict SPF conditions, breeding is not easy, expensive |
High |
| BALB/c Rag2null/IL2Rγnull (BRG) Rag-2 null/Jak3 null (BRJ) |
No mature T and B cells, no NK cells |
Excellent engraftment of PDX, resistant to stress, easy breeding, radio resistant |
High |
NK: natural killer cells, Mφ: macrophages, DCs: dendritic cells, NOG/NSG: NOD/SCID/IL2Rγnull, NOJ: NOD/SCID/Jak3null, s.c.: subcutaneous.
Figure 5.
Generation process of PDX. Surgical specimen from a patient’s tumor (1) is divided into small pieces (2) and transplanted into an anesthetized immunocompromised mouse (3). Tumor growth takes 1 to 6 months (4). Once tumors are grown in F0 mice, xenografts are resected (5) and cut into small pieces (6). Parts of tumor tissues are analyzed for tumor characteristics, such as whole exome sequencing (WES), RNA sequencing (RNA-seq), and copy number variation (CNV) analysis. The remnant PDX tumor is stored in liquid nitrogen, or further transplanted into immunocompetent mice (7) for expansion. Conventionally, F2 or F3 PDX tumors are used for cancer biology study, such as drug sensitivity screening, identifying biomarkers, etc.
Success rates of PDX establishment vary by tumor origin and disease characteristics such as tumor aggressiveness, relapse/recurrence status, and primary or metastatic tumor. More aggressive, relapsed and highly metastatic tumors tend to show higher transplantation rates [53]. Gastrointestinal cancers, such as colon and pancreatic cancers, seem to have higher engraftment ratios compared with other cancers. Engraftment ratios are also higher in more immunocompromised mice (Nude < SCID < NOD/SCID < NSG) (Table 5) [53]. The engraftment ratio for breast cancer is relatively low, and orthotropic transplantation is needed [64]. Orthotropic and renal capsule engraftment clearly increase the engraftment ratio for some tumors, although special techniques are required [31,65,66]. For hematological malignancies such as leukemia and multiple myeloma, direct engraftment into the bloodstream or into the bone marrow of NOG/NSG mice is necessary. Human hormone replacement supports hormone-dependent tumors such as breast and prostate cancers [67,68,69].
Table 5.
PDX success rates in different immunocompromised mice.
| Tumor Type | Mice Strain | Implantation Site | Number of Sample | Engraftment Ratio | References |
|---|---|---|---|---|---|
| Cholangiocarcinoma | SCID NOD/SCID BRJ |
s.c. * s.c. s.c. |
55 20 16 |
34.5% 5.8% 75% |
Ojima, 2010 [84] Cavalloni, 2016 [85] Vaeteewoottacharn, 2019 [57] |
| Colorectal cancer | Nude NOD/SCID NSG |
s.c. s.c. s.c |
85 85 27 |
63.5% 87% 54% |
Julien, 2012 [86] Bertolini, 2011 [87] Chou, 2013 [88] |
| Pancreatic cancer | Nude SCID NSG |
s.c. s.c. s.c |
69 12 121 |
61% 67% 71.1% |
Garrido-Laguna, 2011 [89] Mattie, 2013 [90] Guo, 2019 [91] |
| Gastric cancer | Nude NOD/SCID Nude/SCID Nude/NOG |
s.c. s.c. s.c s.c |
32 185 83/119 62 |
73.7% 34.1% 16.9%/26.9% 24.2% |
Wang, 2017 [92] Zhu, 2015 [93] Zhang, 2015 [94] Choi, 2016 [95] |
| Head and neck cancer | Nude NSG |
s.c. s.c. |
46 26 |
54% 84.6% |
Keysar, 2013 [96] Kimple, 2013 [97] |
| Breast cancer | Nude Nude Nude NOD/SCID SCID/Beige NSG |
s.c. fat pad ** fat pad fat pad s.c. s.c. |
200 314 109 49 162 32 |
12.5% 2.5% (ER+) 24.3% (ER−) 27% 19% 31.3% |
Marangoni, 2007 [98] Cottu, 2012 [99] DeRose, 2011 [100] Zhang, 2013 [101] |
| Ovarian cancer | Nude Nude SCID SCID NSG |
s.c. r.c. *** s.c. s.c. s.c. |
138 45 34 168 12 |
25% 48.8% 50% 74% 83% |
Ricci, 2014 [102] Heo, 2017 [103] Dobbin, 2014 [104] Weroha, 2014 [105] Topp, 2014 [106] |
| Non-small lung cancer | NOD/SCID NOD/SCID NOD/SCID NSG |
s.c. r.c. s.c. s.c. |
102 527 308 441 |
25% 90% 26% 28.7% |
Fichtner, 2008 [107] Dong, 2010 [108] Chen, 2019 [109] Wang, 2017 [51] |
| Glioblastoma | NSG | orthotopic | 100 | 30% | Brabetz, 2018 [110] |
| Prostate | Nude NOD/SCID SCID SCID SCID NSG |
s.c. s.c. s.c. orthotopic r.c. s.c. |
23 23 86 57 122 27 |
39% 48% 58.1% 71.9% 93.4% 37% |
Priolo, 2010 [111] Wang, 2005 [66] Wetterauer, 2015 [112] |
| Renal cell carcinoma | Nude NOD/SCID NSG |
s.c. r.c. s.c. |
336 94 74 |
8.9% 37.2% 45% |
Lang, 2016 [113] Sivanand, 2013 [114] Dong, 2017 [115] |
| Melanoma | NOG NSG |
s.c. s.c. |
26 694 |
88.4% 65.8% |
Einarsdottir, 2014 [116] Krepler, 2017 [117] |
* s.c., subcutaneous, ** mammarian fat pad, *** r.c., renal capsule.
5. Generation of PDX-Derived Cell Lines
A tumor cell line can be generated from a PDX tissue sample [57,70,71]. The establishment of tumor cell lines from primary tissues is relatively difficult with the conventional protocol because fibroblasts often outgrow and overcome the cancer cell growth in vitro. In PDX tissue, human fibroblasts are substituted by murine fibroblasts. These mouse fibroblasts have shorter lifespans and are more sensitive to mechanical and enzymatic removal, so the elimination of fibroblasts takes less time. As mentioned earlier, fluorescence-emitting immunocompromised mice have also been developed. These mice have superior benefits for distinguishing engrafted tumor cells and mouse-derived cells [46,72] and, thus, may be useful for establishing PDX-derived cancer cell lines. These PDX-derived tumor cell lines are useful for high-throughput drug screening, as they retain the characteristics of the primary tumors. It is worth mentioning that, in some cases, male-derived tumor tissues retain the Y chromosome in PDX but lose it during cell line development. This might imply that at least one more mutation is required to establish PDX-derived cell lines [57].
6. PDX in Humanized Mice
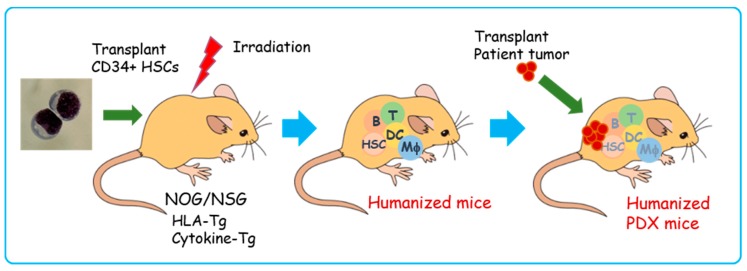
The immune system plays an essential role in tumor control. Recently, cancer immunotherapy using various approaches including antibodies, cancer vaccines, adoptive cell therapies, and immune checkpoint blockade therapies has gained attention as a promising and effective modality with fewer side effects [73,74,75]. However, a mouse model that allows monitoring of the immune response is needed to test these newly developed therapies, because the current PDX model lacks principal immune cells. Mice with a reconstituted human immune system, so-called humanized mice, are available. Humanized mice are generated by transplantation of human hematopoietic stem cells into highly immunocompromised mice such as NOG, NSG, and NOJ [21,22,23,76]. Originally used for pathological studies of human-specific pathogens, these models can mimic the human immune system to a certain degree, but they do not represent a complete and functional human immune system. The mouse bone marrow and thymic microenvironments are different from humans and, therefore, the T cells are not fully developed. Moreover, myeloid and erythroid cell development in these mice are lower than in humans. The humanized bone marrow–liver–thymus (BLT) mouse model can be generated by engraftment of human fetal liver and thymus along with human hematopoietic stem cells into an immunocompromised mouse renal capsule [77]. Since the BTL mouse harbors a nearly complete human immune system including functional T cell response, it is a powerful tool to study human immunology and immunotherapy. However, BLT usage is greatly limited by ethical issues including a restricted supply of the human fetal thymus and liver tissues needed to generate these mice. Human leukocyte antigen (HLA) class I and class II transgenic mice, and several types of NOG/NSG mice with human cytokine transgenes, have been developed to overcome some of these limitations [78,79] (Figure 6). The NSG mice that express human stem cell factor, granulocyte-macrophage colony stimulating factor, and interleukin-3, termed NSG-SGM3, showed robust human hematopoietic reconstitution, a higher frequency of human myeloid cells, and increased regulatory T-cell development [80]. The HLA-expressing humanized mice develop functional HLA-restricted T cells [81,82]. Attempts have also been made to introduce the human hematopoietic microenvironment into immunocompromised mice [83]. These mice represent a very useful model to reconstitute a more accurate human immune system against human malignancies.
Figure 6.
Schematic illustration of humanized PDX model generation. First, CD34+ human hematopoietic stem cells are transplanted into irradiated human leukocyte antigen (HLA)/human cytokine transgenic (Tg) NOG/NSG mice. Then, human hematopoietic and immune systems are reconstituted within 8–12 weeks (humanized mice). Patient-derived tumors are transplanted into humanized mice (humanized PDX mice). T: T lymphocytes, B: B lymphocytes, DCs: dendritic cells, Mφ: macrophages, HSCs: hematopoietic stem cells, HLA-Tg: human leukocyte antigen class I and II transgenic mice, Cytokine-Tg: human cytokine (stem cell factor (SCF), IL-3, granulocyte-monocyte colony stimulating factor (GM-CSF), thrombopoietin (TPO), etc.) transgenic mice.
The PDX in humanized mice (humanized PDX) has been established for several types of tumors [91,118,119,120]. The humanized PDX mice model offers a unique platform for examining human acquired and innate immune responses to clinically-relevant tumors and for evaluating immune therapy [121]. However, current models of humanized PDX mice still have several limitations: 1) the balance of hematopoietic and immune cells remains different from that in humans; 2) since the source of HSC is not the same as that of the tumor, it is challenging to match the HLA. These issues require attention for the development of a patient-similar immune response PDX model.
A specific population of immune cells can be reconstituted in highly immunocompromised mice. Human mature T lymphocytes can reconstitute in the classical PBMC transplanted model, although the duration is relatively short (4–8 weeks) and most of the T lymphocytes are activated [11]. We succeeded in reconstituting human B and T cells and immune response by transplantation of PBMCs into the spleens of NOJ mice [122]. Functional human NK cells and γδT cells can be reconstituted in severe immunocompromised mice and have been used to evaluate the anti-tumor effects of these cells [123,124]. These systems can be applied for the evaluation of cancer immunotherapies such as adoptive cell therapy (NK, gamma delta T (γδT), NK T (NKT), chimeric antigen receptor T (CAR T) cell therapies), antibody therapy (direct killing activity, antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC)), and immune check point blockade therapy.
7. Perspective
Developments in xenograft technology and highly immunocompromised mice such as NOG/NSG allow us to broaden the application of the PDX platform. Nevertheless, PDX models still require optimization for clinical relevance. The human stromal components are rapidly lost and replaced by the murine microenvironment during engraftment [125]. Recently, it was reported that PDX models undergo mouse-specific tumor evolution with rapid accumulation of copy number alterations during PDX passaging that differed from those acquired during tumor evolution in patients by the strong selection pressures in the mice [126], and only selected clones remain after passages. Thus, current PDX models are not complete “Avatar” models of human cancer. In spite of these findings, PDX models are still the most relevant in vivo cancer model for precision medicine, as they keep consistency with their patients’ primary tumor relative to conventional tumor models, especially drug response profiles [127]. Humanized mice with PDX are expected to offer a novel platform for examining immunotherapy (Figure 6) [121]. Despite several limitations, humanized PDX mice have already provided several benefits in studies of cancer behaviors and the functions of immunocompetent cells in tumor microenvironments [118,119,121]. Several attempts have been made to establish humanized microenvironments and generate more comprehensive and functional immune systems in immunocompromised mice [83]. Further development and improvement of these systems will provide an unprecedented platform for personalized cancer medicine, particularly cancer immunotherapy.
The PDX mice models have emerged as important tools for cancer research, with the potential to allow a personalized approach using gene expression and drug sensitivity profiles. However, they have several limitations that should be noted. Establishment of PDX is time consuming (6 months to 2 years), the success rate varies (10–90%), and it is difficult to retrieve complete patient data. Therefore, many institutions and organizations are focused on creating a large stock of PDX or PDX libraries. European institutions established EurOPDX, a consortium to store PDX, and have already accumulated more than 1500 samples in a PDX bank [128,129]. Jackson Laboratory provides more than 450 samples for researchers [60]. Mega-pharmacies are also establishing their own PDX libraries, and Novartis recently published data on drug screening using 1000 PDX [55]. These PDX biobanks are excellent in vivo platforms for precision medicine [31]. The PDX biobanks with patients’ clinical data, pathologies, gene profiles, and drug response data (Figure 4) are critically important for drug response prediction and validation to generate drug response information for tumors from similar genetic backgrounds. Currently, PDX resources are available in the USA and Europe, and most PDX are derived from common cancers. This might create a bias of information. Hence, PDX biobanks in Asia and rare cancer PDX are essential, as is the requirement for a sharing system among PDX biobanks around the world.
Acknowledgments
We thank S. Fujikawa for her technical assistance and Y. Kanagawa for her secretarial work.
Author Contributions
Conceptualization, S.O. and K.V.; methodology, R.K.; writing—original draft preparation, S.O.; writing—review and editing, S.O. and K.V.; visualization, S.O.; funding acquisition, S.O. and K.V.
Funding
This research was funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture and Sport Science and Technology (MEXT) of Japan (grant number 16K08742); the National Science and Technology Development Agency and the e-Asia Joint Research Program (grant number P1950436, 19jm0210062h0002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alteri E., Guizzaro L. Be open about drug failures to speed up research. Nature. 2018;563:317–319. doi: 10.1038/d41586-018-07352-7. [DOI] [PubMed] [Google Scholar]
- 2.DiMasi J.A., Reichert J.M., Feldman L., Malins A. Clinical approval success rates for investigational cancer drugs. Clin. Pharmacol. Ther. 2013;94:329–335. doi: 10.1038/clpt.2013.117. [DOI] [PubMed] [Google Scholar]
- 3.Ledford H. Us cancer institute overhauls cell lines. Veteran cells to be replaced by human tumours grown in mice. Nature. 2016;530:391. doi: 10.1038/nature.2016.19364. [DOI] [PubMed] [Google Scholar]
- 4.Mestas J., Hughes C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 5.Kojima Y., Hayakawa F., Morishita T., Sugimoto K., Minamikawa Y., Iwase M., Yamamoto H., Hirano D., Imoto N., Shimada K., et al. Ym155 induces apoptosis through proteasome-dependent degradation of mcl-1 in primary effusion lymphoma. Pharmacol. Res. 2017;120:242–251. doi: 10.1016/j.phrs.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Flanagan S.P. ’Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8:295–309. doi: 10.1017/S0016672300010168. [DOI] [PubMed] [Google Scholar]
- 7.Budzynski W., Radzikowski C. Cytotoxic cells in immunodeficient athymic mice. Immunopharmacol. Immunotoxicol. 1994;16:319–346. doi: 10.3109/08923979409007097. [DOI] [PubMed] [Google Scholar]
- 8.Giovanella B.C., Fogh J. The nude mouse in cancer research. Adv. Cancer Res. 1985;44:69–120. doi: 10.1016/s0065-230x(08)60026-3. [DOI] [PubMed] [Google Scholar]
- 9.Bosma G.C., Custer R.P., Bosma M.J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 10.McCune J.M., Namikawa R., Kaneshima H., Shultz L.D., Lieberman M., Weissman I.L. The scid-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.2971269. [DOI] [PubMed] [Google Scholar]
- 11.Mosier D.E., Gulizia R.J., Baird S.M., Wilson D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 12.Taghian A., Budach W., Zietman A., Freeman J., Gioioso D., Ruka W., Suit H.D. Quantitative comparison between the transplantability of human and murine tumors into the subcutaneous tissue of ncr/sed-nu/nu nude and severe combined immunodeficient mice. Cancer Res. 1993;53:5012–5017. [PubMed] [Google Scholar]
- 13.Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979;278:451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- 14.Mosier D.E., Stell K.L., Gulizia R.J., Torbett B.E., Gilmore G.L. Homozygous scid/scid;beige/beige mice have low levels of spontaneous or neonatal t cell-induced b cell generation. J. Exp. Med. 1993;177:191–194. doi: 10.1084/jem.177.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen M., Galvani S., Canivet C., Kamar N., Bohler T. Reconstitution of immunodeficient scid/beige mice with human cells: Applications in preclinical studies. Toxicology. 2008;246:18–23. doi: 10.1016/j.tox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. Exp. Anim. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 17.Kikutani H., Makino S. The murine autoimmune diabetes model: Nod and related strains. Adv. Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 18.Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B., McKenna S., Mobraaten L., Rajan T.V., Greiner D.L., et al. Multiple defects in innate and adaptive immunologic function in nod/ltsz-scid mice. J. Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 19.Notarangelo L.D., Giliani S., Mazza C., Mella P., Savoldi G., Rodriguez-Perez C., Mazzolari E., Fiorini M., Duse M., Plebani A., et al. Of genes and phenotypes: The immunological and molecular spectrum of combined immune deficiency. Defects of the gamma(c)-jak3 signaling pathway as a model. Immunol. Rev. 2000;178:39–48. doi: 10.1034/j.1600-065X.2000.17812.x. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K., Nakajima H., Saito Y., Saito T., Leonard W.J., Iwamoto I. Janus kinase 3 (jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: Comparative analysis of gamma(c), jak3, and gamma(c) and jak3 double-deficient mice. Int. Immunol. 2000;12:123–132. doi: 10.1093/intimm/12.2.123. [DOI] [PubMed] [Google Scholar]
- 21.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., et al. Nod/scid/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 22.Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S., Kotb M., Gillies S.D., King M., Mangada J., et al. Human lymphoid and myeloid cell development in nod/ltsz-scid il2r gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 23.Okada S., Harada H., Ito T., Saito T., Suzu S. Early development of human hematopoietic and acquired immune systems in new born nod/scid/jak3null mice intrahepatic engrafted with cord blood-derived cd34 + cells. Int. J. Hematol. 2008;88:476–482. doi: 10.1007/s12185-008-0215-z. [DOI] [PubMed] [Google Scholar]
- 24.McDermott S.P., Eppert K., Lechman E.R., Doedens M., Dick J.E. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 25.Nagatani M., Kodera T., Suzuki D., Igura S., Fukunaga Y., Kanemitsu H., Nakamura D., Mochizuki M., Kemi M., Tamura K., et al. Comparison of biological features between severely immuno-deficient nod/shi-scid il2rg(null) and nod/ltsz-scid il2rg(null) mice. Exp. Anim. 2019 doi: 10.1538/expanim.19-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro-Alvarez N., Yang Y.G. Cd47: A new player in phagocytosis and xenograft rejection. Cell. Mol. Immunol. 2011;8:285–288. doi: 10.1038/cmi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takenaka K., Prasolava T.K., Wang J.C., Mortin-Toth S.M., Khalouei S., Gan O.I., Dick J.E., Danska J.S. Polymorphism in sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T., Takenaka K., Urata S., Shima T., Kikushige Y., Tokuyama T., Iwamoto C., Nishihara M., Iwasaki H., Miyamoto T., et al. Polymorphic sirpa is the genetic determinant for nod-based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121:1316–1325. doi: 10.1182/blood-2012-06-440354. [DOI] [PubMed] [Google Scholar]
- 29.Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J.C., Lanzavecchia A., Manz M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 30.Ono A., Hattori S., Kariya R., Iwanaga S., Taura M., Harada H., Suzu S., Okada S. Comparative study of human hematopoietic cell engraftment into balb/c and c57bl/6 strain of rag-2/jak3 double-deficient mice. J. Biomed Biotechnol. 2011;2011:539748. doi: 10.1155/2011/539748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada S., Vaeteewoottacharn K., Kariya R. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine. Chem. Pharm. Bull. 2018;66:225–230. doi: 10.1248/cpb.c17-00789. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto C., Takenaka K., Urata S., Yamauchi T., Shima T., Kuriyama T., Daitoku S., Saito Y., Miyamoto T., Iwasaki H., et al. The balb/c-specific polymorphic sirpa enhances its affinity for human cd47, inhibiting phagocytosis against human cells to promote xenogeneic engraftment. Exp. Hematol. 2014;42:163–171.e161. doi: 10.1016/j.exphem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Goto H., Kariya R., Matsuda K., Kudo E., Katano H., Okada S. A potential role of the nod genetic background in mouse peritoneal macrophages for the development of primary effusion lymphoma. Leuk Res. 2016;42:37–42. doi: 10.1016/j.leukres.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Goto H., Kojima Y., Matsuda K., Kariya R., Taura M., Kuwahara K., Nagai H., Katano H., Okada S. Efficacy of anti-cd47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur. J. Cancer. 2014;50:1836–1846. doi: 10.1016/j.ejca.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Strowig T., Rongvaux A., Rathinam C., Takizawa H., Borsotti C., Philbrick W., Eynon E.E., Manz M.G., Flavell R.A. Transgenic expression of human signal regulatory protein alpha in rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. USA. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kariya R., Matsuda K., Gotoh K., Vaeteewoottacharn K., Hattori S., Okada S. Establishment of nude mice with complete loss of lymphocytes and nk cells and application for in vivo bio-imaging. Vivo. 2014;28:779–784. [PubMed] [Google Scholar]
- 37.Tanaka A., Takeda S., Kariya R., Matsuda K., Urano E., Okada S., Komano J. A novel therapeutic molecule against htlv-1 infection targeting provirus. Leukemia. 2013;27:1621–1627. doi: 10.1038/leu.2013.46. [DOI] [PubMed] [Google Scholar]
- 38.Benavides F., Oberyszyn T.M., VanBuskirk A.M., Reeve V.E., Kusewitt D.F. The hairless mouse in skin research. J. Dermatol. Sci. 2009;53:10–18. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiniger H.J., Meier H., Kaliss N., Cherry M., Chen H.W., Stoner R.D. Hereditary immunodeficiency and leukemogenesis in hrs-j mice. Cancer Res. 1974;34:201–211. [PubMed] [Google Scholar]
- 40.Crottes D., Rapetti-Mauss R., Alcaraz-Perez F., Tichet M., Gariano G., Martial S., Guizouarn H., Pellissier B., Loubat A., Popa A., et al. Sigmar1 regulates membrane electrical activity in response to extracellular matrix stimulation to drive cancer cell invasiveness. Cancer Res. 2016;76:607–618. doi: 10.1158/0008-5472.CAN-15-1465. [DOI] [PubMed] [Google Scholar]
- 41.Smee D.F., Dagley A., Downs B., Hagloch J., Tarbet E.B. Enhanced efficacy of cidofovir combined with vaccinia immune globulin in treating progressive cutaneous vaccinia virus infections in immunosuppressed hairless mice. Antimicrob. Agents Chemother. 2015;59:520–526. doi: 10.1128/AAC.04289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai Y., Wei X., Lin S., Qin L., Cheng L., Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J. Hematol. Oncol. 2017;10:106. doi: 10.1186/s13045-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman R. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet. Oncol. 2002;3:546–556. doi: 10.1016/S1470-2045(02)00848-3. [DOI] [PubMed] [Google Scholar]
- 44.Yang M., Reynoso J., Jiang P., Li L., Moossa A.R., Hoffman R.M. Transgenic nude mouse with ubiquitous green fluorescent protein expression as a host for human tumors. Cancer Res. 2004;64:8651–8656. doi: 10.1158/0008-5472.CAN-04-3118. [DOI] [PubMed] [Google Scholar]
- 45.Niclou S.P., Danzeisen C., Eikesdal H.P., Wiig H., Brons N.H., Poli A.M., Svendsen A., Torsvik A., Enger P.O., Terzis J.A., et al. A novel egfp-expressing immunodeficient mouse model to study tumor-host interactions. FASEB J. 2008;22:3120–3128. doi: 10.1096/fj.08-109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotoh K., Kariya R., Matsuda K., Hattori S., Vaeteewoottacharn K., Okada S. A novel egfp-expressing nude mice with complete loss of lymphocytes and nk cells to study tumor-host interactions. Biosci. Trends. 2014;8:202–205. doi: 10.5582/bst.2014.01049. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman R.M., Bouvet M. Imaging the microenvironment of pancreatic cancer patient-derived orthotopic xenografts (pdox) growing in transgenic nude mice expressing gfp, rfp, or cfp. Cancer Lett. 2016;380:349–355. doi: 10.1016/j.canlet.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Vaeteewoottacharn K., Kariya R., Dana P., Fujikawa S., Matsuda K., Ohkuma K., Kudo E., Kraiklang R., Wongkham C., Wongkham S., et al. Inhibition of carbonic anhydrase potentiates bevacizumab treatment in cholangiocarcinoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016;37:9023–9035. doi: 10.1007/s13277-016-4785-8. [DOI] [PubMed] [Google Scholar]
- 49.Tentler J.J., Tan A.C., Weekes C.D., Jimeno A., Leong S., Pitts T.M., Arcaroli J.J., Messersmith W.A., Eckhardt S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassidy J.W., Caldas C., Bruna A. Maintaining tumor heterogeneity in patient-derived tumor xenografts. Cancer Res. 2015;75:2963–2968. doi: 10.1158/0008-5472.CAN-15-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D., Pham N.A., Tong J., Sakashita S., Allo G., Kim L., Yanagawa N., Raghavan V., Wei Y., To C., et al. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int. J. Cancer. 2017;140:662–673. doi: 10.1002/ijc.30472. [DOI] [PubMed] [Google Scholar]
- 52.Gargiulo G. Next-generation in vivo modeling of human cancers. Front. Oncol. 2018;8:429. doi: 10.3389/fonc.2018.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins A.T., Lang S.H. A systematic review of the validity of patient derived xenograft (pdx) models: The implications for translational research and personalised medicine. PeerJ. 2018;6:e5981. doi: 10.7717/peerj.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bleijs M., van de Wetering M., Clevers H., Drost J. Xenograft and organoid model systems in cancer research. EMBO J. 2019;38:e101654. doi: 10.15252/embj.2019101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao H., Korn J.M., Ferretti S., Monahan J.E., Wang Y., Singh M., Zhang C., Schnell C., Yang G., Zhang Y., et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015;21:1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 56.Pompili L., Porru M., Caruso C., Biroccio A., Leonetti C. Patient-derived xenografts: A relevant preclinical model for drug development. J. Exp. Clin. Cancer Res. Cr. 2016;35:189. doi: 10.1186/s13046-016-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaeteewoottacharn K., Pairojkul C., Kariya R., Muisuk K., Imtawil K., Chamgramol Y., Bhudhisawasdi V., Khuntikeo N., Pugkhem A., Saeseow O.T., et al. Establishment of highly transplantable cholangiocarcinoma cell lines from a patient-derived xenograft mouse model. Cells. 2019:8. doi: 10.3390/cells8050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin K., Teng L., Shen Y., He K., Xu Z., Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: A systematic review. Clin. Transl. Oncol. 2010;12:473–480. doi: 10.1007/s12094-010-0540-6. [DOI] [PubMed] [Google Scholar]
- 59.Chijiwa T., Kawai K., Noguchi A., Sato H., Hayashi A., Cho H., Shiozawa M., Kishida T., Morinaga S., Yokose T., et al. Establishment of patient-derived cancer xenografts in immunodeficient nog mice. Int. J. Oncol. 2015;47:61–70. doi: 10.3892/ijo.2015.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shultz L.D., Goodwin N., Ishikawa F., Hosur V., Lyons B.L., Greiner D.L. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb. Protoc. 2014;2014:694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown K.M., Xue A., Mittal A., Samra J.S., Smith R., Hugh T.J. Patient-derived xenograft models of colorectal cancer in pre-clinical research: A systematic review. Oncotarget. 2016;7:66212–66225. doi: 10.18632/oncotarget.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu C., Li X., Liu P., Li M., Luo F. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine. Oncol. Lett. 2019;17:3–10. doi: 10.3892/ol.2018.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sittithumcharee G., Suppramote O., Vaeteewoottacharn K., Sirisuksakun C., Jamnongsong S., Lapanuwat P., Suntiparpluacha M., Matha A., Chusorn P., Buraphat P., et al. Dependency of cholangiocarcinoma on cyclin d-dependent kinase activity. Hepatology. 2019 doi: 10.1002/hep.30704. [DOI] [PubMed] [Google Scholar]
- 64.Murayama T., Gotoh N. Patient-derived xenograft models of breast cancer and their application. Cells. 2019;8 doi: 10.3390/cells8060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffman R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y., Revelo M.P., Sudilovsky D., Cao M., Chen W.G., Goetz L., Xue H., Sadar M., Shappell S.B., Cunha G.R., et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate. 2005;64:149–159. doi: 10.1002/pros.20225. [DOI] [PubMed] [Google Scholar]
- 67.Cho S.Y., Kang W., Han J.Y., Min S., Kang J., Lee A., Kwon J.Y., Lee C., Park H. An integrative approach to precision cancer medicine using patient-derived xenografts. Mol. Cells. 2016;39:77–86. doi: 10.14348/molcells.2016.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whittle J.R., Lewis M.T., Lindeman G.J., Visvader J.E. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. Bcr. 2015;17:17. doi: 10.1186/s13058-015-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centenera M.M., Hickey T.E., Jindal S., Ryan N.K., Ravindranathan P., Mohammed H., Robinson J.L., Schiewer M.J., Ma S., Kapur P., et al. A patient-derived explant (pde) model of hormone-dependent cancer. Mol. Oncol. 2018;12:1608–1622. doi: 10.1002/1878-0261.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oyama R., Takahashi M., Yoshida A., Sakumoto M., Takai Y., Kito F., Shiozawa K., Qiao Z., Arai Y., Shibata T., et al. Generation of novel patient-derived cic- dux4 sarcoma xenografts and cell lines. Sci. Rep. 2017;7:4712. doi: 10.1038/s41598-017-04967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borodovsky A., McQuiston T.J., Stetson D., Ahmed A., Whitston D., Zhang J., Grondine M., Lawson D., Challberg S.S., Zinda M., et al. Generation of stable pdx derived cell lines using conditional reprogramming. Mol. Cancer. 2017;16:177. doi: 10.1186/s12943-017-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shima K., Mizuma M., Hayashi H., Nakagawa K., Okada T., Sakata N., Omura N., Kitamura Y., Motoi F., Rikiyama T., et al. Potential utility of egfp-expressing nog mice (nog-egfp) as a high purity cancer sampling system. J. Exp. Clin. Cancer Res. Cr. 2012;31:55. doi: 10.1186/1756-9966-31-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waldmann T.A. Immunotherapy: Past, present and future. Nat. Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 74.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H., Chen J. Current status and future directions of cancer immunotherapy. J. Cancer. 2018;9:1773–1781. doi: 10.7150/jca.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shultz L.D., Ishikawa F., Greiner D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 77.Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and cd34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 78.Morton J.J., Bird G., Refaeli Y., Jimeno A. Humanized mouse xenograft models: Narrowing the tumor-microenvironment gap. Cancer Res. 2016;76:6153–6158. doi: 10.1158/0008-5472.CAN-16-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De La Rochere P., Guil-Luna S., Decaudin D., Azar G., Sidhu S.S., Piaggio E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39:748–763. doi: 10.1016/j.it.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Billerbeck E., Barry W.T., Mu K., Dorner M., Rice C.M., Ploss A. Development of human cd4+foxp3+ regulatory t cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing nod-scid il2rgamma(null) humanized mice. Blood. 2011;117:3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shultz L.D., Saito Y., Najima Y., Tanaka S., Ochi T., Tomizawa M., Doi T., Sone A., Suzuki N., Fujiwara H., et al. Generation of functional human t-cell subsets with hla-restricted immune responses in hla class i expressing nod/scid/il2r gamma(null) humanized mice. Proc. Natl. Acad. Sci. USA. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeng Y., Liu B., Rubio M.T., Wang X., Ojcius D.M., Tang R., Durrbach A., Ru Z., Zhou Y., Lone Y.C. Creation of an immunodeficient hla-transgenic mouse (humamice) and functional validation of human immunity after transfer of hla-matched human cells. PLoS ONE. 2017;12:e0173754. doi: 10.1371/journal.pone.0173754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Theocharides A.P., Rongvaux A., Fritsch K., Flavell R.A., Manz M.G. Humanized hemato-lymphoid system mice. Haematologica. 2016;101:5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ojima H., Yoshikawa D., Ino Y., Shimizu H., Miyamoto M., Kokubu A., Hiraoka N., Morofuji N., Kondo T., Onaya H., et al. Establishment of six new human biliary tract carcinoma cell lines and identification of mageh1 as a candidate biomarker for predicting the efficacy of gemcitabine treatment. Cancer Sci. 2010;101:882–888. doi: 10.1111/j.1349-7006.2009.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cavalloni G., Peraldo-Neia C., Sassi F., Chiorino G., Sarotto I., Aglietta M., Leone F. Establishment of a patient-derived intrahepatic cholangiocarcinoma xenograft model with kras mutation. Bmc Cancer. 2016;16:90. doi: 10.1186/s12885-016-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Julien S., Merino-Trigo A., Lacroix L., Pocard M., Goere D., Mariani P., Landron S., Bigot L., Nemati F., Dartigues P., et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 2012;18:5314–5328. doi: 10.1158/1078-0432.CCR-12-0372. [DOI] [PubMed] [Google Scholar]
- 87.Bertotti A., Migliardi G., Galimi F., Sassi F., Torti D., Isella C., Cora D., Di Nicolantonio F., Buscarino M., Petti C., et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies her2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 88.Chou J., Fitzgibbon M.P., Mortales C.L., Towlerton A.M., Upton M.P., Yeung R.S., McIntosh M.W., Warren E.H. Phenotypic and transcriptional fidelity of patient-derived colon cancer xenografts in immune-deficient mice. PLoS ONE. 2013;8:e79874. doi: 10.1371/journal.pone.0079874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garrido-Laguna I., Uson M., Rajeshkumar N.V., Tan A.C., de Oliveira E., Karikari C., Villaroel M.C., Salomon A., Taylor G., Sharma R., et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin. Cancer Res. 2011;17:5793–5800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mattie M., Christensen A., Chang M.S., Yeh W., Said S., Shostak Y., Capo L., Verlinsky A., An Z., Joseph I., et al. Molecular characterization of patient-derived human pancreatic tumor xenograft models for preclinical and translational development of cancer therapeutics. Neoplasia. 2013;15:1138–1150. doi: 10.1593/neo.13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo S., Gao S., Liu R., Shen J., Shi X., Bai S., Wang H., Zheng K., Shao Z., Liang C., et al. Oncological and genetic factors impacting pdx model construction with nsg mice in pancreatic cancer. FASEB J. 2019;33:873–884. doi: 10.1096/fj.201800617R. [DOI] [PubMed] [Google Scholar]
- 92.Wang H., Lu J., Tang J., Chen S., He K., Jiang X., Jiang W., Teng L. Establishment of patient-derived gastric cancer xenografts: A useful tool for preclinical evaluation of targeted therapies involving alterations in her-2, met and fgfr2 signaling pathways. BMC Cancer. 2017;17:191. doi: 10.1186/s12885-017-3177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y., Tian T., Li Z., Tang Z., Wang L., Wu J., Li Y., Dong B., Li N., Zou J., et al. Establishment and characterization of patient-derived tumor xenograft using gastroscopic biopsies in gastric cancer. Sci. Rep. 2015;5:8542. doi: 10.1038/srep08542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang T., Zhang L., Fan S., Zhang M., Fu H., Liu Y., Yin X., Chen H., Xie L., Zhang J., et al. Patient-derived gastric carcinoma xenograft mouse models faithfully represent human tumor molecular diversity. PLoS ONE. 2015;10:e0134493. doi: 10.1371/journal.pone.0134493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi Y.Y., Lee J.E., Kim H., Sim M.H., Kim K.K., Lee G., Kim H.I., An J.Y., Hyung W.J., Kim C.B., et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci. Rep. 2016;6:22172. doi: 10.1038/srep22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keysar S.B., Astling D.P., Anderson R.T., Vogler B.W., Bowles D.W., Morton J.J., Paylor J.J., Glogowska M.J., Le P.N., Eagles-Soukup J.R., et al. A patient tumor transplant model of squamous cell cancer identifies pi3k inhibitors as candidate therapeutics in defined molecular bins. Mol. Oncol. 2013;7:776–790. doi: 10.1016/j.molonc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kimple R.J., Harari P.M., Torres A.D., Yang R.Z., Soriano B.J., Yu M., Armstrong E.A., Blitzer G.C., Smith M.A., Lorenz L.D., et al. Development and characterization of hpv-positive and hpv-negative head and neck squamous cell carcinoma tumorgrafts. Clin. Cancer Res. 2013;19:855–864. doi: 10.1158/1078-0432.CCR-12-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marangoni E., Vincent-Salomon A., Auger N., Degeorges A., Assayag F., de Cremoux P., de Plater L., Guyader C., De Pinieux G., Judde J.G., et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin. Cancer Res. 2007;13:3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 99.Cottu P., Marangoni E., Assayag F., de Cremoux P., Vincent-Salomon A., Guyader C., de Plater L., Elbaz C., Karboul N., Fontaine J.J., et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res. Treat. 2012;133:595–606. doi: 10.1007/s10549-011-1815-5. [DOI] [PubMed] [Google Scholar]
- 100.DeRose Y.S., Wang G., Lin Y.C., Bernard P.S., Buys S.S., Ebbert M.T., Factor R., Matsen C., Milash B.A., Nelson E., et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X., Claerhout S., Prat A., Dobrolecki L.E., Petrovic I., Lai Q., Landis M.D., Wiechmann L., Schiff R., Giuliano M., et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73:4885–4897. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ricci F., Bizzaro F., Cesca M., Guffanti F., Ganzinelli M., Decio A., Ghilardi C., Perego P., Fruscio R., Buda A., et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014;74:6980–6990. doi: 10.1158/0008-5472.CAN-14-0274. [DOI] [PubMed] [Google Scholar]
- 103.Heo E.J., Cho Y.J., Cho W.C., Hong J.E., Jeon H.K., Oh D.Y., Choi Y.L., Song S.Y., Choi J.J., Bae D.S., et al. Patient-derived xenograft models of epithelial ovarian cancer for preclinical studies. Cancer Res. Treat. 2017;49:915–926. doi: 10.4143/crt.2016.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dobbin Z.C., Katre A.A., Steg A.D., Erickson B.K., Shah M.M., Alvarez R.D., Conner M.G., Schneider D., Chen D., Landen C.N. Using heterogeneity of the patient-derived xenograft model to identify the chemoresistant population in ovarian cancer. Oncotarget. 2014;5:8750–8764. doi: 10.18632/oncotarget.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weroha S.J., Becker M.A., Enderica-Gonzalez S., Harrington S.C., Oberg A.L., Maurer M.J., Perkins S.E., AlHilli M., Butler K.A., McKinstry S., et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin. Cancer Res. 2014;20:1288–1297. doi: 10.1158/1078-0432.CCR-13-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Topp M.D., Hartley L., Cook M., Heong V., Boehm E., McShane L., Pyman J., McNally O., Ananda S., Harrell M., et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol. Oncol. 2014;8:656–668. doi: 10.1016/j.molonc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fichtner I., Rolff J., Soong R., Hoffmann J., Hammer S., Sommer A., Becker M., Merk J. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 2008;14:6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 108.Dong X., Guan J., English J.C., Flint J., Yee J., Evans K., Murray N., Macaulay C., Ng R.T., Gout P.W., et al. Patient-derived first generation xenografts of non-small cell lung cancers: Promising tools for predicting drug responses for personalized chemotherapy. Clin. Cancer Res. 2010;16:1442–1451. doi: 10.1158/1078-0432.CCR-09-2878. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y., Zhang R., Wang L., Correa A.M., Pataer A., Xu Y., Zhang X., Ren C., Wu S., Meng Q.H., et al. Tumor characteristics associated with engraftment of patient-derived non-small cell lung cancer xenografts in immunocompromised mice. Cancer. 2019 doi: 10.1002/cncr.32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brabetz S., Leary S.E.S., Grobner S.N., Nakamoto M.W., Seker-Cin H., Girard E.J., Cole B., Strand A.D., Bloom K.L., Hovestadt V., et al. A biobank of patient-derived pediatric brain tumor models. Nat. Med. 2018;24:1752–1761. doi: 10.1038/s41591-018-0207-3. [DOI] [PubMed] [Google Scholar]
- 111.Priolo C., Agostini M., Vena N., Ligon A.H., Fiorentino M., Shin E., Farsetti A., Pontecorvi A., Sicinska E., Loda M. Establishment and genomic characterization of mouse xenografts of human primary prostate tumors. Am. J. Pathol. 2010;176:1901–1913. doi: 10.2353/ajpath.2010.090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wetterauer C., Vlajnic T., Schuler J., Gsponer J.R., Thalmann G.N., Cecchini M., Schneider J., Zellweger T., Pueschel H., Bachmann A., et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate. 2015;75:585–592. doi: 10.1002/pros.22939. [DOI] [PubMed] [Google Scholar]
- 113.Lang H., Beraud C., Bethry A., Danilin S., Lindner V., Coquard C., Rothhut S., Massfelder T. Establishment of a large panel of patient-derived preclinical models of human renal cell carcinoma. Oncotarget. 2016;7:59336–59359. doi: 10.18632/oncotarget.10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sivanand S., Pena-Llopis S., Zhao H., Kucejova B., Spence P., Pavia-Jimenez A., Yamasaki T., McBride D.J., Gillen J., Wolff N.C., et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci. Transl. Med. 2012;4:137ra175. doi: 10.1126/scitranslmed.3003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong Y., Manley B.J., Becerra M.F., Redzematovic A., Casuscelli J., Tennenbaum D.M., Reznik E., Han S., Benfante N., Chen Y.B., et al. Tumor xenografts of human clear cell renal cell carcinoma but not corresponding cell lines recapitulate clinical response to sunitinib: Feasibility of using biopsy samples. Eur. Urol. Focus. 2017;3:590–598. doi: 10.1016/j.euf.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Einarsdottir B.O., Bagge R.O., Bhadury J., Jespersen H., Mattsson J., Nilsson L.M., Truve K., Lopez M.D., Naredi P., Nilsson O., et al. Melanoma patient-derived xenografts accurately model the disease and develop fast enough to guide treatment decisions. Oncotarget. 2014;5:9609–9618. doi: 10.18632/oncotarget.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krepler C., Sproesser K., Brafford P., Beqiri M., Garman B., Xiao M., Shannan B., Watters A., Perego M., Zhang G., et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Rep. 2017;21:1953–1967. doi: 10.1016/j.celrep.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao Y., Shuen T.W.H., Toh T.B., Chan X.Y., Liu M., Tan S.Y., Fan Y., Yang H., Lyer S.G., Bonney G.K., et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut. 2018;67:1845–1854. doi: 10.1136/gutjnl-2017-315201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yao L.C., Aryee K.E., Cheng M., Kaur P., Keck J.G., Brehm M.A. Creation of pdx-bearing humanized mice to study immuno-oncology. Methods Mol. Biol. 2019;1953:241–252. doi: 10.1007/978-1-4939-9145-7_15. [DOI] [PubMed] [Google Scholar]
- 120.Buque A., Galluzzi L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer. 2018;4:599–601. doi: 10.1016/j.trecan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Choi Y., Lee S., Kim K., Kim S.H., Chung Y.J., Lee C. Studying cancer immunotherapy using patient-derived xenografts (pdxs) in humanized mice. Exp. Mol. Med. 2018;50:99. doi: 10.1038/s12276-018-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Satoh M., Saito M., Tanaka K., Iwanaga S., Ali S.N., Seki T., Okada S., Kohara M., Harada S., Kai C., et al. Evaluation of a recombinant measles virus expressing hepatitis c virus envelope proteins by infection of human pbl-nod/scid/jak3null mouse. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:e81–e88. doi: 10.1016/j.cimid.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harada H., Suzu S., Ito T., Okada S. Selective expansion and engraftment of human cd16+ nk cells in nod/scid mice. Eur. J. Immunol. 2005;35:3599–3609. doi: 10.1002/eji.200535125. [DOI] [PubMed] [Google Scholar]
- 124.Goto H., Matsuda K., Srikoon P., Kariya R., Hattori S., Taura M., Katano H., Okada S. Potent antitumor activity of zoledronic acid-induced vgamma9vdelta2 t cells against primary effusion lymphoma. Cancer Lett. 2013;331:174–182. doi: 10.1016/j.canlet.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y., Chanana P., Davila J.I., Hou X., Zanfagnin V., McGehee C.D., Goode E.L., Polley E.C., Haluska P., Weroha S.J., et al. Gene expression differences between matched pairs of ovarian cancer patient tumors and patient-derived xenografts. Sci. Rep. 2019;9:6314. doi: 10.1038/s41598-019-42680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ben-David U., Ha G., Tseng Y.Y., Greenwald N.F., Oh C., Shih J., McFarland J.M., Wong B., Boehm J.S., Beroukhim R., et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 2017;49:1567–1575. doi: 10.1038/ng.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aparicio S., Hidalgo M., Kung A.L. Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer. 2015;15:311–316. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]
- 128.Hidalgo M., Amant F., Biankin A.V., Budinska E., Byrne A.T., Caldas C., Clarke R.B., de Jong S., Jonkers J., Maelandsmo G.M., et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Byrne A.T., Alferez D.G., Amant F., Annibali D., Arribas J., Biankin A.V., Bruna A., Budinska E., Caldas C., Chang D.K., et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]