Abstract
Purpose
The Jundiaí Zika Cohort (JZC) is a prospective pregnancy and birth cohort setup in the State of São Paulo, Brazil, to investigate the epidemic of cases of microcephaly and other neurological disorders, presumed to be associated with Zika virus (ZIKV) infection.
Participants
A total of 748 women with high-risk pregnancies were recruited in the period of March 2016 to August 2017.
Findings to date
Baseline sociodemographic and medical data were collected at recruitment from 737 pregnant women. Biological samples (ie, blood, saliva and urine) were collected from 695 of the pregnant women (94.3%), of whom 53 (7.6%) were ZIKV-positive on subsequent testing by reverse transcription polymerase chain reaction (RT-PCR) in urine. Biological sample (ie, blood, saliva, urine and cerebrospinal fluid) were collected within 10 days of birth from 409 (57.4%) of the liveborn infants, of whom 19 (4.6%) were ZIKV-positive on subsequent testing by RT-PCR in urine. All remaining biological specimens, as well as colostrum, umbilical cord and placental samples, have been stored in a secure biorepository. Antenatal and postnatal imaging studies and neonatal anthropometry were carried out.
Future plans
The JZC provides a unique data set which will continue to be explored to study the effects of pregnancy comorbidities on Zika virus infection during pregnancy, the long-term outcomes of children with congenital Zika infection and how physiotherapy and group interventions can improve outcomes for congenitally-infected children. All women in the cohort have reached the end of their pregnancy and currently the oldest children are 2 years old. The study will continue until all the children reach their third birthday (April 2021).
Keywords: zika virus, flavivirus, congenital infection, pregnancy
Strengths and limitations of this study.
The Jundiaí Zika Cohort (JZC) is one of the few prospective Zika cohort studies that has recruited both asymptomatic and symptomatic pregnant women and therefore benefits from having a large control group.
The high-risk profile of the pregnant women provides a unique opportunity to study comorbidities that may contribute to, or potentially be protective for, the development of negative sequelae associated with Zika virus (ZIKV) exposure.
The prevalence of ZIKV reverse transcription polymerase chain reaction positivity was relatively low in this study population resulting in small numbers for estimating absolute and relative risks.
Because high-risk pregnant women are at higher risk of developing some of the outcomes of interest, this may have reduced the power to detect differences between Zika-exposed and unexposed dyads.
Introduction
The Jundiaí Zika Cohort (JZC) is an ongoing multidisciplinary longitudinal study which is following a cohort of pregnant women and their children to study the effects of prenatal Zika virus (ZIKV) exposure. The cohort was set-up in response to the clusters of cases of severe microcephaly and associated neurological disorders that were reported in areas affected by ZIKV in the Northeast of Brazil in October 20151 and that provoked the Public Health Emergency of International Concern declared by the WHO on 1st February 2016.2 At this point in time, the São Paulo Research Foundation (FAPESP) encouraged all researchers who had been part of the Brazilian Genome Project to submit thematic research project proposals addressing the potential causes of the cluster of cases of microcephaly.
Laboratory confirmation of autochthonous ZIKV transmission in Brazil was first established in the north-eastern states of Pernambuco, Rio Grande do Norte and Bahia, and later in other states of the central-west and south-eastern regions of Brazil. When the JZC was set-up, there was no published data about the ZIKV epidemic in São Paulo state. It was not known how the differences in climate and socioeconomic status between this south-eastern state and the poorer and more tropical north-eastern regions, where the ZIKV epicentre was focused, would influence the epidemic. The study site of Jundiaí in the south-eastern state of São Paulo was therefore chosen in order to explore these variations.
This cohort profile aims to describe the Jundiaí Zika Cohort including: The context behind its creation, materials and methodology, recruitment and follow-up of pregnant women and children as well as some of its preliminary results. The oldest children of the cohort, as of November 2018, are 2 years old; all the children will be followed up until their third birthday.
Cohort description
Study site
The JZC is housed in the paediatric department of the Jundiaí Medical School in the city of Jundiaí, São Paulo state. Jundiaí is 50 km northwest of the city of São Paulo (figure 1) and has a population of 405 740 inhabitants.3 It has a relatively high Human Development Index ranking 11th out of the total 5565 municipalities in the country.3 The climate in the area is humid subtropical, according to the Köppen classification, with a mean annual temperature of 20.9°C. The majority (64%) of the land in the municipality of Jundiaí is considered rural and 31% of this is made up of the Japi Mountain, a Biosphere Reserve of Atlantic Forest recognised by UNESCO since 1994.4 Jundiaí University Hospital is the only public maternity facility in the municipality of Jundiaí and is the local referral centre for high-risk pregnancies. In 2017, 37.6% of babies born in the municipality were born in this hospital; the remaining 62.4% were born in one of three private hospitals in Jundiaí.5
Figure 1.
Map showing the location of the municipality of Jundiaí in the State of São Paulo, Brazil.19 CSF, cerebrospinal fluid; PCR, polymerase chain reaction; USS, ultrasound scan; ZIKV, Zika virus.
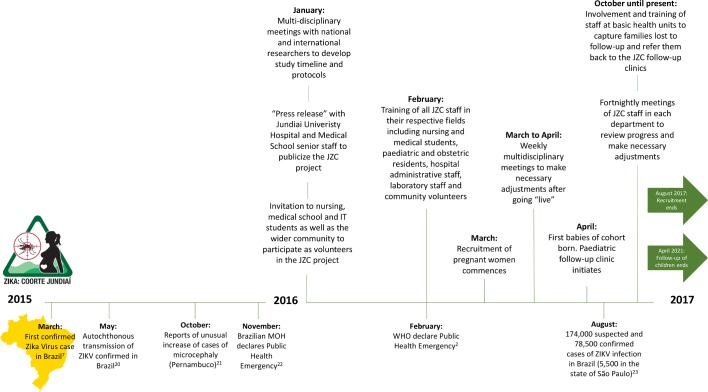
Given the severity of the phenotype assumed to be associated with prenatal exposure to ZIKV and the urgent need to establish a cause, the JZC, like many other Zika Cohorts in Brazil, commenced without any formal funding on 1st March 2016. It later received seed funding from The London School of Hygiene and Tropical Medicine prior to obtaining formal FAPESP funding for a thematic research project over a year after the initiation of the project on 1st April 2017 (figure 2).
Figure 2.
The context of the initiation of the Jundiaí Zika Cohort presented as a timeline of events related to the introduction of ZIKV in Brazil in the period 2015 to 2017 and beyond.2 20–23 ZIKV, Zika virus.
Following ethical approval for the study from the research ethics committee of Jundiaí Medical School (protocol number 1446577), written informed consent was obtained from participating women for themselves and for future follow-up of their child.
This cohort profile describes the recruitment and follow-up of the pregnant women and children in the JZC. The oldest children of the cohort, as of November 2018, are 2 years old; all the children will be followed up until their third birthday.
Cohort description
Eligibility and recruitment
All pregnant women attending the high-risk pregnancy clinic at Jundiaí University Hospital (referred from one of the 38 basic health units in the Jundiaí area due to the presence of risk factors that could affect the well-being of themselves or their unborn child)6 between 1st March 2016 and 23rd August 2017 were invited to participate. Common risk factors included diabetes, hypertension, twin pregnancy and adolescent pregnancy. The only exclusion criteria at this stage were women who had life-threatening conditions and women who had severe learning difficulties. The reasons for choosing this study population of high-risk pregnant women were (i) to try to maximise recruitment efficiency and pregnancy follow-up adherence, (ii) to provide an appropriate location for the examination of the women and collection of clinical samples and (iii) to optimise newborn data quality by ensuring a large proportion would be born in Jundiaí University Hospital. Over the duration of follow-up, clinical teams cared for pregnant women in accordance with the Brazilian Ministry of Health protocols.
As the study commenced in the midst of the ZIKV outbreak in Brazil, and seroprevalence studies had not been carried out, it was not known what the true incidence or prevalence of ZIKV infection was in the Jundiaí area; or indeed what the prevalence of microcephaly was in either ZIKV exposed or unexposed pregnant women. In 2016, in the State of São Paulo, 9845 cases of ZIKV infection were reported to the Brazilian Notifiable Disease Registry (SINAN) of which 5056 were considered probable, giving a crude incidence of 11.3 cases of ZIKV per 100 000 inhabitants in the State of São Paulo in the year 2016.7 However, this is likely to be a gross underestimation.
The sample size for the cohort was calculated using an estimated prevalence of cases of microcephaly among neonates of ZIKV reverse transcription polymerase chain reaction (RT-PCR) positive pregnant women of 2%. A final analytical cohort size of n=531 would give us 80% power to detect a crude relative risk of 2 with a probability of type I error (α) of 5%.8 Although initially the JZC aimed to enrol 500 pregnant women, recruitment continued for longer than initially anticipated to try to capture possible seasonal differences in the incidence of ZIKV disease.
Study participant characteristics
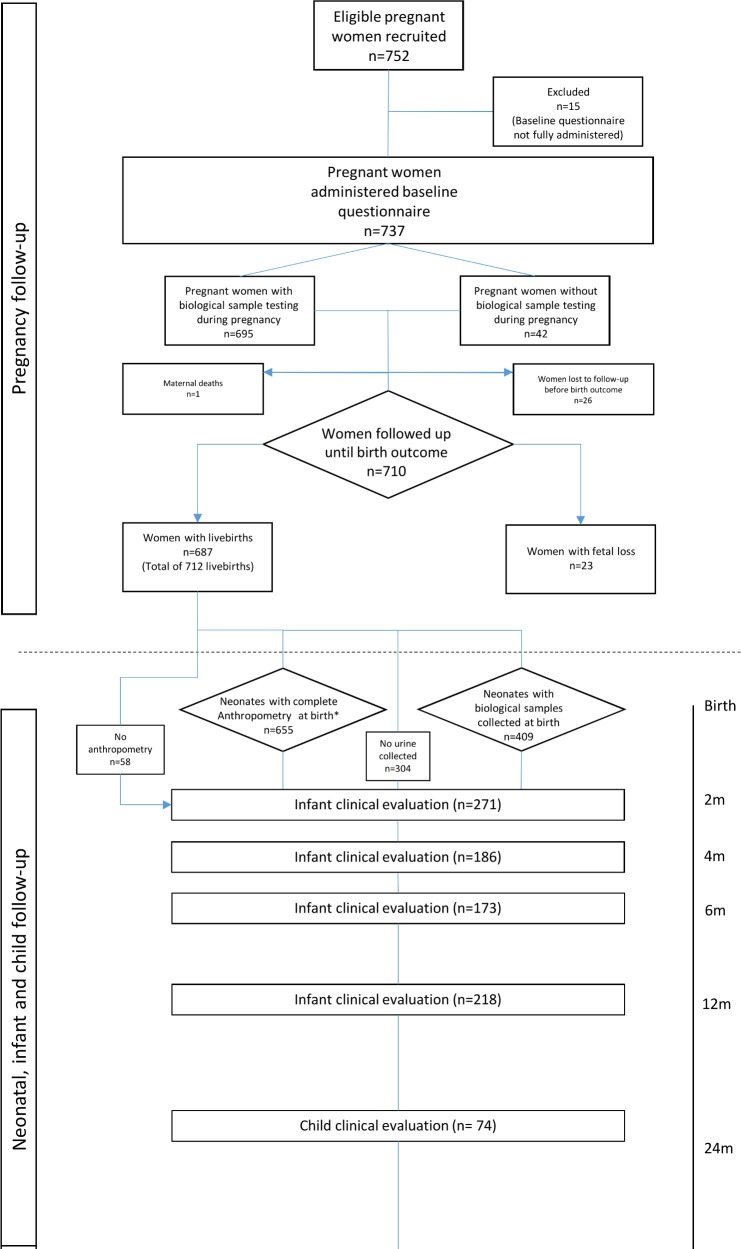
During the recruitment period, 752 women were enrolled in the study (figure 3). Of these, 15 (2%) were excluded as the baseline questionnaire was not fully administered. The mean age of women was 27.5 years (13 to 46). Three hundred and sixty-eight (53.7%) of the women were of white ethnicity, 238 (34.7%) mixed race or brown (known as ‘parda’ in Portuguese), 66 (9.6%) black, 11 (1.6%) Asian and 2 (0.3%) indigenous. Five hundred and sixty-nine (77.2%) of the women reported being married or living with their partner (table 1). During pregnancy, 231 (33.9%) of the women had diabetes and 129 (18.9%) had hypertension. The presence of other risk factors, or comorbidities, is detailed in table 2.
Figure 3.
Flow diagram showing participants of the Jundiaí Zika Cohort at each stage of the study, recruitment period: 01 March 2016 to 23 August 2017, Jundiaí, São Paulo, Brazil.
Table 1.
Description of women in the Jundiaí Zika cohort
| Pregnant women (n=737) | |
| Age in years (mean and range) | 27.5 (13–46) |
| Ethnicity White | 53.7 % (n = 368) |
| Mixed race | 34.7 % (n = 238) |
| Black | 9.6 % (n = 66) |
| Asian | 1.6 % (n = 11) |
| Indigenous | 0.3 % (n = 2) |
| Unknown | 7.1% (n=52) |
| Education >12 years | 15.4% (n=106) |
| 12 years | 44.4 % (n = 305) |
| 9–11 years | 23.7 % (n = 163) |
| ≤8 years | 16.4 % (n = 113) |
| Unknown | 6.8 % (n = 50) |
| Married/living with partner | 77.2% (n=569) |
| Biological sample tested for ZIKV RT-PCR during pregnancy | 94.3% (n=695) |
| Positive maternal urine ZIKV RT-PCR during pregnancy | 7.6% (n=53) |
| Lost to follow-up before birth outcome | 3.5% (n=26) |
| Maternal deaths | 0.1% (n=1) |
| Women with live births (of those followed up) | 97.6% (n=687) |
| Foetal deaths | 3.2% (n=23) |
*Note: Percentages for all categories were calculated with exclusion of those with missing data from the denominator.
RT-PCR, reverse transcription polymerase chain reaction; ZIKV, Zika virus.
Table 2.
Presence of risk factors (comorbidities) among pregnant women in the Jundiaí Zika cohort
| Risk factor | Proportion (n) of women with risk factor (total n=682) |
| Diabetes | 33.9% (231) |
| Hypertension | 18.9% (129) |
| Adolescent | 10.0% (68) |
| Urinary tract infection | 5.6% (38) |
| Twin pregnancy | 4.3% (29) |
| Hypothyroidism | 4.1% (28) |
| Obesity or previous bariatric surgery | 3.2% (22) |
| Pre-eclampsia | 2.6% (18) |
| Syphilis | 2.3% (16) |
| Asthma | 2.1% (14) |
| Previous preterm labour or foetal death | 1.8% (12) |
| Epilepsy | 1.8% (12) |
| Threatened preterm labour | 1.6% (11) |
| Heart disease | 1.5% (10) |
| Anaemia (including sickle cell and thalassaemia) | 1.3% (9) |
| Illicit drug use | 1.0% (7) |
| Psychiatric illness | 0.7% (5) |
| HIV | 0.4% (3) |
Note: Categories are not mutually exclusive.
Of the 737 pregnant women with full baseline information collected, 695 (94.3%) provided biological samples (ie, blood, saliva and urine from each) for ZIKV testing. Of the 695 women whose urine was tested by RT-PCR, 53 (7.6%) were ZIKV-positive. Due to financial constraints during the outbreak, blood and saliva collected from both women and neonates have been stored in a biorepository for future testing. Twenty-six (3.4%) women were lost to follow-up during pregnancy (ie, before the birth outcome), and there was one first trimester maternal death. Of the 710 women who were followed until the birth outcome, 23 (3.2%) had foetal losses and 687 (96.9%) had live births, of which 25 (3.6%) were twin pregnancies.
Of the 712 live births, 376 (52.7%) were female (table 3). Of these, 655 (92.0%) had anthropometry at birth (a minimum of weight and head circumference measured at birth); the mean birth weight among live births was 3001 g (590 g to 4525 g), mean length at birth was 47.5 cm (28.5 cm to 58.5 cm) and mean head circumference was 33.7 cm (22 cm to 38.5 cm). The number of liveborn infants who had urine tested for ZIKV RT-PCR within 10 days of birth was 409 (57.4%). Of these, 19 (4.6%) were positive. Notably, no neonates presented with symptoms consistent with postnatal ZIKV infection in the first 10 days. Of the 712 live births, 271 (38.1%) babies were seen in the JZC paediatric clinic between 0 to 2 months of age, 186 (26.1%) between 3 to 4 months of age, 173 (24.3%) between 5 to 6 months of age, 218 (30.6%) between 7 to 12 months of age and 74 (10.4%) between 13 to 24 months of age. So far, no infants have been followed up beyond 2 years of age.
Table 3.
Description of liveborn infants in the Jundiaí Zika cohort
| Liveborn infants (n=712) | |
| Twin pairs | 3.5% (n=25) |
| Sex (female) | 52.8% (n=376) |
| Delivery method | |
| Vaginal | 47.1 % (n = 330) |
| Caesarean section | 50.3 % (n = 352) |
| Forceps | 2.6 % (n = 18) |
| Unknown | 1.7 % (n = 13) |
| Weight at birth in grams (mean and range) | 3001 (590 – 4525) |
| Length at birth in cm (mean and range) | 47.5 (28.5–58.5) |
| Head circumference at birth (mean and range) | 33.7 (22–38.5) |
| Biological sample tested for ZIKV RT-PCR at<10 days | 57.4% (n=409) |
| Positive urine ZIKV RT-PCR in first 10 days of life | 4.6% (n=19) |
Note: Percentages for all categories were calculated with exclusion of those with missing data from the denominator.
RT-PCR, reverse transcription polymerase chain reaction; ZIKV, Zika virus.
External validity and possible participation biases
The recruitment of only high-risk pregnant women brought advantages, as discussed earlier, in both logistics and maximisation of follow-up rates, however, these advantages also introduced limitations in external validity when generalising JZC findings to the general pregnant population of Brazil. Moreover, as recruitment was carried out in a specific population of pregnant women that were users of a particular health service, it is possible that there are systematic differences between those recruited and those not recruited, which we were not able to measure, and these may have introduced bias. However, when comparing the sociodemographic profile of the pregnant women in our cohort and the profile of pregnant women living and using public maternity facilities in the State of São Paulo at the time of the study,9 10 we can see that they are quite similar. For example, around half of the women were white, around half had vaginal deliveries and the majority had finished high school and were co-habiting and/or married to their partner (table 1).
Furthermore, as there is no known association between high-risk pregnancies and congenital Zika syndrome (CZS), we do not anticipate that the restricted selection criteria of the cohort would have a significant impact on our interpretation of the underlying biology of congenital ZIKV infections.
Follow-up
Pregnant women
Women who at enrolment reported not having had any symptoms consistent with ZIKV infection during their pregnancy (and who were currently asymptomatic), according to the WHO ZIKV clinical case definition,11 were followed up as per Group 1 and women who were symptomatic at any point during pregnancy were followed up as per Group 2 (figure 3). Women were asked to contact the research team and attend the hospital if they experienced any symptoms consistent with ZIKV infection at any point in their pregnancy so that biological samples could be collected as detailed below. In addition, trained volunteers carried out weekly telephone follow-up consultations at pre-arranged times that were convenient for the women until the time of birth to ask specifically about the occurrence of any ZIKV symptoms and any women who had experienced symptoms were advised to go to the hospital.
Regardless of symptom occurrence, women in both groups were seen 14 to 21 days after enrolment for biological sample collection and then in 2 to 3 monthly intervals thereafter (sample collection details and laboratory procedures are described below). Antenatal ultrasound scanning was carried out in months 3, 5, 7 and 8 in asymptomatic (Group 1) women and monthly in symptomatic (Group 2) women at the São Paulo foetal medicine centre (CPMF). Additionally, where malformations, signs of congenital ZIKV infection or intra-uterine growth restriction were found, ultrasound scanning was carried out weekly at Jundiaí University Hospital or CPMF (figure 4).
Figure 4.
Full follow-up protocol for Jundiaí Zika cohort study (01 March 2016 to 23 August 2017), Jundiaí, São Paulo, Brazil. JZC, Jundiaí Zika Cohort; ZIKV, Zika virus.
Neonates, infants, children
Women whose birth outcomes resulted in a live birth were invited to come to the JZC paediatric follow-up clinic within the neonate’s first month of life. Subsequently, mothers were asked to bring their children back monthly in the first 12 months of life and at 3 monthly intervals thereafter until they completed 36 months.
At each stage of follow-up there was a significant non-response rate (see figure 3). Pregnant women and mothers who did not attend a scheduled follow-up appointment were contacted by phone, mobile or Facebook. In addition, JZC teams visited and spoke to staff at the basic health units in Jundiaí and requested them to refer on any children belonging to the cohort. Despite these efforts, for many families, a complex and precarious social situation precluded them from being able to attend the follow-up appointments.
Data collection
The JZC is a multidisciplinary study containing a rich range of information regarding the follow-up and outcomes of the JZC mothers and children, both exposed and unexposed to ZIKV during pregnancy. The main health, medical and laboratory data collected to date are listed in table 4.
Table 4.
The Jundiaí Zika cohort, summary of health, medical and laboratory data collected from women and their children
| Phase | Measurements |
| Pregnant women at enrolment | Baseline questionnaire - sociodemographic details, past medical history, family history, past obstetric history (parity, miscarriages, mode of delivery, malformations), current obstetric history (if pregnancy was planned, use of tobacco, alcohol, drug and medications and vaccinations received), presence of symptoms/signs consistent with ZIKV infection (fever, rash, non-purulent conjunctivitis, arthritis/arthralgia, lymphadenopathy, myalgia, headache) at any point throughout pregnancy, the woman’s environment (type of housing, number of rooms, number of people per household), preventative measures (use of repellent, protective clothing, window or bed nets, barrier contraception) and their knowledge of ZIKV and its forms of transmission as well as what their sources of information were |
| Pregnant women follow-up 14–21 days after enrolment | Sample collection (blood, saliva, urine) for ZIKV RT-PCR and IgG/IgM Symptoms questionnaire - presence of symptoms/signs consistent with ZIKV infection (timing, duration, intensity, action taken) |
| Pregnant women subsequent 2–3 monthly follow-ups | Sample collection (blood, saliva, urine) for ZIKV RT-PCR and IgG/IgM Symptoms questionnaire - presence of symptoms/signs consistent with ZIKV infection (timing, duration, intensity, action taken) Antenatal ultrasound at São Paulo foetal medicine centre |
| Pregnant women weekly phone follow-up | Symptoms questionnaire - presence of symptoms/signs consistent with ZIKV infection (timing, duration, intensity, action taken) |
| Birth (mother and neonate) | Sample collection (blood, saliva, urine) for ZIKV RT-PCR and IgG/IgM. Colostrum (mother) and cerebrospinal fluid (neonate exposed to ZIKV and/or with microcephaly) for ZIKV PCR. Anthropometry – weight, length, head circumference (neonate) Placenta and umbilical collection - pathology |
| Neonatal, infant and child follow-ups | Sample collection (blood, saliva, urine) for ZIKV RT-PCR and IgG/IgM in months 1, 3, 6 and 15 for neonates. (Women found to be ZIKV RT-PCR positive during pregnancy also had blood, saliva and urine collected during paediatric follow-up appointments). Paediatric follow-up questionnaire – problems, significant events, feeding, vaccinations, developmental milestones reached, review of lab test results (including heel-prick test) Anthropometry – weight, length, head circumference Paediatric physical examination – general, cardiovascular, respiratory, gastrointestinal, neurological, developmental Physiotherapy assessment Speech and language assessment |
| ZIKV exposed infants and/or with microcephaly/other neurological abnormalities | Opthalmology assessment (with Teller-CAT Cambridge Colour Test, funduscopy and extrinsic ocular motility tests) Specialist neurodevelopmental assessment (using Bayley-III developmental scales) Specialist audiology assessment Gastrointestinal assessment Imaging – Cranial ultrasound and CT brain at birth and 12 months |
PCR, polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction; ZIKV, Zika virus.
The initial baseline questionnaire administered to the pregnant women was designed by the JZC researchers before any internationally standardised collection tool had been developed. In August 2016, the WHO created a standardised questionnaire in order to streamline data collected by all the Zika cohorts12; new questions which were not already in the initial JZC tool (mainly related to environmental exposures, for example mosquito repellent and bednet use, type of housing and water sources) were added.
Four specialist foetal medicine doctors carried out the antenatal ultrasound scans at the CPMF with Voluson S10, Voluson E6 e Voluson E8 GE Healthcare equipment. Foetal anthropometry was carried out and gestational age estimated.
Anthropometry was carried out in neonates in the first hour of life unless their condition was unstable. Head circumference was measured using a non-elastic tape measure placed between forehead and occiput, weight was measured using digital scales and length using a recumbent baby length scale. Z-scores for these measurements were then calculated using the INTERGROWTH-21st curves13–15 which take into account the sex and gestational age of the neonate. Gestational age was calculated using first-trimester ultrasound when available and last menstrual period when not available.
Clinical samples and laboratory procedures
Blood, saliva and urine were collected from the women and children by nursing staff, healthcare assistants and auxiliary nurses (all registered at the nursing professional registration body of the State of São Paulo (COREN-SP)) following an in-house standardised protocol. Personal protective measures were taken (gloves and eye protection were worn at all times) and samples were labelled appropriately with the patient name, date of collection, date of birth and sample number). Saliva samples were collected by swabbing 10 times on the inside of each cheek with sterile swabs which were then placed into sterile Falcon 15 mL tubes. Women were asked to provide urine samples into 50 mL sterilised collection tubes. For neonatal and infant urine samples, sterile urine collecting bags with adhesive tape were placed over sterilised skin around the penis/vulva. At birth, blood was collected from the umbilical cord and subsequently, in infants and women, blood samples were obtained by venepuncture in the antecubital fossa or in the dorsum of the hand with a maximum of three attempts. Blood was collected into dry and EDTA tubes which were mixed and labelled appropriately and placed in the fridge.
ELISA for identification of anti-ZIKV immunoglobulins (IgM and IgG)
Detection of anti-ZIKV antibodies (IgM and IgG) was performed by ELISA using commercial Zika IgG and Zika IgM ELISA kits Euroimmun (Euroimmun BR 2015), approved by the National Sanitary Surveillance Agency (ANVISA) for the diagnosis of ZIKV.
Serum, blood or plasma were diluted 1:101 in sample buffer. For IgM detection, the buffer contains an IgG/RF absorbent (preparation of anti-goat IgG). After, 100 µL of this dilution was applied to each well of the plates. The plates were covered with protective film and incubated for 60 min at +37°C. After the first incubation, the plates were washed with 400 µl of wash buffer (1X). One hundred microliters of the enzyme conjugate (peroxidase-labelled human IgG and IgM) were applied to each well, followed by incubation for 30 min at room temperature. Another wash was performed, under the same conditions described previously. In each well 100 µL of the substrate/chromogen was added, followed by incubation for 30 min at room temperature, protected from light. After washing, 100 µL of Stop solution was added and the plates were read in Automatic Biochemical Analyzer, model prietest TOUCH (2009 ROBONIK India), in 450 mn absorbance.
For the detection of IgG, three calibrators were used plus the negative and positive controls contained in the kit. For IgM detection a calibrator plus the positive and negative controls were used. The cut-off was calculated by the ratio between the absorbance of the controls and that of the calibrators. Samples with a cut-off <0.8 and positive samples with a cut-off of ≥1.1 were considered negative. The samples with a cut-off between ≥0.8 and 1.1 were considered equivocal.
Real time PCR for ZIKV detection
ZIKV RNA detection was performed by real time PCR (RT-qPCR), as recommended by the WHO, according to the protocol developed by Lanciotti et al 16 on maternal and neonatal urine samples.
Initially, the total RNA was extracted from 140 µL of urine using the QIAamp Viral RNA Mini Kit (QIAGEN), following manufacturer’s instructions. The final RNA was eluted in 60 µl of ultrapure H2O Nuclease-Free Water (2018 Merck KGaA, Darmstadt, Germany) and RT-qPCR was performed on the same day. The remaining RNA was stored in a freezer at −80°C. RT-qPCR was performed with GoTaq Probe qPCR and RT-qPCR Systems (2018 Promega Corporation Brasil, Ltda). For the final volume of 20 µl reaction, 8 µl of RNA template was used. The Mix was created with 10 µl of GoTaq Probe qPCR Master Mix with dUTP (1x), 0,4 µl of GoScript RT Mix for 1-Step RT-qPCR (1x), 1 µl of Forward primer (10 pmol/µL), 1 µl of Reverse primer (10 pmol/µL), 1 µl of probe (10 pmol/µL) (table 5) and of Nuclease-Free Water to complete the final volume. Two sets of primers and probe were used on the RT-qPCR reaction. The reaction occurred in ABI Prism 7500 SDS Real-Time cycler (Applied Biosystems), where the amplification cycles consisted of: One cycle of 15 min at 45°C for reverse transcription, one cycle of 2 min at 95°C for reverse transcriptase inactivation and for polymerase activation, 40 cycles of 15 s at 95°C for denaturation and 1 min at 60°C for annealing and extension. The primers and probes used for this quantification are complementary to the gene encoding the NSI protein of ZIKV (table 5). The probe contains a fluorescent 6-carboxyfluorescein (FAM) reporter dye at the 5’ end and the fluorescent dye 6-carboxytemethylhydhodamine (TAMRA) at the 3’ end. All reactions followed positive and negative controls previously quantified.
Table 5.
Primer and probe sets used for RT-qPCR ZIKV detection
| Primer/probe set | Primer and/or probe | Genome position | Sequence (5’ – 3’) |
| Set 1 | ZIKV 835 | 835–857 | TTGGTCATGATACTGCTGATTGC |
| ZIKV 860-FAM | 860–886 | CGGCATACAGCATCAGGTGCATAGGAG | |
| ZIKV 911 c | 911–890 | CCTTCCACAAAGTCCCTATTGC | |
| Set 2 | ZIKV 1086 | 1086–1102 | CCGCTGCCCAACACAAG |
| ZIKV 1107-FAM | 1162–1139 | AGCCTACCTTGACAAGCAGTCAGACACTCAA | |
| ZIKV 1162 c | 1107–1137 | CCACTAACGTTCTTTTGCAGACAT |
Primers designed by Lanciotti et al, 2008 (Based on ZIKV MR 766 GenBank accession no. AY632535).
FAM, fluorescent 6-carboxyfluorescein; PCR, polymerase chain reaction; RT-qPCR, real time quantitative PCR; ZIKV, Zika virus.
The placenta and umbilical cord were collected and stored in formaldehyde and sent to the pathology laboratory where they were examined by specialist placental pathologists.
Patient and public involvement
As can be seen in the timeline of the creation of the JZC (figure 2), the wider community were invited to participate in the design of the JZC study and to join the research team to be trained as volunteers. Some of these members of the public were then involved with recruitment to the study and responsible for the dissemination of information about the study to their local communities. As the JZC involves a long follow-up period of pregnant women and their children, personal clinical results have been regularly communicated to patients and their families. In addition, any relevant study findings have also been carefully and appropriately explained.
Findings to date
ZIKV clinical features during pregnancy
The clinical features of ZIKV infection among pregnant women in the JZC have been described. They have been used to assess the sensitivity of the current standard clinical case definitions, and to investigate whether adverse foetal outcomes are more likely to occur among pregnant women with symptomatic ZIKV infection during pregnancy compared with asymptomatic infection.
Foetal outcomes after congenital ZIKV exposure
This includes studies comparing the incidence of negative foetal outcomes, namely low birth weight, small-for-gestational age, prematurity and foetal death among ZIKV-exposed and unexposed women. Studies have also looked at the prevalence of Chikungunya IgG among mothers who had foetal losses.
Congenital Zika syndrome
The spectrum of congenital Zika virus syndrome in the JZC children has been explored as well as visual acuity alterations among ZIKV exposed babies, including among dizygotic twins.
The placenta
Placental histological findings among ZIKV exposed infants with microcephaly have been reported as well as placental histological findings among mothers with Chikungunya and Dengue infection.
Environmental risk factors, prevention, educational and vector control activities
These studies have included investigations into the environmental risk factors for ZIKV infection, assessment of the peri-domicile environment of women in the JZC and identification of favourable conditions for replication evaluation of educational activities for children to help combat the proliferation of Aedes aegypti and assessment of the knowledge around the modes of transmission and prevention of ZIKV infection as well as the practice of preventative measures among pregnant women in the cohort.
Susceptibility of ZIKV infection of neural progenitor cells among dizygotic twins
Analysis of neural progenitor cells (NPCs) of dizygotic twins discordant for CZS have shown that the development of CZS depends on the intrinsic susceptibility of the NPCs.17
Seroepidemiological arbovirus studies
Studies quantifying the seroprevalence of ZIKV, Chikungunya and Dengue IgG antibodies among pregnant women in the JZC have been carried out.
Of note, unless referenced, these manuscripts are awaiting final publication.
Strengths and limitations
The JZC is one of the few prospective Zika cohort studies that has recruited both asymptomatic and symptomatic women and therefore benefits from having a large control group. It also provides the necessary study population to carry out analyses on ZIKV symptomatology. Women were recruited over more than a 1 year period of time (March 2016 to August 2017) and therefore seasonality can be explored. The diversity and frequency of biological samples collected from the women during pregnancy (and after), as well as their children, mean that JZC now has a rich and invaluable biorepository of clinical material. The high-risk profile of the pregnant women also provides an additional unique opportunity to study other factors that may contribute to, or potentially be protective for, the development of negative sequelae associated with ZIKV exposure. The JZC implemented the use of standardised WHO research method tools, as soon as they were available, and therefore has placed itself in an optimal position to collaborate in Brazilian and international consortia that will ultimately be aiming to perform meta-analyses on all Zika cohort study data.
The limitations of the JZC in part relate to the pressing nature of the ZIKV and microcephaly epidemic and the urgency to start the investigation. The JZC, like many other Zika studies, commenced without any formal funding. Recruitment and data collection commenced in paper form, before formal data management systems could be put in place. In addition, as WHO standardised research protocols were produced after the start of the investigation, some variables contained in the WHO protocol were not in our original questionnaire and therefore some of this data is missing for the earliest recruits in our cohort. As this cohort was built in the midst of the ZIKV epidemic with limited financial resources, the scientific leadership team opted to prioritise the testing of urine samples by RT-PCR due to its wider window of detection (eg, up to 2 weeks in urine vs 1 week in serum).16 18 Although important tests (eg, IgG and IgM assays and plaque reduction neutralization test (PRNT)) and the infrastructure required to perform them have continued to be too costly for testing using available resources, the relevant serum samples have been stored in a secure biorepository for future evaluation on procurement of additional funding. An additional limitation related to testing is the possibility of misclassification among a minority of the ZIKV-positive newborns who may have been infected postnatally during the first 10 days of life. The choice of study population (high-risk pregnant women) had several advantages as stated above. However, there are also a few drawbacks that should be highlighted. First, because women were not recruited based on a suspicion of having been exposed to ZIKV, the prevalence of ZIKV RT-PCR positivity was relatively low, and this equated to small numbers for estimating absolute and relative risks. Furthermore, because high-risk pregnant women are at higher risk of developing some of the outcomes of interest, this may have reduced the power for us to detect differences in frequency of outcomes between Zika-exposed and unexposed dyads. The differential follow-up of women who were symptomatic for ZIKV infection in terms of a more intensive antenatal scanning schedule prioritised women with clinical ZIKV infection (consistent with the WHO definition)11 to try to improve our understanding of the sequence of events that occur in utero after ZIKV infection. As we were unable to offer monthly ultrasounds to all the women in the cohort, this differential follow-up may have also had consequences in terms of the detection of problems in the antenatal period. With regards to the neonatal outcomes, the loss to follow-up that we inevitably experienced may also reduce the power for us to detect differences in the frequency of outcomes between Zika-exposed and unexposed neonates.
Collaboration
The JZC headquarters is at the Jundiaí Medical School under the direction of Professor Saulo Duarte Passos. The cohort has a Facebook page: https://www.facebook.com/zikacoortejdi/ which contains information about the functioning and organisation of the JZC, fundraising events, support for Zika affected families and media coverage. Any researcher wanting to use JZC data must apply to the Jundiaí Zika Cohort Group via Professor Passos (sauloduarte@uol.com.br).
Acknowledgments
The authors would like to thank all the patients and patient advisers involved in the Jundiaí Zika Cohort as well as all the research staff without whom this project would have never been possible: Alexandra Siqueira Melo, Anderson Pereira Soares, André Prado Grion, Andrea Cristina Botelho Silva, Antônio Fernandes Moron, Clóvis Antônio Lopes Pinto, Cristiane Martins, Danila Soares Tambalo, Diego Lima, Dora Fix Ventura, Eduardo Massad, Eduardo Roberto Bagni, Fabiana Martins Soares de Souza, Fernanda Cangerana, Fernanda Guerra Velasco, Fernando N. Arita, Francisco del Moral Hernandez, Geovanne Ribeiro dos Santos, Hérbene José Figuinha Milani, Heydi Segundo Tabares, Juliana Paula Gomes de Almeida, Kallene Vidal, Lucas Rodrigues Laranja, Luiz Baran, Magda Maria Sales Carneiro Sampaio, Mayana Zatz, Marcelo Costa, Márcia Borges Machado, Margareth Martha Arilha Silva, Maria de Fátima Rizzo, Marielton P. Cunha, Max Damico, Mirella Barboni, Mirella Nayane B. Leite, Patricia Carvalho Loiola, Paolo Marinho de Andrade Zanotto, Rafael Izbicki, Renata Chrystina Bianchi de Barros, Rita de Cássia Aguirre B. Dezena, Renato Pereira de Souza, Sandra Helena Alves Bonon, Shahab Zaki Pour, Sergio Rosemberg, Sergio Vranjec, Silvia Maria Ribeiro Oyama, Stéphanno Gomes Pereira Sarmento, Tânia Mendes Quintella, Tânia Ritti Ferraretto, Tathiana Ghisi de Souza, Thamirys Cosmo Gillo Fajardo, Valtenice França, Waldinei Merces Rodrigues.
Footnotes
Contributors: NSC, MR, APP, REG, DV: Conceptualisation, data curation, formal analysis, methodology, investigation, validation, visualisation, writing - original draft and review & editing. EBB: Methodology, resources, supervision, validation, visualisation, writing - review & editing. MFA: Formal analysis, funding acquisition, methodology, resources, supervision, validation, visualisation, writing - review & editing. SDP: Conceptualisation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation.
Funding: The Sao Paulo Research Foundation (FAPESP) currently provide core funding for the JZC (grant number 2016/08578-0). The London School of Hygiene and Tropical Medicine provided initial seed funding (grant number ER1605). EBB received funding from the: Wellcome Trust & the UK’s Department for International Development (205377/Z/16/Z; https://wellcome.ac.uk/) and the European Union’s Horizon 2020 research and innovation program (https://ec.europa.eu/programmes/horizon2020/) under ZikaPLAN grant agreement No. 734584 (https://zikaplan.tghn.org/). NSC was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES-Brazil).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data that support the findings of this study are available upon reasonable request from the The Jundiai Zika Cohort Group (email: adm.projetozika@hufmj.com.br). The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Map disclaimer: The depiction of boundaries on the map(s) in this article do not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
References
- 1. Secretaria de Vigilancia em Saude - Ministerio da Saude – Brasil. Boletim Epidemiologico. Brasilia: Ministry of Health. Brazil, 2016. http://portalarquivos2.saude.gov.br/images/pdf/2016/setembro/16/2016-028-Dengue-SE32.pdf (26 Jun 2018). [Google Scholar]
- 2. WHO. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. Geneva, 2016. [Google Scholar]
- 3. Brazilian Institute of Geography and Statistics (IBGE). População. Cidades. Jundiai. Panorama, 2017. https://cidades.ibge.gov.br/brasil/sp/jundiai/panorama (20 Nov 2017). [Google Scholar]
- 4. UNESCO. Atlantic Forest South-East Reserves, 2018. https://whc.unesco.org/en/list/893 (26 Jun 2018).
- 5. Unidade de Gestão de Promoção da Saúde. Perfil dos Partos em Jundiaí 2018. [Google Scholar]
- 6. NIH. NIH - Eunice Kennedy Shriver National Institute of Child Health and Human Development: High Risk Pregnancy, 2017. https://www.nichd.nih.gov/health/topics/high-risk (27 Mar 2018). [Google Scholar]
- 7. Ministério da Saúde (BR). Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 41 de 2017. Brasilia: Ministério da Saúde, 2018. http://portalarquivos2.saude.gov.br/images/pdf/2017/novembro/07/2017-035-Monitoramento-dos-casos-de-dengue-febre-de-chikungunya-e-febre-pelo-virus-Zika-ate-a-Semana-Epidemiologica-41.pdf. [Google Scholar]
- 8. Kelsey JL, Whittemore AS, Evans AS, et al. ; Methods in Observational Epidemiology. 2nd ed Oxford University Press; 1996. [Google Scholar]
- 9. Teixeira JA, Castro TG, Grant CC, et al. . Dietary patterns are influenced by socio-demographic conditions of women in childbearing age: a cohort study of pregnant women. BMC Public Health 2018;18:301 10.1186/s12889-018-5184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DATASUS - Departamento de Informatica do SUS. Informacoes de Saude - Estatisticas vitais - nascidos vivos. Brasilia: Ministry of Health, 2015. http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinasc/cnv/nvsp.def (27 Mar 2018). [Google Scholar]
- 11. WHO. Zika Virus Disease - Interim Case Definition Emergency preparedness. Geneva, 2016. [Google Scholar]
- 12. World Health Organisation. Harmonization of ZIKV Research Protocols to Address Key Public Health Concerns. Geneva, 2016. http://www.who.int/reproductivehealth/zika/ZIKV-Protocol-Summary-for-WHO-v4-2.pdf (07 Oct 2016). [DOI] [PubMed] [Google Scholar]
- 13. Villar J, Cheikh Ismail L, Victora CG, et al. . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384:857–68. 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 14. Intergrowth-21st. INTERGROWTH-21st Newborn Size at Birth Chart. 2015. https://intergrowth21.tghn.org/articles/intergrowth-21st-newborn-size-birth-chart/ (07 Oct 2016).
- 15. Intergrowth-21st. Newborn biometry by Intergrowth-21st standards/references. 2018. Online calculator http://intergrowth21.ndog.ox.ac.uk.
- 16. Lanciotti RS, Kosoy OL, Laven JJ, et al. . Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caires-Júnior LC, Goulart E, Melo US, et al. . Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat Commun 2018;9:475 10.1038/s41467-017-02790-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bingham AM, Cone M, Mock V, et al. . Comparison of Test Results for Zika Virus RNA in Urine, Serum, and Saliva Specimens from Persons with Travel-Associated Zika Virus Disease - Florida, 2016. MMWR Morb Mortal Wkly Rep 2016;65:475–8. 10.15585/mmwr.mm6518e2 [DOI] [PubMed] [Google Scholar]
- 19. de Abreu RL. Mapa de localização do Município de Jundiaí no Estado de São Paulo, Brasil. Wikipedia, 2006. [Google Scholar]
- 20. Zanluca C, Melo VC, Mosimann AL, et al. . First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015;110:569–72. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ECDC. Rapid Risk Assessment. Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barre syndrome. Stockholm, 2015. [Google Scholar]
- 22. Ministério da Saúde (BR). Nota à imprensa: Ministério da Saúde confirma relação entre vírus Zika e microcefalia. Brasilia: Ministério da Saúde, 2015. http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/21014-ministerio-da-saude-confirma-relacao-entre-virus-zika-e-microcefalia (27 Mar 2018). [Google Scholar]
- 23. PAHO. Zika suspected and confirmed cases reported by countries and territories in the Americas Cumulative cases, 2015-2016. Washington DC: PAHO/WHO, 2016. [Google Scholar]