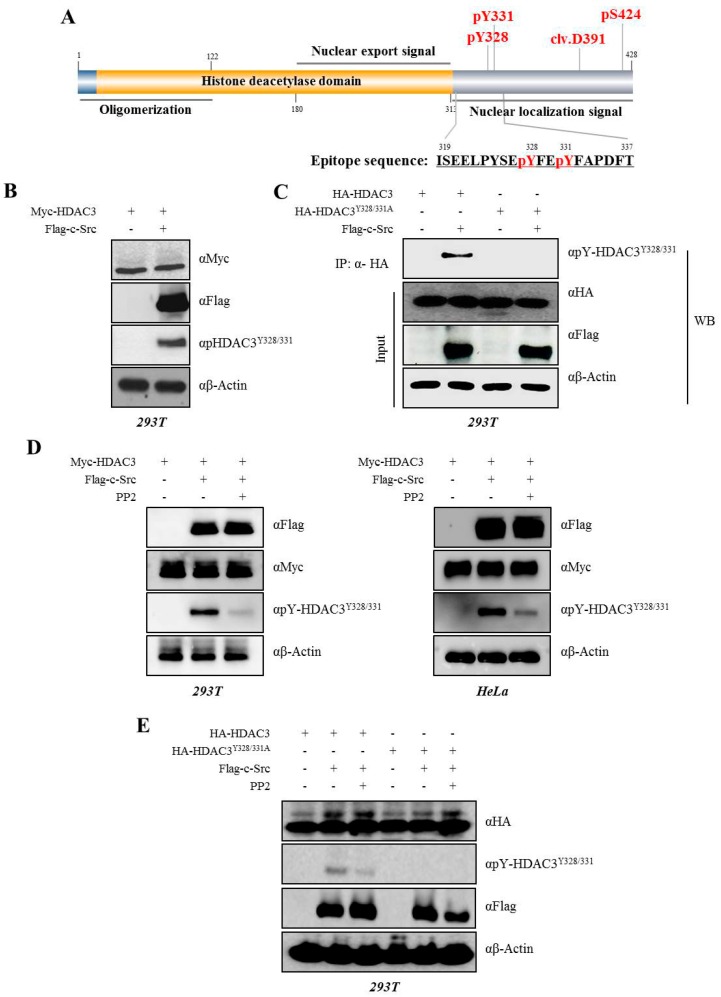
Figure 2.
c-Src phosphorylates Y328 and Y331 on HDAC3 in 293T cells. (A) Modified peptide sequence for the generation of phospho-HDAC3Y328/331 antibody. Schematic diagram of human HDAC3 protein with multiple functional domains. Residues 1 to 122 are necessary for HDAC3 oligomerization. The actual functional nuclear export signal and nuclear localization signal reside between residues 180–313 and 313–428, respectively. pS424, the phosphorylation site of serine 424 on HDAC3; clv.D391, cleavage site of aspartate 391 on HDAC3. (B) c-Src phosphorylates HDAC3 in 293T cells. Myc-tagged HDAC3 was transiently transfected with/without Flag-tagged c-Src in 293T cells. After 48 h, cells were harvested and cell lysates were analyzed by Western blotting with indicated antibodies. (C) Y328 and Y331 are important for the c-Src-mediated phosphorylation of HDAC3. Either HA-HDAC3WT or HA-HDAC3Y328/331A plasmid was transfected with/without Flag-c-Src into 293T cells. After 48 h, various HDAC3 plasmid samples were immunoprecipitated with HA antibody and analyzed by Western blotting with indicated antibodies. (D) The c-Src-mediated phosphorylation of Y328 and Y331 on HDAC3 was interfered by PP2, which is a c-Src inhibitor. Myc-HDAC3 was transfected with/without Flag-c-Src into 293T (left panels) and HeLa (right panels) cells, followed by the treatment of cells with 10 µM of PP2 for 30 min before harvest. Cell lysates were analyzed by Western blotting with indicated antibodies. (E) Either HA-HDAC3WT or HA-HDAC3Y328/331A plasmid was transfected with/without Flag-c-Src into 293T cells, followed by the treatment of cells with PP2, a c-Src inhibitor, at 10-µM concentration for 30 min before harvest. Cell lysates were analyzed by Western blotting with indicated antibodies.