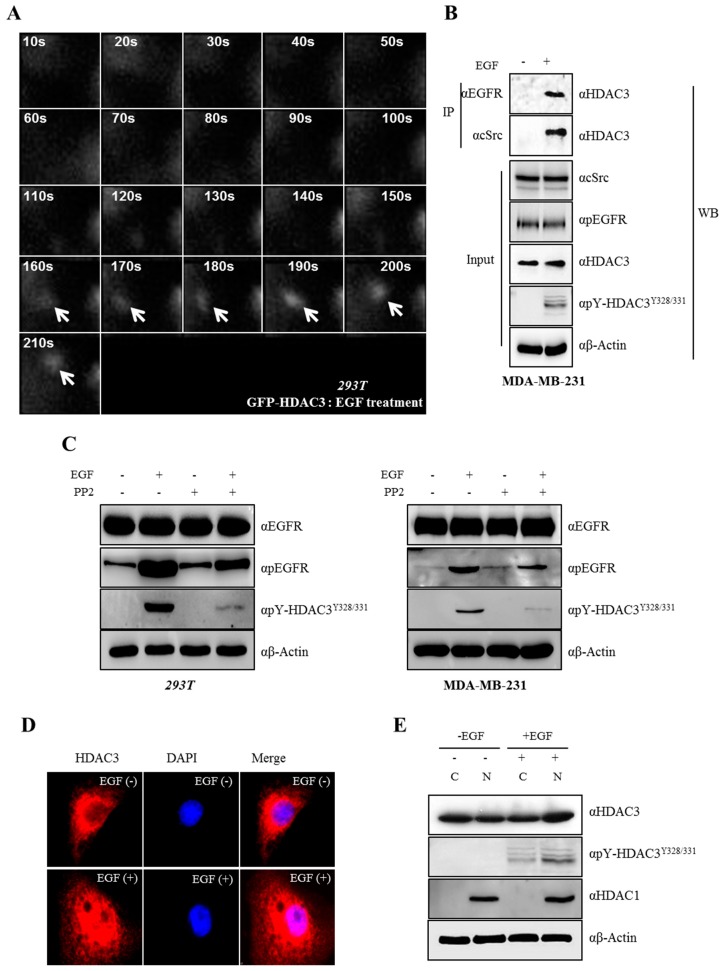
Figure 5.
Epidermal growth factor (EGF) triggers phosphorylation and induces the relocalization of HDAC3. (A) HDAC3 is recruited to the c-Src–EGFR complex for c-Src-mediated phosphorylation upon EGF treatment. The enhanced green fluorescence protein (EGFP)–HDAC3 plasmid was transfected into 293T cells, followed by the treatment of cells with EGF. The GFP–HDAC3 single molecule was visualized using total internal reflection fluorescence (TIRF) microscopy. Images were captured at every minute for 210 s. Arrows indicate the HDAC3 molecule. (B) EGFR–c-Src–HDAC3 forms complexes in the presence of EGF. EGF was treated for 20 min in MDA-MB-231 cells, membrane protein was extracted following the manufacturer’s instruction (see materials and methods), and then immunoprecipitation was carried out with the indicated antibodies. (C) EGF induces the phosphorylation of Y328 and Y331 on HDAC3. 293T (left panel) or MDA-MB-231 cells (right panel) were treated with EGF in the presence or absence of PP2 for 20 min. Cell lysates were analyzed by Western blotting with indicated antibodies. (D) EGF-induced phosphorylation of HDAC3 is required for its nuclear translocation. Cells were treated with EGF for 20 min. Immunofluorescence analysis was performed. (E) Phosphorylation of HDAC3Y328/331 affects the translocation of HDAC3. Cells were treated with EGF for 20 min, and then fractionized into the cytosol and nucleus; then, Western blot assays were performed with the indicated antibodies. HDAC1 was used as a positive control for the nuclear fraction.