Abstract
Obesity is a global pandemic and it is well evident that obesity is associated with the development of many disorders including many cancer types. Breast cancer is one of that associated with a high mortality rate. Adipocytes, a major cellular component in adipose tissue, are dysfunctional during obesity and also known to promote breast cancer development both in vitro and in vivo. Dysfunctional adipocytes can release metabolic substrates, adipokines, and cytokines, which promote proliferation, progression, invasion, and migration of breast cancer cells. The secretion of adipocytes can alter gene expression profile, induce inflammation and hypoxia, as well as inhibit apoptosis. It is known that excessive free fatty acids, cholesterol, triglycerides, hormones, leptin, interleukins, and chemokines upregulate breast cancer development. Interestingly, adiponectin is the only adipokine that has anti-tumor properties. Moreover, adipocytes are also related to chemotherapeutic resistance, resulting in the poorer outcome of treatment and advanced stages in breast cancer. Evaluation of the adipocyte secretion levels in the circulation can be useful for prognosis and evaluation of the effectiveness of cancer therapy in the patients. Therefore, understanding about functions of adipocytes as well as obesity in breast cancer may reveal novel targets that support the development of new anti-tumor therapy. In this systemic review, we summarize and update the effects of secreted factors by adipocytes on the regulation of breast cancer in the tumor microenvironment.
Keywords: adipocytes, breast cancer, obesity, adipokines, hormones, tumor microenvironment
1. Introduction
It is reported that breast cancer is one of the leading cancer types with a high mortality rate, besides lung and skin cancer [1,2]. Among the 36 most common cancer types, breast cancer accounts for 11.6% of incident cases, followed by 6.6% of cancer death in 2018 [2]. In China, the proportional rate of breast cancer has been rapidly increasing and associated with socioeconomic status [3]. Incident rates in urban areas are twice higher than in rural areas, with rates at 0.034% and 0.017%, respectively [3]. In Brazil, about 0.06% of the female population was newly diagnosed with breast cancer in 2018. Japan lists the highest rate with about 0.043% (the data was recorded in 2009) [4]. According to U.S. breast cancer statistics, it was estimated that about 12% of females had a high potency on the development of breast cancer in their life. The number of incidental breast cancer cases will reach 268,600 and more than 41,000 people are predicted to die in 2019 [1]. There are approximately 3.1 million people having breast cancer who are currently being treated, as of January 2019 [2].
Several risk factors have been identified that contribute to the development of breast cancer, including both genetic as well as non-genetic factors. In most cancer cases, environmental factors combined with genetic factors play a major impact on the development of breast cancer [5]. Evidently, obesity has been related to the initiation, development, and mortality of many cancers, especially breast cancer [6,7,8,9,10,11]. A large cohort study reported that BMI was linked to 17 out of 22 most common cancers in UK adults, including uterus, thyroid, gallbladder, kidney, cervix, colon, ovarian, and post-menopausal cancers [12]. In women, obesity increases the risk of death from breast and reproductive organs cancer [13,14,15,16]. A recent finding suggested that high BMI can reversibly regulate breast cancer in pre- and post-menopausal women [17]. High BMI induces breast cancer in pre-menopausal women but inversely in post-menopausal women. Similarly, 34 other studies also confirmed the correlation between high BMI and the risk of breast cancer in post-menopausal women [18]. Additionally, a recent cohort study has shown that there is a 52% increase in the development of breast cancer in post-menopause women by obesity [19]. Moreover, the mortality rate caused by breast cancer is much higher in obese people compared to lean people [11]. Obesity is linked to a higher cost of hospital care after chemotherapy and breast surgery [20]. Additionally, several studies have demonstrated that high weight gain is more important than BMI value for breast cancer development, and weight loss may reduce the incident rate in post-menopause women [4]. However, 35% of breast cancer patients reported increasing their body weight from 1.4 to 5.0 kg during and after breast cancer treatment [11,20,21,22,23]. Overall, in addition to the risk of hypertension and cardiovascular diseases, obesity is of high concern in cancer, especially in breast cancer patients.
Adipocytes are the major cellular components of adipose tissue and play an important role in maintaining the energy balance. Any dysfunction in adipocyte function or the energy balance leads to overweight and obesity. There are three types of adipocytes classified based on the origin, structure, and function including white, brown, and brite adipocytes [24,25,26,27]. In general, white adipocytes are the main storage of energy, whereas brite/brown adipocytes with higher expression levels of uncoupling protein 1, a mitochondrial proton carrier (UCP1), can generate heat by thermogenesis process [24,25,26,27]. Dysfunctional adipose tissue can secrete excessive fatty acid, cholesterol, triglycerides, hormones, and adipokines that are linked to metabolic dysfunction, insulin resistance, and inferior outcome in cancer treatment [28,29,30,31]. There are more than 600 proteins released by adipose tissue, in which about 50 adipokines are produced mainly by adipocytes, which provides a novel pool of biomarkers for the study of metabolic diseases [32]. Accumulated fatty acids will be up-taken into the tumors and generate energy for tumor development through β-oxidation. Fatty acids are the major source of ATP in the tumor. Moreover, insulin resistance and high glucose level in serum can provide nutrients for cancer cell invasion [29,33]. Additionally, adipocytes can contribute to the resistance of the chemotherapeutic drug. In co-culture with fibroblast, adipocytes can switch-off anti-cancer drug effects by metabolizing the drug into a less effective secondary [34]. Adipocytes also release extracellular matrix proteins and recruit other neighbor cells such as macrophages and other immune cells, mimicking the immune infiltrates of the tumor [35].
Thus, fat mass and adipose-tissue mass are strongly correlated with obese status and breast cancer in post-menopausal women. Adipocytes can regulate the tumor microenvironment via secreting energy nutrients which contributes to the risk of breast cancer incidence, proliferation, and metastasis. Beside other obesity-related complications, physical and mental consequences are strongly associated with cancers incidence and proliferation [6,36]. Therefore, it is necessary to understand how adipose tissues and adipocytes can contribute to the risk of breast cancer and mortality.
2. Adipocytes Regulate Breast Cancer via Their Metabolic Substrates
Obesity is strongly related to dysfunctional metabolism in adipocytes leading to several chronic diseases. High levels of free fatty acids, cholesterol, glycerol, and triglycerides get accumulated in serum to impact on the breast tumor initiation, development, and migration process (Table 1) [9,37,38,39,40,41,42,43,44,45,46,47,48,49]. In vitro, co-culture of mature adipocytes with breast cancer cells increased the breast cancer proliferation, that strongly supports the notion that adipocytes directly impact cancer cells by their secreted factors [50].
Table 1.
Adipocytes regulate breast cancer via their metabolic substrates.
| Metabolic Substrates | Released by White/Brite/Brown Adipocytes | Effect on BC Development | Effect on BC Cell Proliferation | Effect on BC Cell Invasion | References | |
|---|---|---|---|---|---|---|
| Free fatty acids | Saturated; (n-6) fatty acids | White | Increase | Increase | Increase | [46,47,53,57,58,59] |
| (n-3) fatty acids | White | Decrease | Decrease | Decrease | [43,45,49] | |
| Lipids, Triglycerides | White | Increase | Increase | Increase | [61] | |
| Cholesterol | Total | White | Increase | Increase | Increase | [60,62,63] |
| HDL | White | Decrease | Decrease | Decrease | [44] | |
| LDL | White | Increase | Increase | [44] | ||
| VLDL | White | Increase | Increase | Increase | [44] | |
| 27-OHC | White | Decrease | Decrease | [41,53] | ||
| Exosome | mir- 3184-5p |
White | - | Increase | Increase | [50] |
| mir- 184c-3p |
White | - | Decrease | Decrease | [50] | |
| Proteases (MMP-9, MMP-11) | White | Increase | Increase | Increase | [74,75,76] | |
Note: 27-Hydroxycholesterol, 27-OHC; matrix metalloproteinase-9, MMP-9; high-density lipoprotein, HDL; low-density lipoprotein, LDL; very-low-density lipoprotein, VLDL; breast cancer, BC.
Free fatty acids (FFA) are released from daily meals, which deposit as lipid droplets in the adipose tissue. Both saturated and unsaturated fatty acids are released from high-fat diet or in original obese status [51,52]. Inflammation-induced obesity is an essential mechanism in the development and invasion of breast cancer [28,38,39]. Saturated fatty acids can activate toll-like receptor 4 to amplify inflammation and contribute to angiogenesis and tumor progression [53]. Inflamed microenvironment promotes adipocyte cell death, recruits macrophages, and forms a crown-like structure (CLS) [28]. The number of CLS is nine times higher in cancer patients with obesity or overweight than lean breast cancer women (70% and 8.3%, respectively) [54]. In addition, the numbers of CLS are related to poor prognosis and the development of breast tumors [55]. A study demonstrates that induced inflammation in white adipocyte tissue and increased CLS reduced the survival rate in patients [55]. Furthermore, saturated fatty acids can activate NF-κB, leading TNF-α production that acts on the breast cancer cell proliferation, invasion, and metastasis [46,56]. Beside saturated fatty acids, unsaturated fatty acids also contribute to breast cancer progression and prognosis. Long-chain (n-6) fatty acids (linoleic acid) are well-known as pro-inflammatory factors, which induce the development of breast cancer [47], whereas other fatty acids reversely impact on the breast cancer patients [43,45,49]. FFAs induce breast cancer invasion by activating the epidermal growth factor receptor, GTP-binding protein, and protein kinase C pathway [57], and by controlling cell proliferation via phosphatidylinositol 3-kinase (PI3K) [58] and cell migration through free fatty acid receptor 1 and 4 and AKT pathway activation [59]. In vitro, David et al. stimulated breast cancer cells proliferation by supplement of FFAs on serum-free medium in a dose-dependent manner, linoleic acid can stimulate breast cancer cell growth at three times higher concentration than oleic acid (0.75 µg/mL) [47]. In contrast, Basil et al. also demonstrated that FFAs can induce apoptosis and lipid peroxidation in the human breast cancer cell culture [49]. Thus, supplement fish oil is proposed for pre-menopausal women to eliminate the risk of breast cancer.
In obesity, cholesterol is significantly induced and released into the bloodstream. However, whether the total cholesterol or high-density lipoprotein (HDL) and low-density lipoprotein (LDL) influence breast cancer growth remains elusive. A meta-analysis study has shown the inverse correlation between total cholesterol and HDL to the risk of breast cancer [60]. Both LDL and very-low-density lipoprotein (VLDL) exposure stimulates the development, migration, and invasion of breast cancer cell through activation PIK3/AKT pathway, especially VLDL promotes lung metastasis and angiogenic activity on the breast cancer cells [44]. Furthermore, hyperlipidemia positively associated with breast cancer risk and total survival rate regardless of BMI [61]. An analysis with over 664,000 women revealed that women above the age of 40 with high serum cholesterol have a 45% higher risk of development of new breast cancer and 40% lower the survival rate and treatment outcome of developed breast cancer [62]. Co-treatment with statins to lower cholesterol level showed the improvement in total survival rate and cancer-specific survival in cancer patients [63]. However, the protective effects of statin are still being considered. A recent meta-analysis study, which collected health data from over 823,000 post-menopausal volunteers, showed a similar invasive rate between former, current, and non-users of statin during treatment of breast cancer [64]. Oxysterol 27-hydroxycholesterol (27-OHC) is known as the metabolite substrate of cholesterol by Cytochrome P450 Family 27 Subfamily A Member 1 (CYP27A1) enzymes. High level of serum cholesterol corresponds to a high level of serum 27-OHC [42,65]. 27-OHC plays as a liver X factor [37] (LXR) and estrogen receptor (ER) agonist [66]. LXR activation lowers cholesterol accumulation and suppresses cell growth in both normal and breast cancer cells [53,67]. Whereas ER activation promotes breast cell proliferation in estrogen receptor-positive breast cancer (ER+) cells but not in negative cell types [41]. Considering the impacts of 27-OHC on ER+ breast cancer cells, Lu et al. conducted a case-control study and demonstrated that treatment 27-OHC on breast cancer differs by menopausal status. The opposite effects are seen in the pre-menopausal and post-menopausal status [68]. Thus, it is necessary to conduct additional studies on the effect of 27-OHC on breast cancer.
Additionally, adipose tissue also releases exosomes. Adipocyte exosome contains proteins related to fatty acid oxidation (FAO) [48]. Obesity induces exosomes modulating FAO and further contributes to tumor migration [48,69]. Other investigations also demonstrated that microRNAs that are released from adipose exosomes can support breast cancer tumor growth and invasive capacity [50,70]. Among 98 secreted miRNAs, miR-3184-5p is the most upregulated, whereas miR-181c-3p is the most downregulated one in breast cancer. Both target on FOXP4 and PPARα [50]. There are eight miRNAs associated with BMI value, where miR-191-5p, miR-17-5p were identified involving in tumor progression. In detail, miR-191-5p upregulated by 17β-estradiol protected ERα-positive tumors against apoptosis. miR-17-5p was inversely the impact of inflammatory cytokines, resulting in suppress tumor growth [71]. A recent paper has reported miR-144 and miR-126 secreted by adipocytes induced brown differentiation and tumor progression [72].
Matrix metalloproteinases (MMPs) family is known to play vital roles in the invasion and metastasis of tumor cells. MMP-9 is highly expressed in breast tumors compared to normal tissue. A high level of MMP-9 has been reported to have a positive correlation to brain metastases in breast cancer patients [73,74,75]. MMP-11 and MMP13 expression were also promising markers linked to the poor outcome of breast cancer which may be a novel target for the treatment of breast cancer [76].
3. Adipocytes Regulate Breast Cancer via Their Released Hormones
Obesity also affects breast cancer in women by secretion of some adipokines, which are also called as “released hormones”, especially estrogen, adiponectin, leptin, and insulin (Table 2) [77,78,79]. Estrogen and estrogen derivatives are significantly induced in post-menopausal obese women with increased BMI [80,81,82]. A random evaluation of post-menopausal women reported that there was a 35% higher estrogen level and 130% higher estradiol level in obese people compared to the low BMI/athletic ones [81]. Estrogen is mostly produced in the ovary in pre-menopausal women, but in adipose tissues in post-menopausal women [83]. Cytochrome P450 aromatase, catalyzed to biosynthesized estrogen by converting androgens to estrogens, is highly expressed in adipose tissue [84]. Therefore, estrogen levels in breast tumors associated with adipose tissue are 10 times higher than in the blood [85]. In addition, cytokines produced in adipose tissues can also promote cytochrome P450 aromatase secretion to induce estrogen biosynthesis [86,87,88]. Estrogen receptors (ERs) are important for estrogen function, ERα is linked to tumor cell proliferation whereas ERβ contributes to a favorable prognosis [89]. ERα positivity is related to obesity-induced breast cancer especially in post-menopausal women [90,91]. Estrogen-ERs complex, stabilized with a co-activator protein, interacts with estrogen response element site on DNA, leading to the regulation gene expression related to growth, differentiation, and other dysfunctions [92]. This complex can also bind to the promoter region to modify that gene expression [93]. Furthermore, estrogen can act by mediating the ER-membrane pathway including GPCR-like protein, G proteins, MAPK/ERK, and PI3K/AKT pathway [94,95,96]. These signaling pathways contribute to the proliferation and survival of breast cancer via increasing Bcl-2, cyclin D1, number of G0/G1 cells [97,98,99]. Lastly, estrogens promote breast cancer invasion and migration. In vitro, estrogen treatment remodels cytoskeleton and acts on GPCR-like protein and estrogen receptor signaling pathway to induce metastatic on breast cell [100,101].
Table 2.
Adipocytes regulate breast cancer via their released hormones.
| Hormone | Released by White/Brite/Brown Adipocytes | Effect on BC Development | Effect on BC Cell Proliferation | Effect on BC Cell Invasion | Reference |
|---|---|---|---|---|---|
| Estrogen | White | Increase | Increase | Increase | [97,98,99,100,101] |
| Adiponectin | White | Decrease | Decrease | Decrease | [108,109,110,111,112,128] |
| Leptin | White | Increase | Increase | Increase | [111,112,113,114,118,119,128,132] |
| Insulin | White | Increase | Increase | Increase | [40,120,121,125,126] |
| Visfatin | White | Increase | Increase | Increase | [128] |
| PAI-1 | White | Increase | Increase | Increase | [130,133] |
| Resistin | White | Increase | Increase | Increase | [128,129,134] |
| White | Decrease | Decrease | Decrease | [135] |
Note: Plasminogen activator inhibitor-1, PAI-1; breast cancer, BC.
Adiponectin is secreted from white adipocyte tissue before binding to specific receptors to act on insulin resistance, glucose uptake, and FFA oxidation. However, obese people have a lower expression of adiponectin receptors, that can cause adiponectin resistance [102]. In the report published in 2004, breast cancer women who have low serum adiponectin may have a higher risk of angiogenesis and metastasis [103]. In contrast, high adiponectin level can lower the incident rate of breast cancer in women [104]. However, a recent stratified case-control study revealed that adiponectin levels are not associated with breast cancer in pre-menopausal women but negatively affected post-menopausal women [105]. Adiponectin reduces insulin resistance and inflammation properties in obesity, which can improve breast tumor microenvironment [106,107]. In contrast to leptin, adiponectin decrease proliferation and stimulate the cell death program in breast cancer cells by several pathways including AMP-activated protein kinase, mTOR, and NF-κB pathways [108,109,110]. Morad et al. conducted an in vivo test in human breast tissue showing estrogen treatment increased leptin secretion, whereas tamoxifen treatment increased adiponectin and adiponectin/leptin ratio after six weeks treatment [111]. Adiponectin treatment reduces tumor size in ER-negative breast cancer but induces in ER-positive breast cancer because of the strong effects of estrogen on breast cells [112]. In summary, adiponectin can be considered for breast cancer therapy in combination with other drugs.
The adipokine, leptin is strongly accumulated in obesity and promotes the development of breast tumors [113,114]. Leptin acts directly on the leptin receptors, leading to inflammation in adipocyte microenvironment and increases the risk of metastatic potential [115]. Thus, the impact of leptin depends on site of leptin receptors expression. Distant leptin-derived metastatic in breast cancer is associated with 34% in the leptin receptor-positive patients, but none in the leptin receptor-negative patients [116]. Leptin activates ERα to stimulate estrogen-related breast cancer pathway, resulting in the proliferation and migration [117]. Furthermore, leptin can increase aromatase activity at high concentration and induce estradiol and estrogen in obese women [118]. Mechanically, activation of leptin receptor stimulates downstream ERK1/2, AP1, STAT3, PI3K, and MAPK pathways [117,119]. Additionally, leptin also promotes tumor growth/migration via VEGF signaling (endothelial growth factor signaling), HIF-1α stabilization, which induces hypoxia condition in tumors [119]. The authors also reported that leptin can promote pancreatic cancer burden by inducing MMP-13 production in human [119].
Insulin resistance is most common in obesity and results in hyperinsulinemia. High serum insulin level increases breast cancer growth and invasion [40,120,121] via activation of the PI3K pathway [122], and improved insulin resistance can reduce metastasis in mice [121]. High fasting insulin reduces survival rate, results in poor outcome to anticancer therapy [123], and induces a 2.4 times higher incident rate in post-menopausal women (the results were evaluated by random selection) [40]. In addition, insulin itself induces aromatase activity, which is important to estrogen synthesis in adipose tissue [124]. On the other hand, hyperinsulinemia is highly associated with the amount of insulin-like growth factor 1 (IGF-1) locally, thus, excess IGF-1 in tumor cells [122]. IGF-1 binds to selected receptors (IGF-1R) and upregulates MAPK signaling pathway, resulting in proliferation, development, and progression [125,126]. In the outcome of tamoxifen treatment, IGF-1R is considered to evaluate the effectiveness of therapy. Higher expression of IGF-1R implies poorer outcome and survival rate in breast cancer patients [127].
In addition, other hormones such as visfatin, plasminogen activator inhibitor-1(PAI-1), and resistin slightly contribute to breast cancer initiation and progression. High levels of serum resistin and visfatin were found in post-menopausal women with breast cancer, which correlated with tumor size and a high risk of lymph node metastasis [128]. Resistin promotes breast cancer development via enhanced toll-like receptor 4-mediated transition and NF-κB activation [129]. Secreted PAI-1 by adipose tissue contributes to increase proliferation, angiogenesis, cell migration, and decrease apoptosis, which supports tumor invasion in the breast [130].
Overall, the adipokines significantly contribute to tumor cell proliferation, progression, and migration, which influence the outcome of prognosis in breast cancer patients. Leptin/adiponectin ratio is highly induced in breast cancer, and more interestingly, the ratio correlated to the level of obesity. People with high BMI together with high leptin/adiponectin ratio have a significantly higher risk of breast cancer incidence and metastasis [131].
4. Adipocytes Regulate Breast Cancer via Released Cytokines
Cytokines, generally produced by immune cells, play a vital role in pro-inflammation, anti-infection, and other cellular signaling. Along with white adipocyte, there are immunocytes, vascular, and stromal cells which exist in the adipose tissue, which are involved in the cytokines production (Table 3) [136]. In addition, adipocytes can also secrete several cytokines to regulate the surrounding cells and itself. In vitro co-culture of adipocytes and breast cancer cells results in the secretion of cytokines into the culture supernatant. The findings showed five (IL6, IL8, IFNᵧ-inducible protein 10, CCL2, and CCL5) out of 200 cytokines which were significantly increased after one-week culture, with immature adipocytes having higher cytokine secretion potential than mature adipocytes. Cytokines, produced by immature adipocytes, were reported to increase tumor initiation and metastasis burden in breast cancer [137]. A follow-up study with 534 patients demonstrated IL6, IL8, and IL10 may consider as indicators for poor prognosis and metastatic of breast cancer [138]. Several investigations also emphasized IL6, IL8, CCL2, and CCL5 as stimulators of the survival, proliferation, and invasion of breast cancer cells [139,140,141,142]. Moreover, serum IL6, IL8, and TNFα are related to an advanced stage and metastatic status of breast cancers, whereas IL6 and IL8 are most favorable for prognosis [143].
Table 3.
Adipocytes regulate breast cancer via released cytokines.
| Hormone | Released by White/Bright/Brown Adipocytes | Effect on BC Development | Effect on BC Cell Proliferation | Effect on BC Cell Invasion | Reference | |
|---|---|---|---|---|---|---|
| TNFα | Visceral, subcutaneous white | Increase | Increase | Increase | [132,143,144,145,147,148,149,150] | |
| Interleukins | IL-1b | White | Increase | Increase | Increase | [142,157,158,159] |
| IL-6 | White | Increase | Increase | Increase | [132,138,141,142,143,161] | |
| IL-8 | Visceral, subcutaneous white | Increase | Increase | Increase | [138,139,141,142,143] | |
| IL10 | White | [138] | ||||
| Chemokines | CCL2 | White | Increase | Increase | Increase | [140,142] |
| CCL5 | White | Increase | Increase | Increase | [140,142] | |
| CXCL18 | White | [169,170] | ||||
| CXCL12 | White | Increase | Increase | Increase | [191,192] | |
| CXCL10/IP-10 | White | Increase | Increase | Increase | [174,175] | |
Note: Interleukin, IL; C-X-C motif chemokine 10, CXCL10 or interferon γ-induced protein 10 kDa, IP-10; Tumor Necrosis Factor-alpha, TNFα; C-C Motif Chemokine Ligand 2, CCL2; breast cancer, BC.
High levels of TNFα causes chronic inflammation and insulin resistance, that in turn provides positive effects on the development of cancers [144,145]. TNFα functions by interacting with TNF receptors, including TNFRI and TNFRII [146]. Several studies were conducted to determine the effects of TNFα both in vitro and in vivo experiments and in clinical trials. In vitro, TNFα promotes cell development in both ER-positive and ER-negative breast cancer cell lines [147,148]. In ER-positive cell lines, TNFα inhibits apoptosis, but not in ER-negative cell lines [148]. The authors showed that TNFα can activate NF-κB, ERK, AKT, and JNK pathways which are necessary for cell proliferation [145,147,149,150] and positively modulate estrogen metabolic pathway, leading to high amounts of estrogen in tumors [151]. Furthermore, TNFα also promotes tumor migration by inducing cytokines such as MMP-9 [149] and chemokines receptors CCR9 and CCR5 [152]. In vivo, mice treated with TNFα developed tumors compared to control mice group with PBS treatment [147,150]. Blocking TNFα significantly reduces breast tumor size [153]. In addition, TNFα regulates interleukin synthesis, adiponectin secretion, and aromatase expression in adipose tissue [154,155,156].
Numerous reviews have also reported the strong association between interleukins and breast cancers, notably IL1β, IL6, and IL8 [142]. Firstly, IL1β activates inflammatory NF-κB signaling pathway in the tumor cells to promote the development of breast cancer [157,158]. More importantly, IL1β strongly linked to migration in breast cancer. IL1β increases the phosphorylation of focal adhesion kinases and expression of MMP9 which are related to the adhesion and migration of cancer cells [159]. IL1β also induces cyclooxygenase-2, hypoxia-inducible factor 1α which promotes angiogenesis, inflammation, and metastasis in cancer [158,160]. Secondly, together with TNFα, IL6 is a major inflammation inducer which activates STAT3, resulting in cancer progression [161]. Interestingly, abrogation of STAT3 signaling by knocking out the STAT3 gene did not influence the tumor formation but greatly suppressed the lung metastasis in mice [162]. A recent study reports that IL6 triggers the expression of VEGF promoted cancer cell multiplication [142]. Thus, it is strongly evident that STAT3 regulates metastasis. High circulating IL6 correlated with poorer prognosis and high risk of metastatic burden [163]. In vitro, over-expression IL6 induces genes involved in epithelial-mesenchymal transition and lowers E-cadherin, supporting that IL6 can regulate adhesion and migration of breast cancer cells [164]. Together with other cytokines, IL8 is angiogenic in cancers [139]. IL6 and IL8 promote oncogene Ras transformation, and then actively induce cell development by many signal pathway [137]. IL6, IL8, IL1β, IP10, CCL2, and CCL5 contribute to the activation of Src kinases and signal to NF-κB pathway [137,165]. The patients with better treatment outcome had significantly low levels of IL8 compared to that of pre-treated patients [138].
Chemokines are small protein molecules that regulate leucocyte transportation in response to inflammation and/or homeostatic conditions. Chemokines such as CCL2, CCL5, CCL4, and CXCL8 are also involved in the incidence and development of breast cancer [166,167]. CCL2 and CCL5 induce tumor-associated macrophages (TAMs), thereby inhibiting T-cell activation and promoting angiogenesis in breast tumors [140]. In addition, CXCL18 is highly accumulated in TAMs in breast tumors [168]. CXCL18 induces IL4, IL13, and IL10 in TAMs promoting initiation and invasion of breast carcinoma cells [169,170] via NF-κB and AP-1 pathways [166]. Both CCL2 and CCL4 suppress infiltration of macrophages and immune cells to the tumor site and are related to an advanced stage and poor prognosis [171,172,173]. CXCl10, also called IP10, stimulates the release of other chemokines, tumor progression, and metastasis via CRXR3 and activation of NF-κB signaling pathway [174,175]. Chemokines directly bind to chemokine receptors, alters the expression and/or function of secondary messengers to stimulate angiogenesis, suppress anti-tumor immune cells’ infiltration [166]. Chemokine receptors CXCR1, CXCR2, CXCR4, CXCR5, and CX3CR1 are critical in the recruitment of macrophage in the breast tumor microenvironment. Therefore, anti-chemokine receptors can bring the therapeutic potential for the treatment of breast cancer. In fact, CXCR1 and CXCR2 antagonists are widely researched on the treatment of autoimmune diseases and prevention metastasis cancer. The clinical trials for breast cancer therapy still need further investigations in individual therapy or combination with chemotherapy or immunotherapy [176]. Moreover, tumor microenvironment in breast cancer continuously produces chemokines that may develop to a higher stage of disease and metastasis [166].
Furthermore, adipocytes also impact on the surrounding cells in the breast tumor microenvironment including immune cells, cancer-associated fibroblasts, endothelial cells, and mesenchymal stem cells [177]. Adipocytes can act as immune regulatory cells. Adipocytes release minimal amounts of cytokines that enhance cytokines/chemokines production in immune cells at different levels in the tumor [178,179]. Damage-associated chemicals released by dead and dying adipocytes stimulate recruitment of macrophages and other immune cells, which can be observed by the presence of crown-like structure within adipocytes [31]. In addition, the secreted cytokines can stimulate differentiation of breast cancer mesenchymal stem cells into adipocytes as well as cancer-associated fibroblasts that amplifies the impacts of adipocytes in the tumor microenvironment. Cancer-associated fibroblasts, an important IL-6 source, strongly related to tumor growth and therapy resistance which can be targeted for the development of potential drugs on treatment and prevention of breast cancer [180,181]. CXCL12 secreted by cancer-associated fibroblasts promotes the proliferation of breast tumor cells. The level of serum CXCL12 is associated with a high mortality rate in cancer patients [182,183,184]. Adipocytes surrounding tumor cells exhibit phenotypical changes termed as adipocyte-derived fibroblasts (ADFs). ADFs enhance release fibronectin and collagen I, increase gene expression of adipokines and adipocytokines (TNF-α, IL-6 and IL-1β) which induce invasive abilities of breast tumor cells [185,186]. Among tumor microenvironment, endothelial cells are converted to fibroblast-like cells in the presence of TNF-α. These cells enhance the production of chemokines CXCL1/2 that promote tumor cells survival and metastasis as well as contribute to chemotherapeutic resistance [187]. It was reported that exosomes secreted by preadipocytes regulated early-stage breast cancer via miR140/Sox2/Sox9 pathway which is critical in stem cell renewal, differentiation, and cell migration in the tumor microenvironment [188].
It is true that the number of publications about the effects of brite/brown adipocytes on breast cancer is limited and the impacts of brite/brown adipocytes on breast cancer remain poorly understood. In the tumor microenvironment, brown/brite adipocytes impact positively on breast cancer. Enrichment of those cells in xenograft led to larger tumor size in mice [189]. Brown adipose tissue activity was reported to be significantly higher in breast cancer group, especially in young women with an increase of 25.6% compared to non-breast cancer group [190]. On the other hand, breast cancer cells can promote the differentiation of adipose stem cells in the tumor microenvironment [72].
5. Conclusions
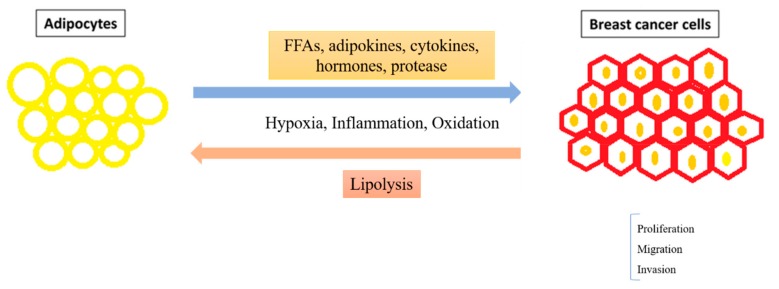
Adipocytes are strongly linked to obesity-driven breast cancer through their secreted metabolic substrates, adipokines, and cytokines (Figure 1). Accumulated FFA, cholesterol, triglycerides, estrogen, leptin, insulin, interleukins, and chemokines together promote breast cancer initiation, proliferation, and invasion. In contrast, the adiponectin secreted by adipocytes is anti-tumorigenic in breast cancer. Evaluation of levels of each soluble factor secreted by adipocytes supports the prediction of the prognosis and anti-cancer treatment efficiencies in patients. Hyperinsulinemia induces insulin resistance in tumor and is linked to lower the IGF-1 expression and aromatase activity resulting in poor prognosis. In addition, obesity induces inflammatory microenvironment by adipocyte-released cytokines to promote cancer cell progression. Leptin, an adipokine, can de-stable HIF-1α and stimulate hypoxia condition in breast tumors. White adipose tissue can produce estrogen by aromatase, which is the most important enzyme for estrogen synthesis in obese post-menopausal women. High levels of estrogen in breast tissue promote cancer development and metastasis. Moreover, the estrogen-progesterone combination as a hormone replacement therapy or contraceptive preparation increases the incidence and mortality rate of breast cancer. Interestingly, tumor cells can regulate lipidation and lipolysis in adipocytes to provide energy and nutrients for tumor development [193]. Therefore, control of body weight, as well as weight gain in menopausal women, is necessary to reduce the risk of breast cancer.
Figure 1.
Adipocytes regulation of breast cancer.
Acknowledgments
We would like to thank Phuong Linh Nguyen (an English editor), Tran Uyen Ngoc (Nong Lam University, Ho Chi Minh, Vietnam) for critical reading and checking to improve the manuscript.
Author Contributions
D.-T.C., T.N.T.P., V.H.P., and T.C.D. conceptualized the manuscript; T.C.D., T.N.T.P., L.B.M., D.-K.T., N.L.B.T., P.T.N., T.-T.N., V.V.T., V.H.P., and D.T.C. drafted the manuscript in equal parts; and D.T.C., V.H.P., V.T.N.N., K.K., and T.C.-D. revised and edited the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DeSantis C.E., Ma J., Sauer A.G., Newman L.A., Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA A Cancer J. Clin. 2017;67:439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Fan L., Strasser-Weippl K., Li J.-J., St Louis J., Finkelstein D.M., Yu K.-D., Chen W.-Q., Shao Z.-M., Goss P.E. Breast cancer in China. Lancet Oncol. 2014;15:e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 4.Kops N.L., Bessel M., Caleffi M., Ribeiro R.A., Wendland E.M. Body Weight and Breast Cancer: Nested Case–Control Study in Southern Brazil. Clin. Breast Cancer. 2018;18:e797–e803. doi: 10.1016/j.clbc.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 5.De Silva S., Tennekoon K.H., Karunanayake E.H. Overview of the genetic basis toward early detection of breast cancer. Breast Cancer Targets Ther. 2019;11:71. doi: 10.2147/BCTT.S185870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu D.-T., Nguyet N.T.M., Dinh T.C., Lien N.V.T., Nguyen K.-H., Ngoc V.T.N., Tao Y., Le D.-H., Nga V.B., Jurgoński A. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;12:1095–1100. doi: 10.1016/j.dsx.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Schottenfeld D., Beebe-Dimmer J.L., Buffler P.A., Omenn G.S. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu. Rev. Public Health. 2013;34:97–117. doi: 10.1146/annurev-publhealth-031912-114350. [DOI] [PubMed] [Google Scholar]
- 8.Picon-Ruiz M., Morata-Tarifa C., Valle-Goffin J.J., Friedman E.R., Slingerland J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA A Cancer J. Clin. 2017;67:378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engin A. Obesity-associated Breast Cancer: Analysis of risk factors. In: Engin A.B., Engin A., editors. Obesity and Lipotoxicity. Springer International Publishing; Cham, Germany: 2017. pp. 571–606. [DOI] [PubMed] [Google Scholar]
- 10.Kolb R., Phan L., Borcherding N., Liu Y., Yuan F., Janowski A.M., Xie Q., Markan K.R., Li W., Potthoff M.J., et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat. Commun. 2016;7:13007. doi: 10.1038/ncomms13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D.S.M., Vieira A.R., Aune D., Bandera E.V., Greenwood D.C., McTiernan A., Navarro Rosenblatt D., Thune I., Vieira R., Norat T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhaskaran K., Douglas I., Forbes H., dos-Santos-Silva I., Leon D.A., Smeeth L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh P., Kapil U., Shukla N.K., Deo S.V.S., Dwivedi S.N. Association of overweight and obesity with breast cancer in India. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2011;36:259. doi: 10.4103/0970-0218.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabiepoor S., Khalkhali H.R., Sadeghi E. What kind of sexual dysfunction is most common among overweight and obese women in reproductive age? Int. J. Impot. Res. 2017;29:61. doi: 10.1038/ijir.2016.46. [DOI] [PubMed] [Google Scholar]
- 15.Veronelli A., Mauri C., Zecchini B., Peca M.G., Turri O., Valitutti M.T., Dall’Asta C., Pontiroli A.E. Sexual Dysfunction Is Frequent in Premenopausal Women with Diabetes, Obesity, and Hypothyroidism, and Correlates with Markers of Increased Cardiovascular Risk. A Preliminary Report. J. Sex. Med. 2009;6:1561–1568. doi: 10.1111/j.1743-6109.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 16.Freeman E.W., Gracia C.R., Sammel M.D., Lin H., Lim L.C.-L., Strauss Iii J.F. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil. Steril. 2007;87:101–106. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 17.Kerr J., Anderson C., Lippman S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017;18:e457–e471. doi: 10.1016/S1470-2045(17)30411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renehan A.G., Roberts D.L., Dive C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute . SEER Stat Fact Sheets: Female Breast Cancer. National Cancer Institute; Bethesda, MD, USA: [(accessed on 1 June 2019)]. Available online: https://seer.cancer.gov/statfacts/html/breast.html. [Google Scholar]
- 20.Winkels R.M., Beijer S., van Lieshout R., van Barneveld D., Hofstede J., Kuiper J., Vreugdenhil A., van Warmerdam L.J.C., Schep G., Blaisse R., et al. Changes in body weight during various types of chemotherapy in breast cancer patients. e-SPEN J. 2014;9:e39–e44. doi: 10.1016/j.clnme.2013.10.004. [DOI] [Google Scholar]
- 21.Pedersen B., Delmar C., Lörincz T., Falkmer U., Grønkjær M. Investigating Changes in Weight and Body Composition Among Women in Adjuvant Treatment for Breast Cancer: A Scoping Review. Cancer Nurs. 2019;42:91–105. doi: 10.1097/NCC.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 22.Caan B.J., Kwan M.L., Shu X.O., Pierce J.P., Patterson R.E., Nechuta S.J., Poole E.M., Kroenke C.H., Weltzien E.K., Flatt S.W., et al. Weight Change and Survival after Breast Cancer in the After Breast Cancer Pooling Project. Cancer Epidemiol. Biomark. Prev. 2012;21:1260–1271. doi: 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy S.M., Sadim M., Li J., Yi N., Agarwal S., Mantzoros C.S., Kaklamani V.G. Clinical and genetic predictors of weight gain in patients diagnosed with breast cancer. Br. J. Cancer. 2013;109:872. doi: 10.1038/bjc.2013.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu D.-T., Tao Y., Taskén K. OPA1 in Lipid Metabolism: Function of OPA1 in Lipolysis and Thermogenesis of Adipocytes. Horm. Metab. Res. 2017;49:276–285. doi: 10.1055/s-0043-100384. [DOI] [PubMed] [Google Scholar]
- 25.Chu D.-T., Tao Y. Human thermogenic adipocytes: A reflection on types of adipocyte, developmental origin, and potential application. J. Physiol. Biochem. 2017;73:1–4. doi: 10.1007/s13105-016-0536-y. [DOI] [PubMed] [Google Scholar]
- 26.Chu D.-T., Tao Y., Son L.H., Le D.-H. Cell source, differentiation, functional stimulation, and potential application of human thermogenic adipocytes in vitro. J. Physiol. Biochem. 2016;73:315–321. doi: 10.1007/s13105-017-0567-z. [DOI] [PubMed] [Google Scholar]
- 27.Chu D.-T., Gawronska-Kozak B. Brown and brite adipocytes: Same function, but different origin and response. Biochimie. 2017;138:102–105. doi: 10.1016/j.biochi.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman S.E., Blackburn O.A., Marchildon F., Cohen P. Insights into the Link Between Obesity and Cancer. Curr. Obes. Rep. 2017;6:195–203. doi: 10.1007/s13679-017-0263-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Scherer P.E. The dysfunctional adipocyte—a cancer cell’s best friend. Nat. Rev. Endocrinol. 2018;14:132. doi: 10.1038/nrendo.2017.174. [DOI] [PubMed] [Google Scholar]
- 30.Lengyel E., Makowski L., DiGiovanni J., Kolonin M.G. Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–384. doi: 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cozzo A.J., Fuller A.M., Makowski L. Contribution of Adipose Tissue to Development of Cancer. Compr. Physiol. 2017;8:237–282. doi: 10.1002/cphy.c170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehr S., Hartwig S., Sell H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. PROTEOMICS–Clin. Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin P.J., Stambolic V. Impact of the obesity epidemic on cancer. Annu. Rev. Med. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 34.Sheng X., Parmentier J.-H., Tucci J., Pei H., Cortez-Toledo O., Dieli-Conwright C.M., Oberley M.J., Neely M., Orgel E., Louie S.G. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol. Cancer Res. 2017;15:1704–1713. doi: 10.1158/1541-7786.MCR-17-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun K., Tordjman J., Clément K., Scherer P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu D.T., Nguyet N.T.M., Nga V.T., Thai Lien N.V., Vo D.D., Lien N., Nhu Ngoc V.T., Son L.H., Le D.-H., Nga V.B., et al. An update on obesity: Mental consequences and psychological interventions. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13:155–160. doi: 10.1016/j.dsx.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Baek A.E., Nelson E.R. The Contribution of Cholesterol and Its Metabolites to the Pathophysiology of Breast Cancer. Horm. Cancer. 2016;7:219–228. doi: 10.1007/s12672-016-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Estevez L., Moreno-Bueno G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21:35. doi: 10.1186/s13058-019-1124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert C.A., Slingerland J.M. Cytokines, obesity, and cancer: New insights on mechanisms linking obesity to cancer risk and progression. Annu. Rev. Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 40.Gunter M.J., Hoover D.R., Yu H., Wassertheil-Smoller S., Rohan T.E., Manson J.E., Li J., Ho G.Y.F., Xue X., Anderson G.L., et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S., Nelson E.R. 27-Hydroxycholesterol, an endogenous selective estrogen receptor modulator. Maturitas. 2017;104:29–35. doi: 10.1016/j.maturitas.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimbung S., Chang C.-Y., Bendahl P.-O., Dubois L., Thompson J.W., McDonnell D.P., Borgquist S. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr. Relat. Cancer. 2017;24:339–349. doi: 10.1530/ERC-16-0533. [DOI] [PubMed] [Google Scholar]
- 43.Liu J., Ma D.W.L. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014;6:5184–5223. doi: 10.3390/nu6115184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu C.-W., Lo Y.-H., Chen C.-H., Lin C.-Y., Tsai C.-H., Chen P.-J., Yang Y.-F., Wang C.-H., Tan C.-H., Hou M.-F., et al. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017;388:130–138. doi: 10.1016/j.canlet.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 45.Monk J.M., Turk H.F., Liddle D.M., De Boer A.A., Power K.A., Ma D.W.L., Robinson L.E. n-3 polyunsaturated fatty acids and mechanisms to mitigate inflammatory paracrine signaling in obesity-associated breast cancer. Nutrients. 2014;6:4760–4793. doi: 10.3390/nu6114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parrales A., Ranjan A., Iwakuma T. Unsaturated fatty acids regulate stemness of ovarian cancer cells through NF-κB. Stem Cell Investig. 2017;4:49. doi: 10.21037/sci.2017.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose D.P., Connolly J.M. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990;50:7139–7144. [PubMed] [Google Scholar]
- 48.Thomou T., Mori M.A., Dreyfuss J.M., Konishi M., Sakaguchi M., Wolfrum C., Rao T.N., Winnay J.N., Garcia-Martin R., Grinspoon S.K. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoll B.A. N-3 fatty acids and lipid peroxidation in breast cancer inhibition. Br. J. Nutr. 2002;87:193–198. doi: 10.1079/BJN2001512. [DOI] [PubMed] [Google Scholar]
- 50.Rajarajan D., Selvarajan S., Charan Raja M.R., Kar Mahapatra S., Kasiappan R. Genome-wide analysis reveals miR-3184-5p and miR-181c-3p as a critical regulator for adipocytes-associated breast cancer. J. Cell. Physiol. 2019 doi: 10.1002/jcp.28428. [DOI] [PubMed] [Google Scholar]
- 51.Liu T.-W., Heden T.D., Matthew Morris E., Fritsche K.L., Vieira-Potter V.J., Thyfault J.P. High-Fat Diet Alters Serum Fatty Acid Profiles in Obesity Prone Rats: Implications for In Vitro Studies. Lipids. 2015;50:997–1008. doi: 10.1007/s11745-015-4061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raatz S.K., Bibus D., Thomas W., Kris-Etherton P. Total Fat Intake Modifies Plasma Fatty Acid Composition in Humans. J. Nutr. 2001;131:231–234. doi: 10.1093/jn/131.2.231. [DOI] [PubMed] [Google Scholar]
- 53.Vigushin D.M., Dong Y., Inman L., Peyvandi N., Alao J.P., Sun C., Ali S., Niesor E.J., Bentzen C.L., Coombes R.C. The nuclear oxysterol receptor LXRα is expressed in the normal human breast and in breast cancer. Med Oncol. 2004;21:123–131. doi: 10.1385/MO:21:2:123. [DOI] [PubMed] [Google Scholar]
- 54.Morris P.G., Hudis C.A., Giri D., Morrow M., Falcone D.J., Zhou X.K., Du B., Brogi E., Crawford C.B., Kopelovich L., et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyengar N.M., Ghossein R.A., Morris L.G., Zhou X.K., Kochhar A., Morris P.G., Pfister D.G., Patel S.G., Boyle J.O., Hudis C.A., et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer. 2016;122:3794–3802. doi: 10.1002/cncr.30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subbaramaiah K., Howe L.R., Bhardwaj P., Du B., Gravaghi C., Yantiss R.K., Zhou X.K., Blaho V.A., Hla T., Yang P., et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev. Res. 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Soto-Guzman A., Navarro-Tito N., Castro-Sanchez L., Martinez-Orozco R., Salazar E.P. Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin. Exp. Metastasis. 2010;27:505–515. doi: 10.1007/s10585-010-9340-1. [DOI] [PubMed] [Google Scholar]
- 58.Hardy S., Langelier Y., Prentki M. Oleate Activates Phosphatidylinositol 3-Kinase and Promotes Proliferation and Reduces Apoptosis of MDA-MB-231 Breast Cancer Cells, Whereas Palmitate Has Opposite Effects 1. Cancer Res. 2000;60:6353. [PubMed] [Google Scholar]
- 59.Cleofas M.-M., Alejandra O.-M., Christian G.-R., Pedro C.-R., Eduardo Perez S. Oleic acid induces migration through a FFAR1/4, EGFR and AKT-dependent pathway in breast cancer cells. Endocr. Connect. 2019;8:252–265. doi: 10.1530/EC-18-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Touvier M., Fassier P., His M., Norat T., Chan D.S.M., Blacher J., Hercberg S., Galan P., Druesne-Pecollo N., Latino-Martel P. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 2015;114:347–357. doi: 10.1017/S000711451500183X. [DOI] [PubMed] [Google Scholar]
- 61.His M., Dartois L., Fagherazzi G., Boutten A., Dupré T., Mesrine S., Boutron-Ruault M.-C., Clavel-Chapelon F., Dossus L. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes Control. 2017;28:77–88. doi: 10.1007/s10552-016-0832-4. [DOI] [PubMed] [Google Scholar]
- 62.Carter P.R., Uppal H., Chandran S., Bainey K.R., Potluri R. Patients with a diagnosis of hyperlipidaemia have a reduced risk of developing breast cancer and lower mortality rates: A large retrospective longitudinal cohort study from the UK ACALM registry. Eur. Heart J. 2017;38(Suppl. 1):644–645. [Google Scholar]
- 63.Zhong S., Zhang X., Chen L., Ma T., Tang J., Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat. Rev. 2015;41:554–567. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Borgquist S., Tamimi R.M., Chen W.Y., Garber J.E., Eliassen A.H., Ahern T.P. Statin use and breast cancer risk in the nurses’ health study. Cancer Epidemiol. Prev. Biomark. 2016;25:201–206. doi: 10.1158/1055-9965.EPI-15-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borgquist S., Bjarnadottir O., Kimbung S., Ahern T.P. Statins: A role in breast cancer therapy? J. Intern. Med. 2018;284:346–357. doi: 10.1111/joim.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DuSell C.D., Nelson E.R., Abdo J., McDonnell D.P., Gesty-Palmer D., Wang X., Khosla S., Mödder U.I., Umetani M., Javitt N.B. The Endogenous Selective Estrogen Receptor Modulator 27-Hydroxycholesterol Is a Negative Regulator of Bone Homeostasis. Endocrinology. 2010;151:3675–3685. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu X., Menke J.G., Chen Y., Zhou G., MacNaul K.L., Wright S.D., Sparrow C.P., Lund E.G. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 68.Lu D.-L., Le Cornet C., Sookthai D., Johnson T.S., Kaaks R., Fortner R.T. Circulating 27-hydroxycholesterol and breast cancer risk: Results from the EPIC-Heidelberg cohort. JNCI J. Natl. Cancer Inst. 2018;111:365–371. doi: 10.1093/jnci/djy115. [DOI] [PubMed] [Google Scholar]
- 69.Lazar I., Clement E., Dauvillier S., Milhas D., Ducoux-Petit M., LeGonidec S., Moro C., Soldan V., Dalle S., Balor S. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: A novel mechanism linking obesity and cancer. Cancer Res. 2016;76:4051–4057. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 70.Kasiappan R., Rajarajan D. Role of MicroRNA Regulation in Obesity-Associated Breast Cancer: Nutritional Perspectives. Adv. Nutr. 2017;8:868–888. doi: 10.3945/an.117.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams B.D., Arem H., Hubal M.J., Cartmel B., Li F., Harrigan M., Sanft T., Cheng C.J., Pusztai L., Irwin M.L. Exercise and weight loss interventions and miRNA expression in women with breast cancer. Breast Cancer Res. Treat. 2018;170:55–67. doi: 10.1007/s10549-018-4738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Q., Li J., Li Z., Sun S., Zhu S., Wang L., Wu J., Yuan J., Zhang Y., Sun S., et al. Exosomes from the tumour–adipocyte interplay stimulate beige/brown differentiation and reprogram metabolism in stromal adipocytes to promote tumour progression. J. Exp. Clin. Cancer Res. 2019;38:223. doi: 10.1186/s13046-019-1210-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors. 2018;18:3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gautam J., Banskota S., Lee H., Lee Y.-J., Jeon Y.H., Kim J.-A., Jeong B.-S. Down-regulation of cathepsin S and matrix metalloproteinase-9 via Src, a non-receptor tyrosine kinase, suppresses triple-negative breast cancer growth and metastasis. Exp. Mol. Med. 2018;50:118. doi: 10.1038/s12276-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lafleur M.A., Drew A.F., De Sousa E.L., Blick T., Bills M., Walker E.C., Williams E.D., Waltham M., Thompson E.W. Upregulation of matrix metalloproteinases (MMPs) in breast cancer xenografts: A major induction of stromal MMP-13. Int. J. Cancer. 2005;114:544–554. doi: 10.1002/ijc.20763. [DOI] [PubMed] [Google Scholar]
- 76.Roscilli G., Cappelletti M., De Vitis C., Ciliberto G., Di Napoli A., Ruco L., Mancini R., Aurisicchio L. Circulating MMP11 and specific antibody immune response in breast and prostate cancer patients. J. Transl. Med. 2014;12:54. doi: 10.1186/1479-5876-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhardwaj P., Au C.C., Benito-Martin A., Ladumor H., Oshchepkova S., Moges R., Brown K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019;189:161–170. doi: 10.1016/j.jsbmb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue W., Wang J.-P., Li Y., Fan P., Liu G., Zhang N., Conaway M., Wang H., Korach K.S., Bocchinfuso W., et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int. J. Cancer. 2010;127:1748–1757. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russo J., Russo I.H. The role of estrogen in the initiation of breast cancer. The J. Steroid Biochem. Mol. Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lukanova A., Lundin E., Zeleniuch-Jacquotte A., Muti P.C., Mure A.J., Rinaldi S., Dossus L., Micheli A., Arslan A., Lenner P., et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: A cross-sectional study in healthy women. Eur. J. Endocrinol. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 81.McTiernan A., Wu L., Chen C., Chlebowski R., Mossavar-Rahmani Y., Modugno F., Perri M.G., Stanczyk F.Z., Van Horn L., Wang C.Y., et al. Relation of BMI and Physical Activity to Sex Hormones in Postmenopausal Women. Obesity. 2006;14:1662–1677. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 82.Boyapati S.M., Shu X.O., Gao Y.-T., Dai Q., Yu H., Cheng J.R., Jin F., Zheng W. Correlation of Blood Sex Steroid Hormones with Body Size, Body Fat Distribution, and Other Known Risk Factors for Breast Cancer in Post-Menopausal Chinese Women. Cancer Causes Control. 2004;15:305–311. doi: 10.1023/B:CACO.0000024256.48104.50. [DOI] [PubMed] [Google Scholar]
- 83.Purohit A., Reed M.J. Regulation of estrogen synthesis in postmenopausal women. Steroids. 2002;67:979–983. doi: 10.1016/S0039-128X(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 84.Sebastian S., Bulun S.E. A Highly Complex Organization of the Regulatory Region of the Human CYP19 (Aromatase) Gene Revealed by the Human Genome Project. J. Clin. Endocrinol. Metab. 2001;86:4600–4602. doi: 10.1210/jcem.86.10.7947. [DOI] [PubMed] [Google Scholar]
- 85.Van Landeghem A.A.J., Poortman J., Nabuurs M., Thijssen J.H.H. Endogenous Concentration and Subcellular Distribution of Androgens in Normal and Malignant Human Breast Tissue. Cancer Res. 1985;45:2907. [PubMed] [Google Scholar]
- 86.Purohit A., Newman S.P., Reed M.J. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res. BCR. 2002;4:65–69. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y., Nichols J.E., Valdez R., Mendelson C.R., Simpson E.R. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]
- 88.Richards J.A., Brueggemeier R.W. Prostaglandin E2 Regulates Aromatase Activity and Expression in Human Adipose Stromal Cells via Two Distinct Receptor Subtypes. J. Clin. Endocrinol. Metab. 2003;88:2810–2816. doi: 10.1210/jc.2002-021475. [DOI] [PubMed] [Google Scholar]
- 89.Omoto Y., Kobayashi S., Inoue S., Ogawa S., Toyama T., Yamashita H., Muramatsu M., Gustafsson J.Å., Iwase H. Evaluation of oestrogen receptor β wild-type and variant protein expression, and relationship with clinicopathological factors in breast cancers. Eur. J. Cancer. 2002;38:380–386. doi: 10.1016/S0959-8049(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 90.Rose D.P., Komninou D., Stephenson G.D. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes. Rev. 2004;5:153–165. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 91.Kawai M., Minami Y., Kuriyama S., Kakizaki M., Kakugawa Y., Nishino Y., Ishida T., Fukao A., Tsuji I., Ohuchi N. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: The Miyagi Cohort Study. Br. J. Cancer. 2010;103:1443–1447. doi: 10.1038/sj.bjc.6605885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall J.M., Couse J.F., Korach K.S. The Multifaceted Mechanisms of Estradiol and Estrogen Receptor Signaling. J. Biol. Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 93.McKenna N.J., Lanz R.B., O’Malley B.W. Nuclear Receptor Coregulators: Cellular and Molecular Biology. Endocr. Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 94.Wong C.-W., McNally C., Nickbarg E., Komm B.S., Cheskis B.J. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc. Natl. Acad. Sci. USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Schiff R., Massarweh S.A., Shou J., Bharwani L., Mohsin S.K., Osborne C.K. Cross-Talk between Estrogen Receptor and Growth Factor Pathways as a Molecular Target for Overcoming Endocrine Resistance. Clin. Cancer Res. 2004;10:331s. doi: 10.1158/1078-0432.CCR-031212. [DOI] [PubMed] [Google Scholar]
- 96.Migliaccio A., Di Domenico M., Castoria G., de Falco A., Bontempo P., Nola E., Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. doi: 10.1002/j.1460-2075.1996.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acconcia F., Totta P., Ogawa S., Cardillo I., Inoue S., Leone S., Trentalance A., Muramatsu M., Marino M. Survival versus apoptotic 17β-estradiol effect: Role of ERα and ERβ activated non-genomic signaling. J. Cell. Physiol. 2005;203:193–201. doi: 10.1002/jcp.20219. [DOI] [PubMed] [Google Scholar]
- 98.Marino M., Acconcia F., Bresciani F., Weisz A., Trentalance A. Distinct nongenomic signal transduction pathways controlled by 17beta-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol. Biol. Cell. 2002;13:3720–3729. doi: 10.1091/mbc.e02-03-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doisneau-Sixou S.F., Sergio C.M., Carroll J.S., Hui R., Musgrove E.A., Sutherland R.L. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr. Relat. Cancer. 2003;10:179–186. doi: 10.1677/erc.0.0100179. [DOI] [PubMed] [Google Scholar]
- 100.Giretti M.S., Fu X.-D., De Rosa G., Sarotto I., Baldacci C., Garibaldi S., Mannella P., Biglia N., Sismondi P., Genazzani A.R., et al. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE. 2008;3:e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Q.-F., Wu T.-T., Yang J.-Y., Dong C.-R., Wang N., Liu X.-H., Liu Z.-M. 17β-Estradiol promotes the invasion and migration of nuclear estrogen receptor-negative breast cancer cells through cross-talk between GPER1 and CXCR1. J. Steroid Biochem Mol. Biol. 2013;138:314–324. doi: 10.1016/j.jsbmb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 102.Kadowaki T., Yamauchi T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 103.Mantzoros C., Markopoulos C., Chavelas C., Alexe D.M., Dalamaga M., Dessypris N., Trichopoulos D., Petridou E., Papadiamantis Y., Spanos E., et al. Adiponectin and Breast Cancer Risk. J. Clin. Endocrinol. Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 104.Körner A., Pazaitou-Panayiotou K., Kelesidis T., Kelesidis I., Williams C.J., Kaprara A., Bullen J.W., Neuwirth A.K., Tseleni S., Mitsiades N.S., et al. Total and high-molecular-weight adiponectin in breast cancer: In vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2007;2:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 105.Ye J., Jia J., Dong S., Zhang C., Yu S., Li L., Mao C., Wang D., Chen J., Yuan G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014;23:158–165. doi: 10.1097/CEJ.0b013e328364f293. [DOI] [PubMed] [Google Scholar]
- 106.Berg A.H., Combs T.P., Du X., Brownlee M., Scherer P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 107.Kadowaki T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and its receptors in insulin resistance, diabetes, and metabolic syndrome, and obesity. J. Clin. Investig. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dalamaga M., Diakopoulos K.N., Mantzoros C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim A.Y., Lee Y.S., Kim K.H., Lee J.H., Lee H.K., Jang S.-H., Kim S.-E., Lee G.Y., Lee J.-W., Jung S.-A., et al. Adiponectin Represses Colon Cancer Cell Proliferation via AdipoR1-and-R2-Mediated AMPK Activation. Mol. Endocrinol. 2010;24:1441–1452. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bråkenhielm E., Veitonmäki N., Cao R., Kihara S., Matsuzawa Y., Zhivotovsky B., Funahashi T., Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA. 2004;101:2476. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abrahamsson A., Morad V., Dabrosin C. Estradiol Affects Extracellular Leptin: Adiponectin Ratio in Human Breast Tissue in Vivo. J. Clin. Endocrinol. Metab. 2014;99:3460–3467. doi: 10.1210/jc.2014-1129. [DOI] [PubMed] [Google Scholar]
- 112.Mauro L., Pellegrino M., Giordano F., Ricchio E., Rizza P., De Amicis F., Catalano S., Bonofiglio D., Panno M.L., Andò S. Estrogen receptor-α drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015;29:2150–2160. doi: 10.1096/fj.14-262808. [DOI] [PubMed] [Google Scholar]
- 113.Vaisse C., Halaas J.L., Horvath C.M., Darnell J.E., Stoffel M., Friedman J.M. Leptin activation of Stat3 in the hypothalamus of wild–type and ob/ob mice but not db/db mice. Nature Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 114.Guo S., Liu M., Wang G., Torroella-Kouri M., Gonzalez-Perez R.R. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim. Biophys. Acta. 2012;1825:207–222. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Delort L., Rossary A., Farges M.-C., Vasson M.-P., Caldefie-Chézet F. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015;140:37–48. doi: 10.1016/j.lfs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 116.Ishikawa M., Kitayama J., Nagawa H. Enhanced Expression of Leptin and Leptin Receptor (OB-R) in Human Breast Cancer. Clin. Cancer Res. 2004;10:4325. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 117.Catalano S., Mauro L., Marsico S., Giordano C., Rizza P., Rago V., Montanaro D., Maggiolini M., Panno M.L., Andó S. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor α in MCF-7 cells. J. Biol. Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- 118.Geisler J., Haynes B., Ekse D., Dowsett M., Lønning P.E. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. J. Steroid Biochem. Mol. Biol. 2007;104:27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 119.Fan Y., Gan Y., Shen Y., Cai X., Song Y., Zhao F., Yao M., Gu J., Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120–16134. doi: 10.18632/oncotarget.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coughlin S.S., Giovannucci E.L. Diabetes and Cancer. Diabetes. 2012:294–305. doi: 10.1002/9781118405550.ch14. [DOI] [Google Scholar]
- 121.Ferguson R.D., Novosyadlyy R., Fierz Y., Alikhani N., Sun H., Yakar S., LeRoith D. Hyperinsulinemia enhances c-Myc-mediated mammary tumor development and advances metastatic progression to the lung in a mouse model of type 2 diabetes. Breast Cancer Res. 2012;14:R8. doi: 10.1186/bcr3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ulanet D.B., Ludwig D.L., Kahn C.R., Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc. Natl. Acad. Sci. USA. 2010;107:10791–10798. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goodwin P.J., Ennis M., Pritchard K.I., Trudeau M.E., Koo J., Madarnas Y., Hartwick W., Hoffman B., Hood N. Fasting Insulin and Outcome in Early-Stage Breast Cancer: Results of a Prospective Cohort Study. J. Clin. Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 124.Rose D.P., Gracheck P.J., Vona-Davis L. The Interactions of Obesity, Inflammation and Insulin Resistance in Breast Cancer. Cancers. 2015;7:2147–2168. doi: 10.3390/cancers7040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lopez T., Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/S1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 126.Christopoulos P.F., Msaouel P., Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peiró G., Adrover E., Sánchez-Tejada L., Lerma E., Planelles M., Sánchez-Payá J., Aranda F.I., Giner D., Gutiérrez-Aviñó F.J. Increased insulin-like growth factor-1 receptor mRNA expression predicts poor survival in immunophenotypes of early breast carcinoma. Mod. Pathol. 2010;24:201. doi: 10.1038/modpathol.2010.191. [DOI] [PubMed] [Google Scholar]
- 128.Assiri A.M.A., Kamel H.F.M., Hassanien M.F.R. Resistin, visfatin, adiponectin, and leptin: Risk of breast cancer in pre-and postmenopausal saudi females and their possible diagnostic and predictive implications as novel biomarkers. Dis. Markers. 2015;2015:253519. doi: 10.1155/2015/253519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang C.H., Wang P.J., Hsieh Y.C., Lo S., Lee Y.C., Chen Y.C., Tsai C.H., Chiu W.C., Chu-Sung Hu S., Lu C.W., et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2017;37:589. doi: 10.1038/onc.2017.357. [DOI] [PubMed] [Google Scholar]
- 130.Carter J.C., Church F.C. Obesity and breast cancer: The roles of peroxisome proliferator-activated receptor-γ and plasminogen activator inhibitor-1. PPAR Res. 2009;2009:345320. doi: 10.1155/2009/345320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen D.-C., Chung Y.-F., Yeh Y.-T., Chaung H.-C., Kuo F.-C., Fu O.-Y., Chen H.-Y., Hou M.-F., Yuan S.-S.F. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 132.Bougaret L., Delort L., Billard H., Le Huede C., Boby C., De la Foye A., Rossary A., Mojallal A., Damour O., Auxenfans C., et al. Adipocyte/breast cancer cell crosstalk in obesity interferes with the anti-proliferative efficacy of tamoxifen. PloS ONE. 2018;13:e0191571. doi: 10.1371/journal.pone.0191571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carter J.C., Church F.C. Mature breast adipocytes promote breast cancer cell motility. Exp. Mol. Pathol. 2012;92:312–317. doi: 10.1016/j.yexmp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 134.Dalamaga M., Sotiropoulos G., Karmaniolas K., Pelekanos N., Papadavid E., Lekka A. Serum resistin: A biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin. Biochem. 2013;46:584–590. doi: 10.1016/j.clinbiochem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 135.Georgiou G.P., Provatopoulou X., Kalogera E., Siasos G., Menenakos E., Zografos G.C., Gounaris A. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast. 2016;29:163–169. doi: 10.1016/j.breast.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 136.Coppack S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2007;60:349–356. doi: 10.1079/PNS2001110. [DOI] [PubMed] [Google Scholar]
- 137.Picon-Ruiz M., Pan C., Drews-Elger K., Jang K., Besser A.H., Zhao D., Morata-Tarifa C., Kim M., Ince T.A., Azzam D.J., et al. Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/miR-302b–Mediated Malignant Progression. Cancer Res. 2016;76:491. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 138.Wang H., Yang X. Association between serum cytokines and progression of breast cancer in Chinese population. Medicine. 2017;96:e8840. doi: 10.1097/MD.0000000000008840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waugh D.J.J., Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 140.Soria G., Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 141.Nicolini A., Carpi A., Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 142.Fasoulakis Z., Kolios G., Papamanolis V., Kontomanolis E.N. Interleukins Associated with Breast Cancer. Cureus. 2018;10:e3549. doi: 10.7759/cureus.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma Y., Ren Y., Dai Z.-J., Wu C.-J., Ji Y.-H., Xu J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017;26:421–426. doi: 10.17219/acem/62120. [DOI] [PubMed] [Google Scholar]
- 144.Sheen-Chen S.-M., Chen W.-J., Eng H.-L., Chou F.-F. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res. Treat. 1997;43:211–215. doi: 10.1023/A:1005736712307. [DOI] [PubMed] [Google Scholar]
- 145.Cai X., Cao C., Li J., Chen F., Zhang S., Liu B., Zhang W., Zhang X., Ye L. Inflammatory factor TNF-α promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-κB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget. 2017;8:58338–58352. doi: 10.18632/oncotarget.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aggarwal B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003;3:745. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 147.Rivas M.A., Carnevale R.P., Proietti C.J., Rosemblit C., Beguelin W., Salatino M., Charreau E.H., Frahm I., Sapia S., Brouckaert P. TNFα acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-κB-dependent pathways. Exp. Cell Res. 2008;314:509–529. doi: 10.1016/j.yexcr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Rubio M.F., Werbajh S., Cafferata E.G.A., Quaglino A., Coló G.P., Nojek I.M., Kordon E.C., Nahmod V.E., Costas M.A. TNF-α enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene. 2005;25:1367. doi: 10.1038/sj.onc.1209176. [DOI] [PubMed] [Google Scholar]
- 149.Kim S., Choi J.H., Kim J.B., Nam S.J., Yang J.-H., Kim J.-H., Lee J.E. Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules. 2008;13:2975–2985. doi: 10.3390/molecules13122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Varela L.M., Stangle-Castor N.C., Shoemaker S.F., Shea-Eaton W.K., Ip M.M. TNFα induces NFκB/p50 in association with the growth and morphogenesis of normal and transformed rat mammary epithelial cells. J. Cell. Physiol. 2001;188:120–131. doi: 10.1002/jcp.1103. [DOI] [PubMed] [Google Scholar]
- 151.Kamel M., Shouman S., El-Merzebany M., Kilic G., Veenstra T., Saeed M., Wagih M., Diaz-Arrastia C., Patel D., Salama S. Effect of tumour necrosis factor-alpha on estrogen metabolic pathways in breast cancer cells. J. Cancer. 2012;3:310. doi: 10.7150/jca.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valdivia-Silva J.E., Franco-Barraza J., Silva A.L.E., Pont G.D., Soldevila G., Meza I., García-Zepeda E.A. Effect of pro-inflammatory cytokine stimulation on human breast cancer: Implications of chemokine receptor expression in cancer metastasis. Cancer Lett. 2009;283:176–185. doi: 10.1016/j.canlet.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 153.Warren M.A., Shoemaker S.F., Shealy D.J., Bshara W., Ip M.M. Tumor necrosis factor deficiency inhibits mammary tumorigenesis and a tumor necrosis factor neutralizing antibody decreases mammary tumor growth in neu/erbB2 transgenic mice. Mol. Cancer Ther. 2009;8:2655. doi: 10.1158/1535-7163.MCT-09-0358. [DOI] [PubMed] [Google Scholar]
- 154.Macci A., Madeddu C. Obesity, Inflammation, and Postmenopausal Breast Cancer: Therapeutic Implications. Sci. World J. 2011;11:17. doi: 10.1100/2011/806787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grisouard J., Dembinski K., Mayer D., Keller U., Müller B., Christ-Crain M. Targeting AMP-activated protein kinase in adipocytes to modulate obesity-related adipokine production associated with insulin resistance and breast cancer cell proliferation. Diabetol. Metab. Syndr. 2011;3:16. doi: 10.1186/1758-5996-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim K.-Y., Kim J.K., Jeon J.H., Yoon S.R., Choi I., Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-α in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005;327:460–467. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 157.Dinarello C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002;20:S1–S13. [PubMed] [Google Scholar]
- 158.Howe L.R. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. BCR. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang F.-M., Liu H.-Q., Liu S.-R., Tang S.-P., Yang L., Feng G.-S. SHP-2 promoting migration and metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1 βin vivo andin vitro. Breast Cancer Res. Treat. 2005;89:5–14. doi: 10.1007/s10549-004-1002-z. [DOI] [PubMed] [Google Scholar]
- 160.Liao D., Johnson R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 161.Grivennikov S., Karin E., Terzic J., Mucida D., Yu G.-Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell. 2009;15:241. doi: 10.1016/j.ccr.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ranger J.J., Levy D.E., Shahalizadeh S., Hallett M., Muller W.J. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69:6823–6830. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang G.J., Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- 164.Sullivan N.J., Sasser A.K., Axel A.E., Vesuna F., Raman V., Ramirez N., Oberyszyn T.M., Hall B.M. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Penson R.T., Kronish K., Duan Z., Feller A.J., Stark P., Cook S.E., Duska L.R., Fuller A.F., Goodman A.K., Nikrui N., et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int. J. Gynecol. Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 166.Ali S., Lazennec G. Chemokines: Novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26:401–420. doi: 10.1007/s10555-007-9073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luboshits G., Shina S., Kaplan O., Engelberg S., Nass D., Lifshitz-Mercer B., Chaitchik S., Keydar I., Ben-Baruch A. Elevated Expression of the CC Chemokine Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) in Advanced Breast Carcinoma. Cancer Res. 1999;59:4681. [PubMed] [Google Scholar]
- 168.Chen J., Yao Y., Gong C., Yu F., Su S., Chen J., Liu B., Deng H., Wang F., Lin L., et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010;11:889. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 170.DeNardo D.G., Barreto J.B., Andreu P., Vasquez L., Tawfik D., Kolhatkar N., Coussens L.M. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chavey C., Bibeau F., Gourgou-Bourgade S., Burlinchon S., Boissière F., Laune D., Roques S., Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. BCR. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lebrecht A., Grimm C., Lantzsch T., Ludwig E., Hefler L., Ulbrich E., Koelbl H. Monocyte chemoattractant protein-1 serum levels in patients with breast cancer. Tumor Biol. 2004;25:14–17. doi: 10.1159/000077718. [DOI] [PubMed] [Google Scholar]
- 173.Goede V., Brogelli L., Ziche M., Augustin H.G. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int. J. Cancer. 1999;82:765–770. doi: 10.1002/(SICI)1097-0215(19990827)82:5<765::AID-IJC23>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 174.Jin W.J., Kim B., Kim D., Park Choo H.-Y., Kim H.-H., Ha H., Lee Z.H. NF-κB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells. Exp. Mol. Med. 2017;49:e295. doi: 10.1038/emm.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ejaeidi A.A., Craft B.S., Puneky L.V., Lewis R.E., Cruse J.M. Hormone receptor-independent CXCL10 production is associated with the regulation of cellular factors linked to breast cancer progression and metastasis. Exp. Mol. Pathol. 2015;99:163–172. doi: 10.1016/j.yexmp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 176.Kitamura T., Pollard J.W. Therapeutic potential of chemokine signal inhibition for metastatic breast cancer. Pharmacol. Res. 2015;100:266–270. doi: 10.1016/j.phrs.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mittal S., Brown N.J., Holen I. The breast tumor microenvironment: Role in cancer development, progression and response to therapy. Expert Rev. Mol. Diagn. 2018;18:227–243. doi: 10.1080/14737159.2018.1439382. [DOI] [PubMed] [Google Scholar]
- 178.Vielma S.A., Klein R.L., Levingston C.A., Young M.R.I. Adipocytes as immune regulatory cells. Int. Immunopharmacol. 2013;16:224–231. doi: 10.1016/j.intimp.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Korkaya H., Liu S., Wicha M.S. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Investig. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mao Y., Keller E.T., Garfield D.H., Shen K., Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shiga K., Hara M., Nagasaki T., Sato T., Takahashi H., Takeyama H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers. 2015;7:2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 183.Chu Q.D., Panu L., Holm N.T., Li B.D.L., Johnson L.W., Zhang S. High Chemokine Receptor CXCR4 Level in Triple Negative Breast Cancer Specimens Predicts Poor Clinical Outcome. J. Surg. Res. 2010;159:689–695. doi: 10.1016/j.jss.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 184.Burger J.A., Kipps T.J. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 185.Okumura T., Ohuchida K., Kibe S., Iwamoto C., Ando Y., Takesue S., Nakayama H., Abe T., Endo S., Koikawa K., et al. Adipose tissue-derived stromal cells are sources of cancer-associated fibroblasts and enhance tumor progression by dense collagen matrix. Int. J. Cancer. 2019;144:1401–1413. doi: 10.1002/ijc.31775. [DOI] [PubMed] [Google Scholar]
- 186.Lopes-Coelho F., Gouveia-Fernandes S., Serpa J. Metabolic cooperation between cancer and non-cancerous stromal cells is pivotal in cancer progression. Tumor Biol. 2018;40:1010428318756203. doi: 10.1177/1010428318756203. [DOI] [PubMed] [Google Scholar]
- 187.Bussard K.M., Mutkus L., Stumpf K., Gomez-Manzano C., Marini F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. BCR. 2016;18:84. doi: 10.1186/s13058-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gernapudi R., Yao Y., Zhang Y., Wolfson B., Roy S., Duru N., Eades G., Yang P., Zhou Q. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 2015;150:685–695. doi: 10.1007/s10549-015-3326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh R., Parveen M., Basgen J.M., Fazel S., Meshesha M.F., Thames E.C., Moore B., Martinez L., Howard C.B., Vergnes L. Increased expression of beige/brown adipose markers from host and breast cancer cells influence xenograft formation in mice. Mol. Cancer Res. 2016;14:78–92. doi: 10.1158/1541-7786.MCR-15-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cao Q., Hersl J., La H., Smith M., Jenkins J., Goloubeva O., Dilsizian V., Tkaczuk K., Chen W., Jones L. A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer. 2014;14:126. doi: 10.1186/1471-2407-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ahirwar D.K., Nasser M.W., Ouseph M.M., Elbaz M., Cuitiño M.C., Kladney R.D., Varikuti S., Kaul K., Satoskar A.R., Ramaswamy B., et al. Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation. Oncogene. 2018;37:4428–4442. doi: 10.1038/s41388-018-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee Y., Jung W.H., Koo J.S. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res. Treat. 2015;153:323–335. doi: 10.1007/s10549-015-3550-9. [DOI] [PubMed] [Google Scholar]
- 193.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17:1498. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]