Abstract
Foxp3-expressing regulatory T (Treg) cells can suppress the activity of various types of immune cells and play key roles in the maintenance of self-tolerance and in the regulation of immune responses against pathogens and tumor cells. Treg cells consist of heterogeneous subsets that have distinct phenotypes and functions. Upon antigen stimulation, naïve-like thymus-derived Treg cells, which circulate in secondary lymphoid organs, can differentiate into effector Treg (eTreg) cells and migrate to and control immune homeostasis of peripheral tissues. eTreg cells are heterogeneous in terms of their ability to localize to specific tissues and suppress particular types of immune responses. Differentiation and function of diverse eTreg subsets are regulated by a variety of transcription factors that are activated by antigens and cytokines. In this article, we review the current understanding of the transcriptional regulation of differentiation and function of eTreg cells.
Keywords: Treg subsets, effector Treg, transcriptional regulation
1. Introduction
Immune suppressive CD4 T cells expressing the transcription factor Forkhead box protein 3 (Foxp3), known as regulatory T (Treg) cells, play an essential role in maintaining immune tolerance and tissue homeostasis [1]. Foxp3 is indispensable for Treg development, maintenance, and function, and ectopic expression of Foxp3 is sufficient to provide CD4 conventional T cells with immune suppressive functions and Treg phenotypes [1,2,3]. Foxp3 deficiency causes severe autoimmune diseases in both human and mice as shown in IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) patients and scurfy mice, respectively [4,5,6].
Treg cells can be subdivided into various unique subsets, and this heterogeneity is essential for Treg-mediated immune homeostasis. The first level of Treg subset classification is based on the developmental pathways. Thymus-derived Treg (tTreg) cells differentiate from CD4/CD8 double-positive or CD4 single-positive thymocytes in the thymus, depending on recognition of tissue-restricted self-antigens expressed in medullary thymic epithelial cells [7,8,9,10]. On the other hand, peripherally derived Treg (pTreg) cells differentiate from naïve CD4 T cells upon antigen stimulation in the presence of TGF-β in the secondary lymphoid tissues [8,11]. Helios and neuropilin-1 (Nrp1) have been widely used as markers to distinguish tTreg (Helios+ Nrp1+) and pTreg (Helios- Nrp1-) cells [12,13]. The roles of tTreg and pTreg cells are distinct, as mice deficient for a Foxp3 cis regulatory element—conserved non-coding sequence (CNS) 1, in which generation of pTreg cells, but not tTreg cells, is specifically impaired—develop spontaneous inflammation in lung and gastrointestinal tissues [14].
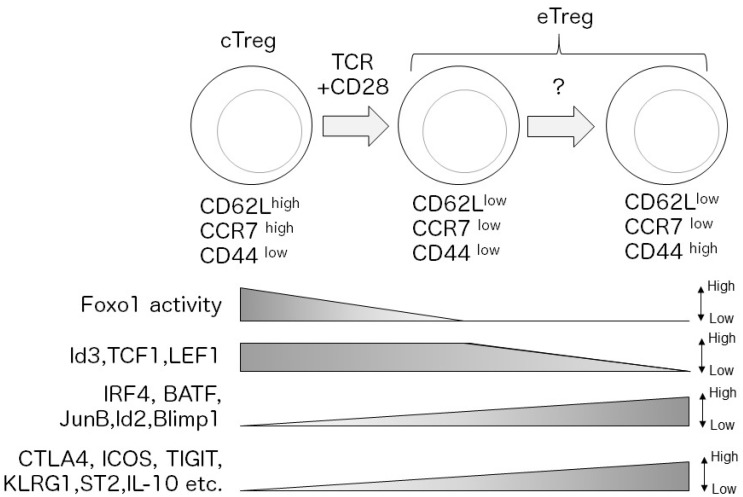
The second level of Treg subset classification is based on Treg’s activation status. tTreg cells can be subdivided into naïve-like central Treg (cTreg) cells (also known as resting Treg cells) and activated effector Treg (eTreg) cells (also known as activated Treg cells or effector memory Treg cells) [15,16,17,18,19,20]. After maturation, tTreg cells egress the thymus as cTreg cells, which are defined by CD62Lhigh(hi)CD44low(lo) or CC chemokine receptor (CCR7)hiCD44lo phenotypes, circulate in secondary lymphoid organs, depending on the functions of homing receptors CD62L and CCR7 [15,16]. Upon antigen stimulation, cTreg cells differentiate into eTreg cells, which are defined by CD62LloCD44hi or CCR7loCD44hi phenotypes [15,16,21] (Figure 1). eTreg cells express higher levels of Treg effector molecules, such as cytotoxic T cell antigen 4 (CTLA4) and inducible T cell costimulator (ICOS) compared to cTreg cells, which likely contribute to enhanced suppressive activity of eTreg cells [15,16,17,21,22,23,24].
Figure 1.
Upon antigen stimulation, central Treg (cTreg) cells (CD62Lhi CCR7hi CD44lo) differentiate into effector Treg (eTreg) cells (CD62Llo CCR7lo CD44hi) depending on TCR and CD28 signaling. After activation, TCR-dependent transcription factors, such as interferon regulatory factor 4 (IRF4), are induced and regulate the eTreg transcriptional program. In contrast, Foxo1 is inactivated by Akt-signaling, which decreases expression of cTreg-related molecules. Loss of Id2, transcription factor 1 (TCF1), and lymphoid enhancer binding factor 1 (LEF1) expression is a signature of mature eTreg cells. Mature eTreg cells highly express immune suppressive molecules, such as cytotoxic T cell antigen 4 (CTLA4) and inducible T cell costimulator (ICOS).
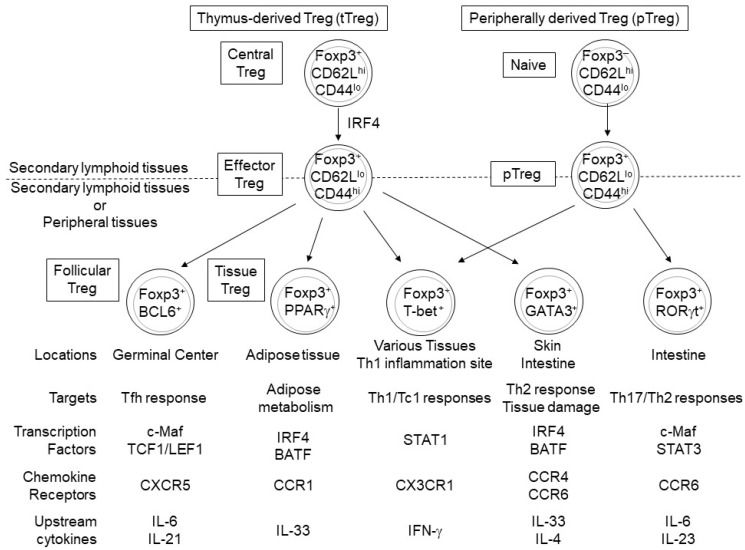
The majority of eTreg cells migrate to and accumulate in non-lymphoid peripheral tissues and inflamed sites, probably due to a decrease in CD62L and CCR7 expression and a concomitant increase in the expression of various chemokine receptors (e.g., CCR4, CCR6, and CCR10) and adhesion molecules (e.g., KLRG1, CD103) [16,17,22,23]. eTreg cells can reside in non-lymphoid peripheral tissues, as tissue Treg cells, and play a role not only in the maintenance of immune homeostasis but also in tissue repair and regeneration [25,26,27,28,29,30,31]. For example, visceral adipose tissue (VAT)-Treg cells accumulate in and suppress the inflammation of adipose tissue, thereby regulating insulin resistance [32,33,34]. In the colon, Treg subsets expressing RAR-related orphan receptor (ROR)γt and GATA-binding protein 3 (GATA3) are involved in inhibiting inflammation and tissue repair, respectively [35,36,37]. Thus, each tissue-specific Treg subset exhibits distinct functions and phenotypes, likely due to tissue-specific environmental cues (Figure 2).
Figure 2.
Peroxisome-proliferator-activated receptor γ (PPARγ), B cell lymphoma 6 (BCL6), T-bet, GATA-binding protein 3 (GATA3), and RORγt regulate the differentiation and function of tissue Treg cells derived from eTreg and peripherally derived Treg (pTreg) cells. Treg cells expressing PPARγ, BCL6, or GATA3 mainly differentiate from tTreg cells, while RORγt+ Treg cells differentiate from naïve CD4+ T cells [34,35,36,43,44] and T-bet+ Treg cells likely differentiate from both eTreg and pTreg cells [45,46,47]. These tissue-specific Treg subsets express different functional molecules and play different roles in maintaining tissue homeostasis by suppressing specific immune responses and regulating lipid metabolism and tissue repair.
A subset of eTreg cells expressing CXC chemokine receptor 5 (CXCR5), known as follicular regulatory T (Tfr) cells, accumulate in the germinal center (GC) of lymphoid organs and suppress GC reactions that are required for high affinity antibody production of B cells [38,39,40]. In contrast, some CXCR5+ Tfr cells with naïve-like phenotypes are present in the blood [41]. Unlike other Treg subsets, GC Tfr cells do not express the α chain of the interleukin (IL)-2 receptor, CD25 [42]. The TCR repertoire of Tfr cells resembles that of Treg cells rather than Tfh cells, consistent with the notion that most Tfr cells do not differentiate from naïve CD4 T cells, unlike Tfh cells [43].
cTreg and eTreg cell maintenance relies on IL-2 and ICOS signals, respectively, in secondary lymphoid tissues [15]; however, some tissue-specific Treg cells, including colonic Treg cells, are maintained in an IL-2-dependent manner [48]. Although most eTreg cells require TCR signals for their maintenance, a small subset of antigen-experienced Treg cells known as memory Treg cells can survive even in the absence of cognate antigens in an IL-7-dependent manner [49,50].
Recent studies have revealed substantial heterogeneity in gene expression profiles within each Treg subset, suggesting a considerable functional diversity of Treg cells. In fact, single-cell RNA-sequencing (RNA-seq) analysis of Treg cells has demonstrated the complexity of cellular states in both cTreg and eTreg cells and that eTreg cell diversity is affected by TCR signal strength [51]. In addition, comparative single cell RNA-seq analysis of Treg cells in lymphoid and non-lymphoid (skin and colon) tissues suggested that Treg heterogeneity is associated with the progressive trajectory of Treg states from the lymph nodes to non-lymphoid tissues and the adaptation of Treg cells to each peripheral tissue [52]. Furthermore, mounting evidence indicates that differentiation and functional states of distinct Treg subsets are regulated by various transcription factors (Figure 1 and Figure 2, Table 1). In this review, we focus on the transcription factors that play crucial roles in eTreg differentiation and functions.
Table 1.
A summary of eTreg-related transcription factor expression and functions.
| Name | Expression | Function | Target | Upstream Regulator | Ref. |
|---|---|---|---|---|---|
| IRF4 | eTreg | Regulates eTreg differentiation | ICOS, Blimp1, FGL2, ST2, GATA3, CCR8, CTLA4, KLRG1, IL-10, CCR6 | TCR+CD28+IL-2 | [16,53,54] |
| BATF | eTreg | Promotes expression of eTreg-related genes | ICOS, GATA3, ST2, KLRG1, CCR4, CCR6 |
TCR+CD28+IL-2 | [17,54,55] |
| JunB | eTreg | Promotes expression of eTreg-related genes | ICOS, FGL2, CTLA4, TIGIT, KLRG1 |
TCR+CD28+IL-2 | [23,56,57] |
| Blimp-1 | eTreg | Promotes expression of eTreg-related genes | IL-10, KLRG1, Ebi3, CCR6, BCL2 |
TCR+CD28+IL-2 | [58] |
| Myb | eTreg | Promotes expression of eTreg-related genesin tTreg | ICOS, TIGIT, KLRG1, BCL2, MYC |
TCR+CD28+IL-2 | [22] |
| RelA | All Tregs | Promotes expression of eTreg-related genes | TIGIT, ST2, KLRG1, CD103, CD30 | TNF-α, GITR | [59,60] |
| c-Rel | All Tregs | Promotes expression of eTreg-related genes | TIGIT, ST2, ICOS, EBI3,Blimp-1,IL-10 | Unknown | [59,61] |
| Id2 | eTreg | Suppresses Tfr differentiation | CXCR5, IL-10, HIF1-α, IKZF3, Myb, IL-10 rα, E2F2 |
TCR+CD28+IL-2 | [62,63,64] |
| Id3 | cTreg, CD44lo eTreg |
TCR+CD28+IL-2- Erk,PI3K/mTOR | [62,63] | ||
| E2A/ HEB |
eTreg | Suppresses eTreg differentiation | ICOS, IRF4, CD103, KLRG1, RORγt |
TCR+CD28 | [65] |
| TCF1/ LEF1 |
cTreg, CD44lo eTreg |
Regulates Tfr differentiationSuppresses expression of eTreg-related genes? | CD44, ICOS, TIGIT, IRF4, GATA3, Blimp-1, T-bet, Bcl6, CXCR5 |
Unknown | [66,67] |
| Foxo1 | All Treg | Maintains expression of cTreg-related genes | CD62L, CCR7, Bim, GzmB | TCR+CD28-Akt | [18,68] |
| T-bet | eTreg, Tissue Tregs |
Regulates migration to Th1-inflammatory sites | CXCR3 | IFNγ-STAT1 | [45,69] |
| GATA3 | Skin and Intestinal tTreg | Maintains Treg homeostasis Suppresses skin inflammation | ST2, Foxp3 | TCR, IL-4, IL-33 | [37,70,71] |
| RORγt | Intestinal pTreg | Regulates migration to intestinal tissue | CCR6 | IL-6/IL-23-STAT3, Microbiota | [35,36,72] |
| BCL6 | Follicular Treg | Regulates Tfr differentiation | CXCR5, PD-1 | IL-21, IL-6 | [73,74] |
| c-Maf | eTreg | Regulates RORγτ+ Treg and Tfr differentiations | RORγt, CXCR5, IL-10 | TCR+CD28+IL-2, STAT3, Microbiota, Notch1/2 | [75,76,77] |
| STAT3 | All Tregs | Regulates RORγt+ Treg and Tfr differentiation | TCF1, RORγt | IL-6,IL-23,IL-21 | [35,74,75,78,79] |
| PPARγ | VAT-Treg | Regulates VAT-Treg differentiation | CCR1, PCYT1A, DGAT1, IL-10 |
TCR,IL-33, Adipose tissue-derived factor? | [32,34,44,54] |
| RORα | Skin and Colon Tregs | Promotes expression of eTreg-related genes | CCR6, CD73, DR3 | Unknown | [52,80] |
2. Transcription Factors in a Core eTreg Transcriptional Program
Although eTreg cells, particularly tissue Treg cells, heterogeneously express gene subsets including Treg effector molecules and chemokine receptors (Figure 2), it has been suggested that the differentiation of most eTreg cells depends on a common transcriptional program. In this section, we outline the transcription factors that play a role in the core eTreg transcriptional program.
2.1. IRF4
A member of the interferon regulatory factor (IRF) family of transcription factors, IRF4, regulates differentiation of a variety of immune cells [81]. IRF4 is expressed in eTreg and pTreg cells, but not in cTreg cells, depending on TCR signal [16,21,23]. IRF4 is essential for eTreg generation, as IRF4-deficient Treg cells fail to acquire eTreg phenotypes, including decreased CD62L expression and increased ICOS expression [53,58]. Treg-specific IRF4-deficient mice develop a lethal autoimmune disease characterized by loss of body weight, splenomegaly, lymphadenopathy, and inflammation in lung, stomach, and pancreas [48]. Notably, T helper 2 (Th2)-dependent immune responses are specifically activated in Treg-specific IRF4-deficient mice, indicating an essential role for eTreg cells in suppression of Th2-dependent immunity [53]. Furthermore, IRF4 is required for eTreg generation not only under Th2 inflammatory conditions but also under Th1 inflammatory conditions, suggesting a generalized role for IRF4 in eTreg differentiation [58]. Loss of IRF4 in Treg cells results in defective expression of the majority of eTreg-related molecules, including inducible T cell costimulatory (Icos), Il10, Il-1 receptor 11 (Il1rl1), c-maf, fibrinogen-like protein 2 (Fgl2), Ccr8, and PR domain containing 1 (Prdm1), confirming the necessity of IRF4 in eTreg differentiation [53,58]. Importantly, IRF4 can interact physically and functionally with Foxp3 [53,58]. The colocalization of IRF4 and Foxp3 at the Icos locus [53] suggests that IRF4 may cooperate with Foxp3 to regulate eTreg differentiation and function. Thus, IRF4 plays a central role in the eTreg transcriptional program.
2.2. BATF and JunB
IRF4 is thought to interact with AP-1 transcription factors, such as basic leucine zipper ATF-like transcription factor (BATF) and JunB [82], which contain an alpha-helical basic region leucine zipper (bZIP) domain, thereby regulating expression of genes containing AP-1/IRF4 composite elements (AICEs) [83]. Indeed, IRF4, BATF, and JunB colocalize at AICE-containing gene loci in several T cell subsets including Th17 and CD8 cytotoxic T cells [84,85,86]. Furthermore, loss of IRF4 and BATF similarly impair differentiation of Th17 cells, CD8 cytotoxic T cells and T follicular helper (Tfh) cells [86,87,88,89,90,91].
Analysis of BATF-deficient mice has shown that BATF promotes Treg accumulation in the colon and visceral adipose tissues (VAT) [54,92]. Furthermore, a recent analysis of mice bearing Foxp3 A384T mutation, which is found in IPEX patients, revealed a critical role for BATF in eTreg cells [17]. The Foxp3 A384T mutation in mice causes severe inflammation in the skin, liver, and colon and aberrant activation of Th2 and Th17 immune responses, partly due to reduced expression of BATF. Both Foxp3 A384T mutant and BATF-deficient Treg cells fail to differentiate into eTreg cells and to accumulate in peripheral tissues [17].
We have recently reported that JunB regulates an IRF4-dependent eTreg transcriptional program [23]. Like BATF and IRF4, JunB is highly expressed in CD62L–ICOS+ eTreg cells in vivo and can be induced by stimulation with anti-CD3 and anti-CD28 antibodies in the presence of IL-2 in vitro [23]. Treg-specific JunB-deficient mice exhibit severe inflammation in the colon and lung, aberrant activation of Th1, Th2, and Th17 cells, and enhanced humoral immune responses [23]. Unlike IRF4, JunB is not essential for eTreg generation, but JunB is required for homeostasis, colonic accumulation, and TCR-dependent suppressive functions of eTreg cells [23]. JunB regulates expression of a subset of eTreg-related genes, such as Icos, Klrg1, T cell immunoreceptor with Ig and ITIM domains (Tigit), Ctla4, and Gzmb in Treg cells in BATF-dependent and BATF-independent manners [23]. Mechanistically, JunB promotes DNA-binding of IRF4 at loci of eTreg-related genes containing AICE motifs, on which JunB colocalizes with BATF and IRF4, such as Icos and Ctla4. Recently, using different JunB-deficient mouse models, other groups also demonstrated that JunB is essential for the expression of eTreg-related molecules [56,57], although the autoimmune phenotypes of their mice are different from those of our mice [56].
2.3. Blimp1
B lymphocyte induced maturation protein 1, Blimp-1 (encoded by Prdm1) is transcriptionally induced by IRF4 and regulates eTreg functions [50]. Blimp-1 is highly expressed in a subset of eTreg cells producing IL-10 and promotes expression of a subset of IRF4 target genes, including Il10, Klrg1, Epstein-Barr virus induced gene 3 (Ebi3), Ccr6, and B cell leukemia/lymphoma 2 (Bcl2), suggesting an important role for Blimp1 in an IRF4-dependent eTreg transcriptional program [58]. Treg-specific Blimp1-deficient mice are healthy at young ages, but they develop autoimmune inflammation in salivary glands and pancreas with age [93,94]. In an experimental autoimmune encephalomyelitis model, Blimp1 was expressed in Treg cells which accumulate in the inflamed central nervous system and contribute to Treg stability by preventing IL-6-dependent induction of DNA methyltransferase 3 α (Dnmt3a), which inhibits Foxp3 expression by methylating the CNS2 of Foxp3 [95]. Blimp-1 is also preferentially expressed in and is required for the suppressive activity of RORγt+ Treg cells by preventing the production of IL-17 inflammatory cytokine [96].
2.4. Myb
The proto-oncogene myeloblastosis (Myb) has been reported as an important regulator of thymus-derived eTreg cells but not pTreg cells [22]. Myb expression is upregulated in ICOS+ eTreg subset in vivo and is induced in Treg cells activated with TCR/CD28 stimulation in the presence of IL-2 in vitro [22]. In Myb-deficient mice, expression of eTreg-related molecules, such as ICOS, TIGIT, and KLRG1 is severely diminished in Nrp1+ tTreg cells [22]. In contrast, Myb is neither expressed in nor required for differentiation of pTreg cells [22]. Unlike Myb, IRF4 is essential not only for eTreg differentiation but also for pTreg differentiation. Furthermore, although IRF4 is critical for the generation of all eTreg cells, Myb is likely required for differentiation of ICOS+ eTreg cells from ICOS- eTreg cells, by regulating expression of genes that are associated with eTreg cell survival and proliferation [22]. As in T cell development and Th2 differentiation [97,98], Myb promotes expression of GATA3, but not T-box expressed in T cells (T-bet), in ICOS+ eTreg cells [22], suggesting that Myb may also play a role in deciding the fate of GATA3-expressing eTreg subsets. Thus, Myb functions suggest a stepwise transcriptional regulatory mechanism of eTreg differentiation and a key difference between eTreg and pTreg transcriptional regulatory mechanisms.
2.5. NF-κB
The nuclear factor-κB (NF-κB) family is composed of NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel [99]. Two distinct signaling pathways can turn on NF-κB-dependent transcription programs by the inducing active form of NF-κB (dimerized NF-κB): the canonical NF-κb pathway activates RelA/p65 and c-Rel/p65 heterodimers, while the non-canonical NF-κB pathway activates RelB/p52 heterodimers [99]. TCR signal and a co-stimulatory signal cooperate to activate the canonical NF-κB signaling pathway, which promotes conventional T cell activation [100]. The canonical NF-κB signaling pathway has been shown to be critical for the development of Treg cells. Furthermore, recent studies have shown that canonical NF-κB subunits play important roles in eTreg cells [60,101,102].
c-Rel is not only essential for thymic Treg development but also for eTreg functions [59,61]. Deletion of c-Rel in mature Treg cells causes only mild autoimmune phenotypes in aged mice, but it promotes anti-tumor immunity mediated by CTLs [59,61]. In addition, pharmacological inhibition of c-Rel can improve cancer immunotherapy based on immune checkpoint blockage, suggesting that c-Rel is a potential target for cancer immunotherapy [61]. c-Rel-dependent regulation of expression of eTreg-associated molecules, such as Integrin α E (Itgae), Tigit, Klrg1, Il1r2, and TNF receptor superfamily member 8 (Tnfrsf8), may contribute to eTreg-mediated anti-tumor functions, rather than immune homeostasis [61].
RelA also appears to be important for the differentiation and maintenance of eTreg cells. Treg-specific deletion of RelA induces systemic autoimmune disease [59,60,103]. In a competitive setting under non-inflammatory conditions, RelA-deficiency results in significant reduction in eTreg, Tfr, VAT-Treg, and intestinal RORγt+ Treg cells [60]. RelA promotes expression of eTreg-related molecules, such as Il10, Tigit, Icos, Ebi3, Prdm1, and Il1rl1. RelA can be activated by TNF-α and GITR, which may promote eTreg survival [60]. RelA is not required for IRF4 induction and likely works independently of IRF4 in eTreg cells [60].
2.6. Id2, Id3, E2A, and HEB
E proteins (E2A, HEB, E2-2), which belong to the basic-helix-loop-helix (bHLH) transcription factor family, bind to and regulate genes containing E-box motifs [104]. On the other hand, Id proteins (Id1-Id4) can form heterodimers with E-proteins and suppress their transcriptional activity [104]. Id2 and Id3 are essential for Treg maintenance, and Treg-specific Id2/Id3 double knockout mice are lethal due to multi-organ autoimmunity and show increased Tfr number [62]. Although the specific roles of Id2 and Id3 in eTreg cells are not clear, expression of these proteins is dynamically regulated during eTreg differentiation [62,63]. Almost all cTreg cells express Id3, but eTreg cells can be subdivided into Id3+ and Id3- subsets. Id3- eTreg cells highly express ICOS, KLRG1, TIGIT, and CTLA-4, suggesting that loss of Id3 expression is a signature for mature eTreg cells [63]. On the other hand, eTreg cells, but not cTreg cells, highly express Id2 [63]. TCR signaling decreases expression of Id3 and increases expression of Id2 in Treg cells in vitro. A tight regulation of Id protein expression is likely important for Treg cells, as Id2 overexpression impairs stability and the immune suppressive functions of Treg cells [64]. In contrast, a recent study has revealed that E-proteins suppress differentiation of eTreg cells, as E2A/HEB double knockout Treg cells exhibit increased expression of effector Treg signature molecules, such as IRF4, ICOS, CD103, RORγt, and KLRG1, and enhanced eTreg stability and suppressive function. Thus, interaction of Id and E proteins likely control eTreg differentiation [65].
2.7. TCF1 and LEF1
T cell specific transcription factor 1 (TCF1 encoded by Tcf7) and lymphoid enhancer binding factor 1 (LEF1) are members of the high-mobility group (HMG) family, which has highly conserved HMG DNA-binding domains [105]. These molecules have been shown to regulate not only T cell development in the thymus [105] but also eTreg differentiation [66,76]. cTreg cells homogenously express high levels of TCF1 and LEF1, while eTreg cells heterogeneously express these molecules [66]. TCF1− LEF1− eTreg cells, but not TCF1+ LEF1+ eTreg cells, highly express eTreg-related molecules, such as Cd44, Icos, Tigit, Irf4, Gata3, Prdm1, and Tbx21, suggesting that loss of TCF1 and LEF1 correlates with maturation of eTreg cells [66]. On the other hand, a subset of TCF1+LEF1+ eTreg cells express Tfr signature genes, Bcl6 and Cxcr5 [66]. Indeed, Tfr cells can be differentiated from TCF1+ eTreg cells [66]. TCF1 binds directly to the Bcl6 gene locus and regulates its expression, thereby playing an essential role in Tfr differentiation [66]. Treg-specific TCF1/LEF1 double knockout mice show spontaneous systemic inflammation in thyroid and salivary glands, lung, and small and large intestines [66,76].
2.8. Foxo1
Foxo1, as well as Foxp3, belongs to the Forkhead box family of transcription factors [90]. Inactivation of Foxo1 is an essential process for cTreg cells to differentiate into eTreg cells. In cTreg cells, Foxo1 is constitutively expressed, localized in the nucleus, and positively regulates expression of cTreg-related genes such as Sell, which encodes CD62L, and Ccr7 [18,68]. In contrast, in eTreg cells, AKT-dependent phosphorylation destabilizes Foxo1 and prevents it from accumulating in the nucleus, resulting in the inhibition of expression of cTreg-related genes [18,68]. Indeed, Treg cells expressing a constitutive active form of (Akt-insensitive) Foxo1 mutant cannot downregulate expression of CD62L and CCR7 in Treg cells and thereby loses the ability to accumulate in peripheral tissues, which leads to CD8 T cell-dependent autoimmunity and enhanced anti-tumor immunity [18].
3. Transcription Factors Regulating Differentiation of Distinct Subsets of eTreg Cells
eTreg cells generated in lymphoid tissue adopt distinct fates, including differentiating into various tissue Treg and Tfr cells. The differentiation and function of distinct eTreg subpopulations are regulated by various transcriptional programs. In this section, we focus on the transcription factors that contribute to the diversity of eTreg cells.
3.1. T-Bet, GATA3, RORγt, Bcl6 and STAT5
T-bet, GATA3, RORγt, and B cell lymphoma 6 (Bcl6) define cell lineages of Th1, Th2, Th17, and Tfh cells, respectively [106,107,108]. eTreg cells heterogeneously express these transcription factors, which endow eTreg cells with suppressive functions specifically against T helper cells expressing the same transcription factors (Figure 2).
T-bet is a member of the T-box family of transcription factors [109]. Analysis of T-bet fate mapping reporter mice has shown that T-bet is stably expressed in 50–60% of colonic Treg cells and 20–40% of Treg cells in the lymphoid organs, lungs, small intestine, and liver [45]. Depletion of T-bet+ Treg cells in mice results in aberrant activation of Th1 cells without affecting Th2 and Th17 cells [96]. Conversely, specific depletion of T-bet- Treg cells induces aberrant activation of Th2 and Th17 cells but not Th1 cells [45]. Although T-bet-expressing Treg cells have unique immune suppressive functions, Treg-specific deletion of T-bet in mice is not sufficient to develop spontaneous autoimmune inflammation, suggesting that T-bet+ Treg’s suppressive activity is independent of T-bet expression under steady-state conditions [45]. However, T-bet-deficient Treg cells exhibit impaired proliferation under Th1 inflammatory conditions [46]. In Treg cells, T-bet can be induced by IFN-γ-STAT1 signaling and regulate the expression of CX3CR3, which likely promotes migration of Treg cells to specific tissues [45,46].
GATA3, which belongs to the GATA family that bind to GATA motifs [110], is highly expressed in skin Treg cells (~80%) [70,71] and colonic Treg cells (~30%) [37,71]. A recent study has shown that Treg-specific GATA3-deficient mice develop severe skin inflammation with aberrant activation of a Type 2 immune responses [70]. Another study has shown that GATA3 and T-bet double knockout mice, but not their single knockout mice, develop multi-organ autoimmunity [47]. Like in Th2 cells, IL-4 and IL-33 can upregulate GATA3 expression, which in turn promotes expression of IL-33 receptor ST2 in Treg cells. GATA3 is required for stable Foxp3 expression in GATA3+ Treg cells [37,71,111].
RORγt is a member of the nuclear receptor family [112]. RORγt is expressed in colonic Treg cells (~65%) and small intestinal Treg cells (~35%) [72]. The majority of RORγt+ Treg cells are Nrp1− Helios1− pTreg cells, which are induced by commensal microbiota [35,36]. Probably depending on different environmental cues, Treg-specific RORγt-deficient mice have shown impaired control of distinct types of immune responses [35,36]. A report has shown that Treg-specific RORγt-deficient mice have increased levels of serum IgE at the steady state, and their Th2 immune responses are accelerated under Th2-inducing conditions [35]. Another report has shown that Treg-specific RORγt-deficient mice have elevated production of Th17 and Th1 cytokines, but not Th2 cytokines, in the colon and are more susceptible to trinitrobenzenesulfonic acid-induced colitis [36]. Furthermore, a recent study has shown that a unique property of RORγt+ Treg cells, but not Treg’s RORγt itself, is critical for suppression of Th17-dependent gut chronic inflammation [113]. Colonization of mice by Helicobacter hepaticus results in accumulation of colonic RORγt+ Treg cells bearing TCRs specific to H. hepaticus [113]. In Treg-specific RORγt-deficient mice, although there is a mild increase in colonic H. hepaticus-specific Th17 cells, gut inflammation is not induced upon H. hepaticus colonization [113]. However, depletion of RORγt+ Treg cells by deleting the transcription factor c-Maf (further discussed below), which is critical for generation of this cell population, causes significant increase of H. hepaticus-specific Th17 cells and severe gut inflammation [113].
Bcl6 is expressed in and required for differentiation of Tfr cells, which control germinal center reactions [38,39]. In Treg-specific Bcl6-deficient mice, in which Tfr cells are not generated, humoral immune responses during virus infection are significantly enhanced, and humoral autoimmunity is spontaneously induced in aged mice [114]. Bcl6 directly regulates CXCR5 expression in both Tfh and Tfr cells [115], which enables Tfr cells to accumulate in the germinal centers together with Tfh cells and to suppress Tfh-dependent humoral immunity [38,39,40]. Signal transducer and activator transcription 5 (STAT5) can inhibit Bcl6 expression directly or indirectly by inducing Blimp-1 and is a negative regulator of Tfh differentiation [116,117,118]. Like Tfh cells, Tfr differentiation is inhibited by IL-2-dependent STAT5 activation. Indeed, significant Tfr differentiation suppression has been observed during viral infection when large amounts of IL-2 are produced [73]. Furthermore, loss of CD25 significantly decreases both the frequency and numbers of cTreg cells, but it does not affect the eTreg population [15,119], suggesting that the homeostasis of cTreg cells, but not eTreg cells, is dependent on IL-2/STAT5 signal. However, whether STAT5 promotes cTreg survival or inhibits eTreg differentiation remains unclear.
3.2. c-Maf
c-Maf (encoded by Maf), which belongs to the AP-1 family of transcription factors, regulates the function and development of various T cells [82,120]. c-Maf is essential for induction and maintenance of RORγt+ Treg cells and Tfr cells [75,76,77]. c-Maf is highly expressed in CD62L- eTreg cells and intestinal Treg cells and is induced by CD3, CD28 and IL-2 stimulation in vitro [76,77]. As mentioned above, in mice colonized with H. hepaticus, generation of H. hepaticus-specific RORγt+ Treg cells depends on c-Maf [75]. Furthermore, c-Maf-dependent suppression of colonic Th17 responses and IgA production contribute to the maintenance of a healthy gut microbiota [76]. In Treg cells, c-Maf facilitates expression of IL-10, while inhibiting expression of IL-17 [75].
3.3. STAT3
c-Maf and RORγt expression in Treg cells, as in CD4 T conventional cells, is regulated by signal transducer and activator transcription 3 (STAT3) [35,75]. IL-6 and IL-23, both of which activate STAT3, promote generation of colonic RORγt+ Treg cells [35]. Furthermore, IL-27, IL-6, and IL-21 can induce c-Maf expression in Treg cells in vitro in a STAT3-dependent manner [75]. Similar to c-Maf, Treg-specific ablation of STAT3 results in the development of spontaneous colitis with abnormal increase of colonic Th17 cells [78]. STAT3 is also involved in Tfr differentiation, with Treg-specific STAT3-deficient mice exhibiting a severely reduced Tfr cell number [75,79].Mammalian target of rapamycin (mTOR) signal, which is required for Tfr generation, phosphorylates STAT3, which then binds to and likely promotes the expression of Tcf1, which is essential for Tfr differentiation [79].
3.4. PPARγ and RORα
In addition to the above-mentioned lineage-defining transcription factors for T helper differentiation and their regulatory transcription factors, eTreg cells heterogeneously express peroxisome-proliferator-activated receptor γ (PPARγ) and retinoic acid receptor-related orphan receptor α (RORα) [33,80], which regulate tissue-specific eTreg functions (Figure 2).
PPARγ, which is an essential transcription factor for adipocyte differentiation, is required for the differentiation and functions of VAT-Treg cells [33]. VAT-Treg cells have been identified as a unique subset of tissue Treg cells that accumulate in and maintain inflammation of adipose tissues, thereby regulating insulin resistance [32,33]. In Treg-specific PPARγ-deficient mice, VAT-Treg cells are not generated, and the effect of pioglitazone, an insulin-sensitizing drug, is diminished, suggesting that VAT-Treg cells are a therapeutic target of diabetes [33]. VAT-Treg differentiation proceeds in a stepwise manner: PPARγlo Treg cells, which are induced by unknown mechanisms in the secondary lymphoid organs, migrate into adipose tissues and further differentiate into PPARγhi VAT-Treg cells, depending on adipose tissue-specific signals as well as TCR, Foxp3, and IL33-ST2 signals [44]. TCR-dependent activation of IRF4 and BATF is required for generation of PPARγ+ Treg cells, probably due to their essential roles in ST2+ eTreg differentiation [54].
A nuclear receptor RORα is also involved in the regulation of tissue-specific functions of eTreg cells. Skin Treg cells express high RORα levels, with Treg-specific Rorα deletion accelerating the inflammation induced by innate lymphocyte 2 (ILC2) and Th2 cells in an atopic dermatitis mouse model [80]. In skin Treg cells, RORα promotes the expression of death receptor 3 (DR3), which is also expressed in and promotes activity of ILC2. RORα-dependent DR3 expression allows skin Treg cells to compete with ILC2 for the DR3 ligand, thereby suppressing ILC2-dependent immunity [80]. It has also been suggested that the RORα-dependent suppression of IL-4 expression is required for the immune suppressive activity of skin Treg cells. Like skin Treg cells, colonic Treg cells express higher RORα levels than lymphoid tissue Treg cells [52], suggesting that RORα may regulate Treg functions in non-lymphoid tissues other than skin.
4. Conclusions and Perspectives
A central driver of eTreg differentiation is a transcriptional program mediated by IRF4, which is activated by TCR. In concert with BATF, JunB, and probably other AP-1 transcription factors, IRF4 regulates expression of a majority of eTreg-related genes. IRF4-induced Blimp1 promotes expression of a subset of eTreg-related genes, such as IL-10. In addition, Myb and NF-κB are also crucial for the unfolding of the TCR-dependent eTreg transcriptional program. Furthermore, inactivation of cTreg-related transcription factor Foxo1 is also required for eTreg differentiation. Heterogeneous expression of these transcription factors in each eTreg cell, probably due to different strength and/or duration of TCR signal, may contribute to the diversity of eTreg phenotypes. Future studies are needed to reveal how these TCR-dependent transcription factors cooperate with each other in eTreg differentiation.
eTreg cells migrate to targeted tissues, adapt to the tissue environments, and exert specific suppressive functions, depending on the transcription factors that are essential for differentiation of T helper cell lineages (T-bet, GATA3, RORγt, and Bcl6) and tissue-specific cell types (e.g., PPAR-γ). Expression of these transcription factors in eTreg cells can be induced in specific cytokine environments, but the in vivo mechanisms regulating the expression of these transcription factors are not fully understood. Particularly, how diverse eTreg subsets are generated and maintained at the steady state remains an open question.
Like well-characterized CD8 memory T cells, a subset of antigen-experienced Treg cells can survive for a long time after the removal of their cognate antigens and mount stronger suppressive activity upon re-exposure to the same antigens [20,49,50,121]. Like eTreg cells, memory Treg cells also exhibit decreased expression of the homing receptors CD62L and CCR7, and increased expression of CTLA4 and ICOS [49,50]. This phenotypic similarity suggests a possible association between the transcriptional programs of eTreg and memory Treg cells; however, the transcriptional mechanisms underlying memory Treg differentiation remain largely unexplored.
In addition to the transcription factors discussed in this review, epigenetic modifications are closely associated with eTreg differentiation [55,122,123,124,125,126,127,128]; however, the molecular links between the Treg transcriptional program and epigenetic regulation remain poorly understood. Further elucidation of the roles of eTreg-related transcription factors in gene expression, chromatin accessibility, and histone modifications at the single cell level will allow us to identify the missing link between transcription factors and epigenetic regulation in eTreg cells.
It has also been suggested that metabolic control is important in eTreg differentiation and function [79,129,130]. For example, mTOR-dependent cholesterol biosynthesis likely promotes the proliferation and expression of CTLA4 and ICOS in TCR-stimulated Treg cells [129], suggesting that mTOR-dependent metabolic pathways play a role in eTreg energy generation. Interestingly, recent studies have demonstrated that specific metabolic pathways and/or metabolites can regulate the epigenetic status of T cells [113,131,132]. Mitochondrial respiration levels are higher in Treg cells than in other T cell subsets, and the loss of mitochondria respiratory chain complex III results in decreased Treg suppressive activity and increased DNA methylation [133], although the relevance of this in eTreg populations remains unclear. It is important to determine how, and to what extent, cellular metabolic pathways or metabolites regulate the activity of eTreg-related transcription factors and epigenetic regulators.
Thus, further elucidation of transcriptional regulatory mechanisms and their crosstalk with epigenetics and metabolism in the differentiation of eTreg, tissue Treg, and memory Treg cells may contribute towards the development of drugs that target specific Treg functions.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author Contributions
Conceptualization and writing, S.-i.K. and H.I.
Funding
This work was supported by KAKENHI grant (16K19164, 18K15201) from JSPS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Brunkow M.E., Jeffery E.W., Hjerrild K.A., Paeper B., Clark L.B., Yasayko S.A., Wilkinson J.E., Galas D., Ziegler S.F., Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 6.Wildin R.S., Ramsdell F., Peake J., Faravelli F., Casanova J.L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot J.D., Dooley J.L., Farr A.G., Rudensky A.Y. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas A.K., Benoist C., Bluestone J.A., Campbell D.J., Ghosh S., Hori S., Jiang S., Kuchroo V.K., Mathis D., Roncarolo M.G., et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat. Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh C.S., Lee H.M., Lio C.W. Selection of regulatory T cells in the thymus. Nat. Rev. Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 10.Klein L., Robey E.A., Hsieh C.S. Central CD4(+) T cell tolerance: Deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 2019;19:7–18. doi: 10.1038/s41577-018-0083-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav M., Louvet C., Davini D., Gardner J.M., Martinez-Llordella M., Bailey-Bucktrout S., Anthony B.A., Sverdrup F.M., Head R., Kuster D.J., et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss J.M., Bilate A.M., Gobert M., Ding Y., Curotto de Lafaille M.A., Parkhurst C.N., Xiong H., Dolpady J., Frey A.B., Ruocco M.G., et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefowicz S.Z., Niec R.E., Kim H.Y., Treuting P., Chinen T., Zheng Y., Umetsu D.T., Rudensky A.Y. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smigiel K.S., Richards E., Srivastava S., Thomas K.R., Dudda J.C., Klonowski K.D., Campbell D.J. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine A.G., Arvey A., Jin W., Rudensky A.Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayatsu N., Miyao T., Tachibana M., Murakami R., Kimura A., Kato T., Kawakami E., Endo T.A., Setoguchi R., Watarai H., et al. Analyses of a Mutant Foxp3 Allele Reveal BATF as a Critical Transcription Factor in the Differentiation and Accumulation of Tissue Regulatory T Cells. Immunity. 2017;47:268–283.e9. doi: 10.1016/j.immuni.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Luo C.T., Liao W., Dadi S., Toure A., Li M.O. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529:532–536. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liston A., Gray D.H. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 20.Li M.O., Rudensky A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vahl J.C., Drees C., Heger K., Heink S., Fischer J.C., Nedjic J., Ohkura N., Morikawa H., Poeck H., Schallenberg S., et al. Continuous T Cell Receptor Signals Maintain a Functional Regulatory T Cell Pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Dias S., D’Amico A., Cretney E., Liao Y., Tellier J., Bruggeman C., Almeida F.F., Leahy J., Belz G.T., Smyth G.K., et al. Effector Regulatory T Cell Differentiation and Immune Homeostasis Depend on the Transcription Factor Myb. Immunity. 2017;46:78–91. doi: 10.1016/j.immuni.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi S.I., Sasaki D., Hsieh T.H., Taira N., Arakaki N., Yamasaki S., Wang K., Sarkar S., Shirahata H., Miyagi M., et al. JunB regulates homeostasis and suppressive functions of effector regulatory T cells. Nat. Commun. 2018;9:5344. doi: 10.1038/s41467-018-07735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X., Zhang J., Gu Q., Huang M., Zhang W., Guo J., Zhou X. Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Rep. 2017;21:1853–1869. doi: 10.1016/j.celrep.2017.10.090. [DOI] [PubMed] [Google Scholar]
- 25.Panduro M., Benoist C., Mathis D. Tissue Tregs. Annu. Rev. Immunol. 2016;34:609–633. doi: 10.1146/annurev-immunol-032712-095948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M., Komai K., Nakamura T., Srirat T., Yoshimura A. Tissue regulatory T cells and neural repair. Int. Immunol. 2019;31:361–369. doi: 10.1093/intimm/dxz031. [DOI] [PubMed] [Google Scholar]
- 27.Arpaia N., Green J.A., Moltedo B., Arvey A., Hemmers S., Yuan S., Treuting P.M., Rudensky A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuswanto W., Burzyn D., Panduro M., Wang K.K., Jang Y.C., Wagers A.J., Benoist C., Mathis D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali N., Zirak B., Rodriguez R.S., Pauli M.L., Truong H.A., Lai K., Ahn R., Corbin K., Lowe M.M., Scharschmidt T.C., et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell. 2017;169:1119–1129.e11. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M., Komai K., Mise-Omata S., Iizuka-Koga M., Noguchi Y., Kondo T., Sakai R., Matsuo K., Nakayama T., Yoshie O., et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 31.Mathur A.N., Zirak B., Boothby I.C., Tan M., Cohen J.N., Mauro T.M., Mehta P., Lowe M.M., Abbas A.K., Ali N., et al. Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity. 2019;50:655–667.e4. doi: 10.1016/j.immuni.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A.B., Benoist C., Shoelson S., et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipolletta D., Feuerer M., Li A., Kamei N., Lee J., Shoelson S.E., Benoist C., Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodin D., van Panhuys N., Li C., Magnuson A.M., Cipolletta D., Miller C.M., Wagers A., Germain R.N., Benoist C., Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohnmacht C., Park J.H., Cording S., Wing J.B., Atarashi K., Obata Y., Gaboriau-Routhiau V., Marques R., Dulauroy S., Fedoseeva M., et al. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 36.Sefik E., Geva-Zatorsky N., Oh S., Konnikova L., Zemmour D., McGuire A.M., Burzyn D., Ortiz-Lopez A., Lobera M., Yang J., et al. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiering C., Krausgruber T., Chomka A., Fröhlich A., Adelmann K., Wohlfert E.A., Pott J., Griseri T., Bollrath J., Hegazy A.N., et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung Y., Tanaka S., Chu F., Nurieva R.I., Martinez G.J., Rawal S., Wang Y.H., Lim H., Reynolds J.M., Zhou X.H., et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linterman M.A., Pierson W., Lee S.K., Kallies A., Kawamoto S., Rayner T.F., Srivastava M., Divekar D.P., Beaton L., Hogan J.J., et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollenberg I., Agua-Doce A., Hernandez A., Almeida C., Oliveira V.G., Faro J., Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 41.Fonseca V.R., Agua-Doce A., Maceiras A.R., Pierson W., Ribeiro F., Romao V.C., Pires A.R., da Silva S.L., Fonseca J.E., Sousa A.E., et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci. Immunol. 2017:2. doi: 10.1126/sciimmunol.aan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wing J.B., Kitagawa Y., Locci M., Hume H., Tay C., Morita T., Kidani Y., Matsuda K., Inoue T., Kurosaki T., et al. A distinct subpopulation of CD25(-) T-follicular regulatory cells localizes in the germinal centers. Proc. Natl. Acad. Sci. USA. 2017;114:E6400–E6409. doi: 10.1073/pnas.1705551114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maceiras A.R., Almeida S.C.P., Mariotti-Ferrandiz E., Chaara W., Jebbawi F., Six A., Hori S., Klatzmann D., Faro J., Graca L. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat. Commun. 2017;8:15067. doi: 10.1038/ncomms15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C., DiSpirito J.R., Zemmour D., Spallanzani R.G., Kuswanto W., Benoist C., Mathis D. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell. 2018;174:285–299.e12. doi: 10.1016/j.cell.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine A.G., Medoza A., Hemmers S., Moltedo B., Niec R.E., Schizas M., Hoyos B.E., Putintseva E.V., Chaudhry A., Dikiy S., et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546:421–425. doi: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch M.A., Tucker-Heard G., Perdue N.R., Killebrew J.R., Urdahl K.B., Campbell D.J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu F., Sharma S., Edwards J., Feigenbaum L., Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat. Immunol. 2015;16:197–206. doi: 10.1038/ni.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L., Chu C., Teng F., Bessman N.J., Goc J., Santosa E.K., Putzel G.G., Kabata H., Kelsen J.R., Baldassano R.N., et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568:405–409. doi: 10.1038/s41586-019-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenblum M.D., Gratz I.K., Paw J.S., Lee K., Marshak-Rothstein A., Abbas A.K. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenblum M.D., Way S.S., Abbas A.K. Regulatory T cell memory. Nat. Rev. Immunol. 2016;16:90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zemmour D., Zilionis R., Kiner E., Klein A.M., Mathis D., Benoist C. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 2018;19:291–301. doi: 10.1038/s41590-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miragaia R.J., Gomes T., Chomka A., Jardine L., Riedel A., Hegazy A.N., Whibley N., Tucci A., Chen X., Lindeman I., et al. Single-Cell Transcriptomics of Regulatory T Cells Reveals Trajectories of Tissue Adaptation. Immunity. 2019;50:493–504.e7. doi: 10.1016/j.immuni.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y., Chaudhry A., Kas A., deRoos P., Kim J.M., Chu T.T., Corcoran L., Treuting P., Klein U., Rudensky A.Y. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasanthakumar A., Moro K., Xin A., Liao Y., Gloury R., Kawamoto S., Fagarasan S., Mielke L.A., Afshar-Sterle S., Masters S.L., et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 55.Delacher M., Imbusch C.D., Weichenhan D., Breiling A., Hotz-Wagenblatt A., Trager U., Hofer A.C., Kagebein D., Wang Q., Frauhammer F., et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat. Immunol. 2017;18:1160–1172. doi: 10.1038/ni.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J., Ma S., Hotz-Wagenblatt A., Angel P., Mohr K., Schlimbach T., Schmitt M., Cui G. Regulatory T cells sense effector T-cell activation through synchronized JunB expression. FEBS Lett. 2019;593:1020–1029. doi: 10.1002/1873-3468.13393. [DOI] [PubMed] [Google Scholar]
- 57.Katagiri T., Yamazaki S., Fukui Y., Aoki K., Yagita H., Nishina T., Mikami T., Katagiri S., Shiraishi A., Kimura S., et al. JunB plays a crucial role in development of regulatory T cells by promoting IL-2 signaling. Mucosal Immunol. 2019 doi: 10.1038/s41385-019-0182-0. [DOI] [PubMed] [Google Scholar]
- 58.Cretney E., Xin A., Shi W., Minnich M., Masson F., Miasari M., Belz G.T., Smyth G.K., Busslinger M., Nutt S.L., et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 59.Oh H., Grinberg-Bleyer Y., Liao W., Maloney D., Wang P., Wu Z., Wang J., Bhatt D.M., Heise N., Schmid R.M., et al. An NF-κB Transcription-Factor-Dependent Lineage-Specific Transcriptional Program Promotes Regulatory T Cell Identity and Function. Immunity. 2017;47:450–465.e5. doi: 10.1016/j.immuni.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasanthakumar A., Liao Y., Teh P., Pascutti M.F., Oja A.E., Garnham A.L., Gloury R., Tempany J.C., Sidwell T., Cuadrado E., et al. The TNF Receptor Superfamily-NF-kappaB Axis Is Critical to Maintain Effector Regulatory T Cells in Lymphoid and Non-lymphoid Tissues. Cell Rep. 2017;20:2906–2920. doi: 10.1016/j.celrep.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 61.Grinberg-Bleyer Y., Oh H., Desrichard A., Bhatt D.M., Caron R., Chan T.A., Schmid R.M., Klein U., Hayden M.S., Ghosh S. NF-κB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer. Cell. 2017;170:1096–1108.e13. doi: 10.1016/j.cell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyazaki M., Miyazaki K., Chen S., Itoi M., Miller M., Lu L.F., Varki N., Chang A.N., Broide D.H., Murre C. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat. Immunol. 2014;15:767–776. doi: 10.1038/ni.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan J.M., Höllbacher B., Campbell D.J. Cutting Edge: Dynamic Expression of Id3 Defines the Stepwise Differentiation of Tissue-Resident Regulatory T Cells. J. Immunol. 2019;202:31–36. doi: 10.4049/jimmunol.1800917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang S.M., Sharma G., Verma R., Byun S., Rudra D., Im S.H. Inflammation-induced Id2 promotes plasticity in regulatory T cells. Nat. Commun. 2018;9:4736. doi: 10.1038/s41467-018-07254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han X., Huang H., Gao P., Zhang Q., Liu X., Jia B., Strober W., Hou B., Zhou X., Gao G.F., et al. E-protein regulatory network links TCR signaling to effector Treg cell differentiation. Proc. Natl. Acad. Sci. USA. 2019 doi: 10.1073/pnas.1800494116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang B.H., Wang K., Wan S., Liang Y., Yuan X., Dong Y., Cho S., Xu W., Jepsen K., Feng G.S., et al. TCF1 and LEF1 Control Treg Competitive Survival and Tfr Development to Prevent Autoimmune Diseases. Cell Rep. 2019;27:3629–3645.e6. doi: 10.1016/j.celrep.2019.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xing S., Gai K., Li X., Shao P., Zeng Z., Zhao X., Zhao X., Chen X., Paradee W.J., Meyerholz D.K., et al. Tcf1 and Lef1 are required for the immunosuppressive function of regulatory T cells. J. Exp. Med. 2019;216:847–866. doi: 10.1084/jem.20182010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ouyang W., Liao W., Luo C.T., Yin N., Huse M., Kim M.V., Peng M., Chan P., Ma Q., Mo Y., et al. Novel Foxo1-dependent transcriptional programs control Treg cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koch M.A., Thomas K.R., Perdue N.R., Smigiel K.S., Srivastava S., Campbell D.J. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison O.J., Linehan J.L., Shih H.-Y., Bouladoux N., Han S.-J., Smelkinson M., Sen S.K., Byrd A.L., Enamorado M., Yao C., et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science. 2019;363:eaat6280. doi: 10.1126/science.aat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wohlfert E.A., Grainger J.R., Bouladoux N., Konkel J.E., Oldenhove G., Ribeiro C.H., Hall J.A., Yagi R., Naik S., Bhairavabhotla R., et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J. Clin. Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim K.S., Hong S.W., Han D., Yi J., Jung J., Yang B.G., Lee J.Y., Lee M., Surh C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 73.Botta D., Fuller M.J., Marquez-Lago T.T., Bachus H., Bradley J.E., Weinmann A.S., Zajac A.J., Randall T.D., Lund F.E., Leon B., et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat. Immunol. 2017;18:1249–1260. doi: 10.1038/ni.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H., Xie M.M., Liu H., Dent A.L. Stat3 Is Important for Follicular Regulatory T Cell Differentiation. PLoS ONE. 2016;11:e0155040. doi: 10.1371/journal.pone.0155040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu M., Pokrovskii M., Ding Y., Yi R., Au C., Harrison O.J., Galan C., Belkaid Y., Bonneau R., Littman D.R. C-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature. 2018;554:373–377. doi: 10.1038/nature25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neumann C., Blume J., Roy U., Teh P.P., Vasanthakumar A., Beller A., Liao Y., Heinrich F., Arenzana T.L., Hackney J.A., et al. c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nat. Immunol. 2019;20:471–481. doi: 10.1038/s41590-019-0316-2. [DOI] [PubMed] [Google Scholar]
- 77.Wheaton J.D., Yeh C.H., Ciofani M. Cutting Edge: C-Maf Is Required for Regulatory T Cells To Adopt RORgammat(+) and Follicular Phenotypes. J. Immunol. 2017;199:3931–3936. doi: 10.4049/jimmunol.1701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudhry A., Rudra D., Treuting P., Samstein R.M., Liang Y., Kas A., Rudensky A.Y. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu L., Huang Q., Wang H., Hao Y., Bai Q., Hu J., Li Y., Wang P., Chen X., He R., et al. The Kinase mTORC1 Promotes the Generation and Suppressive Function of Follicular Regulatory T Cells. Immunity. 2017;47:538–551.e5. doi: 10.1016/j.immuni.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Malhotra N., Leyva-Castillo J.M., Jadhav U., Barreiro O., Kam C., O’Neill N.K., Meylan F., Chambon P., von Andrian U.H., Siegel R.M., et al. RORalpha-expressing T regulatory cells restrain allergic skin inflammation. Sci. Immunol. 2018:3. doi: 10.1126/sciimmunol.aao6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tamura T., Yanai H., Savitsky D., Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 82.Eferl R., Wagner E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 83.Murphy T.L., Tussiwand R., Murphy K.M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 84.Hasan Z., Koizumi S.I., Sasaki D., Yamada H., Arakaki N., Fujihara Y., Okitsu S., Shirahata H., Ishikawa H. JunB is essential for IL-23-dependent pathogenicity of Th17 cells. Nat. Commun. 2017;8:15628. doi: 10.1038/ncomms15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carr T.M., Wheaton J.D., Houtz G.M., Ciofani M. JunB promotes Th17 cell identity and restrains alternative CD4(+) T-cell programs during inflammation. Nat. Commun. 2017;8:301. doi: 10.1038/s41467-017-00380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurachi M., Barnitz R.A., Yosef N., Odorizzi P.M., DiIorio M.A., Lemieux M.E., Yates K., Godec J., Klatt M.G., Regev A., et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 2014;15:373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brustle A., Heink S., Huber M., Rosenplanter C., Stadelmann C., Yu P., Arpaia E., Mak T.W., Kamradt T., Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 88.Schraml B.U., Hildner K., Ise W., Lee W.L., Smith W.A., Solomon B., Sahota G., Sim J., Mukasa R., Cemerski S., et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao S., Buzo B.F., Pham D., Jiang L., Taparowsky E.J., Kaplan M.H., Sun J. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–845. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Man K., Miasari M., Shi W., Xin A., Henstridge D.C., Preston S., Pellegrini M., Belz G.T., Smyth G.K., Febbraio M.A., et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 91.Ise W., Kohyama M., Schraml B.U., Zhang T., Schwer B., Basu U., Alt F.W., Tang J., Oltz E.M., Murphy T.L., et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C., Thangamani S., Kim M., Gu B.H., Lee J.H., Taparowsky E.J., Kim C.H. BATF is required for normal expression of gut-homing receptors by T helper cells in response to retinoic acid. J. Exp. Med. 2013;210:475–489. doi: 10.1084/jem.20121088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cretney E., Leung P.S., Trezise S., Newman D.M., Rankin L.C., Teh C.E., Putoczki T.L., Gray D.H., Belz G.T., Mielke L.A., et al. Characterization of Blimp-1 function in effector regulatory T cells. J. Autoimmun. 2018;91:73–82. doi: 10.1016/j.jaut.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Bankoti R., Ogawa C., Nguyen T., Emadi L., Couse M., Salehi S., Fan X., Dhall D., Wang Y., Brown J., et al. Differential regulation of Effector and Regulatory T cell function by Blimp1. Sci Rep. 2017;7:12078. doi: 10.1038/s41598-017-12171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garg G., Muschaweckh A., Moreno H., Vasanthakumar A., Floess S., Lepennetier G., Oellinger R., Zhan Y., Regen T., Hiltensperger M., et al. Blimp1 Prevents Methylation of Foxp3 and Loss of Regulatory T Cell Identity at Sites of Inflammation. Cell Rep. 2019;26:1854–1868.e5. doi: 10.1016/j.celrep.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogawa C., Bankoti R., Nguyen T., Hassanzadeh-Kiabi N., Nadeau S., Porritt R.A., Couse M., Fan X., Dhall D., Eberl G., et al. Blimp-1 Functions as a Molecular Switch to Prevent Inflammatory Activity in Foxp3(+)RORgammat(+) Regulatory T Cells. Cell Rep. 2018;25:19–28.e5. doi: 10.1016/j.celrep.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maurice D., Hooper J., Lang G., Weston K. c-Myb regulates lineage choice in developing thymocytes via its target gene Gata3. EMBO J. 2007;26:3629–3640. doi: 10.1038/sj.emboj.7601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakata Y., Brignier A.C., Jin S., Shen Y., Rudnick S.I., Sugita M., Gewirtz A.M. c-Myb, Menin, GATA-3, and MLL form a dynamic transcription complex that plays a pivotal role in human T helper type 2 cell development. Blood. 2010;116:1280–1290. doi: 10.1182/blood-2009-05-223255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taniguchi K., Karin M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 100.Gerondakis S., Fulford T.S., Messina N.L., Grumont R.J. NF-kappaB control of T cell development. Nat. Immunol. 2014;15:15–25. doi: 10.1038/ni.2785. [DOI] [PubMed] [Google Scholar]
- 101.Long M.X., Park S.G., Strickland I., Hayden M.S., Ghosh S. Nuclear Factor-kappa B Modulates Regulatory T Cell Development by Directly Regulating Expression of Foxp3 Transcription Factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 102.Ruan Q., Kameswaran V., Tone Y., Li L., Liou H.C., Greene M.I., Tone M., Chen Y.H. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Messina N., Fulford T., O’Reilly L., Loh W.X., Motyer J.M., Ellis D., McLean C., Naeem H., Lin A., Gugasyan R., et al. The NF-kappaB transcription factor RelA is required for the tolerogenic function of Foxp3(+) regulatory T cells. J. Autoimmun. 2016;70:52–62. doi: 10.1016/j.jaut.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 104.Kee B.L. E and ID proteins branch out. Nat. Rev. Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 105.Steinke F.C., Xue H.H. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol. Res. 2014;59:45–55. doi: 10.1007/s12026-014-8545-9. [DOI] [PubMed] [Google Scholar]
- 106.Zhu J., Yamane H., Paul W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stockinger B., Omenetti S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017;17:535–544. doi: 10.1038/nri.2017.50. [DOI] [PubMed] [Google Scholar]
- 108.Vinuesa C.G., Linterman M.A., Yu D., MacLennan I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 109.Pritchard G.H., Kedl R.M., Hunter C.A. The evolving role of T-bet in resistance to infection. Nat. Rev. Immunol. 2019;19:398–410. doi: 10.1038/s41577-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tindemans I., Serafini N., DiSanto J.P., Hendriks R.W. GATA-3 function in innate and adaptive immunity. Immunity. 2014;41:191–206. doi: 10.1016/j.immuni.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y., Su M.A., Wan Y.Y. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eberl G. RORgammat, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 2017;10:27–34. doi: 10.1038/mi.2016.86. [DOI] [PubMed] [Google Scholar]
- 113.Xu T., Stewart K.M., Wang X., Liu K., Xie M., Ryu J.K., Li K., Ma T., Wang H., Ni L., et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature. 2017;548:228–233. doi: 10.1038/nature23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu W., Liu X., Lin X., Feng H., Sun L., Li S., Chen H., Tang H., Lu L., Jin W., et al. Deficiency in T follicular regulatory cells promotes autoimmunity. J. Exp. Med. 2018;215:815–825. doi: 10.1084/jem.20170901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatzi K., Nance J.P., Kroenke M.A., Bothwell M., Haddad E.K., Melnick A., Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oestreich K.J., Mohn S.E., Weinmann A.S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnston R.J., Choi Y.S., Diamond J.A., Yang J.A., Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDonald P.W., Read K.A., Baker C.E., Anderson A.E., Powell M.D., Ballesteros-Tato A., Oestreich K.J. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat. Commun. 2016;7:10285. doi: 10.1038/ncomms10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fan M.Y., Low J.S., Tanimine N., Finn K.K., Priyadharshini B., Germana S.K., Kaech S.M., Turka L.A. Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep. 2018;25:1204–1213.e4. doi: 10.1016/j.celrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gabrysova L., Alvarez-Martinez M., Luisier R., Cox L.S., Sodenkamp J., Hosking C., Perez-Mazliah D., Whicher C., Kannan Y., Potempa K., et al. c-Maf controls immune responses by regulating disease-specific gene networks and repressing IL-2 in CD4(+) T cells. Nat. Immunol. 2018;19:497–507. doi: 10.1038/s41590-018-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gratz I.K., Truong H.A., Yang S.H., Maurano M.M., Lee K., Abbas A.K., Rosenblum M.D. Cutting Edge: Memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DuPage M., Chopra G., Quiros J., Rosenthal W.L., Morar M.M., Holohan D., Zhang R., Turka L., Marson A., Bluestone J.A. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang D., Quiros J., Mahuron K., Pai C.C., Ranzani V., Young A., Silveria S., Harwin T., Abnousian A., Pagani M., et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep. 2018;23:3262–3274. doi: 10.1016/j.celrep.2018.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanaka S., Pfleger C., Lai J.F., Roan F., Sun S.C., Ziegler S.F. KAP1 Regulates Regulatory T Cell Function and Proliferation in Both Foxp3-Dependent and -Independent Manners. Cell Rep. 2018;23:796–807. doi: 10.1016/j.celrep.2018.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hou S., Clement R.L., Diallo A., Blazar B.R., Rudensky A.Y., Sharpe A.H., Sage P.T. FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program. J. Exp. Med. 2019;216:605–620. doi: 10.1084/jem.20181134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yue X., Lio C.J., Samaniego-Castruita D., Li X., Rao A. Loss of TET2 and TET3 in regulatory T cells unleashes effector function. Nat. Commun. 2019;10:2011. doi: 10.1038/s41467-019-09541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakatsukasa H., Oda M., Yin J., Chikuma S., Ito M., Koga-Iizuka M., Someya K., Kitagawa Y., Ohkura N., Sakaguchi S., et al. Loss of TET proteins in regulatory T cells promotes abnormal proliferation, Foxp3 destabilization and IL-17 expression. Int. Immunol. 2019;31:335–347. doi: 10.1093/intimm/dxz008. [DOI] [PubMed] [Google Scholar]
- 128.Obata Y., Furusawa Y., Endo T.A., Sharif J., Takahashi D., Atarashi K., Nakayama M., Onawa S., Fujimura Y., Takahashi M., et al. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat. Immunol. 2014;15:571–579. doi: 10.1038/ni.2886. [DOI] [PubMed] [Google Scholar]
- 129.Zeng H., Yang K., Cloer C., Neale G., Vogel P., Chi H. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chapman N.M., Zeng H., Nguyen T.M., Wang Y., Vogel P., Dhungana Y., Liu X., Neale G., Locasale J.W., Chi H. mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat. Commun. 2018;9:2095. doi: 10.1038/s41467-018-04392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peng M., Yin N., Chhangawala S., Xu K., Leslie C.S., Li M.O. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354:481–484. doi: 10.1126/science.aaf6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bailis W., Shyer J.A., Zhao J., Canaveras J.C.G., Al Khazal F.J., Qu R., Steach H.R., Bielecki P., Khan O., Jackson R., et al. Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature. 2019;571:403–407. doi: 10.1038/s41586-019-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weinberg S.E., Singer B.D., Steinert E.M., Martinez C.A., Mehta M.M., Martinez-Reyes I., Gao P., Helmin K.A., Abdala-Valencia H., Sena L.A., et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–499. doi: 10.1038/s41586-018-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]