Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is a highly heritable and prevalent neurodevelopmental disorder that frequently persists into adulthood. Strong evidence from genetic studies indicates that single nucleotide polymorphisms (SNPs) harboured in the ADGRL3 (LPHN3), SNAP25, FGF1, DRD4, and SLC6A2 genes are associated with ADHD. We genotyped 26 SNPs harboured in genes previously reported to be associated with ADHD and evaluated their potential association in 386 individuals belonging to 113 nuclear families from a Caribbean community in Barranquilla, Colombia, using family-based association tests. SNPs rs362990-SNAP25 (T allele; p = 2.46 × 10−4), rs2282794-FGF1 (A allele; p = 1.33 × 10−2), rs2122642-ADGRL3 (C allele, p = 3.5 × 10−2), and ADGRL3 haplotype CCC (markers rs1565902-rs10001410-rs2122642, OR = 1.74, Ppermuted = 0.021) were significantly associated with ADHD. Our results confirm the susceptibility to ADHD conferred by SNAP25, FGF1, and ADGRL3 variants in a community with a significant African American component, and provide evidence supporting the existence of specific patterns of genetic stratification underpinning the susceptibility to ADHD. Knowledge of population genetics is crucial to define risk and predict susceptibility to disease.
Keywords: ADHD, ADGRL3, LPHN3, SNAP25, FGF1, genetics, Caribbean community, FBAT, predictive genomics
1. Introduction
Attention deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental behavioural disorder that affects ~5% (with figures reaching 17%) of children and adolescents of different cohorts worldwide [1,2,3,4,5,6]. Although in some patients this condition tends to resolve by adulthood, in other cases it can persist and can have serious life-long health and socio-economic adverse consequences [7]. Affected individuals are at increased risk of poor educational achievement, low-income, underemployment, legal difficulties, and impaired social relationships [8,9]. ADHD increases the risk for disruptive (externalizing) symptoms of conduct disorder (CD), oppositional defiant disorder (ODD), and substance use disorder (SUD) [8,9].
Genetic factors are strongly implicated in the aetiology of ADHD, CD, ODD, and SUD [6,10,11,12]. In particular, common single nucleotide polymorphisms (SNPs) harboured in the Adhesion G-protein-coupled receptor L3 (ADGRL3, also known as Latrophilin 3 or LPHN3; markers rs2345039, rs6551665, and rs1947274), the Synaptosomal-associated protein of molecular weight 25 kDa (SNAP25), the Fibroblast growth factor 1 (FGF1), the Solute carrier family 6 (neurotransmitter transporter, noradrenalin) member 2 (SLC6A2), and the Dopamine receptor D4 (DRD4) genes predispose one to ADHD [6,13], as confirmed by worldwide replications [2,3,6,14,15,16,17,18,19,20,21].
In this study, we explored the association of ADHD with SNPs within these genes in a family-based sample of 386 individuals ascertained from a community inhabiting the city of Barranquilla located in the Caribbean coast of Colombia. Barranquilla, with a population of ~2.4 million, is a cosmopolitan city that represents the confluence of many populations (e.g., aboriginal Amerindian, African, and a complex admixture of European (Spain), Syrian–Lebanese, Sephardic Jew, German, Italian, and English communities). These communities settled in the Atlantic coast of Colombia during the last five centuries [22] and established a pattern of genetic flow [23] that is very different from other communities in Colombia, i.e., the Paisa community [24,25] or the Andean communities surrounding Bogota [26,27]. It has been determined that the Caribbean community has one of the largest African American admixture in Colombia and in general in the Central and South American regions [28].
Our overarching goal was to evaluate the effect of genomic variants, already associated with ADHD, in the susceptibility to develop this condition in families ascertained from this Caribbean community and compare those with genomic variants predictors of ADHD in other Colombian communities. This will allow us to define specific biological predictors and study the role of genetic flow as a pivotal factor shaping the susceptibility to this neuropsychiatric disease.
2. Subjects and Methods
2.1. Subjects
We studied 386 individuals (218 (56.5%) males and 168 (43.5%) females) from 113 nuclear families whose members were born in the Barranquilla, Colombia, metropolitan area (Table 1). A total of 224 (58%) individuals were diagnosed with ADHD and 162 (42%) were diagnosed as unaffected; 94 (26.2%) were children (6–11 years), 34 (9.4%) adolescents (12–17 years) and 232 (64.4%) adults (>18 years). No children or adults were under medication. The average family size was 3.4 ± 0.65 members, with 74 (65.4%) trios, 33 (29.2%) families with four members, 4 (3.5%) with five members, and 2 (1.8%) with six members [29]. Families belonged to the medium socio-economic stratum, with an average monthly income of ~US$1000–3000. All individuals participated voluntarily and written informed consent was obtained from all of them either directly or from their parents (in the case of children <18 years old). The study was approved by the Ethics Committee of Universidad Simón Bolívar at Barranquilla, Colombia (approval # 00032, October 13, 2011).
Table 1.
Demographic characteristics of individuals included in this study. Unaffected individuals were also ascertained from the 113 nuclear families but are clinically undiagnosed with ADHD.
| Affected | Unaffected | Statistic Index | p-Value | Effect Size | |
|---|---|---|---|---|---|
| n = 221 | n = 165 | ||||
| Gender | Frequency (%) | Frequency (%) | χ2 | ||
| Male | 151 (68.32) | 70 (42.42) | 24.849 | <0.00001 | — |
| Female | 70 (31.68) | 95 (57.58) | |||
| Mean (SD) | Mean (SD) | Mann–Whitney’s U | |||
| Age | 21.4 (15.31) | 33.9 (12.69) | 26435 | <0.0001 | 0.883 |
2.2. Clinical Assessment
The Grupo de Neurociencias del Caribe carried out an extensive clinical, neurological, and neuropsychological evaluation to define ADHD status and the presence of other comorbidities, using a multi-stage scheme. The full evaluation protocol is described elsewhere [30]. Briefly, we employed the Diagnostic Interview for Children and Adolescents version IV (DICA-IV) [31,32] as the gold standard to assess the diagnosis of ADHD and/or ADHD-related comorbidities including CD and ODD in children, adolescents, and adults. For children and adolescents, the DICA-IV structured interview was completed by their parents who reported children’s symptoms and consequences in the academic, legal, and work-related areas, as well as alcohol and tobacco consumption, and its consequences [31,32,33]. This information was subsequently used to define the index case (proband). Presumptive ADHD diagnosis in childhood was assessed by obtaining a self-report retrospectively reporting on parents’ behaviour during grades 1 to 11 using the DICA-IV [34]. Persistent symptoms impacting family, social, and work-related environments were also recorded. Following the C criteria of DSM-IV, ADHD symptoms in children and adolescents were evaluated by their parents and teachers using the Colombian version of the Behavioural Assessment System for Children (BASC) [35] and the ADHD checklist [36,37]. Initially, 124 nuclear families with at least one child affected with ADHD were sequentially ascertained from patients attending a research program in ADHD [38], advertised in the Grupo Neurociencias del Caribe’s website.
2.3. SNPs Selection, DNA Extraction, and Genotyping
Twenty-six SNPs were selected from the ADHD gene data base (http://adhd.psych.ac.cn/) [39] as well as from previously reported associations to ADHD, ADHD endophenotypes, SUD, or ADHD comorbidities [2,3,6,18,40]. The full list of SNPs genotyped in this cohort is presented in Table S1 of the Supplementary Material.
Genomic DNA was isolated from blood samples using the MasterPure®DNA Purification Kit (Epicentre Biotechnologies, Chicago, IL, USA) according to the manufacturer’s protocol and stored at −80 °C. DNA concentrations were measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of DNA samples was verified via 260/280 absorbance ratios. Genotyping was performed using multiplex Sequenom® Technology on the Agena Bioscience MassARRAY® MALDI-TOF instrument at the University of Arizona Genetics Core (UAGC). Primers designed for PCR amplification and extension were prepared by the UAGC. To avoid potential biases, samples were placed randomly, and laboratory personnel were blinded to the identity and source of DNA samples.
3. Statistical Analysis
3.1. Quality Control
SNPs with a minor allele frequency (MAF) ≥ 0.05 were classified as common and as rare otherwise [41]. Rare SNPs were excluded from the analysis. Additional parameters for excluding genetic markers from the analyses included (i) deviations from Hardy–Weinberg equilibrium with p-value < 0.05/m, where m is the number of SNPs being tested (26 in total, see Table S1 in the Supplementary Material); (ii) a minimum genotype call rate of 80% [42,43,44,45]; (iii) the presence of more than two alleles; and (iv) MAF < 0.05. Allele and genotype frequencies were estimated using maximum likelihood. Mendelian errors, a common feature in SNP-based genotyping, were detected and subsequently corrected with the methods available in Golden Helix’s® SNP variation suite (SVS) 8.4.0 (Golden Helix, Inc. Bozeman, MT, USA).
3.2. Genetic- and Haplotype-Based Association Analyses
Given that this cohort is comprised by nuclear families of size four (n = 33; 29.2%), five (n = 4; 3.5%) and six (n = 2; 1.8%), and not only by trios (n = 74; 65.4%), we used the family-based association test (FBAT) to study the association of SNPs and ADHD. The FBAT provides a unified framework to generalize the transmission disequilibrium test (TDT) [46,47], initially proposed to disclose genetic associations based on trios information. The FBAT accounts for different genetic models, sampling of family-based ascertainment designs, disease phenotypes, missing parents, and different null hypotheses [46]. The FBAT, as implemented in the PBAT module of SVS 8.4.0, allows testing combination of phenotypes (as a group) and genotypes that have the highest power by those predicted from the parents’ genotypes. As age and sex are known to impact ADHD susceptibility [48,49,50,51], both variables were included as covariates under the hypothesis of no linkage and no association. Adding these covariates substantially increases FBAT power [52,53]. Additive, dominant, recessive, and heterozygous advantage models of inheritance were explored. When testing the association between a particular biallelic marker and ADHD, using both the dominant and recessive genetic models of inheritance is equivalent to testing either of them.
The PBAT module also performs the FBAT and haplotype tests for selected combinations of phenotypes and markers on the actual patients’ genotypes, both as a group and individually, automatically controlling the Type I error rate to adjust for multiple comparisons [54], and the problem of population stratification that can lead to spurious associations [46,55,56,57,58]. FBAT screening methods are minimally affected by non-causal SNPs, since the final decision is based on the FBAT statistic [59].
FBATs use affected subjects as cases, and family members, parents, or siblings as “controls” (referred to as “unaffected individuals” from now on). Furthermore, low genotype call rates are compensated by the existence of parents’ genotypes, and paternity and Mendelian inconsistencies are also controlled [52,53]. All these features were essential to analyze our cohort, which includes complex family structures with multiple affected individuals and, in some cases, several probands, which introduces complex patterns of ascertainment. For interpretation purposes, the sign of the p-value of the FBAT indicates the direction of the effect; a positive p-value indicates susceptibility to ADHD, while a negative p-value indicates a protective effect.
Haplotype-based association analyses were also performed using the Parent TDT algorithm available in HaploView [60] including only SNPs located in the same chromosomal region.
4. Results
4.1. Family-Based Association Tests
Out of the 26 SNPs genotyped, 14 had one or more than two alleles, six had a MAF < 0.05, and six passed filters and quality control (Table 2). The total genotyping rate was 87.9% in all samples and 88.1% in the set of markers finally included for genetic analysis (Table 2).
Table 2.
Statistics for genotyped markers passing quality control in 386 individuals segregating ADHD and belonging to 113 nuclear families from a Caribbean community.
| Chr | Marker | Position a | Gene | Marker Information | |||
|---|---|---|---|---|---|---|---|
| Alleles b | MAF c | HW p-Value | %Genotyping | ||||
| 4 | rs1565902 | 61,542,902 | ADGRL3 | C/T | 0.473 | 0.048 | 89.4 |
| 4 | rs10001410 | 61,608,511 | ADGRL3 | C/T | 0.372 | 0.841 | 92.0 |
| 4 | rs2122642 | 61,832,546 | ADGRL3 | C/T | 0.330 | 0.326 | 91.5 |
| 5 | rs2282794 | 142,602,144 | FGF1 | G/A | 0.458 | 0.244 | 85.8 |
| 11 | rs916457 | 637,014 | DRD4 | C/A | 0.074 | 0.546 | 88.1 |
| 20 | rs362990 | 10,295,573 | SNAP25 | T/G | 0.120 | 0.788 | 80.3 |
a UCSC GRCh37/hg19 coordinates. b Minor allele reported in bold. c Sample-based estimate. Chr: Chromosome; MAF: Minor allele frequency; HW: Hardy–Weinberg. Additional details on these intronic markers are provided in Table S1. Note: The p-value for the HW disequilibrium test was calculated for the full sample.
We found that markers SNAP25-rs362990, FGF1-rs2282794, and ADGRL3-rs2122642 reached statistically significant FBAT statistics and confer susceptibility to ADHD (Table 3a). In particular, the T allele of marker SNAP25-rs362990 was found to confer susceptibility to ADHD under two different inheritance models (additive model, p = 2.46 × 10−4; heterozygous advantage (HA) model, p = 5.21 × 10−4; Table 3a). Furthermore, the two alleles of marker FGF1-rs2282794 confer susceptibility to ADHD in our cohort under three different inheritance models (A allele, dominant model p = 0.013; G allele, recessive model, p = 0.013; G allele, HA model, p = 0.016; Table 3a). Finally, the C and T alleles of marker ADGRL3-rs2122642 confer susceptibility to ADHD under a recessive (p = 0.035; Table 3a) or dominant (p = 0.035; Table 3a) model of inheritance, respectively.
Table 3.
Results of (a) family- and (b) haplotype-based association tests for ADHD in 386 individuals belonging to 113 nuclear families from a Caribbean community.
(a)
| Chr | Marker | Gene | Position a | Marker Information | FBAT Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Observed | (Counts) [Frequency] |
Allele | Cohort Frequency | Model | NIF | p-Value | ||||
| 20 | rs362990 | SNAP25 | 10,276,221 | A | A/T | (4145/863) [0.828/0.172] |
T | 0.094 | Additive | 55 | 2.46 × 10−4 |
| HA | 55 | 5.21 × 10−4 | |||||||||
| 5 | rs2282794 | FGF1 | 141,981,709 | G | A/G | (520/4488) [0.104/0.896] |
A | 0.458 | Dominant | 44 | 0.013 |
| G | 0.542 | Recessive | 44 | 0.013 | |||||||
| HA | 64 | 0.016 | |||||||||
| 4 | rs2122642 | ADGRL3 | 62,698,264 | G | C/T | (2722/2286) [0.543/0.457] |
C | 0.744 | Recessive | 45 | 0.035 |
| T | 0.256 | Dominant | 45 | 0.035 | |||||||
(b)
| Markers | Haplotype | Frequency | OR (T:U) | χ2 | p-Value | |
|---|---|---|---|---|---|---|
| Raw | Permuted | |||||
| rs1565902-rs10001410-rs2122642 | CCC | 0.411 | 1.74 (74.1:42.5) | 8.5 | 0.004 | 0.021 |
a UCSC GRCh37/hg19 coordinates. Chr: Chromosome; HA: Heterozygous advantage; NIF: Number of informative families; FBAT: Family-based association test; OR: Odds ratio; T: Transmitted; UT: Untransmitted. Permuted p-values were obtained using 10,000 permutations as implemented in the HaploView’s ParentTDT algorithm [60]. All markers are intronic. Additional details on these markers are provided in Table S1.
4.2. Haplotype Block within ADGRL3 Confer Susceptibility to ADHD
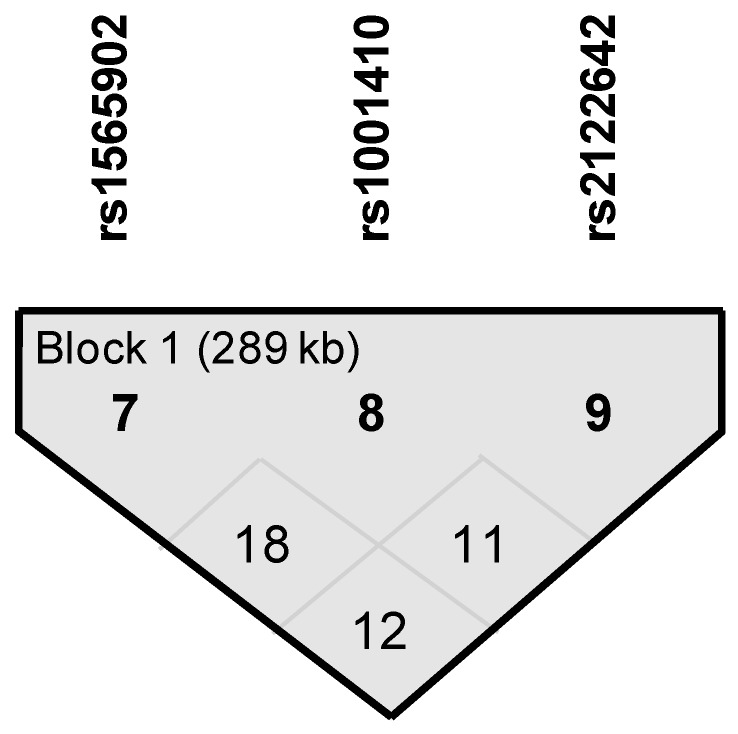
We identified a haplotype block within the ADGRL3 gene that confers susceptibility to ADHD (ppermuted < 0.05, Table 3b); this block spans 189 kb and is comprised by markers rs1565902, rs10001410, and rs2122642 (Figure 1). This CCC haplotype has a frequency of 41.1% in the full sample and is ~1.7 times more likely to be present in ADHD affected individuals than in unaffected individuals from these nuclear families (Table 3b).
Figure 1.
Linkage disequilibrium blocks for haplotype-based association analyses in 113 nuclear families segregating ADHD from a Caribbean community. See Table 3b for more information.
5. Discussion
Family-based designs are robust against population admixture and stratification, and allow conducting complex segregation analysis and linkage and association studies that would be next to impossible in case/control-based designs [32,61,62,63]. With few exceptions [64,65,66,67], recent genetic studies on ADHD have primarily focused on case/control-based designs to study genetic contributions to ADHD susceptibility [15,16,18,68,69,70].
Here we evaluated the association between ADHD and intronic SNPs harboured in, among others, the SNAP25, ADGRL3, FGF1, DRD4, and SLC6A2 genes (Table S1, Supplementary Material) in 113 nuclear families ascertained from the metropolitan area of Barranquilla, Colombia. Despite that some of the genotyped markers have been reported as associated to ADHD [71], ADHD comorbidities [72,73], and ADHD endophenotypes [40], most genetic variants associated to ADHD have mainly been identified in populations with no African American background [14,15,74,75,76,77].
We found that markers rs362990-SNAP25, rs2282794-FGF1, and rs2122642-ADGRL3 were also associated with ADHD in this set of nuclear families from the Colombian Caribbean coast (Table 3a). Moreover, we found that haplotype CCC (markers rs1565902-rs10001410-rs2122642, p = 0.021) within the ADGRL3 gene confers susceptibility to ADHD in our set of nuclear families (Table 3b).
SNAP25, previously associated with ADHD and reduced expression in the prefrontal cortex [73], encodes a protein essential for synaptic vesicle fusion and neurotransmitter release and may play an important role in the synaptic function of specific neuronal systems [78]. Mutations within SNAP25 may alter the level or function of the protein and hence may have an effect on the functions of synaptic vesicle fusion and neurotransmitter release [79]. Mouse models with a deletion of SNAP25 show a hyperactive phenotype similar to ADHD in humans [80]. Although the same SNP was not genotyped in our sample, our genetic association result between a SNP harboured in SNAP25 and ADHD is consistent with a case/control study in another Colombian sample with no African American component [81], which reported that individual SNPs and a haplotype within SNAP25 were also associated with ADHD.
It is notable that SNPs in FGF1 and ADGLR3, previously reported to be associated with ADHD endophenotypes and ADHD susceptibility in the Paisa genetic isolate [6,40], were also associated with ADHD in this set of nuclear families from the Colombian Caribbean coast. FGF1 maps to 5q31.3, encodes a protein in the fibroblast growth factor (FGF) family and is expressed in key areas of the brain related to attention and activity (i.e., frontal cortex and the hippocampus) and to major depression (i.e., prefrontal cortex and the anterior cingulate cortex) [82] that may also be relevant to ADHD. The FGF family is involved in several cell survival activities including embryonic development, cell growth, morphogenesis, tissue regeneration, and tumour growth and invasion [83]. The encoded protein functions as a modifier of endothelial cell migration and proliferation, as well as an angiogenic factor, and has an important role in neural survival in Alzheimer’s disease [84,85,86]. The fact that marker rs2282794-FGF1 is associated with ADHD in this Caribbean community reinforces the importance of further studying the FGF1 gene as a novel candidate gene for ADHD [40].
ADGRL3 is a member the latrophilin subfamily of G protein-coupled receptors and has already been implicated in ADHD susceptibility, predicting ADHD severity, disruptive behaviours comorbidity, long-term outcome, response to treatment, and SUD [2,3,6,14,15,16,17,18,19,20,21,74]. Animal models of ADHD have also provided convincing evidence of the critical role of ADGRL3 in shaping the hyperactive/impulsive phenotype [87,88,89,90]. Besides further replicating the genetic contribution of these genes to ADHD, these results also suggest that these genetic effects are not unique to the Paisa community. Subsequent studies are needed to better understand how population stratification and different ethnic backgrounds, particularly African and Syrian–Lebanese, may impact ADHD susceptibility.
Altogether, our results suggest an association between SNP located in the SNAP25, FGF1, and ADGRL3 genes and ADHD in this Caribbean community, which exhibits a strong genetic admixture between Aboriginal Amerindian communities with Spaniards and Africans, and later with other communities [22]. Although some clinical studies have been performed to better understand the manifestations of ADHD in individuals with an African American background [91,92,93,94], our study is the first to show that variants harbored in previously reported ADHD genes confer susceptibility to this disorder in such a population. Interestingly, this association is present when different genetic inheritance models are used (Table 3a). Future studies will include conducting complex segregation analyses to determine the inheritance mode of transmission (i.e., major gene, multifactorial contributors, or cohort effect) [10] of ADHD and comorbidities in this set of 113 families, the definition of cognitive and neuropsychological endophenotypes [40,95,96], and to perform linkage and association genetic analysis between common, rare, and functional exomic variants to ADHD, ADHD comorbidities [10,18,97], and reaction times [98]. In the future, we plan to perform genetic association analyses of the already genotyped SNPs (Table S1, Supplementary Material) and ADHD comorbidities in this cohort, as well as in individuals with extreme ADHD phenotypes [3,10,29,99,100,101,102]. Further functional studies and possibly deep sequencing of these genes in this set of families could allow the identification of causal variants, and enhance translational medicine approaches to increase the accuracy of ADHD diagnosis and improve long-term outcomes.
Acknowledgments
We express our highest appreciation to the families enrolled in this study. M.L.C.-H. and E.M.-S. are doctoral students at Universidad del Norte, Barranquilla, Colombia, and Universidad De Flores in Buenos Aires, Argentina, respectively. Some of this work is to be presented in partial fulfilment of the requirements for the PhD degree. P.J.P.-R., M.A.-B. and J.I.V. have full access to all the data in the study, and responsible for submitting this work for publication. The authors assert that all procedures contributing to this work have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/8/8/907/s1, Table S1: Single nucleotide polymorphisms (SNPs) genotyped in 386 individuals belonging to 113 nuclear families from Barranquilla, Colombia.
Author Contributions
Conceptualization: P.J.P.-R., D.A.P.; methodology: G.A.G.-L., M.A.-B., J.I.V.; validation: P.J.P.-R., J.E.A.-L., M.L.C.-H., D.A.P., M.A.-B., J.I.V.; formal analysis: G.A.G.-L., M.A.-B., J.I.V.; investigation: P.J.P.-R., J.E.A.-L., M.L.C.-H., M.L.M.-B., E.M.-S., M.S.-R., M.E.A.-R., A.A.-H., D.A.P.; resources: P.J.P.-R., J.E.A.-L., M.E.A.-R., A.A.-H.; data curation: M.A.-B., J.I.V.; writing—original draft preparation: P.J.P.-R., F.X.C., M.A.-B., J.I.V.; writing—review & editing: P.J.P.-R., J.E.A.-L., M.L.C.-H., M.L.M.-B., E.M.-S., M.S.-R., M.E.A.-R., A.A.-H., G.A.G.-L., C.A.M., D.A.P., F.X.C., M.A.-B., J.I.V.; visualization: M.A.-B., J.I.V.; supervision: P.J.P.-R., J.E.A.-L., C.A.M., D.A.P., F.X.C., M.A.-B., J.I.V.; project administration: P.J.P.-R., J.E.A.-L.; funding acquisition: P.J.P.-R., J.E.A.-L., M.L.C.-H., M.S.-R., D.A.P.
Funding
This study was financed by COLCIENCIAS, project “Fenotipos Complejos y Endofenotipos del Trastorno por Déficit de Atención e Hiperactividad y su Asociación con Genes Mayores y de Susceptibilidad”, grant 1253-5453-1644, contract RC 384-2011. JIV is partially supported by research grant FOFICO 32101 PE0031 from Universidad del Norte, Barranquilla, Colombia. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Visser S., Bitsko R., Danielson M., Perou R. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. Mortal. Morb. Wkly. Rep. 2010;59:1439–1443. [PubMed] [Google Scholar]
- 2.Jain M., Velez J.I., Acosta M.T., Palacio L.G., Balog J., Roessler E., Pineda D., Londono A.C., Palacio J.D., Arbelaez A., et al. A cooperative interaction between lphn3 and 11q doubles the risk for adhd. Mol. Psychiatry. 2011;17:741–747. doi: 10.1038/mp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta M.T., Velez J.I., Bustamante M.L., Balog J.Z., Arco-Burgos M., Muenke M. A two-locus genetic interaction between lphn3 and 11q predicts adhd severity and long-term outcome. Transl. Psychiatry. 2011;1:e17. doi: 10.1038/tp.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukstein O.G. Attention deficit hyperactivity disorder and substance use disorders. Curr. Top. Behav. Neurosci. 2012;9:145–172. doi: 10.1007/7854_2011_148. [DOI] [PubMed] [Google Scholar]
- 5.Pelham W.E., Jr., Fabiano G.A. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J. Clin. Child. Adolesc. Psychol. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- 6.Arcos-Burgos M., Jain M., Acosta M.T., Shively S., Stanescu H., Wallis D., Domene S., Velez J.I., Karkera J.D., Balog J., et al. A common variant of the latrophilin 3 gene, lphn3, confers susceptibility to adhd and predicts effectiveness of stimulant medication. Mol. Psychiatry. 2010;15:1053–1066. doi: 10.1038/mp.2010.6. [DOI] [PubMed] [Google Scholar]
- 7.Sibley M.H., Pelham W.E., Jr., Molina B.S., Gnagy E.M., Waschbusch D.A., Garefino A.C., Kuriyan A.B., Babinski D.E., Karch K.M. Diagnosing adhd in adolescence. J. Consult. Clin. Psychol. 2012;80:139–150. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibley M.H., Pelham W.E., Molina B.S., Gnagy E.M., Waschbusch D.A., Biswas A., MacLean M.G., Babinski D.E., Karch K.M. The delinquency outcomes of boys with adhd with and without comorbidity. J. Abnorm. Child. Psychol. 2011;39:21–32. doi: 10.1007/s10802-010-9443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina B.S., Pelham W.E., Gnagy E.M., Thompson A.L., Marshal M.P. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcohol Clin. Exp. Res. 2007;31:643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain M., Palacio L.G., Castellanos F.X., Palacio J.D., Pineda D., Restrepo M.I., Munoz J.F., Lopera F., Wallis D., Berg K., et al. Attention-deficit/hyperactivity disorder and comorbid disruptive behavior disorders: Evidence of pleiotropy and new susceptibility loci. Biol. Psychiatry. 2007;61:1329–1339. doi: 10.1016/j.biopsych.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Arcos-Burgos M., Castellanos F.X., Pineda D., Lopera F., Palacio J.D., Palacio L.G., Rapoport J.L., Berg K., Bailey-Wilson J.E., Muenke M. Attention-deficit/hyperactivity disorder in a population isolate: Linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am. J. Hum. Genet. 2004;75:998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta M.T., Arcos-Burgos M., Muenke M. Attention deficit/hyperactivity disorder (adhd): Complex phenotype, simple genotype? Genet. Med. 2004;6:1–15. doi: 10.1097/01.GIM.0000110413.07490.0B. [DOI] [PubMed] [Google Scholar]
- 13.Martinez A.F., Muenke M., Arcos-Burgos M. From the black widow spider to human behavior: Latrophilins, a relatively unknown class of g protei-coupled receptors, are implicated in psychiatric disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:1–10. doi: 10.1002/ajmg.b.31137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruxel E.M., Salatino-Oliveira A., Akutagava-Martins G.C., Tovo-Rodrigues L., Genro J.P., Zeni C.P., Polanczyk G.V., Chazan R., Schmitz M., Arcos-Burgos M., et al. Lphn3 and attention-deficit/hyperactivity disorder: A susceptibility and pharmacogenetic study. Genes Brain Behav. 2015;14:419–427. doi: 10.1111/gbb.12224. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Sanchez C.I., Riveiro-Alvarez R., Soto-Insuga V., Rodrigo M., Tirado-Requero P., Mahillo-Fernandez I., Abad-Santos F., Carballo J.J., Dal-Re R., Ayuso C. Attention deficit hyperactivity disorder: Genetic association study in a cohort of spanish children. Behav. Brain Funct. 2016;12:2. doi: 10.1186/s12993-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang I.W., Lim M.H., Kwon H.J., Jin H.J. Association of lphn3 rs6551665 a/g polymorphism with attention deficit and hyperactivity disorder in korean children. Gene. 2015;566:68–73. doi: 10.1016/j.gene.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Ribases M., Ramos-Quiroga J.A., Sanchez-Mora C., Bosch R., Richarte V., Palomar G., Gastaminza X., Bielsa A., Arcos-Burgos M., Muenke M., et al. Contribution of lphn3 to the genetic susceptibility to adhd in adulthood: A replication study. Genes Brain Behav. 2010;10:149–157. doi: 10.1111/j.1601-183X.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- 18.Acosta M.T., Swanson J., Stehli A., Molina B.S., Team M.T.A., Martinez A.F., Arcos-Burgos M., Muenke M. Adgrl3 (lphn3) variants are associated with a refined phenotype of adhd in the mta study. Mol. Genet. Genom. Med. 2016;4:540–547. doi: 10.1002/mgg3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song J., Kim S.W., Hong H.J., Lee M.G., Lee B.W., Choi T.K., Lee S.H., Yook K.H. Association of snap-25, slc6a2, and lphn3 with oros methylphenidate treatment response in attention-deficit/hyperactivity disorder. Clin. Neuropharmacol. 2014;37:136–141. doi: 10.1097/WNF.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 20.Labbe A., Liu A., Atherton J., Gizenko N., Fortier M.E., Sengupta S.M., Ridha J. Refining psychiatric phenotypes for response to treatment: Contribution of lphn3 in adhd. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159:776–785. doi: 10.1002/ajmg.b.32083. [DOI] [PubMed] [Google Scholar]
- 21.Fallgatter A.J., Ehlis A.C., Dresler T., Reif A., Jacob C.P., Arcos-Burgos M., Muenke M., Lesch K.P. Influence of a latrophilin 3 (lphn3) risk haplotype on event-related potential measures of cognitive response control in attention-deficit hyperactivity disorder (adhd) Eur Neuropsychopharmacol. 2013;23:458–468. doi: 10.1016/j.euroneuro.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villalón J. Colonias Extranjeras en Barranquilla, Colombia. Ediciones Uninorte; Barranquilla, Colombia: 2008. [Google Scholar]
- 23.Mathias R.A., Taub M.A., Gignoux C.R., Fu W., Musharoff S., O′Connor T.D., Vergara C., Torgerson D.G., Pino-Yanes M., Shringarpure S.S., et al. A continuum of admixture in the western hemisphere revealed by the african diaspora genome. Nat. Commun. 2016;7:12522. doi: 10.1038/ncomms12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arcos-Burgos M., Muenke M. Genetics of population isolates. Clin. Genet. 2002;61:233–247. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- 25.Bravo M.L., Valenzuela C.Y., Arcos-Burgos O.M. Polymorphisms and phyletic relationships of the paisa community from antioquia (colombia) Gene Geogr. 1996;10:11–17. [PubMed] [Google Scholar]
- 26.De Castro M., Restrepo C.M. Genetics and genomic medicine in colombia. Mol. Genet. Genom. Med. 2015;3:84–91. doi: 10.1002/mgg3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossa H., Aquino J., Pereira R., Ibarra A., Ossa R.H., Perez L.A., Granda J.D., Lattig M.C., Groot H., Fagundes de Carvalho E., et al. Outlining the ancestry landscape of colombian admixed populations. PLoS ONE. 2016;11:e0164414. doi: 10.1371/journal.pone.0164414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mapa Genético de los Colombianos. [(accessed on 8 March 2019)]; Available online: http://historico.unperiodico.unal.edu.co/ediciones/105/15.html.
- 29.Pineda D.A., Acosta-López J.E., Cervantes-Henríquez M.L., Jimenez-Figueroa G., Sánchez-Rojas M., Pineda-Alhucema W., Mejía-Segura E., Puentes-Rozo J. Conglomerados de clases latentes en 408 miembros de 120 familias nucleares de barranquilla con un caso índice afectado de trastorno de atención hiperactividad. Acta Neurol. Colomb. 2016;32:275–284. doi: 10.22379/24224022108. [DOI] [Google Scholar]
- 30.Cervantes-Henriquez M.L., Acosta-Lopez J.E., Martinez-Banfi M.L., Velez J.I., Mejia-Segura E., Lozano-Gutierrez S.G., Sanchez-Rojas M., Zurbaran M.A., Zurek E.E., Arcos-Burgos M., et al. Adhd endophenotypes in caribbean families. J. Atten. Disord. 2018 doi: 10.1177/1087054718763741. [DOI] [PubMed] [Google Scholar]
- 31.Reich W. Diagnostic interview for children and adolescents (dica) J. Am. Acad. Child. Adolesc. Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Palacio J.D., Castellanos F.X., Pineda D.A., Lopera F., Arcos-Burgos M., Quiroz Y.T., Henao G.C., Puerta I.C., Ramirez D.L., Rapoport J.L., et al. Attention-deficit/hyperactivity disorder and comorbidities in 18 paisa colombian multigenerational families. J. Am. Acad. Child. Adolesc. Psychiatry. 2004;43:1506–1515. doi: 10.1097/01.chi.0000142279.79805.dc. [DOI] [PubMed] [Google Scholar]
- 33.Tacchini G., Coppola M.T., Musazzi A., Altamura A.C., Invernizzi G. multinational validation of the composite international diagnostic interview (cidi) Minerva Psichiatr. 1994;35:63–80. [PubMed] [Google Scholar]
- 34.Acosta-Lopez J., Cervantes-Henriquez M.L., Jiménez-Figueroa G., Nunez B.M., Sanchez R.M., Puentes R.P. Uso de una escala comportamental wender utah para evaluar en retrospectiva trastorno de atención-hiperactividad en adultos de la ciudad de barranquilla. Rev. Univ. Salud. 2013;15:45–61. [Google Scholar]
- 35.Pineda D.A., Kamphaus R.W., Mora O., Restrepo M.A., Puerta I.C., Palacio L.G., Jimenez I., Mejia S., Garcia M., Arango J.C., et al. A system of multidimensional behavior assessment. A scale for parents of children from 6 to 11 years of age. Colombian version. Rev. Neurol. 1999;28:672–681. [PubMed] [Google Scholar]
- 36.APA . Diagnostic and Statistical Manual of Mental Disorders (Dsm) 4th ed. American Psychiatric Association; Washington, DC, USA: 2000. [Google Scholar]
- 37.DSM-IV . Manual Diagnóstico y Estadístico de Los Trastornos Mentales: Texto Revisado. Masson; Pontarlier, France: 2002. [Google Scholar]
- 38.Puentes-Rozo P.J., Pineda D.A., Acosta-López J.E., Cervantes-Henríquez M.L., Martinez-Banfi M.L., Jiménez-Figueroa G., Mejía-Segura E., Sánchez-Rojas M., Pineda-Alhucema W., Zurbarán M.A., et al. Attention Deficit/Hyperactivity Disorder and Comorbidities in 120 Nuclear Families From a Caribbean Community. 2017. Unpublished work.
- 39.Zhang L., Chang S., Li Z., Zhang K., Du Y., Ott J., Wang J. Adhdgene: A genetic database for attention deficit hyperactivity disorder. Nucleic Acids Res. 2012;40:D1003–D1009. doi: 10.1093/nar/gkr992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mastronardi C.A., Pillai E., Pineda D.A., Martinez A.F., Lopera F., Velez J.I., Palacio J.D., Patel H., Easteal S., Acosta M.T., et al. Linkage and association analysis of adhd endophenotypes in extended and multigenerational pedigrees from a genetic isolate. Mol. Psychiatry. 2016;21:1434–1440. doi: 10.1038/mp.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bansal V., Libiger O., Torkamani A., Schork N.J. Statistical analysis strategies for association studies involving rare variants. Nat. Rev. Genet. 2010;11:773–785. doi: 10.1038/nrg2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Easton D.F., Pooley K.A., Dunning A.M., Pharoah P.D., Thompson D., Ballinger D.G., Struewing J.P., Morrison J., Field H., Luben R., et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu W., Wang Y., Wang Y., Li R., Lin R., Jin L. Missing call bias in high-throughput genotyping. BMC Genom. 2009;10:106. doi: 10.1186/1471-2164-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter D.J., Kraft P., Jacobs K.B., Cox D.G., Yeager M., Hankinson S.E., Wacholder S., Wang Z., Welch R., Hutchinson A., et al. A genome-wide association study identifies alleles in fgfr2 associated with risk of sporadic postmenopausal breast cancer. Nat. Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittaker P., Bumpstead S., Downes K., Ghori J. Snp analysis by maldi-tof mass spectrometry. In: Celis J., Simons K., Small J., Hunter T., Shotton D., editors. Cell Biology: A Laboratory Handbook. 3rd ed. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 46.Laird N.M., Horvath S., Xu X. Implementing a unified approach to family-based tests of association. Genet. Epidemiol. 2000;19:S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 47.Spielman R.S., McGinnis R.E., Ewens W.J. Transmission test for linkage disequilibrium: The insulin gene region and insulin-dependent diabetes mellitus (iddm) Am. J. Hum. Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 48.Mowlem F.D., Rosenqvist M.A., Martin J., Lichtenstein P., Asherson P., Larsson H. Sex differences in predicting adhd clinical diagnosis and pharmacological treatment. Eur. Child. Adolesc. Psychiatry. 2018;28:481–489. doi: 10.1007/s00787-018-1211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oerbeck B., Overgaard K., Pripp A.H., Aase H., Reichborn-Kjennerud T., Zeiner P. Adult adhd symptoms and satisfaction with life: Does age and sex matter? J. Atten. Disord. 2019;23:3–11. doi: 10.1177/1087054715612257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramtekkar U.P., Reiersen A.M., Todorov A.A., Todd R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for dsm-v and icd-11. J. Am. Acad. Child. Adolesc. Psychiatry. 2010;49:217–228. doi: 10.1097/00004583-201003000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skogli E.W., Teicher M.H., Andersen P.N., Hovik K.T., Oie M. Adhd in girls and boys–Gender differences in co-existing symptoms and executive function measures. BMC Psychiatry. 2013;13:298. doi: 10.1186/1471-244X-13-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lange C., Laird N.M. On a general class of conditional tests for family-based association studies in genetics: The asymptotic distribution, the conditional power, and optimality considerations. Genet. Epidemiol. 2002;23:165–180. doi: 10.1002/gepi.209. [DOI] [PubMed] [Google Scholar]
- 53.Lange C., Laird N.M. Power calculations for a general class of family-based association tests: Dichotomous traits. Am. J. Hum. Genet. 2002;71:575–584. doi: 10.1086/342406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabinowitz D., Laird N. A unified approach to adjusting association tests for population admixture with arbitrary pedigree structure and arbitrary missing marker information. Hum. Hered. 2000;50:211–223. doi: 10.1159/000022918. [DOI] [PubMed] [Google Scholar]
- 55.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 56.Vélez J.I., Correa J.C., Arcos-Burgos M. A new method for detecting significant p-values with applications to genetic data. Rev. Colomb. Estad. 2014;37:67–76. doi: 10.15446/rce.v37n1.44358. [DOI] [Google Scholar]
- 57.Lange C., DeMeo D., Silverman E.K., Weiss S.T., Laird N.M. Pbat: Tools for family-based association studies. Am. J. Hum. Genet. 2004;74:367–369. doi: 10.1086/381563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lunetta K.L., Faraone S.V., Biederman J., Laird N.M. Family-based tests of association and linkage that use unaffected sibs, covariates, and interactions. Am. J. Hum. Genet. 2000;66:605–614. doi: 10.1086/302782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu X., Rakovski C., Xu X., Laird N. An efficient family-based association test using multiple markers. Genet. Epidemiol. 2006;30:620–626. doi: 10.1002/gepi.20174. [DOI] [PubMed] [Google Scholar]
- 60.Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: Analysis and visualization of ld and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 61.Evangelou E., Trikalinos T.A., Salanti G., Ioannidis J.P. Family-based versus unrelated case-control designs for genetic associations. PLoS Genet. 2006;2:e123. doi: 10.1371/journal.pgen.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laird N.M., Lange C. Family-based designs in the age of large-scale gene-association studies. Nat. Rev. Genet. 2006;7:385–394. doi: 10.1038/nrg1839. [DOI] [PubMed] [Google Scholar]
- 63.Ott J., Kamatani Y., Lathrop M. Family-based designs for genome-wide association studies. Nat. Rev. Genet. 2011;12:465–474. doi: 10.1038/nrg2989. [DOI] [PubMed] [Google Scholar]
- 64.Leung P.W., Chan J.K., Chen L.H., Lee C.C., Hung S.F., Ho T.P., Tang C.P., Moyzis R.K., Swanson J.M. Family-based association study of drd4 gene in methylphenidate-responded attention deficit/hyperactivity disorder. PLoS ONE. 2017;12:e0173748. doi: 10.1371/journal.pone.0173748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakur G.A., Sengupta S.M., Grizenko N., Choudhry Z., Joober R. Family-based association study of adhd and genes increasing the risk for smoking behaviours. Arch. Dis. Child. 2012;97:1027–1033. doi: 10.1136/archdischild-2012-301882. [DOI] [PubMed] [Google Scholar]
- 66.Turic D., Williams H., Langley K., Owen M., Thapar A., O’Donovan M.C. A family based study of catechol-o-methyltransferase (comt) and attention deficit hyperactivity disorder (adhd) Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;133B:64–67. doi: 10.1002/ajmg.b.30123. [DOI] [PubMed] [Google Scholar]
- 67.Neale B.M., Lasky-Su J., Anney R., Franke B., Zhou K., Maller J.B., Vasquez A.A., Asherson P., Chen W., Banaschewski T., et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1337–1344. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu X., Yuan F.F., Huang X., Hou Y., Wang M., Lin J., Wu J. Association of pik3cg gene polymorphisms with attention-deficit/hyperactivity disorder: A case-control study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Mora C., Richarte V., Garcia-Martinez I., Pagerols M., Corrales M., Bosch R., Vidal R., Viladevall L., Casas M., Cormand B., et al. Dopamine receptor drd4 gene and stressful life events in persistent attention deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015;168:480–491. doi: 10.1002/ajmg.b.32340. [DOI] [PubMed] [Google Scholar]
- 70.Wiguna T., Ismail R.I., Winarsih N.S., Kaligis F., Hapsari A., Budiyanti L., Sekartini R., Rahayu S., Guerrero A.P.S. Dopamine transporter gene polymorphism in children with adhd: A pilot study in indonesian samples. Asian J. Psychiatry. 2017;29:35–38. doi: 10.1016/j.ajp.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 71.Lasky-Su J., Banaschewski T., Buitelaar J., Franke B., Brookes K., Sonuga-Barke E., Ebstein R., Eisenberg J., Gill M., Manor I., et al. Partial replication of a drd4 association in adhd individuals using a statistically derived quantitative trait for adhd in a family-based association test. Biol. Psychiatry. 2007;62:985–990. doi: 10.1016/j.biopsych.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Brem S., Grunblatt E., Drechsler R., Riederer P., Walitza S. The neurobiological link between ocd and adhd. Atten. Defic Hyperact. Disord. 2014;6:175–202. doi: 10.1007/s12402-014-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawi Z., Matthews N., Wagner J., Wallace R.H., Butler T.J., Vance A., Kent L., Gill M., Bellgrove M.A. DNA variation in the snap25 gene confers risk to adhd and is associated with reduced expression in prefrontal cortex. PLoS ONE. 2013;8:e60274. doi: 10.1371/journal.pone.0060274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arcos-Burgos M., Velez J.I., Martinez A.F., Ribases M., Ramos-Quiroga J.A., Sanchez-Mora C., Richarte V., Roncero C., Cormand B., Fernandez-Castillo N., et al. Adgrl3 (lphn3) variants predict substance use disorder. Transl. Psychiatry. 2019;9:42. doi: 10.1038/s41398-019-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choudhry Z., Sengupta S.M., Grizenko N., Fortier M.E., Thakur G.A., Bellingham J., Joober R. Lphn3 and attention-deficit/hyperactivity disorder: Interaction with maternal stress during pregnancy. J. Child Psychol. Psychiatry. 2012;53:892–902. doi: 10.1111/j.1469-7610.2012.02551.x. [DOI] [PubMed] [Google Scholar]
- 76.Kappel D.B., Schuch J.B., Rovaris D.L., da Silva B.S., Cupertino R.B., Winkler C., Teche S.P., Vitola E.S., Karam R.G., Rohde L.A., et al. Further replication of the synergistic interaction between lphn3 and the ntad gene cluster on adhd and its clinical course throughout adulthood. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;79:120–127. doi: 10.1016/j.pnpbp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Kappel D.B., Schuch J.B., Rovaris D.L., da Silva B.S., Muller D., Breda V., Teche S.P., Riesgo R.S., Schuler-Faccini L., Rohde L.A., et al. Adgrl3 rs6551665 as a common vulnerability factor underlying attention-deficit/hyperactivity disorder and autism spectrum disorder. Neuromol. Med. 2019;21:60–67. doi: 10.1007/s12017-019-08525-x. [DOI] [PubMed] [Google Scholar]
- 78.Sollner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. Snap receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 79.Brophy K., Hawi Z., Kirley A., Fitzgerald M., Gill M. Synaptosomal-associated protein 25 (snap-25) and attention deficit hyperactivity disorder (adhd): Evidence of linkage and association in the irish population. Mol. Psychiatry. 2002;7:913–917. doi: 10.1038/sj.mp.4001092. [DOI] [PubMed] [Google Scholar]
- 80.Hess E.J., Collins K.A., Wilson M.C. Mouse model of hyperkinesis implicates snap-25 in behavioral regulation. J. Neurosci. 1996;16:3104–3111. doi: 10.1523/JNEUROSCI.16-09-03104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galvez J.M., Forero D.A., Fonseca D.J., Mateus H.E., Talero-Gutierrez C., Velez-van-Meerbeke A. Evidence of association between snap25 gene and attention deficit hyperactivity disorder in a latin american sample. Atten. Defic. Hyperact. Disord. 2014;6:19–23. doi: 10.1007/s12402-013-0123-9. [DOI] [PubMed] [Google Scholar]
- 82.Evans S.J., Choudary P.V., Neal C.R., Li J.Z., Vawter M.P., Tomita H., Lopez J.F., Thompson R.C., Meng F., Stead J.D., et al. Dysregulation of the fibroblast growth factor system in major depression. Proc. Natl. Acad. Sci. USA. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yun Y.R., Won J.E., Jeon E., Lee S., Kang W., Jo H., Jang J.H., Shin U.S., Kim H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010;2010:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mashayekhi F., Hadavi M., Vaziri H.R., Naji M. Increased acidic fibroblast growth factor concentrations in the serum and cerebrospinal fluid of patients with alzheimer’s disease. J. Clin. Neurosci. 2010;17:357–359. doi: 10.1016/j.jocn.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 85.Tao Q.Q., Sun Y.M., Liu Z.J., Ni W., Yang P., Li H.L., Lu S.J., Wu Z.Y. A variant within fgf1 is associated with alzheimer’s disease in the han chinese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014;165:131–136. doi: 10.1002/ajmg.b.32205. [DOI] [PubMed] [Google Scholar]
- 86.Yamagata H., Chen Y., Akatsu H., Kamino K., Ito J., Yokoyama S., Yamamoto T., Kosaka K., Miki T., Kondo I. Promoter polymorphism in fibroblast growth factor 1 gene increases risk of definite alzheimer’s disease. Biochem. Biophys. Res. Commun. 2004;321:320–323. doi: 10.1016/j.bbrc.2004.06.142. [DOI] [PubMed] [Google Scholar]
- 87.Lange M., Norton W., Coolen M., Chaminade M., Merker S., Proft F., Schmitt A., Vernier P., Lesch K.P., Bally-Cuif L. The adhd-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiatry. 2012;17:946–954. doi: 10.1038/mp.2012.29. [DOI] [PubMed] [Google Scholar]
- 88.Martinez A.F., Abe Y., Hong S., Molyneux K., Yarnell D., Lohr H., Driever W., Acosta M.T., Arcos-Burgos M., Muenke M. An ultraconserved brain-specific enhancer within adgrl3 (lphn3) underpins attention-deficit/hyperactivity disorder susceptibility. Biol. Psychiatry. 2016;80:943–954. doi: 10.1016/j.biopsych.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orsini C.A., Setlow B., DeJesus M., Galaviz S., Loesch K., Ioerger T., Wallis D. Behavioral and transcriptomic profiling of mice null for lphn3, a gene implicated in adhd and addiction. Mol. Genet. Genom. Med. 2016;4:322–343. doi: 10.1002/mgg3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wallis D., Arcos-Burgos M., Jain M., Castellanos F.X., Palacio J.D., Pineda D., Lopera F., Stanescu H., Pineda D., Berg K., et al. Polymorphisms in the neural nicotinic acetylcholine receptor alpha4 subunit (chrna4) are associated with adhd in a genetic isolate. Atten. Defic. Hyperact. Disord. 2009;1:19–24. doi: 10.1007/s12402-009-0003-5. [DOI] [PubMed] [Google Scholar]
- 91.Adewuya A.O., Famuyiwa O.O. Attention deficit hyperactivity disorder among nigerian primary school children: Prevalence and co-morbid conditions. Eur. Child. Adolesc. Psychiatry. 2007;16:10–15. doi: 10.1007/s00787-006-0569-9. [DOI] [PubMed] [Google Scholar]
- 92.Miller T.W., Nigg J.T., Miller R.L. Attention deficit hyperactivity disorder in african american children: What can be concluded from the past ten years? Clin. Psychol. Rev. 2009;29:77–86. doi: 10.1016/j.cpr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan P.L., Staff J., Hillemeier M.M., Farkas G., Maczuga S. Racial and ethnic disparities in adhd diagnosis from kindergarten to eighth grade. Pediatrics. 2013;132:85–93. doi: 10.1542/peds.2012-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samuel V.J., Biederman J., Faraone S.V., George P., Mick E., Thornell A., Curtis S., Taylor A., Brome D. Clinical characteristics of attention deficit hyperactivity disorder in african american children. Am. J. Psychiatry. 1998;155:696–698. doi: 10.1176/ajp.155.5.696. [DOI] [PubMed] [Google Scholar]
- 95.Castellanos F.X., Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat. Rev. Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 96.Pineda D.A., Lopera F., Puerta I.C., Trujillo-Orrego N., Aguirre-Acevedo D.C., Hincapie-Henao L., Arango C.P., Acosta M.T., Holzinger S.I., Palacio J.D., et al. Potential cognitive endophenotypes in multigenerational families: Segregating adhd from a genetic isolate. Atten. Defic. Hyperact. Disord. 2011;3:291–299. doi: 10.1007/s12402-011-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arcos-Burgos M., Muenke M. Toward a better understanding of adhd: Lphn3 gene variants and the susceptibility to develop adhd. Atten. Defic. Hyperact. Disord. 2010;2:139–147. doi: 10.1007/s12402-010-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jimenez-Figueroa G., Ardila-Duarte C., Pineda D.A., Acosta-Lopez J.E., Cervantes-Henriquez M.L., Pineda-Alhucema W., Cervantes-Gutierrez J., Quintero-Ibarra M., Sanchez-Rojas M., Velez J.I., et al. Prepotent response inhibition and reaction times in children with attention deficit/hyperactivity disorder from a caribbean community. Atten. Defic. Hyperact. Disord. 2017;9:199–211. doi: 10.1007/s12402-017-0223-z. [DOI] [PubMed] [Google Scholar]
- 99.Barnett I.J., Lee S., Lin X. Detecting rare variant effects using extreme phenotype sampling in sequencing association studies. Genet. Epidemiol. 2013;37:142–151. doi: 10.1002/gepi.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Emond M.J., Louie T., Emerson J., Zhao W., Mathias R.A., Knowles M.R., Wright F.A., Rieder M.J., Tabor H.K., Nickerson D.A., et al. Exome sequencing of extreme phenotypes identifies dctn4 as a modifier of chronic pseudomonas aeruginosa infection in cystic fibrosis. Nat. Genet. 2012;44:886–889. doi: 10.1038/ng.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johar A.S., Anaya J.M., Andrews D., Patel H.R., Field M., Goodnow C., Arcos-Burgos M. Candidate gene discovery in autoimmunity by using extreme phenotypes, next generation sequencing and whole exome capture. Autoimmun. Rev. 2015;14:204–209. doi: 10.1016/j.autrev.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Paz-Filho G., Boguszewski M.C., Mastronardi C.A., Patel H.R., Johar A.S., Chuah A., Huttley G.A., Boguszewski C.L., Wong M.L., Arcos-Burgos M., et al. Whole exome sequencing of extreme morbid obesity patients: Translational implications for obesity and related disorders. Genes. 2014;5:709–725. doi: 10.3390/genes5030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.