Abstract
Rothia aeria is a gram-positive amorphous bacillus and was discovered in the Russian space station ‘Mir’ in 1997. It shows phylogenetic similarity to Actinomyces israelii, and as determined using 16 s ribosomal RNA gene analysis R. aeria is classified as a bacteria of the genus Actinomyces. It was found to colonise in the human oral cavity, and there are some infectious reports but none specifies gynaecological infection. A 57-year-old woman, who had been continuously using intrauterine contraceptive device, presented with fever and lower abdominal pain. She was suspected tube-ovarian abscess caused by A. israelii, but the uterine cavity culture revealed R. aeria infection. Considering surgical treatment, conservative treatment by intravenous benzylpenicillin and subsequently oral ampicillin for 6 months improved the abscess, and she has no recurrence for over 1 year.
Keywords: pelvic inflammatory disease; obstetrics, gynaecology and fertility; drugs: obstetrics and gynaecology
Background
Rothia aeria, of the genus Actinomyces, was discovered in the Russian space station ‘Mir’ and was found to colonise in the oral cavity.1 There are some infectious reports caused by R. aeria, but none specifies gynaecological infection. To the best of our knowledge, this is the first report of a gynaecological infection caused by R. aeria.2–4
Case presentation
A 57-year-old woman presented with fever and lower abdominal pain. She had been continuously using intrauterine contraceptive device (IUCD) for 20 years without undergoing medical examination. She has been experiencing abnormal vaginal discharge for the past 1 year. Before admitting in our hospital, she underwent medical examination in an internal medicine clinic and was administered levofloxacin to treat acute cystitis but her symptoms did not improve. Subsequently, in another gynaecological clinic, she underwent transvaginal ultrasound examination that revealed a right adnexa tumour. She was then admitted in our hospital for careful examination and treatment with a suspicion of tube-ovarian abscess, after the removal of her IUCD.
Investigations
Initial examination at our hospital revealed the following results: white blood cell count, 15.7×109/L ; haemoglobin level, 11.2 g/L; platelet count, 36.3×109/L ; procalcitonin level, 0.051 ng/mL and C reactive protein (CRP) level, 31.91 mg/dL.
Subsequent gynaecological examination demonstrated right lower abdominal tenderness with no rebound or cervical tenderness.
Her vital signs were as follows: body temperature, 38.1°C; blood pressure 112/58 mm Hg and heart rate, 86 beats/min.
Vaginal speculum examination revealed a normal discharge and normal vaginal portion of the cervix.
Transvaginal ultrasound examination identified multiple cystic masses in the right adnexa (38×45 mm) and no ascites in the Douglas fossa, with normal uterus and left adnexa.
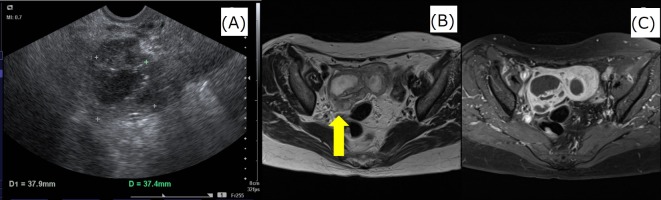
MRI revealed multiple cystic tumours with contrast effect and thickening of the right adnexa wall (figure 1).
Figure 1.
Images obtained at the first examination. Transvaginal ultrasound examination identified multiple cystic masses in the right adnexa. MRI revealed multiple cystic tumours with contrast effect and thickening of the right adnexal wall. (A) Transvaginal ultrasound, (B) MRI T2-weighted image and (C) MRI fat suppression gadolinium contrast T1-weighted image.
Treatment
First, we administered cefmetazole (2 g/day) and clindamycin (1800 mg/day) intravenously according to the treatment protocol or severe pelvic peritonitis. On day 7 of hospitalisation, the antibiotics were switched to ampicillin/sulbactam (12 g/day), which are used for treating Actinomyces and anaerobic infections, as an Actinomyces infection was suspected based on the uterine cavity culture results. On day 12 of hospitalisation, R. aeria was detected in the same culture (figure 2), and thus the antibiotics were switched to benzylpenicillin (24 000 U/day) according to previous reports on R. aeria infection treatment.2–4 The benzylpenicillin treatment improved her fever and inflammation; therefore, this conservative treatment was further continued. After 6 weeks of intravenous benzylpenicillin administration, the patient was discharged with a prescription for oral ampicillin (1500 mg/day). After 6 months of treatment with oral ampicillin, her abdominal tenderness disappeared (figure 3).
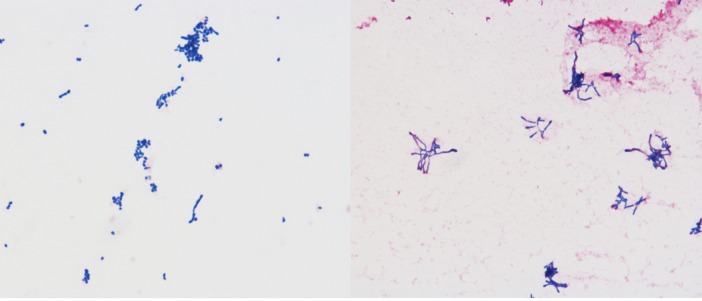
Figure 2.
Rothia aeria as observed microscopically.
Figure 3.
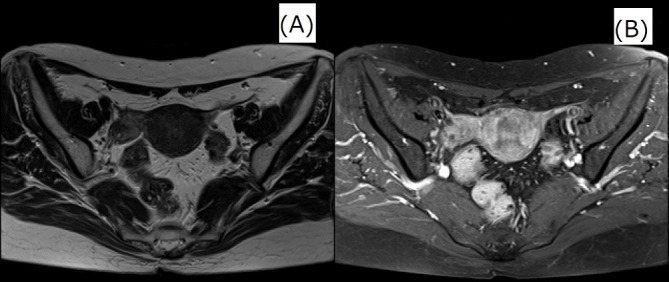
Images obtained after 6 months of the treatment. MRI and transvaginal ultrasonography revealed shrinkage of right adnexal mass. (A)MRI T2-weighted image and (B)MRI fat suppression gadolinium contrast T1-weighted image.
Outcome and follow-up
After 6 months of ampicillin therapy pelvic MRI and transvaginal ultrasound revealed a complete response, and there was no recurrence for over 1 year.
Discussion
R. aeria was isolated from the air of the Russian space station ‘Mir’; it is a gram-positive amorphous bacillus that was isolated in 2004. Unlike Actinomyces israelii, a gram-positive amorphous bacillus, R. aeria grows aerobically. However, as it shows phylogenetic similarity to A. israelii, as determined using 16 s ribosomal RNA gene analysis and as it is also a gram-positive amorphous bacillus, R. aeria is classified as a bacteria of the genus Actinomyces. Gram-positive amorphous bacilli are classified into six genera: Corynebacterium, Propionibacterium, Eubacterium, Actinomyces, Bifidobacterium and Rothia. Furthermore, Rothia is classified into R. aeria (Li et al, 20041), Rothia amarae (Fan et al, 20025), Rothia dentocariosa (Onishi, 19496; Georg and Brown, 19677), Rothia endophytica (Xiong et al, 20138), Rothia mucilaginosa (Collins et al, 2000), Rothia nasimurium (Collins et al, 20009) and Rothia terrae (Chou et al, 200810).
R. aeria is considered to reside in the oral cavity and pharynx, similar to other Actinomyces species. Previously, Rothia had been reported to cause infective endocarditis,2 neonatal bacteremias3 and respiratory infections4 in compromised hosts as opportunistic infections. Additionally, airway infection and haematogenous dissemination by intrinsic infection were reported to be the infection routes.3 4
However, there are no reports on gynaecological infections caused by R. aeria. In this case R. aeria was not detected in the oral cavity, and the patient had no history of steroid administration or immunodeficiency from HIV infection.
Generally, Actinomyces infection in the gynaecological organs manifests as chronic pyogenic granuloma caused by Actinomyces israelii. Intrauterine devices are a known risk factor for such infections11.
However, the long-term use of intrauterine devices is only the common risk factor for Actinomyces infection in gynaecological organs. The transanal and transperineal infectious routes are believed to be responsible with regard to such infections.
The causative bacterium in this case was identified using mass spectrophotometry (VITEK MS, Sysmex bioMérieux), and the bacterial DNA sequence was confirmed using ABI PRISM 310 Genetic Analyzer. Actinomyces infection is difficult to detect based on bacterial culture tests12; thus, it is often diagnosed histopathologically13. Moreover, Actinomyces species morphologically resembles Nocardia species14 and it is possible that R. aeria infection was treated as Actinomyces or Nocardia infection. Molecular approaches such as mass spectrometry and DNA sequencing are considered to diagnose accurately the infections caused by R. aeria.
Furthermore, Tsuzukibashi et al 15 reported oral Rothia species selective medium as a new culture medium for the isolation and possible easy detection of Rothia species from the oral cavity.
There are no comprehensive data on the drug susceptibility of R. aeria; therefore, we selected antibiotics based on previous case reports. Tarumoto et al 2 reported on R. aeria-induced infectious endocarditis and that it was susceptible to most antibiotics except clindamycin and vancomycin. Monju et al 3 reported that ampicillin and cefotaxime were active against neonatal sepsis caused by R. aeria. Using drug susceptibility tests, R. aeria demonstrated good sensitivity against most antibiotics except for clindamycin. Hiyamuta4 reported on a respiratory infection caused by R. aeria that was successfully treated with benzylpenicillin. They found no reports of drug resistance, and similar to Actinomyces, R. aeria’s sensitivity to benzylpenicillin was good.
Tube-ovarian abscess was treated by the conservative administration of antibiotics, considering the timing of surgical intervention. After removal of the IUCD, which was considered to be the cause of infection in this case, and after initiating antibiotic therapy, leucocyte count, CRP level and fever gradually decreased, and the patient’s entire body condition improved. Therefore, surgical intervention was deemed inessential. However, there was a possibility of reducing the duration of antibiotic treatment by surgical intervention, and we should have made an effort to reduce the risk of incidence of antibiotic-resistant bacteria, considering injury to other organs due to strong adhesions.
Learning points.
This is the first case report to identify Rothia aeria as a causative bacterium for a gynaecological infection.
The sensibility of antibiotics was good.
The advances of the mass spectrometer and analysis of base sequence can identify a bacterial species in detail, possibly facilitating identification of an infection due to an unprecedented bacterial species.
Footnotes
Contributors: YT designed this manuscript and wrote the initial draft. YA contributed to the planning, conduct and reporting of the work described in the article. Both the authors have contributed to data collection and interpretation and critically reviewed the manuscript. Both the authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Li Y, Kawamura Y, Fujiwara N, et al. Rothia aeria sp. nov., Rhodococcus baikonurensis sp. nov. and Arthrobacter russicus sp. nov., isolated from air in the Russian space laboratory Mir. Int J Syst Evol Microbiol 2004;54:827–35. 10.1099/ijs.0.02828-0 [DOI] [PubMed] [Google Scholar]
- 2. Tarumoto N, Sujino K, Yamaguchi T, et al. A first report of Rothia aeria endocarditis complicated by cerebral hemorrhage. Intern Med 2012;51:3295–9. 10.2169/internalmedicine.51.7946 [DOI] [PubMed] [Google Scholar]
- 3. Monju A, Shimizu N, Yamamoto M, et al. First case report of sepsis due to Rothia aeria in a neonate. J Clin Microbiol 2009;47:1605–6. 10.1128/JCM.02337-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hiyamuta H. First case report of respiratory infection with Rothia aeria . the Journal of the Japanese Respiratory Society 2014;48:219–23. [PubMed] [Google Scholar]
- 5. Fan Y, Jin Z, Tong J, et al. Rothia amarae sp. nov., from sludge of a foul water sewer. Int J Syst Evol Microbiol 2002;52:2257–60. 10.1099/00207713-52-6-2257 [DOI] [PubMed] [Google Scholar]
- 6. Onishi M. Study on the Actinomyces isolated from the deeper layer of carious dentine. Shikagaku Zasshi 1949;6:273–82. [Google Scholar]
- 7. Lucille K Georg, June M Brown. Rothia, gen. nov. an aerobic genus of the family Actinomycetaceae. International Journal of Systematic and Evolutionary Microbiology 1967;17:79–88. [Google Scholar]
- 8. Xiong ZJ, Zhang JL, Zhang DF, et al. Rothia endophytica sp. nov., an actinobacterium isolated from Dysophylla stellata (Lour.) Benth. Int J Syst Evol Microbiol 2013;63(Pt 11):3964–9. 10.1099/ijs.0.052522-0 [DOI] [PubMed] [Google Scholar]
- 9. Collins MD, Hutson RA, Båverud V, et al. Characterization of a Rothia-like organism from a mouse: description of Rothia nasimurium sp. nov. and reclassification of Stomatococcus mucilaginosus as Rothia mucilaginosa comb. nov. Int J Syst Evol Microbiol 2000;50 Pt 3:1247–51. 10.1099/00207713-50-3-1247 [DOI] [PubMed] [Google Scholar]
- 10. Chou YJ, Chou JH, Lin KY, et al. Rothia terrae sp. nov. isolated from soil in Taiwan. Int J Syst Evol Microbiol 2008;58(Pt 1):84–8. 10.1099/ijs.0.65172-0 [DOI] [PubMed] [Google Scholar]
- 11. Henderson SR. Pelvic actinomycosis associated with an intrauterine device. Obstet Gynecol 1973;41:726–58. [PubMed] [Google Scholar]
- 12. Hager WD, Douglas B, Majmudar B, et al. Pelvic colonization with Actinomyces in women using intrauterine contraceptive devices. Am J Obstet Gynecol 1979;135:680–4. 10.1016/S0002-9378(16)32995-7 [DOI] [PubMed] [Google Scholar]
- 13. Luff RD, Gupta PK, Spence MR, et al. Pelvic actinomycosis and the intrauterine contraceptive device. A cyto-histomorphologic study. Am J Clin Pathol 1978;69:581–6. 10.1093/ajcp/69.6.581 [DOI] [PubMed] [Google Scholar]
- 14. Kim UJ, Won EJ, Kim JE, et al. Rothia aeria infective endocarditis: a first case in Korea and literature review. Ann Lab Med 2014;34:317–20. 10.3343/alm.2014.34.4.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsuzukibashi O, Uchibori S, Kobayashi T, et al. Isolation and identification methods of Rothia species in oral cavities. J Microbiol Methods 2017;134:21–6. 10.1016/j.mimet.2017.01.005 [DOI] [PubMed] [Google Scholar]