Abstract
Male courtship is provoked by perception of a potential mate. In addition, the likelihood and intensity of courtship are influenced by recent mating experience, which affects sexual drive. Using Drosophila melanogaster, we found that the homolog of mammalian neuropeptide Y, neuropeptide F (NPF), and a cluster of male-specific NPF (NPFM) neurons, regulate courtship through affecting courtship drive. Disrupting NPF signaling produces sexually hyperactive males, which are resistant to sexual satiation, and whose courtship is triggered by sub-optimal stimuli. We found that NPFM neurons make synaptic connections with P1 neurons, which comprise the courtship decision center. Activation of P1 neurons elevates NPFM neuronal activity, which then act through NPF receptor neurons to suppress male courtship, and maintain the proper level of male courtship drive.
Research organism: D. melanogaster
Introduction
To mate is a critical decision that sexually reproductive animals must make to ensure propagation of their species. Although courtship is an innate behavior that can be evoked mostly by stimulatory cues emitted from a conspecific of the opposite sex, the intensity of courtship is largely under control of animal’s internal drive state, which is dictated in part by sexual satiation or deprivation.
The fruit fly, Drosophila melanogaster, has served as an animal model to decipher the genetic and neural basis of courtship behavior (Sturtevant, 1915; Hotta and Benzer, 1976; Dickson, 2008; Villella and Hall, 2008; Robinett et al., 2010; Pavlou and Goodwin, 2013; Yamamoto and Koganezawa, 2013). Distinct male and female courtship rituals (Dickson, 2008) are orchestrated by sexually dimorphic neuronal circuits, which are specified by the master transcriptional regulators – Fruitless and Doublesex (Burtis and Baker, 1989; Ito et al., 1996; Ryner et al., 1996; Lee et al., 2000; Demir and Dickson, 2005; Manoli et al., 2005; Stockinger et al., 2005; Rideout et al., 2010).
A male-specific cluster of P1 neurons, comprise the courtship decision center, which integrates multi-modal sensory inputs, and sends outputs to command neurons directing courtship behavior (Kimura et al., 2008; Yu et al., 2010; Kohatsu et al., 2011; von Philipsborn et al., 2011; Pan et al., 2012; Bath et al., 2014; Inagaki et al., 2014; Clowney et al., 2015; Kallman et al., 2015; Kohatsu and Yamamoto, 2015; Zhou et al., 2015). Besides integrating external stimuli, P1 neuronal activity is also influenced by the male’s internal drive state, in part through dopaminergic neurons (Zhang et al., 2016).
Male flies exhibit escalated levels of courtship following periods of sexual deprivation. Conversely, courtship decreases once males become sexually satiated, following mating with an abundance of female partners (Zhang et al., 2016). Sexual deprivation induces higher excitability of P1 neurons, while excitability of P1 neurons is down-regulated in sexually satiated flies (Inagaki et al., 2014). Proper activation of P1 neurons allows male flies to display courtship when external sensory cues from potential mates match with their internal drive states. However, we have a poor understanding concerning the identities of the neuromodulators and associated neurons that impact on the courtship decision center, which responds to mating experience.
Neuropeptide F (NPF) (Brown et al., 1999) is a neuromodulator, and is a candidate for fine-tuning courtship by the internal state since NPF neural circuitry is sexually dimorphic (Lee et al., 2006; Kim et al., 2013), and because NPF expression levels and intracellular Ca2+ activity in NPF neurons are altered by the animal’s mating status (Shohat-Ophir et al., 2012; Gao et al., 2015). However, it is not known whether NPF is essential for male courtship, and the subsets of NPF neurons critical for courtship regulation have not been identified. Moreover, the neurons that interact with the sexually dimorphic NPF neurons to regulate courtship have not been defined.
Here, we show that a cluster of male-specific NPF neurons (NPFM) are essential for regulating male courtship. Disrupting NPF signaling, either by knocking out npf, or by suppressing the activity of NPF neurons, reduces inhibition on courtship behavior in sexually satiated males, and evokes hypersexual activity in deprived males towards inappropriate targets. By combining anatomical, chemogenetic manipulation and Ca2+ imaging approaches, we found that P1 neurons directly activate NPFM neurons, which then act through NPF receptor (NPFR) neurons to suppress male courtship. Our findings indicate that NPF signaling impinges on a dedicated male circuit and is critical for fine-tuning male courtship, in accordance with the internal sexual drive state.
Results
Disruption of NPF signaling elevates male courtship
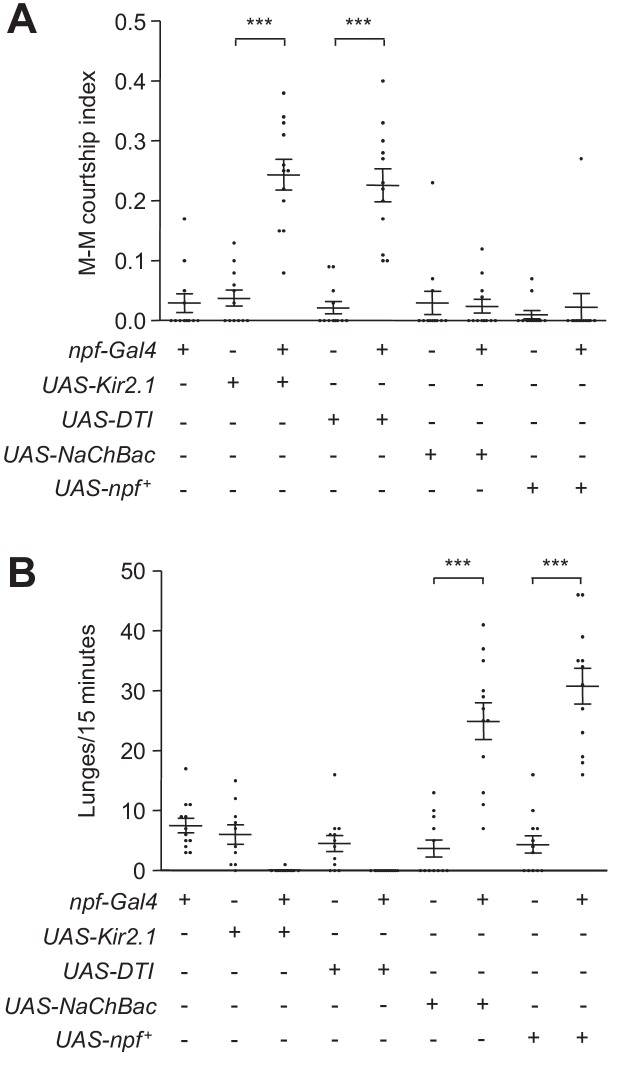
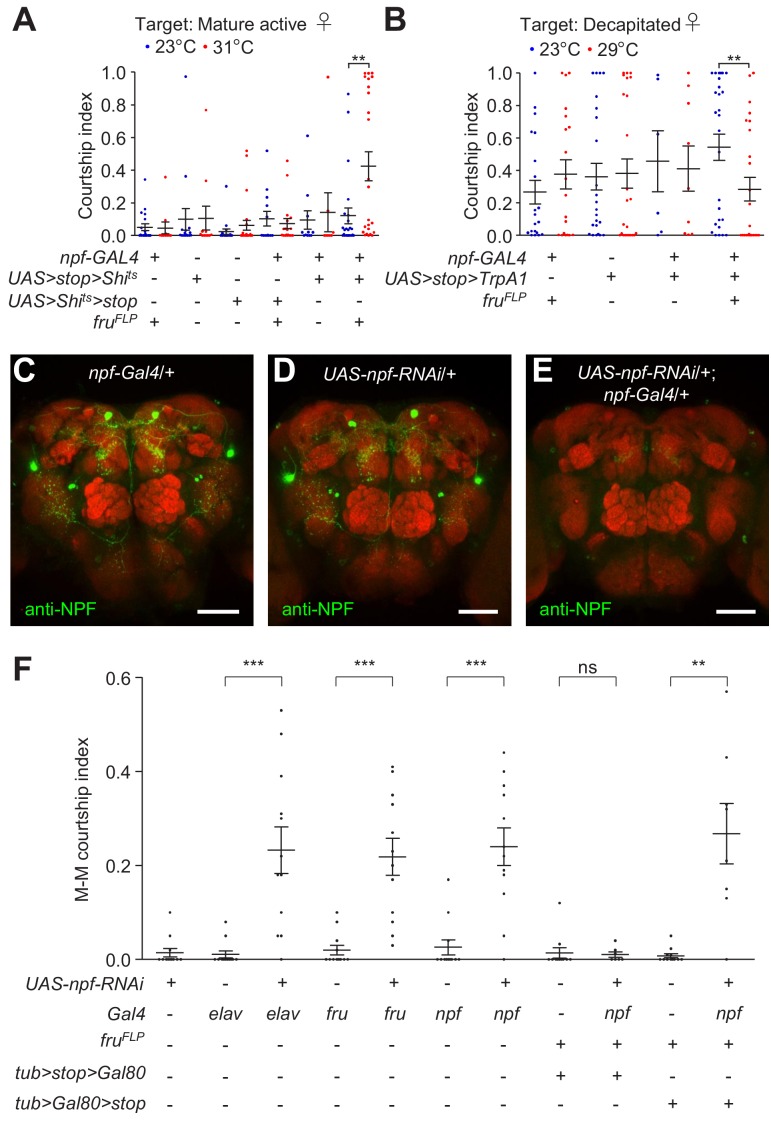
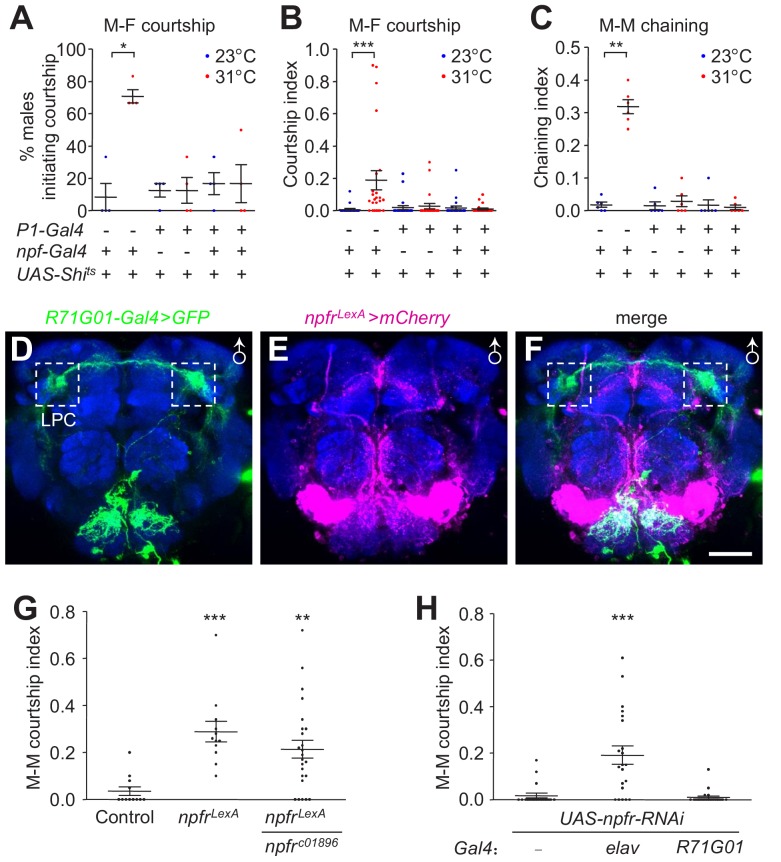
To explore the potential function of NPF in modulating male sex drive, we first inhibited NPF neurons, and assayed its effects on male-female (M–F) and male-male (M–M) courtship. To inactivate NPF neurons, we employed three approaches in conjunction with the Gal4/UAS binary system (Brand and Perrimon, 1993). First, we used flies expressing Shibirets (Shits),which prevents synaptic transmission above 28 °C, due to depletion of synaptic vesicles (Grigliatti et al., 1973; Poodry and Edgar, 1979; van der Bliek and Meyerowitz, 1991; Kuromi and Kidokoro, 1998). We conducted the analysis using males that were group-housed with females, and experienced courtship and mating. Consequently, the males were sexually satiated and had lower courtship drive. When we disrupted synaptic transmission from NPF neurons, a higher percentage of males initiated courtship towards female targets, and their courtship index (ratio of time displaying courtship) was elevated significantly (Figure 1A and B). Courtship was not increased simply by raising the temperature, because flies expressing only the npf-Gal4 or the UAS-Shits exhibited similar courtship at both 23°C and 31°C (Figure 1A and B). At the restrictive temperature (31°C), npf-Gal4/+;UAS-Shits/+ males also vigorously engaged in courting other males and frequently formed courtship chains in which multiple males simultaneously court the preceding male (Huang et al., 2016) (Figure 1C). In contrast, we rarely detected chaining events among males kept at the permissive temperature (23°C; Figure 1C). We observed similar increases in M–M courtship when we inhibited the activity of NPF neurons by expressing a gene encoding either a hyperpolarizing K+ channel (Kir2.1) (Baines et al., 2001) or the diphtheria toxin gene, DTI (Han et al., 2000) (Figure 1—figure supplement 1A). Inhibition of NPF neurons with Kir2.1 or DTI also eliminated M–M lunges, indicating suppression of aggression (Figure 1—figure supplement 1B). Conversely, when we increased NPF activity by constitutively expressing a Na+ channel gene (NaChBac) (Nitabach et al., 2006) or by overexpressing the npf-cDNA (Wu et al., 2003) in NPF neurons, tester males exhibited elevated aggression (Figure 1—figure supplement 1B), but very few M–M courtship events (Figure 1—figure supplement 1A).
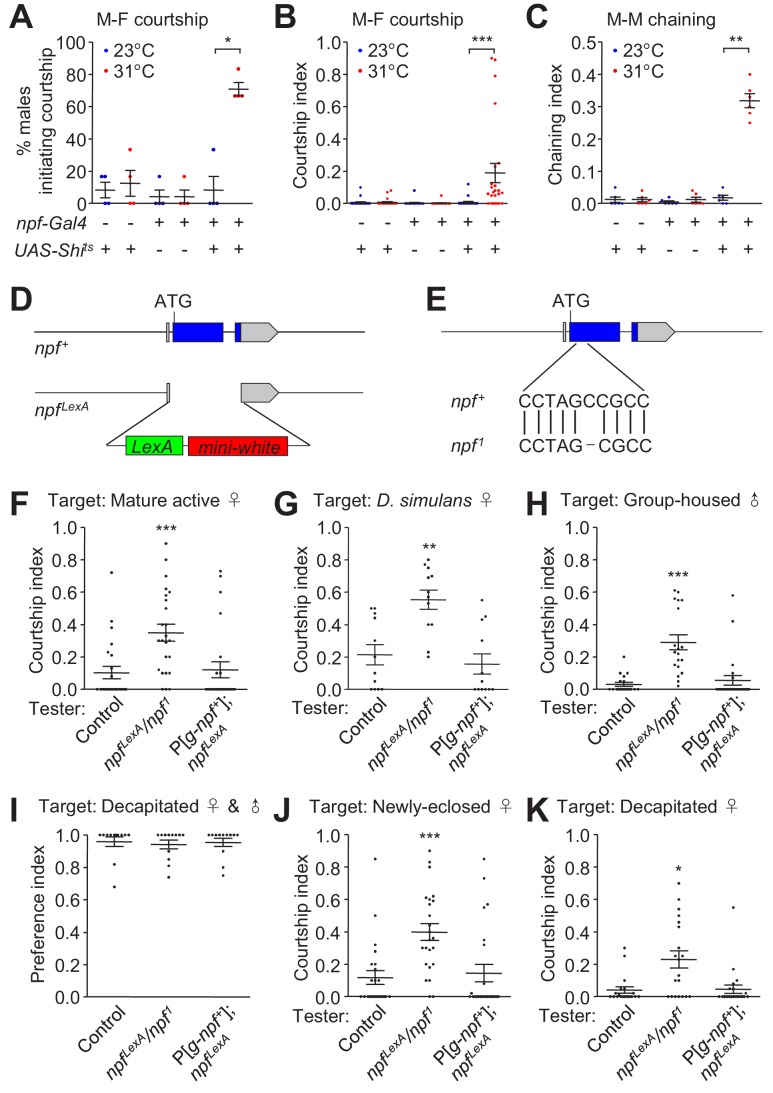
Figure 1. Effects of disruption of NPF neurons and the npf gene on male courtship.
(A and B) Effects of silencing NPF neurons with Shits (npf-Gal4/+;UAS-Shits/+) on courtship of group-housed male flies towards female targets. Male-female (M–F) courtship was assayed at the permissive (23°C) and non-permissive (31 °C) temperatures for Shits. (A) The percentages of males that initiated courtship. n = 4 (6 flies/group). (B) Courtship index (ratio of time that a male fly exhibits courtship behavior out of the total observation time) scored from 20 to 30 min observation time during a 30 min incubation period, n = 24. (C) Silencing NPF neurons with Shits (npf-Gal4/+;UAS-Shits/+) induces male-male (M–M) courtship. Isolation-housed males were assayed for chaining behavior at 23°C and 31°C for 10 min. n = 6 (8—12 flies/group). The chaining index is the proportion of time that ≥ 3 tester males engage in courtship simultaneously out of a 10 min observation time. The bars indicate means ± SEMs. To determine significance, we used the Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001. (D) Schematic illustration of npfLexA knock-in reporter line generated by the CRISPR-HDR method. (E) Schematic illustration of the npf1 allele generated by the CRISPR-NHEJ method. npf1 harbors a single nucleotide deletion in the 2nd position of codon 19. (F) Courtship index of group-housed males towards mature, active females. The control flies are w1118-CS. P[g-npf+] is a transgene encompassing the npf+ genomic region. n = 24. (G) Courtship of isolation-housed males towards Drosophila simulans females. n = 12. (H) Courtship of isolation-housed males towards group-housed w1118 males. n = 19—24. (I) Discrimination of male and female targets by the indicated males. Males of the indicated genotypes were exposed to a decapitated 5 day old male and a decapitated 5 day old virgin female. The preference index indicates the proportion of courtship time directed towards a female target out of the total courtship time in 10 min. A preference index of 1.0 indicates that the male spent 100 % of the time courting the decapitated female. n = 12. (J) Courtship index of group-housed males towards newly-eclosed female targets. n = 24. (K) Courtship of group-housed males towards decapitated female targets. n = 20—22. The bars indicate means ± SEMs. The Kruskal-Wallis test followed by the Dunn’s post hoc test was used to assess significance. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 1—figure supplement 1. Effects of increasing or decreasing NPF signaling on male-male (M–M) courtship and aggression.
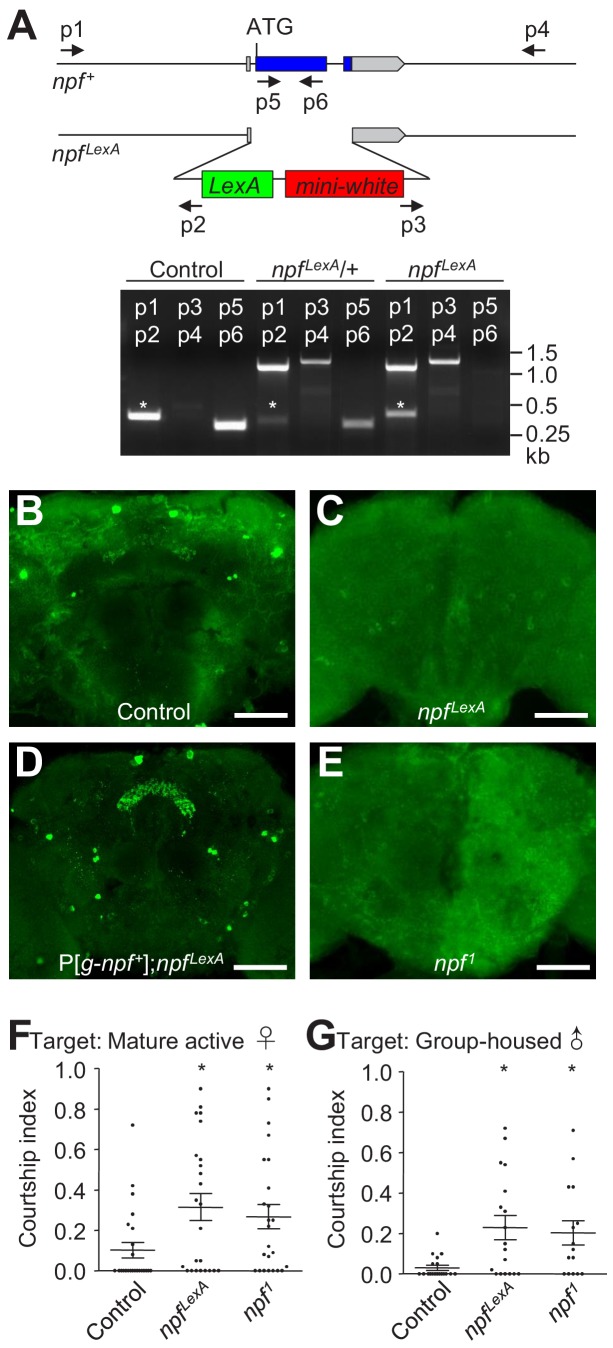
Figure 1—figure supplement 2. Genotyping, testing for NPF expression, and courtship assays using the npf1 and npfLexA mutants.
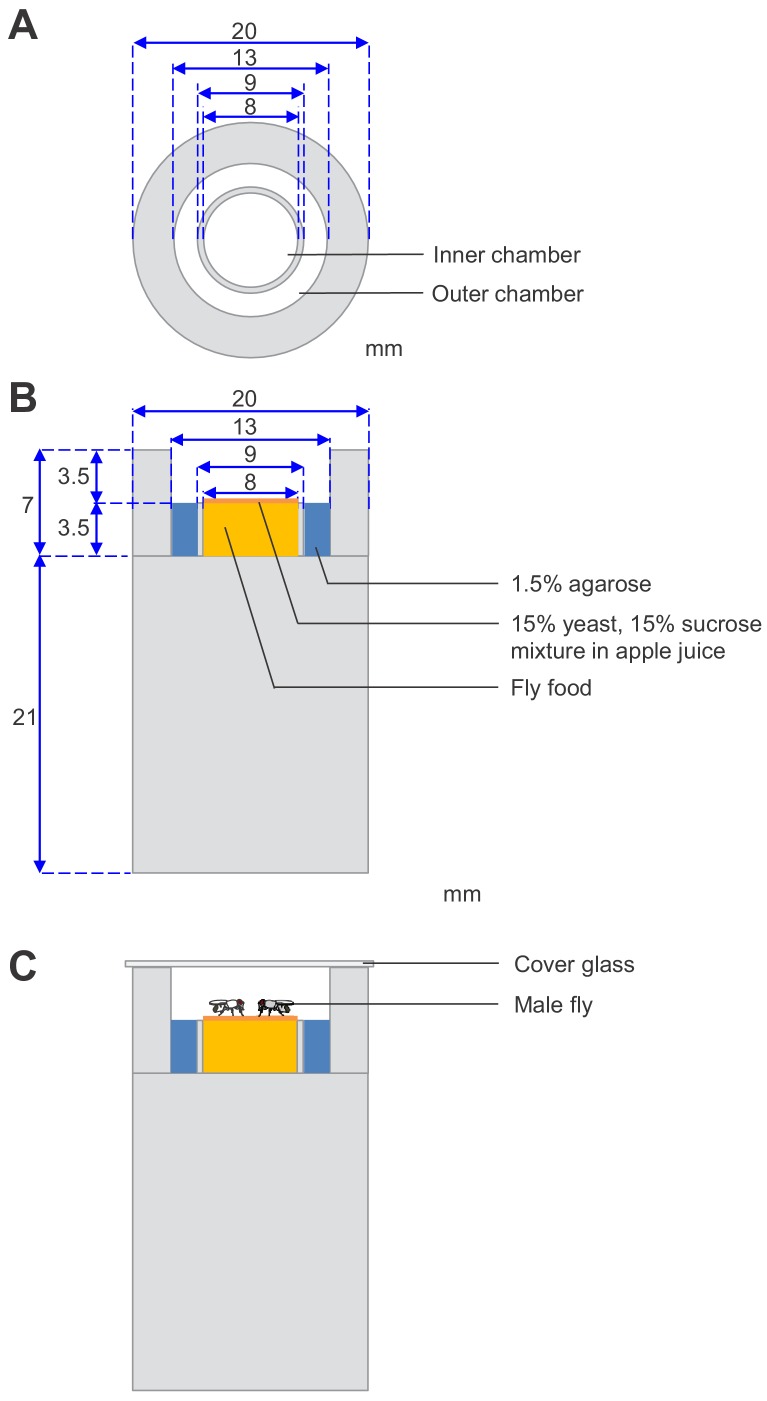
Figure 1—figure supplement 3. Illustration of the aggression chamber.
To clarify if it is the molecule NPF, rather than just NPF neurons, which is responsible for regulating sex drive, we generated two npf null mutants. To create npfLexA, we replaced the npf gene with the LexA reporter using CRISPR-HDR (Figure 1D and Figure 1—figure supplement 2A,B and C) . We also used the CRISPR-NHEJ method to generate an allele with a single nucleotide deletion, thereby changing the reading frame within codon 19, resulting in a null npf allele (npf1; Figure 1E and Figure 1—figure supplement 2B and E).
The courtship index of isolated control males reaches a ceiling level when they were exposed to mature active female targets (Huang et al., 2016). Therefore, to test whether npf mutant males exhibit an increase in courtship, we used mixed sex group-housed males, which in control flies showed a moderate level of courtship activity due to sexual satiation in the presence of an abundance of females. We found that mixed sex group-housed npf mutant males (npfLexA and npf1 and the trans-heterozygous npfLexA/npf1) retained high levels of courtship towards mature active female targets (Figure 1F and Figure 1—figure supplement 2F), indicating the mutants were resistant to sexual satiety induced by group-housing.
We found that the hypersexual activity in npf mutant males was generalized towards normally undesirable targets. These include females of other Drosophila species such as D. simulans females (Clowney et al., 2015) (Figure 1G), and male target flies (Figure 1H and Figure 1—figure supplement 2G). The increased courtship towards males was not due to an inability to discriminate between males and females. When the mutant males were allowed to choose between a decapitated male and a decapitated female, they showed a strong preference for female targets, similar to control males (Figure 1I).
To determine if this increase in courtship is an outcome of sensitized pheromone detection, we introduced newly-eclosed females, which carry negligible cuticular hydrocarbons and are therefore odorless/tasteless targets to tester males (Liu et al., 2011). We found that compared to control males, npf mutant males (npfLexA/npf1) exhibited significantly higher levels of courtship towards these females (Figure 1J). Thus, elevated courtship exhibited by npf mutant males did not appear to be caused by sensitized perception of attractive female pheromones. To test the possibility that the higher courtship levels was due to higher visual alertness in the mutants, we combined the males with motionless decapitated females as targets. Compared to control males, npf mutants exhibited increased courtship towards decapitated females (Figure 1K). To further establish that the courtship phenotype was due to loss of npf, we performed phenotypic rescue experiments with a wild-type npf genomic transgene (P[g-npf+], which restored npf expression to the npf mutant (Figure 1—figure supplement 2B—D). This genomic transgene also rescued normal levels of male courtship behavior to the npf mutant males (Figure 1F—H,J and K). Together, these experiments indicate that loss of npf function stimulates a sexually hyperactive state in males.
Sexually dimorphic NPFM neurons suppress male courtship
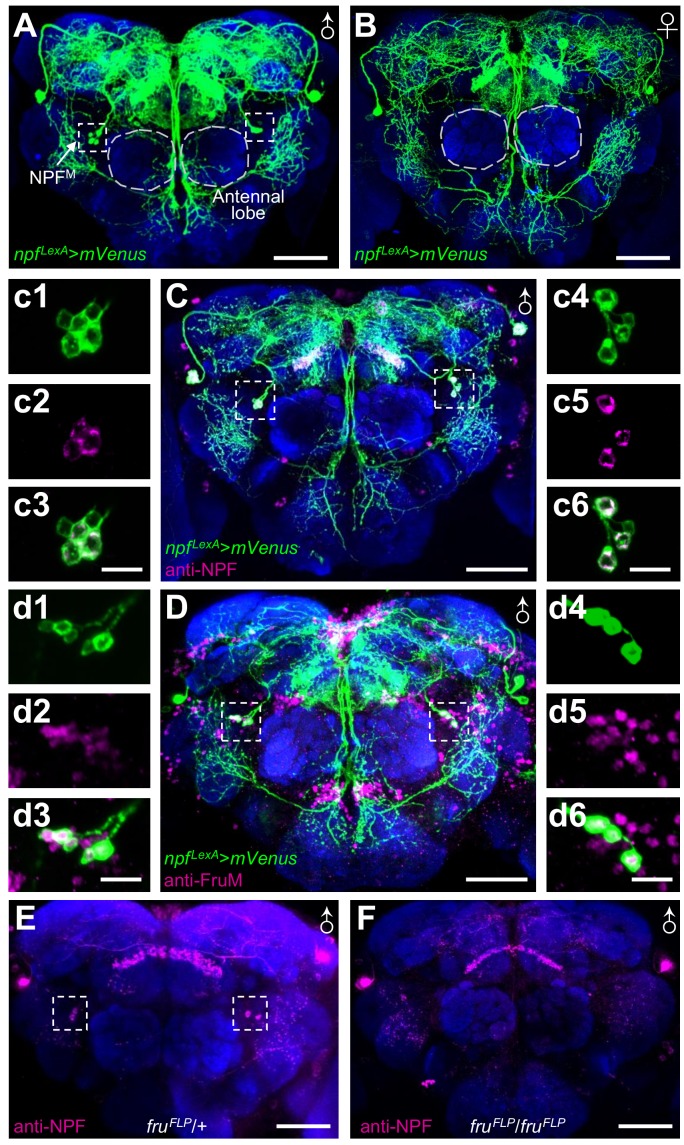
To examine the spatial distribution of the NPF neurons, we expressed lexAop-IVS-mVenus under the control of the LexA that we knocked into the npf gene (npfLexA/+). Among the neurons that were labeled by the npf reporter, was a bilaterally symmetrical cluster of NPF neurons that was male specific (NPFM; Figure 2A and B). The cell bodies of these sexually-dimorphic neurons are dorso-lateral to the antennal lobes and arborize extensively in the superior brain (Figure 2A and B). NPFM neurons can be differentiated from other NPF neurons based on their position in the anterior brain region that is immediately adjacent to antennal lobe. Moreover, NPFM form a cluster of 3— 5 neurons and their cell bodies are smaller than the pair of dorsal medial and the pair of dorsal lateral large NPF neurons. We used anti-NPF antibodies to immunostain the brain and found that the reporter expression pattern recapitulates the spatial distribution of the NPF protein (Figure 2C, c1-c6), confirming that NPFM neurons express NPF.
Figure 2. Identification of male-specific NPFM neurons.
(A and B) npfLexA/LexAop-IVS-mVenus male and female brains immunostained with anti-GFP to detect mVenus. The boxes indicate NPFM neurons, and the circles indicate the antennal lobes. (C) npfLexA/LexAop-IVS-mVenus male brain immunostained with anti-GFP and anti-NPF. NPFM neurons are boxed. (c1—c6) Zoomed in images showing NPFM neurons. (D) npfLexA/LexAop-IVS-mVenus male brain immunostained with anti-GFP and anti-FruM. The boxes indicate NPFM neurons. (d1—d6) Zoomed in images showing NPFM neurons. (E and F) fru mutant (fruFLP/fruFLP) and control fruFLP/+ male brains immunostained with anti-NPF. The boxes indicate NPFM neurons. The scale bars in A—F represent 50 μm. The scale bars in c1—c6 and d1—d6 represent 10 μm.
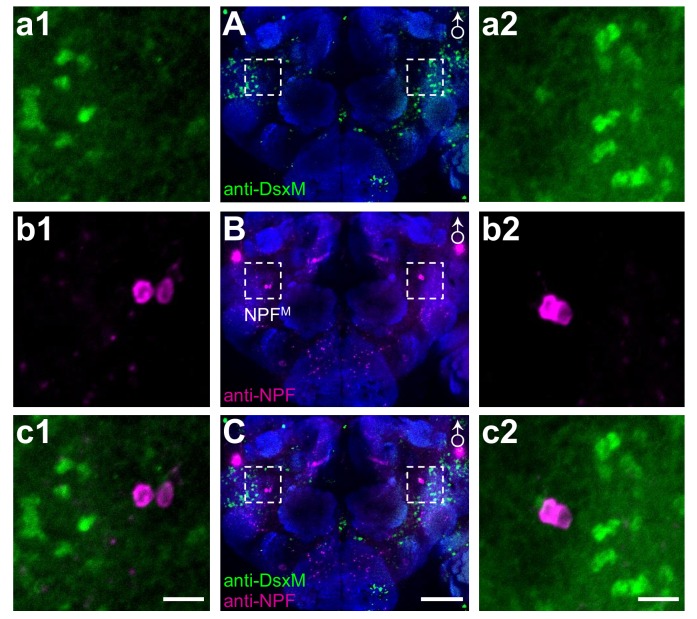
Figure 2—figure supplement 1. w1118-CS male flies stained with anti-NPF and anti-DsxM.
The fruitless (fru) gene is a master regulator of male courtship behavior, and its role is mediated through expression of a male-specific protein, FruM, which is produced through alternative mRNA splicing (Ito et al., 1996; Ryner et al., 1996; Lee et al., 2000; Demir and Dickson, 2005; Manoli et al., 2005; Stockinger et al., 2005). We examined whether NPFM neurons expressed the FruM protein by performing double labeling using anti-FruM, and anti-GFP, which marks the cells expressing mVenus driven by the npfLexA reporter. We found that NPFM neurons expressed FruM (Figure 2D, d1—d6), while they were negative for DsxM (Figure 2—figure supplement 1), another key protein regulating the organization of the male nervous system (Rideout et al., 2010; Robinett et al., 2010).
To determine if FruM is essential for determining the fate of NPFM neurons, we used anti-NPF antibodies to stain the brains of fruFLP mutant (Yu et al., 2010) males. We found that staining of NPFM neurons was eliminated in fruFLP males (Figure 2E and F), indicating that specification of NPFM neurons depends on FruM.
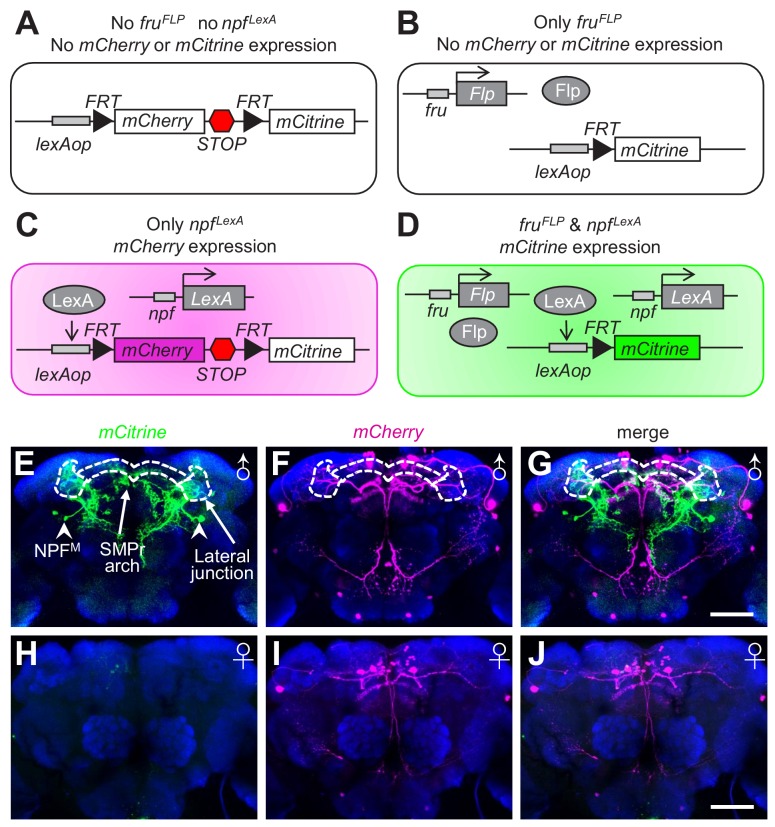
To distinguish the projection pattern of NPFM neurons from the remaining NPF neurons, we used the FlpOut method (Wong et al., 2002) to specifically label NPFM neurons with mCitrine. In the absence of both fruFLP and npfLexA, neither mCherry nor mCitrine is expressed (Figure 3A). If the flies contain the fruFLP transgene but not the npfLexA transgene, the mCherry gene is removed due to expression of Flp (FlpOut), but mCitrine is not expressed (Figure 3B). In flies with npfLexA but no fruFLP, mCherry is expressed, but mCitrine is not expressed due to the transcriptional stop cassette downstream of the coding region for mCherry (Figure 3C). If flies harbor both the fruFLP and npfLexA transgenes, then mCherry is removed by FlpOut in fru-expressing neurons, and mCitrine is expressed (Figure 3D). Therefore, NPFM are the only neurons labeled with mCitrine. Using this intersectional method, we found that NPFM neurons extensively arborize a large proportion of the superior brain of the male (Figure 3E—G and Video 1). In contrast, there were no mCitrine-labeled neurons in the female brain (Figure 3H). Rather, the NPF neurons in females were labeled with mCherry only (Figure 3I and J).
Figure 3. Labeling of NPFM neurons using the FlpOut method.
(A—D) Schematic illustration of the FlpOut method to label NPFM neurons. Only neurons that express both fru (fruFLP) and npf (npfLexA) will express mCitrine. (E—G) Expression patterns of mCitrine (stained with anti-GFP) and mCherry (stained with anti-DsRed) in a male brain. The white dashes outline the SMPr arch and lateral junction regions of the LPC. Arrowheads indicate NPFM soma. (H—J) mCitrine and mCherry expression patterns in a female brain. The scale bars represent 50 μm.
Video 1. Morphology of male-specific NPFM neurons.
A male brain from a LexAop > mCherry > mCitrine/+;npfLexA/fruFLP fly was stained with anti-GFP (recognizes mCitrine) and anti-DsRed (recognizes mCherry). The npf and fru double positive (NPFM) neurons express mCitrine and are labeled by anti-GFP (Figure 3E—G). The remaining npf neurons are labeled by mCherry, and are stained with anti-DsRed. Imaris (Bitplane) software was used to prepare the reconstruction and animation.
To address whether NPFM neurons are exclusively responsible for regulating male courtship, we expressed a conditional repressor or activator specifically in NPFM neurons. To inhibit NPFM neurons, we used the temperature sensitive Shits, which we expressed in NPFM neurons only using the FlpOut method. We employed a transgene that encodes Shits downstream of a 5’ transcriptional stop cassette that is flanked by FRT sites ( UAS > stop > Shits ; note that ‘ >” indicates FRT sites), and removed the stop cassette specifically in fru neurons by expressing flippase exclusively in fru neurons with the fruFLP. After the stops are removed, we expressed UAS-Shits under control of the npf-Gal4, thereby restricting Shits to NPFM neurons only. When we performed courtship assays at the non-permissive temperature for Shits (31 °C), the males showed elevated courtship relative to flies with the same genotype that were assayed at the permissive temperature (23°C) for Shits (Figure 4A). We then tested the effects of inhibiting neurons except for NPFM neurons, using npf-Gal4/+;fruFLP/ UAS > Shits > stop flies, which removes Shits just in NPFM neurons. These males displayed similar levels of courtship at both the permissive temperature and non-permissive temperatures for Shits (Figure 4A).
Figure 4. Specificity of NPFM neurons in regulating male courtship.
(A) Single tester males of the indicated genotypes were assayed for male-female (M–F) courtship at both permissive (23°C) and non-permissive (31°C) temperatures for Shits. Newly-eclosed male flies were isolated for 5 days, after which they were housed with 5—7 w1118 virgin female flies for 4 hr prior to the experiment. 7—15 day-old mature active mated w1118 female flies were used as targets. The courtship index is the mean ratio of time spent by the tester male in courtship within 30 min following a 10 min incubation period. n = 8—24. Bars indicate means ± SEMs. Significance was determined using Mann-Whitney test. **p < 0.01. (B) Single tester males of the indicated genotypes were assayed for courtship at two different temperatures (23°C and 29°C). Newly-eclosed males that were isolated for 2 days were used as testers. Decapitated w1118 female flies were used as the targets. Courtship index represents the mean ratio of time the male flies spent in courting within 10 min following a 5 min incubation period. n = 6—27. Bars indicate means ± SEMs. Significance was determined using Mann-Whitney test. **p < 0.01. (C—E) Immunohistochemistry showing the effect of npf RNAi knock down on NPF protein expression in male brains. Control genotypes of npf-Gal4/+ and UAS-npf-RNAi male brains and experimental genotype of npf-Gal4/+;UAS-npf-RNAi/+ male brains were immuno-stained with anti-NPF. Scale bars indicate 50 μm. (F) Effects on male-male (M–M) courtship due to RNAi knock down of npf in all neurons (elav), fru neurons, npf neurons, non-NPFM npf neurons and NPFM neurons. n = 7—12. The bars indicate means ± SEMs. Mann-Whitney test was used to determine significance. **p < 0.01.
To activate NPFM neurons, we employed a similar FlpOut approach, using UAS > stop > trpA1/npf-Gal4; fruFLP/ +flies, to express the thermally-activated TRPA1-A isoform in NPFM neurons only. This TRPA1 isoform is a Na+ and Ca2+-permeable channel, which is activated at temperatures above ~ 27 °C (Viswanath et al., 2003). To perform these assays, we used decapitated females since intact females stimulate ceiling levels of male courtship, which are resistant to down-regulation, while decapitated females induce moderate levels, which facilitate detecting subtle decreases in male courtship. We found that courtship levels in UAS > stop > trpA1/npf-Gal4;fruFLP/+ males were suppressed at 29°C relative to 23°C (Figure 4B). In contrast, none of the three types of control flies exhibited lower male courtship at 29°C (Figure 4B). Nevertheless, because the CI exhibited by the UAS > stop > trpA1/npf-Gal4;fruFLP/+ males at 29°C was not elevated relative to the CIs displayed by the control males at 29°C, the results preclude the conclusion that activation of sexually dimorphic NPFM neurons inhibits male courtship.
To test whether the NPF produced in NPFM neurons is responsible for inhibiting male courtship, we knocked down NPF expression in distinct groups of neurons. To conduct these experiments, we used UAS-npf-RNAi, which was effective as it greatly reduced NPF levels (Figure 4C—E). We found that knocking down npf expression with the fru-Gal4 induced a dramatic increase in M–M courtship, and did so to a similar extent as when we used a pan-neuronal (elav) Gal4 or the npf-Gal4 (Figure 4F).
To specifically interrogate a requirement for NPF in NPFM neurons, we used FlpOut to introduce Gal80 (which binds and inhibits Gal4 activity) in either fru+ or fru- neurons, thereby confining UAS-npf-RNAi expression to fru- NPF neurons or NPFM neurons, respectively. To knockdown npf specifically in NPFM neurons, we used the following flies that cause excision of Gal80 in fru neurons only, thereby allowing Gal4 expression and RNA knockdown in NPFM neurons: npf-Gal4/tub > Gal 80 >stop;UAS-npf-RNAi/fruFLP flies. Conversely, to prevent npf knockdown in NPFM neurons, we expressed Gal80 specifically in these neurons using npf-Gal4/tub > stop > Gal80;UAS-npf-RNAi/fruFLP flies. We found that knocking down npf exclusively in NPFM neurons elevated M–M courtship while npf knock down in fru- NPF neurons did not change the level of male courtship (Figure 4F). These results indicate that sexually dimorphic NPFM neurons are the subset of NPF neurons that are exclusively required for suppressing male courtship, and the effect is dependent on NPF produced in NPFM neurons.
P1 neurons directly activate NPFM neurons
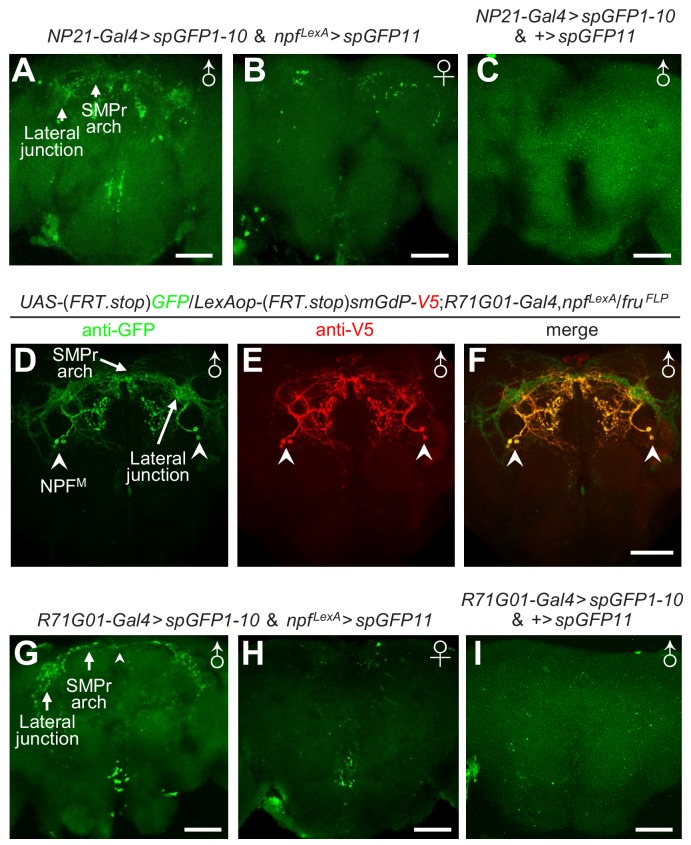
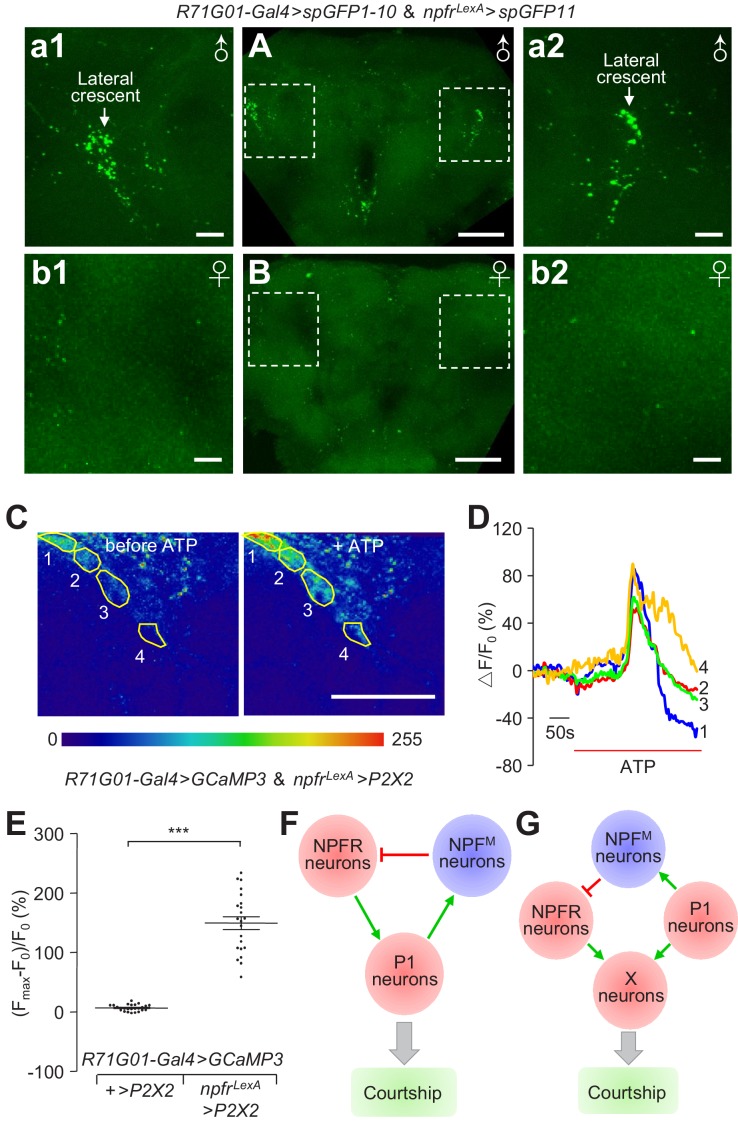
To address how NPFM neurons are integrated into the fru circuit, we adopted the GRASP (GFP Reconstitution Across Synaptic Partners) (Feinberg et al., 2008; Gordon and Scott, 2009) method to detect potential contact loci between NPF neurons and fru neurons. This approach employs a dual binary expression system to synthesize two complementary but non-functional parts of GFP (spGFP1-10 and spGFP11) on the cell membranes of distinct neurons. When the neurons are in close proximity, GFP is reconstituted and fluorescence is produced. We expressed spGFP1-10 and spGFP11 in fru and NPF neurons, respectively and detected strong bouton-shaped GFP signals in the male brain (Figure 5A) but only sparse signals in the female brain (Figure 5B) and no specific reconstituted GFP signals in control male brains missing the driver for the LexAop-spGFP11 (Figure 5C). The reconstituted GFP signals in the male brain reconstruct a distinctive male-specific brain structure – the lateral protocerebral complex (LPC), which includes several neuropils: the lateral junction, superior medial protocerebrum (SMPr) arch, lateral crescent and the ring structure (Figure 5—figure supplement 1A) (Yu et al., 2010). The LPC structure is formed by neural projections from a cluster of male-specific P1 neurons which function as the integrative hub controlling male courtship behavior (Kimura et al., 2008; Yu et al., 2010; Kohatsu et al., 2011; von Philipsborn et al., 2011; Pan et al., 2012; Bath et al., 2014; Inagaki et al., 2014; Clowney et al., 2015; Kallman et al., 2015; Zhou et al., 2015; Zhang et al., 2016).
Figure 5. Anatomical and functional interactions between P1 and NPFM neurons.
(A—C) GRASP approach to examine close interactions between NPF and fru neurons in UAS-spGFP1-10, LexAop-spGFP11/NP21-Gal4, npfLexA flies. GFP fluorescent signals indicate close associations. (A) Reconstituted GFP signals in a male brain. The arrows indicate the SMPr arch and lateral junction structures. (B) Reconstituted GFP signals in a female brain. (C) Negative control for GRASP showing a UAS-spGFP1-10, LexAop-spGFP11/NP21-Gal4 male brain. Scale bars indicate 50 μm. A portion of the brain stacks, including the LPC structure, is shown. The full brain stacks are presented in the source data files. (D—F) FlpOut approach to differentially label P1 neurons and NPFM neurons. (D) Anti-GFP stained fru-positive P1 (due to smGdP expression) and NPFM neurons. Arrows indicate the SMPr arch and the lateral junction. Arrowheads indicate the soma of NPFM neurons. (E) Anti-V5 exclusively labels NPFM neurons. The arrowheads indicate soma of NPFM neurons. (F) Composite of P1 and NPFM neurons. The arrowheads indicate NPFM soma. The scale bar represents 50 μm. (G—I) GRASP approach to examine close interactions between NPF and P1 neurons in UAS-spGFP1-10,LexAop-spGFP11/R71G01-Gal4,npfLexA flies. GFP fluorescent signals indicate close associations. (G) Reconstituted GFP signals in a male brain. Arrows indicate lateral junction and SMPr arch of the LPC. The arrowhead indicates an example of a reconstituted GFP signal. (H) Reconstituted GFP signals in a female brain. (I) Negative control for GRASP showing a UAS-spGFP1-10, LexAop-spGFP11/R71G01-Gal4 male brain. Scale bars indicate 50 μm. A portion of the brain stacks, including the LPC structure, is shown. The full brain stacks are presented in the source data files.
Figure 5—figure supplement 1. Cartoons of male brains showing the approximate positions of selected brain regions and neurons.
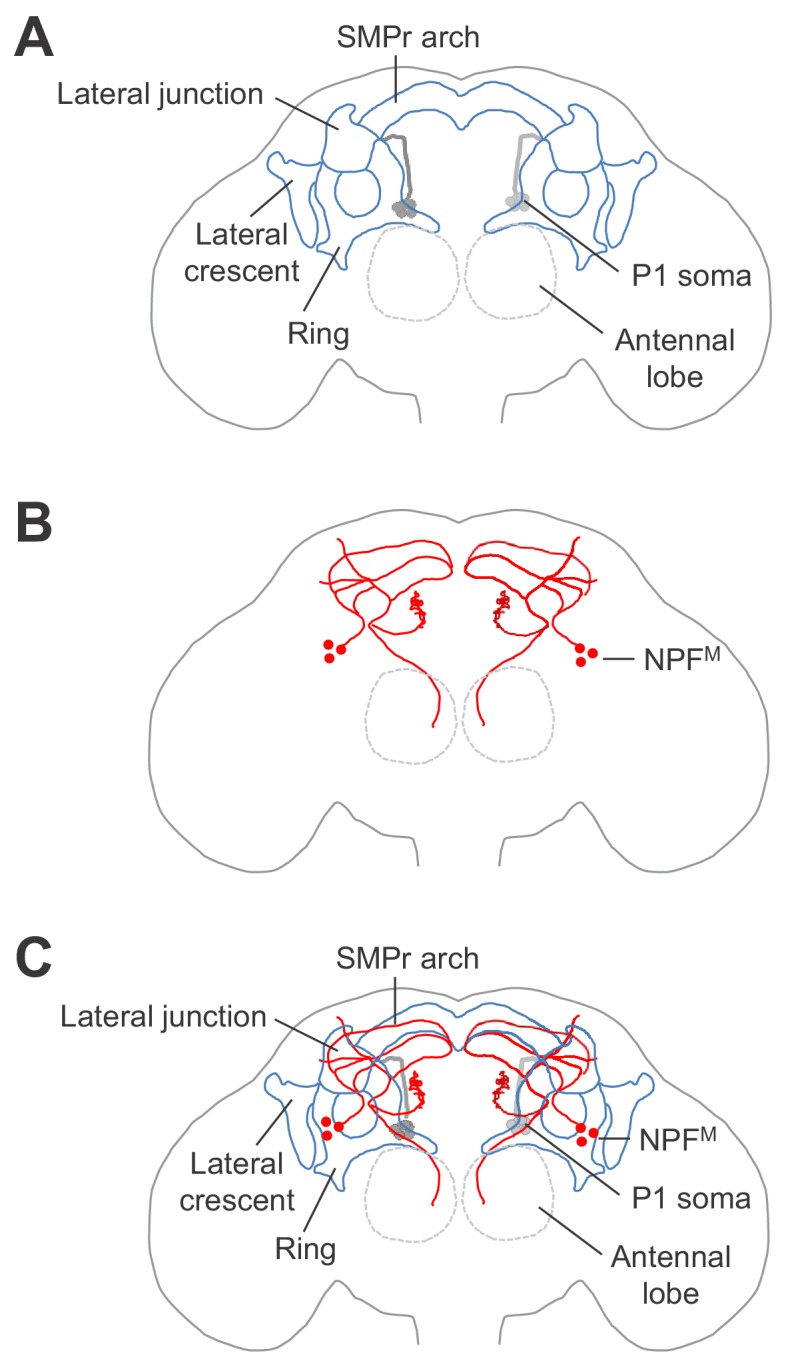
Figure 5—figure supplement 2. Comparison of the projection patterns of NPF and P1 neurons in a male brain.
Figure 5—figure supplement 3. Directionality of connections between P1 and NPFM neurons.
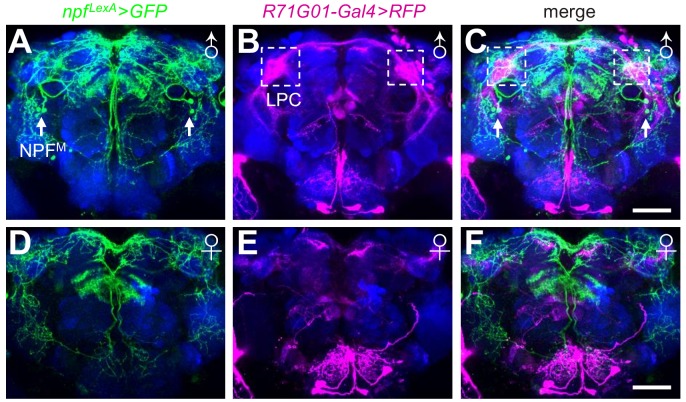
To compare the projection patterns of NPF and P1 neurons, we expressed GFP and RFP in NPF and P1 neurons, respectively, using two binary expression systems. We found that the projections from NPF neurons overlapped with the LPC structure in the male brain (Figure 5—figure supplement 2A—C). However, the female brain does not include an LPC structure (Figure 5—figure supplement 2D—F). We further combined the FlpOut method and dual binary expression systems to exclusively label NPFM and P1 neurons, and found that the projections from these two clusters of neurons overlapped intensely in LPC region (Figure 5D—F and Figure 5—figure supplement 1 and Video 2).
Video 2. Animated representation of projections of NPFM and P1 neurons.
UAS > stop > mCD8::GFP/ LexAop > stop > myr::smGdP-V5;R71G01-Gal4,npfLexA/fruFLP male brain stained with anti-GFP and anti-V5. The P1 neurons were singly labeled with anti-GFP, while NPFM neurons were double labeled with anti-GFP and anti-V5. Imaris (Bitplane) software was used to prepare the reconstruction and animation.
To address if the projections of NPF and P1 neurons form direct connections, we used the R71G01-Gal4 (which is expressed in P1 neurons and a few other neurons) to drive expression of spGFP1-10, and npfLexA to drive expression of spGFP11. We detected strong GFP signals reconstructing the LPC structure in the male brain (Figure 5G), but not in the corresponding brain regions of female brains or control male brains that do not have the driver for LexAop-spGFP11 (Figure 5H and I). The GRASP GFP signals appear to be due to expression of the two parts of the split GFP in NPFM and P1 neurons for the following reasons. First, NPFM and P1 neurons are both male-specific, and the GRASP signals are primarily in the male brain and not in the female brain (Figure 5G and H). Second, the GRASP signals label two LPC structures: the lateral junction and SMPr arch (Figure 5G). Third, the projections of NPFM and P1 overlap extensively in the lateral junction and SMPr arch (Figure 5D—F and Video 2), while fru- NPF projections do not innervate the LPC region (Figure 3F and G and Video 1). Thus, the GRASP signals in the LPC structure appear to be formed by connections between NPFM and P1 neurons.
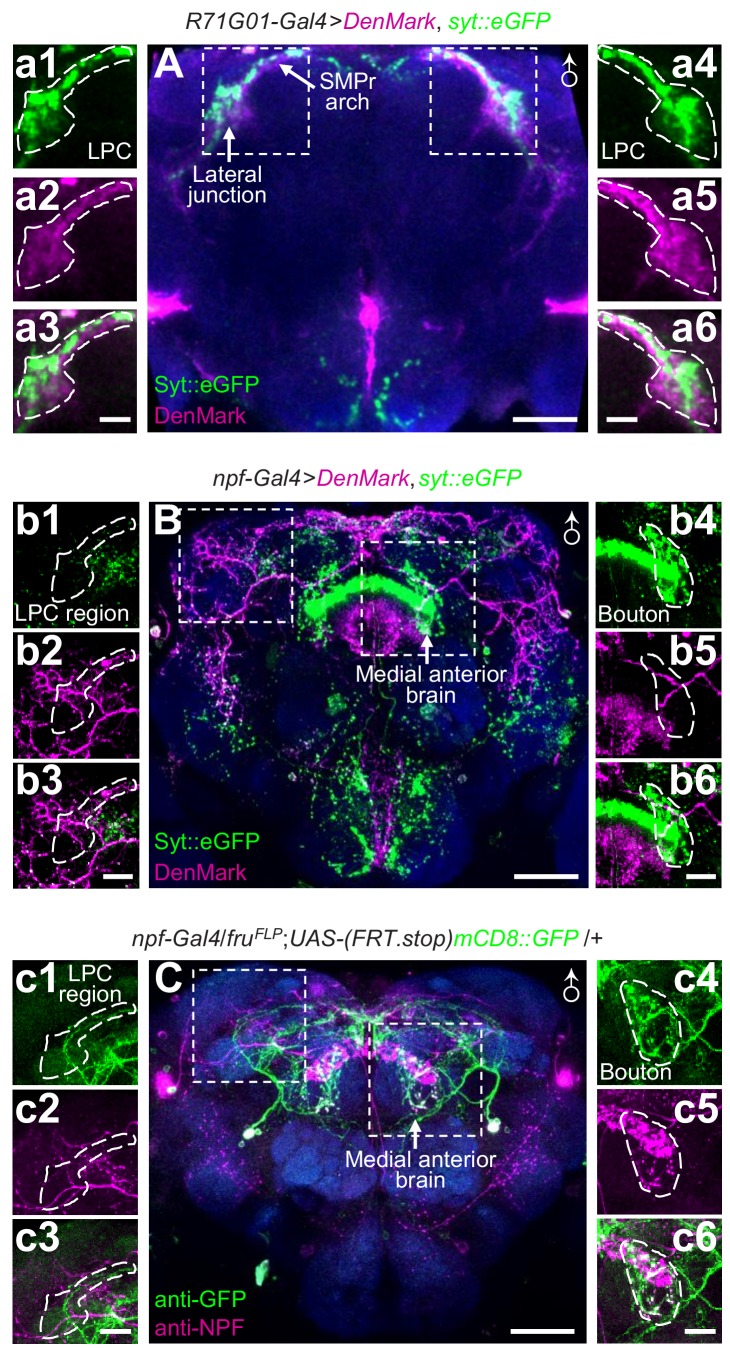
To clarify the directionality of the synaptic connections between NPFM and P1 neurons, we employed genetically encoded markers to label the dendritic (UAS-DenMark) and axonal (UAS-syt::eGFP) branches of NPF and P1 neurons (Wang et al., 2007; Nicolaï et al., 2010). The P1 neurons that extend processes to the lateral junction and SMPr arch within the LPC structure were stained with both Denmark and Syt::eGFP, suggesting that P1 neurons send and receive signals within these neuropils (Figure 5—figure supplement 3A, a1-a6). However, in the corresponding lateral junction and SMPr arch within the LPC region, NPF neurons were labeled with DenMark only (Figure 5—figure supplement 3B, b1-b3), suggesting that NPF neurons mainly receive signals within this region. The NPF axons that stained with Syt::eGFP occurred in several brain regions other than the LPC region (Figure 5—figure supplement 3B, b1-b6).
To distinguish the boutons formed by NPFM neurons from other NPF neurons, we used the FlpOut approach to specifically label projections of NPFM neurons. We stained the brains of male UAS > stop > mCD8::GFP/+;fruFLP/npf-Gal4 flies (Yu et al., 2010) with anti-GFP and anti-NPF so that the boutons formed by NPFM neurons would be double labeled. We found that the double-labeled boutons were concentrated in the medial anterior brain, but not in the lateral superior brain (Figure 5—figure supplement 3C, c1-c6), indicating that the release site of NPFM neurons was outside the LPC region. These results demonstrate that NPFM neurons do not directly act on P1 neurons. Rather, the synaptic connections between NPFM and P1 neurons in the LPC region are formed by pre-synaptic P1 neurons and post-synaptic NPFM neurons.
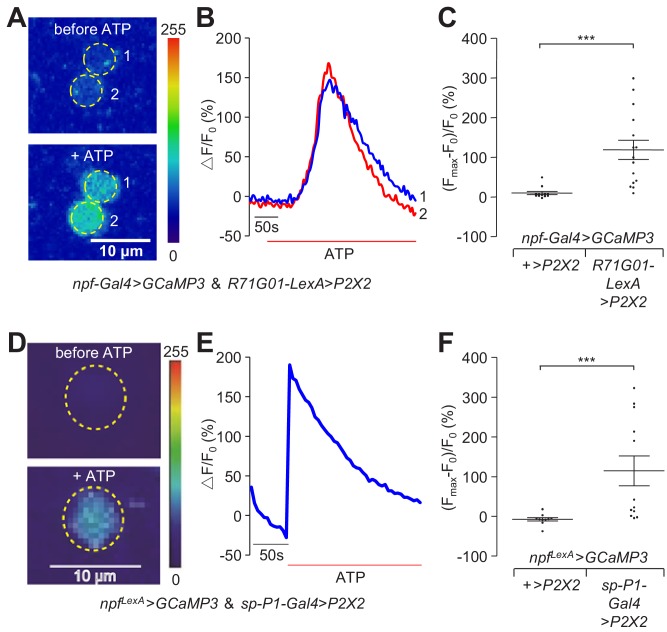
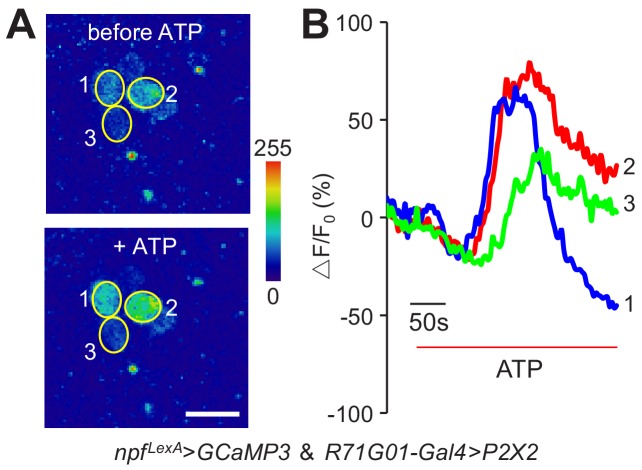
To determine the impact of activation of P1 neurons on the activity of the NPFM neurons, we combined chemogenetics and GCaMP imaging to monitor Ca2+ dynamics (Yao et al., 2012) as an indicator of neural activation. We expressed P2X2 (encoding an ATP-gated cation channel) (Lima and Miesenböck, 2005) in P1 neurons, and expressed GCaMP3 in NPF neurons. We used R71G01-LexA, which is expressed in P1 neurons and a few other neurons, to drive P2X2 expression, and npf-Gal4 to drive UAS-GCaMP3. In a complementary experiment, we switched the two binary systems, and used the R71G01-Gal4 and npfLexA to drive P2X2 and GCaMP3, respectively. Because the diffusion rate and final concentration of ATP that reaches the brain varies across samples, we calculated the maximum fold changes of the GCaMP3 responses after ATP application relative to the basal levels of GCaMP3 before ATP application. We found that ATP-induced activation of P1 neurons led to robust GCaMP3 signals in NPFM neurons (Figure 6A—C and Figure 6—figure supplement 1 and Videos 3 and 4).
Figure 6. Neural activity changes in NPFM neurons in response to activation of P1 neurons.
(A—C) UAS-GCaMP3, LexAop- P2X2/R71G01-LexA;npf-Gal4/+ male brains were imaged for GCaMP3 responses. Cell bodies of NPFM neurons were imaged. (A) Representative heat maps indicating GCaMP3 fluorescence before and during ATP application. The numbers indicate NPFM neurons. (B) Representative traces showing dynamic changes in GCaMP3 fluorescence in NPFM neurons (circled in panel A). (C) Largest GCaMP3 fluorescence changes [(Fmax-F0)/ F0 (%)] in response to ATP application in the control and experimental group. GCaMP3 fluorescence was recorded from 12 NPFM neurons from eight control brains, and 15 NPFM neurons from nine experimental brains. (D—F) UAS- P2X2 , LexAop-GCaMP3/R15A01-AD; npfLexA / R 71 G01-DBD male brains were imaged for GCaMP3 responses. The cell bodies of NPFM neurons were imaged. (D) Representative heat maps indicating GCaMP3 fluorescence before and during ATP application. The numbers indicate NPFM neurons. (E) Representative traces showing dynamic changes in GCaMP3 fluorescence in NPFM neurons (circled in panel D). (F) Largest GCaMP3 fluorescence changes [(Fmax-F0)/ F0 (%)] in response to ATP application in the control and experimental group. GCaMP3 fluorescence was recorded from 10 NPFM neurons from three control brains, and 12 NPFM neurons from three experimental brains. The scale bars in (A and D) represent 10 μm. The bars in (C and F) indicate means ± SEMs. Significance was assessed using the Mann Whitney test, ***p < 0.001.
Figure 6—figure supplement 1. Ca2+ imaging of NPFM neurons in response to activation of P1 neurons.
Video 3. Activation of P1 neurons causes a significant increase in GCaMP3 fluorescence in NPFM neurons.
The NPFM neurons were imaged in a UAS-GCaMP3, LexAop P2X2/R71G01-LexA;npf-Gal4/+ male brain. ATP was applied to the brain sample as indicated.
Video 4. Activation of P1 neurons causes a significant increase in GCaMP3 fluorescence in NPFM neurons following application of ATP to the brain sample.
The NPFM neurons were imaged in a UAS- P2X2,LexAopGCaMP3/+;R71G01-Gal4,npfLexA/+ male brain. ATP was applied to the brain sample as indicated.
In order to exclude the impact from other neurons, we expressed P2X2 in P1 neurons only using a split-P1-Gal4 comprised of R15A01-AD (activation domain) and R71G01-DBD (DNA-binding domain). We imaged Ca2+ dynamics in NPFM neurons in response to ATP application, and detected large increases in GCaMP3 fluorescence in response to activation of P1 neurons (Figure 6D—F), further supporting the conclusion that P1 neurons directly activate NPFM neurons.
Increase in courtship by inhibiting NPFM neurons depends indirectly on P1 neurons
To determine whether the function of NPFM neurons in courtship regulation is dependent on P1 neurons, we tested if silencing P1 neurons would prevent the courtship elevation induced by disruption of NPF neurons. We expressed UAS-Shits in both NPF and P1 neurons (npf-Gal4 and R71G01-Gal4) and assayed male courtship at both permissive and non-permissive temperatures. We found that the courtship dis-inhibition caused by disrupting NPF neurons was eliminated by simultaneous disruption of P1 neurons (Figure 7A—C). The results suggest that NPFM neurons appear to act through P1 neurons to regulate male courtship. Alternatively, NPFM and P1 neurons may act in parallel and serve opposing inputs onto a common neuronal target.
Figure 7. Effects of inactivating NPF and P1 neurons on male courtship, characterization of npfr reporter expression, and impact of npfr on male courtship.
(A—C) Effects of silencing both NPF and P1 neurons with Shits (npf-Gal4/+;R71G01-Gal4/UAS-Shits) on courtship of group-housed males towards female targets. Male-female (M–F) courtship was assayed at the permissive (23°C) and non-permissive (31°C) temperatures for Shits. (A) The percentages of males that initiated courtship. n = 4 (6 flies/group). (B) The courtship indexes were scored based on 20—30 min of observation during a 30 min incubation period. n = 24. (C) Effect of silencing both NPF and P1 neurons with Shits (npf-Gal4/+;R71G01-Gal4/UAS-Shits) on male-male (M–M) courtship. Isolation-housed males were assayed for chaining behavior at 23°C and 31°C for 10 min. n = 6 (8—12 flies/group). The bars indicate means ± SEMs. Significance was assessed using the Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001. (D—F) Spatial distribution of npfr (mCherry) and P1 (GFP) reporters in a male brain (UAS-mCD8::GFP/+;R71G01-Gal4/npfrLexA,LexAop-mCherry). The reporters were detected with GFP and DsRed antibodies. The boxed regions indicate the LPC. The scale bar represents 50 μm. (G) npfrLexA homozygous and npfrLexA/npfrc01896 trans-heterozygous mutants were assayed for M–M courtship. The control flies are w1118-CS. n = 12—24. (H) Effects on M–M courtship due to knock down of npfr pan-neuronally (elav-Gal4) or in P1 neurons. n = 21—23. The bars indicate means ± SEMs. To determine significance, we used the Kruskal-Wallis test followed by the Dunn’s post hoc test. **p < 0.01, ***p < 0.001.
Figure 7—figure supplement 1. npfrLexA mutant.
NPF binds to a G protein-coupled receptor—the NPF receptor (NPFR), which couples to a Gi signaling pathway to inhibit npfr-expressing neurons (Garczynski et al., 2002). To address the roles of the npfr gene and NPFR neurons in regulating male courtship, we replaced a portion of the npfr coding region with LexA, thereby generating an npfr mutant and a reporter (Figure 7—figure supplement 1). We then used the R71G01-Gal4 and npfLexA/+ to label P1 neurons and NPFR neurons with GFP and mCherry, respectively. We found that they primarily stain distinct neuronal populations (Figure 7D—F), indicating that P1 neurons are not the npfr-expressing neurons. These results further support our data suggesting that NPFM axons do not send signals directly to P1 dendrites, but that P1 neurons signal to NPFM neurons.
We assayed courtship behavior of npfrLexA mutant flies, demonstrating that these mutant animals raised in isolation exhibit significantly higher M–M courtship than control males (Figure 7G). We observed similar results with npfrLexA/npfrc01896 trans-heterozygous flies (Figure 7G). RNAi-mediated knockdown of npfr using a pan-neuronal Gal4 (elav) also increased M–M courtship behavior (Figure 7H). In contrast, knocking down npfr expression in P1 neurons had no effect (Figure 7H).
We took advantage of the GRASP method to investigate whether NPFR and P1 neurons make direct connections. We used R71G01-Gal4 and npfrLexA drivers to express spGFP1-10 and spGFP11 respectively. We detected GRASP signals in the lateral crescent within the LPC region of the male brain (Figure 8A,a1,a2). In contrast, we did not detect GRASP GFP fluorescence in female brains or in control male brains (Figure 8B,b1,b2 and Figure 5I).
Figure 8. Anatomical and physiological interactions between NPFR and P1 neurons.
(A and B) GRASP analyses to test for close associations between npfr and P1 neurons. UAS-spGFP1-10,LexAop-spGFP11/R71G01-Gal4,npfrLexA male and female brains were imaged for reconstituted GFP signals. (A) Reconstituted GFP signals in a male brain. The boxes indicate the higher magnification images (a1 and a2) showing the bouton-shaped GFP signals in the lateral crescent within the LPC. (B) Reconstituted GFP signals in a female brain. The boxes indicate the zoomed in areas (b1 and b2) showing the lateral regions of the female brain, corresponding approximately to the lateral crescent regions in the male brain. The scale bars represent 50 μm in (A and B), and 10 μm in a1—a2 and b1—b2. A portion of the brain stacks, including the LPC structure, is shown. The full brain stacks are presented in the source data files. (C—E) Assaying effects on P1 neuronal activity with GCaMP3, after stimulating npfr neurons with ATP. GCaMP3 and P2X2 were expressed specifically in P1 and npfr neurons, respectively, in the following flies: UAS-GCaMP3, LeAop P2X2/+;R71G01-Gal4/npfrLexA. GCaMP3 responses were imaged in the LPC structures in male brains. (C) Representative heat maps indicating GCaMP3 fluorescence before and during ATP application. The numbers indicate the regions within the LPC structure measured. (D) Representative traces showing dynamic fluorescence changes in the specified regions circled in (C). (E) Maximal fluorescence increases [(Fmax-F0)/ F0 (%)] in response to ATP application. GCaMP3 fluorescence was recorded from 25 regions from five control brains, and 22 regions from four experimental brains. The scale bar in (C) represents 50 μm. The bars in (E) indicate means ± SEMs. To determine significance, we used the Mann Whitney test. ***p < 0.001. (F) A model illustrating the feedback loop of NPFM neurons in the regulation of P1 neuronal activity. (G) Illustration of a feedforward parallel model, in which target neurons (X neurons) receive parallel input from P1 neurons and NPFR neurons.
To examine whether activation of NPFR neurons affects the activity of P1 neurons, we expressed P2X2 in NPFR neurons, and GCaMP3 in P1 neurons. We found that activation of NPFR neurons with ATP application induced robust GCaMP3 responses in the LPC structure (Figure 8C—E and Video 5). In control flies that did not express P2X2 , application of ATP did not induce elevation of GCaMP3 fluorescence (Figure 8E). The preceding results indicate that at least a subset of NPFR neurons anatomically connect and functionally activate P1 neurons. Together, our results indicate that NPFM, NPFR and P1 neurons form intricate interactions, and ensure proper courtship output in accordance with a male’s internal drive state.
Video 5. Activation of NPFR neurons with ATP causes a significant increase in GCaMP3 fluorescence in the LPC structure of P1 neurons.
The imaging was performed on a UAS-GCaMP3, LexAop P2X2 /+;R71G01-Gal4/npfrLexA male brain. ATP was applied to the brain sample as indicated.
Discussion
Multiple studies report the contribution of external sensory cues in inducing or suppressing male courtship behavior by signaling onto the P1 courtship decision center in the male brain (Kimura et al., 2008; Yu et al., 2010; Kohatsu et al., 2011; von Philipsborn et al., 2011; Pan et al., 2012; Bath et al., 2014; Inagaki et al., 2014; Clowney et al., 2015; Kallman et al., 2015; Kohatsu and Yamamoto, 2015; Zhou et al., 2015). In contrast, much less is known about how the P1 neurons are regulated by the male’s prior mating experience (Inagaki et al., 2014) and how courtship is affected by the internal drive state. An exception is a recent study that identified a group of dopaminergic neurons that changes in activity in proportion to male mating drive, and which directly activates P1 neurons to promote male courtship (Zhang et al., 2016). In the current study, we characterized a cluster of male-specific NPFM neurons which functions antagonistically to dopamine neurons by serving to suppress courtship by responding to sexual satiation. Disruption of NPFM neurons causes dis-inhibition of courtship in satiated males. The internal drive state of males is encoded by opposing excitatory and inhibitory inputs, which enable a male to make an appropriate mating decision in accordance with its internal drive state.
Suppression of NPF neurons or elimination of npf counters sexual satiation
Elimination of npf or knocking down npf expression exclusively in male-specific NPFM neurons causes male flies to exhibit maladaptive, hypersexual activity. In contrast to control males, which are sexually satiated when exposed to an abundance of females, and consequently display very low courtship levels, we found that flies overcome the sexual satiation imposed by mating if we introduce a loss-of-function mutation in npf or inhibit NPF neurons. Thus, satiation of courtship is dis-inhibited by disrupting NPF signaling.
Our findings that suppressing or eliminating NPF neurons elevates male courtship is in contrast to a previous report that genetic disruption or feminization of NPF neurons reduces male courtship activity (Lee et al., 2006). Maintaining males in the presence or absence of females profoundly affects sexual satiation levels, and the housing conditions were not clearly defined in this previous study. Our conclusions are supported by multiple lines of evidence. First, we found that when we inhibit neurotransmission from NPF neurons, using a temperature sensitive dynamin (Shits), the males showed a dramatic increase in courtship towards female conspecifics. This occurred using group-housed males which normally are sexually satiated. Second, introduction of a genetically encoded toxin, or inhibition of NPF neurons by overexpression of a K+ channel, also increases courtship activity. Third, when we disrupted the npf gene, the mutant males displayed a remarkable increase in courtship. This effect was so profound that the males courted females of another species and also displayed a great increase in M–M courtship, even though their gender preferences remained unchanged. Fourth, disruption of the npfr gene resulted in significant elevation in courtship, consistent with the effect of disrupting npf. Fifth, when we specifically silenced male-specific fru+ NPF (NPFM) neurons, male courtship behavior was elevated. In contrast, silencing fru- NPF neurons had no impact on male courtship. Sixth, knocking down npf expression exclusively in NPFM neurons increased male courtship, while knocking down npf in fru - NPF neurons had no effect.
Neuronal circuit models entail P1 neurons activating NPFM neurons
Our anatomical, physiological and functional evidence demonstrate that P1 neurons activate NPFM neurons, and suggest potential models through which these neurons coordinate to regulate male courtship drive. According to one model, P1 and NPFM neurons form a recurrent inhibitory neuronal circuit (Figure 8F). Stimulation of P1 neurons activates NPFM neurons, which act through an intermediate group of NPF receptor (NPFR neurons) and feedback to inhibit P1 neurons. This recurrent inhibitory model posits that P1 neurons are strongly activated when males are exposed to many females, inducing NPFM neurons to release NPF. This neuropeptide acts on the Gi-coupled NPF receptor and inhibits NPFR neurons, leading to a suppression of P1 activity, and attenuation of male courtship. When the activity of P1 neurons is reduced, stimulation of NPFM neurons and NPF release are diminished. This attenuates the feedback inhibition from NPFM to P1 neurons, leading to a return of P1 neuronal activity, and male courtship drive.
We suggest that the recurrent inhibitory neuronal motif proposed here is important for maintaining proper activities of P1 neurons, thus ensuring appropriate behavioral choices that are critical for a male’s reproductive success, depending on the level of sexual satiety. Because P1 neurons integrate multi-modal sensory input, as well as the male’s internal level of sex drive (Kohatsu et al., 2011; Pan et al., 2012; Bath et al., 2014; Inagaki et al., 2014; Clowney et al., 2015; Kallman et al., 2015; Kohatsu and Yamamoto, 2015; Zhou et al., 2015; Zhang et al., 2016), their activity must be under stringent control so that males display the courtship ritual only when both external sensory cues and the internal drive states are appropriate.
The recurrent inhibitory neural motif proposed here is dedicated to ensure appropriate activation of P1 neurons. Disruption of the inhibitory NPF afferents leads to excessive courtship behavior in the male fly that is maladaptive, as it overrides the courtship inhibition normally imposed by recent mating with females, other males, or females of other Drosophila species.
Recurrent inhibitory neural motifs are important in the central nervous system. In the mammalian spinal cord, motor neurons send collateral branches to Renshaw cells, which in turn send inhibitory signals back to motor neurons (Alvarez and Fyffe, 2007). The function of this recurrent inhibition is assumed to restrict excessive activation of motor neurons and contribute to precise recruitment of muscle fibers in order to generate proper force for different tasks (Alvarez and Fyffe, 2007). Recurrent inhibitory loops also occur in the hippocampus and entorhinal cortex. In these systems, principal cells send excitatory outputs to fast-spiking, parvalbumin-positive interneurons, and at the same time receive inhibitory inputs from these interneurons, thus, closing the feedback inhibition loop (Pouille and Scanziani, 2004; de Almeida et al., 2009; Pastoll et al., 2013).
While NPFM, P1 and NPFR neurons are essential for regulating courtship by responding to prior mating experience, and may do so through a recurrent inhibitory loop (Figure 8F), our data do not exclude other models. Part of the argument in favor of the recurrent inhibitory loop model is that the GRASP analysis suggests that NPFR neurons make direct connections with P1 neurons. Moreover, by coupling chemogenetic manipulation and Ca2+ imaging, we found that activation of NPFR neurons activate P1 neurons. However, NPFR neurons are widely distributed, and our data do not resolve whether the NPFR neurons that activate P1 neurons are the same subset of NPFR neurons that are the direct downstream target of NPFM neurons. Thus, one alternative to the recurrent inhibitory motif is a feedforward parallel model, in which target neurons (X neurons) control courtship drive by receiving parallel input from P1 neurons and NPFR neurons (Figure 8G). This latter model posits that P1 neurons activate X neurons, and at the same time, send axonal branches to activate NPFM neurons, which then act through NPFR neurons and suppress the target neurons through a feedforward mechanism. Future experiments that resolve the anatomical and functional diversity of NPFR neurons should distinguish between the recurrent inhibitory versus feedforward parallel model, which ensure proper courtship output in accordance with a male’s internal drive state.
Impact of NPF activity on courtship versus aggression
Courtship and aggression are closely interrelated social behaviors. If males are housed in isolation, they exhibit elevated courtship and aggression (Wang et al., 2008; Liu et al., 2011). This positive relationship is consistent with the observation that the presence of a potential mate promotes a male fly’s propensity to fight a competitor to win a mating competition (Kravitz and Fernandez, 2015). Though the tendency to fight or to court is positively related, the behavioral choice between courtship and aggression is mutually exclusive.
We found that when we disrupt the activity of NPF neurons, M–M courtship is dominant over aggression. We suggest that loss of NPF function diminishes inhibition of P1 neurons. As a result, even sub-optimal stimuli strongly activate P1 neurons and induce male courtship behavior even towards inappropriate targets. Conversely, when we increase the activity of NPF neurons or over-express the npf-cDNA in NPF neurons, M–M aggression is dominant over courtship.
The precise contribution of NPF neurons in regulating aggression is unresolved. One group found that activation of NPF neurons elevates male aggression (Asahina et al., 2014) while another reported that silencing or feminizing NPF neurons elevates aggression (Dierick and Greenspan, 2007). We found that when we overexpressed either the Na+ channel NaChBac or the npf-cDNA in NPF neurons, the males exhibited increased aggression. We propose that excessive NPF activity suppresses P1 neurons, thereby setting a high threshold for P1 activation. Our observations are consistent with previous report that weaker activation of P1 neurons favors aggression while stronger activation of P1 neurons favors courtship (Hoopfer et al., 2015). It remains to be determined if NPF neurons also impact on the aggression modulatory or arousal center (Asahina et al., 2014; Watanabe et al., 2017), independent of its effect on P1 neurons.
Possible relationship of NPF to courtship regulation by mammalian NPY
NPF is the Drosophila counterpart of mammalian NPY, which regulates feeding, reproduction, aggression, anxiety, depression and the alcohol addiction (Nässel and Wegener, 2011). Previous studies indicate that sexually dimorphic NPY neurons innervate the human INAH3 (interstitial nuclei of anterior hypothalamus 3), a region correlated with sexual orientation and gender identity recognition (LeVay, 1991; Byne et al., 2000; Garcia-Falgueras and Swaab, 2008). The discovery that Drosophila NPF regulates courtship depending on the internal drive state raises questions as to whether NPY may serve similar functions in mammals.
Materials and methods
Key resources
Descriptions of the key fly strains, antibodies, plasmids, chemicals, kits, services and software are provided in the Supplementary file 1.
Fly stocks
The following strains were obtained from Bloomington Stock Center (Indiana University): npf-Gal4 (#25681, and #25682 have identical promoters, but are inserted on the 2nd and 3rd chromosomes, respectively), elav-Gal4 (#8765), fru-Gal4 (NP21 #30027), R71G01-Gal4 (P1-Gal4 #39599), R71G01-LexA (P1-LexA #54733), UAS-NaChBac (#9468), UAS-Kir2.1 (#6596), UAS-DTI (#25039), UAS-mCD8::GFP (#5137), UAS-npf-RNAi (VDRC108772), UAS-npfr-RNAi (VDRC107663), UAS-DenMark,UAS-syt::eGFP (#33064), LexAop-mCherry (#52271), LexAop(FRT.mCherry)ReaChR-mCitrine (#53744), UAS-IVS-mCD8::RFP, LexAop-mCD8::GFP (#32229), UAS-CD4-spGFP1-10,LexAop-CD4-spGFP11 (#58755), LexAop-IVS-CsChrimson.mVenus (#55139), Lexop(FRT.stop)myr::smGdP-V5 (#62107) npfrc01896 (#10747), tub(FRT.Gal80)stop (#38880), tub(FRT.stop)Gal80 (#38878).
UAS-npf was a gift from Dr. Ping Shen (Wu et al., 2003) (University of Georgia), UAS- P2X2,LexAop-GCaMP3 and UAS-GCaMP3, LexAop P2X2 were from Dr. Orie Shafer (Yao et al., 2012) (University of Michigan), UAS-Shibirets was from Dr. Christopher Potter (Kitamoto, 2001) (Johns Hopkins University School of Medicine), fruFLP, UAS-(FRT.stop)mCD8::GFP, UAS-(FRT.stop)Shibirets and UAS-(FRT.Shibirets)stop, UAS-(FRT.stop)dTRPA1 were from Dr. Barry Dickson (Yu et al., 2010) (Janelia Research Campus), R71G01-DBD;R15A01-AD was from Dr. David Anderson (California Institute of Technology).
The npfLexA and npfrLexA mutants were outcrossed into a w1118 background for five generations. The controls for comparison to these mutants were w1118 flies in which we exchanged the X chromosome with Canton-S so the flies are w + on the X chromosome (w1118-CS flies). The full genotypes of the flies used in each figure and video are listed in Supplementary file 2.
Behavioral assays
The behavioral assays were recorded using a Samsung SCB-3001 camera. All behavioral analyses were performed using these videos.
M–F, and M–M courtship assays
To perform courtship assays, we added 3 ml of 1.5 % agarose into each well of 24-well cell culture plates (Corning Incorporated, REF353847). 2 mm diameter holes were drilled on the cover over each well. Custom silicone plugs were prepared (435570, StockCap) for blocking the holes. The cover and the plate were taped together to avoid gaps that might allow flies to escape.
Unless otherwise specified, 5—7 days old mixed sex, group-housed males (10 males raised together with 30 virgin w1118 females for 3 days) were used for the courtship assays. Three types of female targets were used: 1) mature active females, 2) newly-eclosed females, or 3) decapitated females. In experiments in which the targets were either grouped-housed w1118 males or Drosophila simulans females, we used 5—7 day old isolation-housed males as the testers. One tester male and one target were ice anesthetized, and transferred together into courtship chambers. The flies were allowed to recover for 10 min, and then male courtship was scored over the next 10 min. The courtship index is the fraction of time that a tester male performs courtship towards the target.
To test the effects of inhibiting npf neurons with Shits, a single tester male (npf-Gal4/+;UAS-Shits/+) and a target female (mature, active w1118, 5—7 days old) were ice-anesthetized, and the pair was transferred into courtship chambers. The assays were performed at 23°C and 31°C, which are the permissive and non-permissive temperatures for Shits, respectively. Courtship indexes were calculated based on 20—30 min observation during a 30 min incubation period.
Male chaining assays
We inserted newly-eclosed tester males into individual vials, and aged them for 5—7 days. We introduced 8—12 males into a 35 mm Petri dish, which was filled with 8 ml 1.5% agarose through a 2 mm diameter hole drilled on the cover. We allowed the flies to recover for 5 min, and then determined the ratio of time over the next 10 min in which ≥ 3 flies engaged in simultaneous courtship (chaining index).
M–M aggression assay
The aggression assays were carried out as described previously (Zhou et al., 2008), using 5—7 day-old isolation-housed tester males, and 5—7 days group-housed w1118 males as the targets. Briefly, one tester was paired with one target in the assay. The custom-designed chambers were based on previous reports (Zhou et al., 2008; Liu et al., 2011), and were fabricated by the Physics Machine Shop at UCSB (Figure 1—figure supplement 3). The chamber consists of two concentric circular chambers. The outer chamber diameter and height are 13 mm and 7 mm, respectively. The inner chamber diameter and height are 8 mm and 3.5 mm, respectively. The outer and inner chambers are separated by 0.5 mm thick, 3.5 mm high walls. 0.3 ml standard corn meal and molasses fly food was added to the inner chamber. 1.5% agarose was used to fill the space between inner and outer chambers. The heights of the food and agarose patches were the same (3.5 mm). We then dissolved 15% sucrose and 15% yeast in apple juice, and added 15 μl liquid to each food patch. Once the liquid mixture has soaked into the food, and the patch is dry at the surface, the aggression chamber is ready to use. w1118 male targets were transferred to the chamber by ice-anesthetization. A 22 × 22 mm microscope cover glass (Fisher Scientific) was used to cover to the chamber. The targets were allowed to recover for 10 min, and the isolation-housed tester males were introduced into the chamber by gentle tapping. After waiting 5 min for the tester males to recover, we scored the number of lunges during the following 15 min.
Male and female preference assay
To test the preference of a male tester for females versus males, we placed one decapitated w1118 virgin female and one decapitated w1118 male in a courtship chamber. The tester males were isolation-housed for 5—7 days since eclosion, and transferred into the chamber by gentle tapping. After 5 min recovery time, we scored the time during which the tester male performed courtship behavior towards either the decapitated female or the decapitated male target over the course of 10 min. The preference index is the ratio of time that male testers spend courting decapitated female targets out of the total courtship time.
Molecular biology
Generation of npf1 strain
To generate the npf1 allele (Figure 1E) we used the CRISPR mediated NHEJ (clustered regularly interspaced short palindromic repeats – non-homologous end joining) method (Kondo and Ueda, 2013; Ren et al., 2013).
We designed the following oligonucleotides:
npf-gRNA1-f: 5’ CTTCGCCCTTGCCCTCCTAGCCGC 3’
npf-gRNA1-r: 5’ AAACGCGGCTAGGAGGGCAAGGGC 3’
npf-gRNA2-f: 5’ CTTCGTTGCCATGGTCGTCTAAAA 3’
npf-gRNA2-r: 5’ AAACTTTTAGACGACCATGGCAAC 3’
We annealed the oligonucleotides to obtain two independent dimers, and ligated the primer dimers into the BbsI site of pU6-BbsI-ChiRNA BbsI (Addgene #45946). The pU6-BbsI-npf-gDNA1 and the pU6-BbsI-npf-gDNA2 plasmids were co-injected into the BDSC strain #51324 as the Cas9 source (BestGene Plan R). Based on DNA sequencing, we found that npf1 harbored a single nucleotide frameshift deletion that changed the 2nd position of codon 19.
Generation of npfLexA strain
To generate the npfLexA line with an insertion of the LexA reporter (Figure 1D), we used the CRISPR-HDR (clustered regularly interspaced short palindromic repeats – homology directed repair) method (Kondo and Ueda, 2013; Ren et al., 2013). We chose upstream and downstream guide RNAs that targeted the npf coding sequences using the CRISPR Optimal Target Finder: http://tools.flycrispr.molbio.wisc.edu/targetFinder/.
We annealed the following upstream and downstream primer dimers, which we inserted into the BbsI site of pU6-BbsI-ChiRNA (Addgene #45946).
npf_up_ChiRNA_F: 5’ CTTCCCAAACAATGCGTTGCATCC 3’
npf_up_ChiRNA_R: 5’ AAACGGATGCAACGCATTGTTTGG 3’
npf_down_ChiRNA_F: 5’ CTTCAGATTTATGTTACAGGCTCG 3’
npf_down_ChiRNA_R: 5’ AAACCGAGCCTGTAACATAAATCT3’
We amplified the npf upstream (1359 bp, nucleotides 3 R:16609779 to 16611137, release = r 6.16) and downstream (1388 bp, nucleotides 3 R:16610753 to 16612140, release = r 6.16) homology arms using the following primers:
npf_LA_KpnI_F: 5’ GACGCATACCAAACGGTACCCATTGTGACACCGTTGCGCTTTCCA 3’
npf_LA_KpnI_R: 5’ TTTTGATTGCTAGCGAGTTCTATAAATGGCTAATGTATGT 3’
npf_RA_NdeI_F: 5’ CTAGGCGCGCCCATATGTCGCGGTTTTAATGAGGAGGAGATATTC 3’
npf_RA_NdeI_R: 5’ GACAAGCCGAACATATGATCGGACTTGGACGTGGTAAGCCAA 3’
We used the In-Fusion cloning kit (Clontech) to clone the upstream and downstream homology arms into the KpnI and NdeI sites of pBPLexA::p65Uw (Addgene #26231), respectively.
The pU6-BbsI-ChiRNA-npf_up, pU6-BbsI-ChiRNA-npf_down, and pBPLexA::p65Uw-npf_LA + RA plasmids were injected into the BDSC #51323 strain, which provided the source of Cas9 (BestGene Plan R).
We used the following primers to genotype the transformants (as shown in Figure 1—figure supplement 2A):
npf[LexA]LA_GT_F (P1): 5’ CTTTCGGCCAACATTTATTCACG 3’
npf[LexA]LA_GT_R (P2): 5’ AAAGCCCAGTCGCTGTGCTATCT 3’
npf[LexA]RA_GT_F (P3): 5’ TCAAATACCCTTGGATCGAAGTA 3’
npf[LexA]RA_GT_R (P4): 5’ AGGGCTGCTGTAAGTATCGGTTG 3’
npf_Deletion_F (P5): 5’ CTTCCCAAACAATGCGTTGCATCC 3’
npf_Deletion_R (P6): 5’ AAACCGAGCCTGTAACATAAATCT 3’
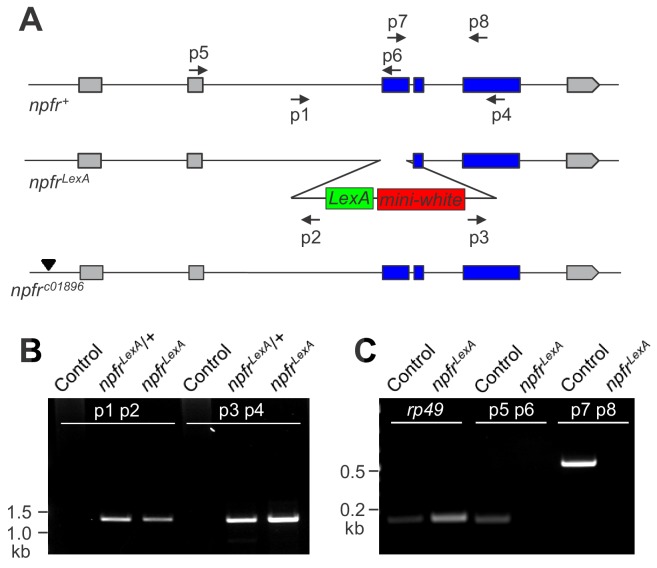
Generation of npfrLexA strain
We employed CRISPR-HDR (Kondo and Ueda, 2013; Ren et al., 2013) to generate the npfrLexA mutant with the LexA knockin. We chose the upstream and a downstream guide RNAs targeting the third exon using the CRISPR Optimal Target Finder.
We annealed the following upstream and downstream primer dimers, which we cloned into the BbsI site of pU6-BbsI-ChiRNA (Addgene #45946).
npfr_up_ChiRNA_F: 5’ CTTC GCAGATGGGGAGCATCTGAG 3’
npfr_up_ChiRNA_R: 5’ AAAC CTCAGATGCTCCCCATCTGC 3’
npfr_down_ChiRNA_F: 5’ CTTC ATTGCGAGCAGTGCGCATGA 3’
npfr_down_ChiRNA_R: 5’ AAAC TCATGCGCACTGCTCGCAAT 3’
We amplified the npfr upstream (1426 bp, nucleotides 3 R:6190969 to 6192394, release = r 6.16) and downstream (1250 bp, nucleotides 3 R:6192051 to 6193300, release = r 6.16) homology arms using the following primers:
npfr_LA_KpnI_F: 5’ GACGCATACCAAACGGTACC TGTGCTGCATAAATTACGGCGACGG 3’
npfr_LA_KpnI_R: 5’ TTTTGATTGCTAGCGGTACC AGATGCTCCCCATCTGCCAGCTGGG 3’
npfr_RA_NdeI_F: 5’ CTAGGCGCGCCCATATG TGCGCACTGCTCGCAATCTGTTCAT 3’
npfr_RA_NdeI_R: 5’ GACAAGCCGAACATATG CGCGCCCACGAACTGCAGGC 3’
We used the In-Fusion cloning kit (Clontech) to clone the upstream and downstream homology arms into the KpnI and NdeI sites of pBPLexA::p65Uw (Addgene #26231).
The pU6-BbsI-ChiRNA-npf_up, and pU6-BbsI-ChiRNA-npf_down, pBPLexA::p65Uw-npf_LA + RA plasmids were co-injected into the BDSC #55821 strain (BestGene Plan R), which provided the source of Cas9.
We used the following primers to genotype the transformants (as shown in Figure 7—figure supplement 1):
npfr[LexA]LA_GT_F (P1): 5’ CATGTCTCGCCTTGATGTGCTGC 3’
npfr[LexA]LA_GT_R (P2): 5’ AAAGCCCAGTCGCTGTGCTATCT 3’
npfr[LexA]RA_GT_F (P3): 5’ TCAAATACCCTTGGATCGAAGTAAA 3’
npfr[LexA]RA_GT_R (P4): 5’ CACAGCGAGAAGATCGAGTAGTAGAA 3’
The following primers were used to amplify npfr cDNA, which we obtained by performing RT-PCR using mRNA extracted from npfrLexA and control flies:
npfr_Deletion_F1 (P5): 5’ CACCTCGGATCTGAATGAGACTGG 3’
npfr_Deletion_R1 (P6): 5’ AGACGATTAGCACGCCGTACATG 3’
npfr_Deletion_F2 (P7): 5’ CACCCTGGTTGTTATAGCCGTCAT 3’
npfr_Deletion_R2 (P8): 5’ ACGCACAGCGAGAAGATCGAGTAG 3’
Generation of P[g-npf+] transgenic flies
We obtained a plasmid covering 20,306 bp of the npf genomic region from P[acman] Resources (http://www.pacmanfly.org/libraries.html). The P[acman] BAC CH322-163E17 plasmid, and a plasmid source of phiC31 were co-injected into a strain (BDSC #9723) with an attP40 site (BestGene Plan H).
Immunohistochemistry
Fly brains were dissected in ice-cold phosphate-buffered saline (PBS, pH 7.4, diluted from a sterile filtered 10x PBS stock, cat#:119-069-131, Quality Biological, Inc. 1x working concentration contains 137 mM NaCl, 2.7 mM KCl, 2 mM KH2PO4, 8 mM Na2HPO4) and fixed in 4 % paraformaldehyde in PBST (0.3 % Triton X-100 in PBS) at room temperature for ~ 20 min. Brains were washed three times in PBST for 20 min each time, and blocked in 5 % normal goat serum in PBST for 1 hr. The brains were incubated with primary antibodies diluted in 5 % normal goat serum in PBST for 24 hr at 4 °C. Samples were washed three times with PBST before applying secondary antibodies for 3 hr at 25 °C in darkness. After washing three times with PBST, the samples were mounted with VectaShield (Vector Labs) on glass slides. The primary antibodies were chicken anti-GFP (1:1000, Invitrogen, A-10262), rabbit anti-DsRed (1:1000, Clontech, 632496), mouse nc82 (1:250, Developmental Studies Hybridoma Bank), rabbit anti-FruM (1:10000) (Stockinger et al., 2005), rat anti-DsxM (1:500) (Hempel and Oliver, 2007) rabbit anti-NPF (1:250 ABIN641365), and mouse anti-V5 (1:500 DyLight549 tagged, MCA2894D549GA BioRad). The secondary antibodies were AlexaFluor 488 goat anti-chicken (1:1000; Invitrogen, A-11039), AlexaFluor 488 goat anti-rat (1:1000; Invitrogen, A-11006), AlexaFluor 568 goat anti-rabbit (1:1000; Invitrogen, A-11011), AlexaFluor 633 goat anti-mouse (1:1000; Invitrogen, A-21050), Rhodamine Red-X goat anti rabbit IgG (1:1000; Molecular Probe, R6394). We adapted a previously described method for anti-V5 and anti-GFP double staining (Nern et al., 2015). Briefly, we first used chicken anti-GFP as the primary antibodies (1:1000, Invitrogen, A-10262) for 24 hr 4 °C. We washed the brains three times with PBST, and then added AlexaFluor 488 goat anti-chicken IgG (1:1000; Invitrogen, A-11039) and DyLight549 tagged mouse anti-V5 antibodies (1:500 DyLight549 tagged, MCA2894D549GA BioRad). The brains were incubated at 25 °C for 3 hr in darkness, washed three times in PBST, and mounted with VectaShield (Vector Labs) on glass slides. We performed the imaging using a Zeiss LSM 700 confocal microscope, and processed the images using ImageJ.
GRASP analysis
To detect native GRASP GFP fluorescence in brains, we used flies aged for ~ 20 days to enhance the reconstructed GFP signals. We dissected the brains in ice-cold PBS, fixed the tissue for 20 min in 4 % paraformaldehyde in PBST at 25 °C, washed three times with PBST, and mounted the brains in PBS for imaging the native fluorescent signals.
Ex vivo Ca2+ imaging
We dissected brains from 7 to 15 day-old males (separated from females for 5 days, raised in ~ 10 male-only group) in cold Drosophila imaging saline (108 mM NaCl, 5 mM KCl, 2 mM CaCl2, 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO45 mM trehalose, 10 mM sucrose, 5 mM HEPES, pH = 7.5 (Inagaki et al., 2014), transferred individual brains to 35 mm plastic Petri dishes (35 3001 Falcon), attached the brain down to the bottom of the dish with a slice harp (SHD-26GH/10, Warner Instruments), and bathed each brain in 2 ml Drosophila imaging saline. We imaged the Ca2+ dynamics using a Zeiss LSM 700 confocal microscope. The images were acquired using a Zeiss 20x water objective (20x/1.0 DIC (uv) VIS-IR, Zeiss) and a 488 nm laser, with the anterior side of the brain facing up to the objective. The images were acquired at a 128 × 128 pixel resolution, and at a frame rate of ~ 10 Hz.~ 10 Z axial sections were imaged in one time-series cycle. The section interval was ~ 1 μm. The time intervals between each cycle were 2 s.
Before stimulating a brain, we imaged the basal GCaMP3 signals for ≥ 10 cycles. We then gently added 200 μl 50 mM ATP (pH adjusted to 7.0, Sigma, A2383-5G) into the Drosophila imaging saline, resulting in a final ATP concentration of 5 mM. We performed a stack registration using the ImageJ Plugins registration module and measured the GCaMP3 intensities using the ImageJ Analyze ROI manager module. ΔF/F0 (%) was calculated as ΔF/F0 (%)=(F-F0)/F0 × 100. Fmax is the maximum fluorescence value following ATP delivery. Fmin is the minimum fluorescence value that occurred during a total of 80 time series cycles after ATP delivery. F0 is the GCaMP3 baseline value averaged for 10 time-series cycles immediately before ATP application.
Statistical analyses
No statistical methods were employed to predetermine sample sizes. Sample sizes were chosen based on previous publications (Demir and Dickson, 2005; Manoli et al., 2005; Stockinger et al., 2005; Pan et al., 2012; Asahina et al., 2014; Clowney et al., 2015; Huang et al., 2016; Zhang et al., 2016). Statistical analysis was performed with Prism5 (GraphPad Software). We performed nonparametric Mann-Whitney test when comparing two groups of data. For comparison of multiple groups of data, we performed Kruskal-Wallis test followed by Dunn’s post hoc test. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. We present the exact number of samples and P values in the figure legends and in the supporting source data files. We present raw data using scatter plots and include exact values in the source data files. When n < 10, individual data points were identified.
Replication
We used only biological replicates throughout this work. To perform the behavioral studies, we defined biological replicates as animals of the same genotype and rearing conditions, exposed to identical treatments. Courtship indexes were calculated using n = 6—27 individual animals. Preference indexes were calculated using n = 12 individual animals. Chaining indexes were calculated using n = 6 groups (8—12 individual animals in each group). Lunging numbers were calculated using n = 10—12 animals. All animals were used once, since their behavioral indexes are sensitive to prior experience. Replicates for the Ca2+ imaging were defined as the number of neurons (Figure 6C and F) or the selected regions (Figure 8E) analyzed per genotype and condition. In all cases we used 3—9 brains/genotype and condition. 2—5 neurons (Figure 6C and F) or 4—7 regions of selection (Figure 8E) were used per brain. Replicates for the immunostaining were defined as brains of the same genotype that underwent identical staining procedures. We stained ≥ 5 brains per experiment. The Gal4/UAS (or LexA/LexAop) binary systems are highly reproducible. Images that were the most intact were selected for display. We did not exclude any data points.
Group allocation
To perform the behavioral assays, the control and experimental groups were reared under the same conditions, collected on the same day, aged in parallel, and assayed on the same day. The control and experimental groups were assayed in an arbitrary order. Behavioral videos were randomly permuted for scoring behavioral indexes. All behavioral analyses were obtained from videos, in which the genotypes were masked. The indexes were calculated blindly.
To perform the Ca2+ imaging, the control and experimental groups were assayed in an arbitrary order. The raw Ca2+ imaging data files were permutated in order and analyzed by Image J software.
Source data files
The raw data for the behavioral assays, Ca2+ imaging assays, summary statistics, and full stacks of the entire brains used in the GRASP experiments are included in the source data files.
Acknowledgements
This work was supported by grants to CM from the National Institute on Deafness and other Communication Disorders (DC007864) and an NIH Director’s Pioneer Award (DP1) supported by the National Institute of Allergy and Infectious Disease (DP1AI124453). We thank Drs. Barry Dickson (Janelia Research Campus), Ping Shen (University of Georgia), Gerald Rubin (Janelia Research Campus), Christopher Potter (Johns Hopkins University School of Medicine), Brian Oliver (NIDDK, NIH), Orie Shafer (University of Michigan), David Anderson (California Institute of Technology) for sharing valuable fly stocks and experimental reagents. We thank Drs. Julie Simpson, Hsiang-Chin Chen, Junjie Luo and Jiangqu Liu (UC Santa Barbara) for critical discussions and technical advice and the Physics Machine Shop (UC Santa Barbara) for fabricating behavioral chambers. We thank Dr. Wen Wang and Ruoping Zhao in the Kunming Institute of Zoology, Chinese Academy of Sciences for providing lab resources during the author’s job transition, which greatly facilitated the completion of this work.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Craig Montell, Email: cmontell@ucsb.edu.
Mani Ramaswami, Trinity College Dublin, Ireland.
Eve Marder, Brandeis University, United States.
Funding Information
This paper was supported by the following grants:
National Institute on Deafness and Other Communication Disorders DC007864 to Craig Montell.
National Institute of Allergy and Infectious Diseases DP1AI124453 to Craig Montell.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Resources, Data curation, Formal analysis, Investigation, Visualization, Methodology, Writing—original draft.
Conceptualization, Formal analysis, Investigation, Writing—review and editing.
Resources, Investigation, Methodology.
Investigation, Methodology, Writing—review and editing.
Resources, Investigation.
Validation, Investigation.
Validation, Investigation.
Conceptualization, Formal analysis, Supervision, Funding acquisition, Writing—original draft, Project administration.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Alvarez FJ, Fyffe REW. The continuing case for the Renshaw cell. The Journal of Physiology. 2007;584:31–45. doi: 10.1113/jphysiol.2007.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, Eyjólfsdóttir EA, Perona P, Anderson DJ. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell. 2014;156:221–235. doi: 10.1016/j.cell.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. The Journal of Neuroscience. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath DE, Stowers JR, Hörmann D, Poehlmann A, Dickson BJ, Straw AD. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nature Methods. 2014;11:756–762. doi: 10.1038/nmeth.2973. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brown MR, Crim JW, Arata RC, Cai HN, Chun C, Shen P. Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides. 1999;20:1035–1042. doi: 10.1016/S0196-9781(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Byne W, Lasco MS, Kemether E, Shinwari A, Edgar MA, Morgello S, Jones LB, Tobet S. The interstitial nuclei of the human anterior hypothalamus: an investigation of sexual variation in volume and cell size, number and density. Brain Research. 2000;856:254–258. doi: 10.1016/S0006-8993(99)02458-0. [DOI] [PubMed] [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron. 2015;87:1036–1049. doi: 10.1016/j.neuron.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. The Journal of Neuroscience. 2009;29:7497–7503. doi: 10.1523/JNEUROSCI.6044-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nature Genetics. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Gao XJ, Riabinina O, Li J, Potter CJ, Clandinin TR, Luo L. A transcriptional reporter of intracellular Ca2+ in Drosophila. Nature Neuroscience. 2015;18:917–925. doi: 10.1038/nn.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Swaab DF. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 2008;131:3132–3146. doi: 10.1093/brain/awn276. [DOI] [PubMed] [Google Scholar]
- Garczynski SF, Brown MR, Shen P, Murray TF, Crim JW. Characterization of a functional neuropeptide F receptor from Drosophila melanogaster. Peptides. 2002;23:773–780. doi: 10.1016/S0196-9781(01)00647-7. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Molecular & General Genetics. 1973;120:107–114. doi: 10.1007/bf00267238. [DOI] [PubMed] [Google Scholar]
- Han DD, Stein D, Stevens LM. Investigating the function of follicular subpopulations during Drosophila oogenesis through hormone-dependent enhancer-targeted cell ablation. Development. 2000;127:573–583. doi: 10.1242/dev.127.3.573. [DOI] [PubMed] [Google Scholar]
- Hempel LU, Oliver B. Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Developmental Biology. 2007;7:113. doi: 10.1186/1471-213X-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife. 2015;4:e11346. doi: 10.7554/eLife.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Benzer S. Courtship in Drosophila mosaics: sex-specific foci for sequential action patterns. PNAS. 1976;73:4154–4158. doi: 10.1073/pnas.73.11.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Liu W, Qi YX, Luo J, Montell C. Neuromodulation of courtship drive through tyramine-responsive neurons in the Drosophila brain. Current Biology. 2016;26:2246–2256. doi: 10.1016/j.cub.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nature Methods. 2014;11:325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. PNAS. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman BR, Kim H, Scott K. Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife. 2015;4:e11188. doi: 10.7554/eLife.11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Jan LY, Jan YN. A PDF/NPF neuropeptide signaling circuitry of male Drosophila melanogaster controls rival-induced prolonged mating. Neuron. 2013;80:1190–1205. doi: 10.1016/j.neuron.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. Journal of Neurobiology. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Yamamoto D. Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nature Communications. 2015;6:6457. doi: 10.1038/ncomms7457. [DOI] [PubMed] [Google Scholar]
- Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195:715–721. doi: 10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA, Fernandez MP. Aggression in Drosophila. Behavioral Neuroscience. 2015;129:549–563. doi: 10.1037/bne0000089. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/S0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. Journal of Neurobiology. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::AID-NEU8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lee G, Bahn JH, Park JH. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. PNAS. 2006;103:12580–12585. doi: 10.1073/pnas.0601171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nature Neuroscience. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: any similarities to vertebrate neuropeptide Y signaling? Peptides. 2011;32:1335–1355. doi: 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Nern A, Pfeiffer BD, Rubin GM. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. PNAS. 2015;112:E2967–E2976. doi: 10.1073/pnas.1506763112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaï LJ, Ramaekers A, Raemaekers T, Drozdzecki A, Mauss AS, Yan J, Landgraf M, Annaert W, Hassan BA. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. PNAS. 2010;107:20553–20558. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. Journal of Neuroscience. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. PNAS. 2012;109:10065–10070. doi: 10.1073/pnas.1207107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H, Solanka L, van Rossum MC, Nolan MF. Feedback inhibition enables θ-nested γ oscillations and grid firing fields. Neuron. 2013;77:141–154. doi: 10.1016/j.neuron.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Pavlou HJ, Goodwin SF. Courtship behavior in Drosophila melanogaster: towards a 'courtship connectome'. Current Opinion in Neurobiology. 2013;23:76–83. doi: 10.1016/j.conb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poodry CA, Edgar L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. The Journal of Cell Biology. 1979;81:520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Ren X, Sun J, Housden BE, Hu Y, Roesel C, Lin S, Liu LP, Yang Z, Mao D, Sun L, Wu Q, Ji JY, Xi J, Mohr SE, Xu J, Perrimon N, Ni JQ. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. PNAS. 2013;110:19012–19017. doi: 10.1073/pnas.1318481110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nature Neuroscience. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett CC, Vaughan AG, Knapp JM, Baker BS. Sex and the single cell. II. there is a time and place for sex. PLOS Biology. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/S0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. Journal of Animal Behavior. 1915;5:351–366. doi: 10.1037/h0074109. [DOI] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Advances in Genetics. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Shaw WR, Tsang HT, Reid E, O'Kane CJ. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nature Neuroscience. 2007;10:177–185. doi: 10.1038/nn1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. PNAS. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Chiu H, Pfeiffer BD, Wong AM, Hoopfer ED, Rubin GM, Anderson DJ. A circuit node that integrates convergent input from neuromodulatory and social Behavior-Promoting neurons to control aggression in Drosophila. Neuron. 2017;95:1112–1128. doi: 10.1016/j.neuron.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/S0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/S0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nature Reviews Neuroscience. 2013;14:681–692. doi: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- Yao Z, Macara AM, Lelito KR, Minosyan TY, Shafer OT. Analysis of functional neuronal connectivity in the Drosophila brain. Journal of Neurophysiology. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Current Biology. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Rogulja D, Crickmore MA. Dopaminergic circuitry underlying mating drive. Neuron. 2016;91:168–181. doi: 10.1016/j.neuron.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nature Neuroscience. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- Zhou C, Franconville R, Vaughan AG, Robinett CC, Jayaraman V, Baker BS. Central neural circuitry mediating courtship song perception in male Drosophila. eLife. 2015;4:e08477. doi: 10.7554/eLife.08477. [DOI] [PMC free article] [PubMed] [Google Scholar]