Introduction
The Zollinger-Ellison Syndrome (ZES) is caused by a gastrin-producing tumor (gastrinoma) that results in the excessive production of gastric acid secretion [1,2]. Two American surgeons, Robert M. Zollinger and Edwin H. Ellison, first explained this syndrome in a landmark 1955 paper that described patients with severe, intractable ulcer disease. It was ten years later that specific radioimmunoassays for gastrin showed the excessive hormone state produced by the tumor was in fact gastrin. Although most descriptions of this syndrome include the presence of multiple post-bulbar ulceration, the majority of patients present with voluminous diarrhea and cramping abdominal pain. This is partially due to the over-the-counter use of potent H2 receptor antagonists, or to the more widespread use of proton pump inhibitors (PPIs), leading to the partial control of the gastric acid hypersecretion.
The actual incidence of ZES in the general population is difficult to accurately determine because of its relative rarity and because of misdiagnosis. In general, however, ZES is estimated to occur in approximately 0.1–3 per million persons in the United States [3]. Two forms of ZES are described: a sporadic form that is not associated with genetic susceptibility, and a genetic form that occurs with the Multiple Endocrine Neoplasia type I (MEN I). ZES occurs in approximately one-third of patients with MEN I at some time during the course of disease [4–6]. MEN I (Wermer’s syndrome) is characterized by the development of hyperparathyroidism, pancreatic islet cell tumors and pituitary tumors that occur with variable degrees of penetrance [7,8]. Therefore, it is recommended that in the evaluation of MEN I that all patients be thoroughly evaluated for ZES (See Diagnosis below).
Because of the strong association of ZES with MEN I, an extensive search for a potential genetic abnormality in ZES has been underway in several laboratories. Impetus for this effort has been heightened by the discovery, in 1988, that the MEN I gene locus was assigned to 11q13, and in 1997 when the gene was identified by Marx and coworkers at the National Institutes of Health [9]. The function of the gene protein, Menin, is uncertain at this time. However, because Menin is located primarily in the nucleus, it may be a nuclear transcriptional regulator [10].
Now that the gene locus is well described, it is possible to determine the patterns of inheritance observed in MEN I and their association with ZES. This is particularly pertinent given the strong association between MEN 1 and the development of pancreatic islet cell tumors such as those that occur with gastrinoma tumors. The genetic link has been particularly well characterized for a large Tasmanian family with MEN-1 (designated Tasman 1), where genetic screening and case follow-ups have identified pancreatic islet cell tumors occurring in up to 60% of affected family members [11]. In one study the potential link between somatic mutations of the Menin gene and the occurrence of pancreatic islet cell tumors was identified However, additional studies involving larger groups of patients will be required to better establish this association [12].
The most obvious pathological derangement in ZES is gastric acid hypersecretion resulting from the gastrin excess produced by the gastrinoma tumor. In the normal state, both central nervous system and peripheral mediators regulate gastric acid secretion; hence, there is a cephalic and a peripheral phase [13]. The cephalic phase is dependent upon vagal innervation of the stomach. The peripheral phase is dependent upon mediators released in the gastric mucosa to either directly stimulate parietal cell function or indirectly by stimulating gastric enterochromaffin-like cells (ECL). Gastrin is normally released from antral G cells in response to meal stimulation, and acts as a potent endocrine regulator of gastric acid secretion by stimulating ECL cell histamine release. The ECL cell possesses a specific receptor for gastrin, the CCKB receptor, which has been cloned from both the human brain and stomach. It has been shown to be a member of the heptahelical, G protein receptor super-family [14]. This receptor is coupled to both transient and steady state elevations in intracellular calcium with histamine release dependent on elevation of steady state calcium [15, 16]. Therefore, the major pathways for the peripheral stimulation of ECL cell function and gastric acid secretion are gastrin, released from antral G cells. The neuropeptide, Pituitary Adenylate Cyclase Activating Polypeptide (PACAP), acting at PACAP receptors expressed on ECL cells, is now thought to be the major neuropeptide regulator of ECL cell histamine release in the cephalic phase of gastric acid secretion [17]. Histamine, released from the ECL cell, is probably the most important direct stimulant of acid secretion through its actions on the H2 histamine receptor expressed on parietal cells, hence, the efficacy of H2 receptor antagonists in controlling gastric acid hypersecretion in patients with ZES [18].
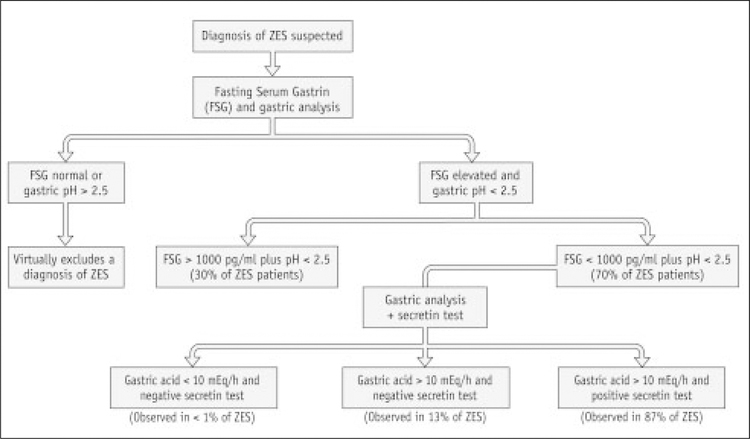
The diagnosis of ZES should be considered in patients with severe gastroduodenal ulcer symptoms. However, diarrhea and abdominal pain are more consistent symptoms. These occur when large amounts of acid enter the duodenum. Malabsorption occurs because of the degradation of pancreatic lipase, and from the large volume of gastric juice entering the small intestine. Generally, the physical examination is not revealing except for showing signs of volume depletion. A diagnosis of ZES is dependent upon the presence of hypergastrinemia (>100 pg/ml) in the absence of achlorhydria, with levels > 900 pg/ml almost diagnostic of the disease. In patients with borderline elevated gastrin values (e.g., 200 to 500 pg/ml), a secretin provocative test may be required to make a diagnosis of ZES [19]. Following the administration of secretin (2 units/kg intravenously as a bolus), a rise in the fasting serum gastrin of >200 pg/ml within 10 minutes is highly sensitive and specific for the diagnosis of ZES [20, 21]. Alternatively, calcium provocative testing can be performed in the equivocal cases, although this test is less sensitive than the secretin infusion test as shown in Figure 1 [19].
Figure 1.
Algorithmic approach if ZES is the suspected diagnosis.
Once a diagnosis of ZES is established, adequate control of gastric acid secretion is required to avoid complications such as severe peptic ulcers and upper gastrointestinal hemorrhage. Gastric analysis is required to follow the adequacy of therapy and is performed by measuring four 15-minute acid collections and using 0.1N NaOH as the titrant. Under basal conditions, the acid output (BAO) is < 10 mEq/hr in normal subjects. Maximal acid output (MAO) can be determined following the administration of pentagastrin subcutaneously and this value directly correlates with the parietal cell mass [20]. Despite curative gastrinoma resection and normalization of serum gastrin, patients may still require long-term gastric analysis to assess the adequacy of gastric antisecretory medication [21].
The use of H2-receptor antagonists for ZES has been nearly completely replaced by the use of proton pump inhibitors. In general, H2-receptor antagonists have a lower efficacy, shorter duration of action and require high doses with a median dose of ranitidine required to fully control gastric acid secretion of 1.2 grams/day. Despite these shortcomings, H2 receptor antagonists are safe, can be used during pregnancy [22], and are the only antisecretory agents currently approved for intravenous use [23]. The substituted benzimidazoles, which block the final step in gastric acid production, have improved gastric acid control in patients with ZES and have replaced H2-receptor antagonists as the first-line agents for the control of gastric acid secretion in patients with ZES [24, 25]. Omeprazole effectively controls gastric acid secretion in nearly 100% of patients with ZES with a dose range of 10–180 mg/24 hours [26]. Omeprazole and lansoprazole have been shown to be equally efficacious [27]. More recently, other substituted benzimidazoles, such as pantoprazole and rabeprazole, have been under investigation for the management of gastric acid hypersecretion in ZES. In early studies, intravenous pantoprazole has been shown to be both efficacious and safe for the rapid and prolonged acid suppression. It may replace IV H2RAs during the perioperative period, during the setting of acute upper gastrointestinal hemorrhage, and during the administration of chemotherapy [27–29].
Treatment
Pharmacologic treatment
Drug therapy for control of gastric acid secretion
The aims of antisecretory therapy are control of gastric acid secretion,10mEq/hr; treatment of peptic ulcer disease; and control of symptoms.
H2 Receptor Antagonists
Ranitidine
| Standard dosage | 900–1200mg every 6 hrs. |
| Contraindications | None. |
| Main drug interactions | None. |
| Main side effects | Lymphopenia (rare). |
| Special points | Shown safe for use during pregnancy and is available in IV form. |
| Cost effectiveness | Monthly cost $500. |
Famotidine
| Standard dosage | 80–120 mg every 6 hrs. |
| Contraindications | None. |
| Main drug interactions | None. |
| Main side effects | Lymphopenia (rare). |
| Special points | Available in IV form. |
| Cost effectiveness | Monthly cost $1,000. |
Proton Pump Inhibitors
Omeprazole
| Standard dosage | 40–80 mg every 12 hrs. |
| Contraindications | Pregnancy. |
| Main drug interactions | None. |
| Main side effects | Headache. |
| Special points | Long-Term safety and efficacy data. |
| Cost effectiveness | Monthly cost $2,000. |
Lansoprazole
| Standard dosage | 30–90 mg every 12 hrs. |
| Contraindications | Pregnancy. |
| Main drug interactions | None. |
| Main side effects | Headache. |
| Special points | Approved by the FDA; alternative to Omeprazole. |
| Cost effectiveness | Monthly cost is $2,000. |
Pantoprazole
| Standard dosage | 40–80 mg every 12 hrs. |
| Contraindications | Pregnancy. |
| Main drug interactions | None. |
| Main side effects | Headache. |
| Special points | Available in IV formulation, not available in United States. |
| Cost effectiveness | Monthly cost is $2,000. |
Diagnosis of primary and metastatic gastrinoma
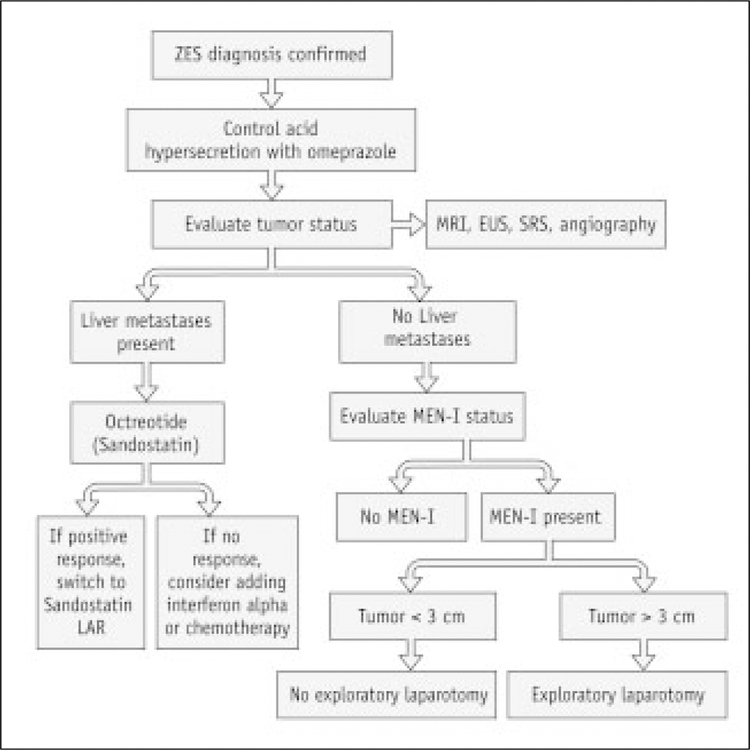
Once the control of gastric acid hypersecretion is achieved, the next most important objective is to localize and treat the gastrinoma tumor (Figure. 2). The first aim in patients with sporadic ZES is to attempt to cure the disease through surgical resection and to reduce the metastatic spread. In patients with the sporadic form of the disease and without liver metastases, it is currently possible to localize and to surgically remove the endocrine tumor(s) and this progress has been improved by refinements in modern medical imaging techniques [30].
Figure 2.
Protocol for Evaluation of ZES.
Diagnostic Imaging Studies
To determine whether there is metastatic spread of tumor to liver or bone.
To localize pancreatic tumor(s).
To localize duodenal tumor(s).
To guide surgical resection.
To assess tumor aggressivity by measuring its growth over time.
Endoscopic ultrasound
| Standard procedure | Olympus ultrasound probe. Imaging of duodenum and pancreas. |
| Contraindications | Similar to upper endoscopy (obstruction, ileus, etc.). |
| Complications | Similar to upper endoscopy (perforation, bleeding). |
| Special points | Sensitivity for detecting pancreatic tumor >80%. |
| Cost effectiveness | Cost is approximately $2,000. |
Computerized tomography (Spiral CT)
| Standard procedure | Imaging with 1–2 mm sections through the pancreas with IV and oral contrast agents. |
| Contraindications | Renal insufficiency, pregnancy. |
| Complications | None. |
| Special points | Sensitivity for detecting pancreatic tumors 70%–80%, sensitivity for hepatic tumors 60%. |
| Cost effectiveness | Cost is approximately $600. |
Magnetic resonance imaging
| Standard procedure | T1, T2, and STIR imaging. |
| Contraindications | Cardiac pacemakers, aneurysm clips, aversion to confinement. |
| Complications | None. |
| Special points | Most sensitive imaging modality for detecting hepatic metastases (>80%); less sensitive for localizing pancreatic tumors. |
| Cost effectiveness | Cost is $1,000. |
Somatostatin receptor scintigraphy (OctreoScan)
| Standard procedure | Injection of Indium-labeled Octreotide to detect the expression of somatostatin receptors on islet cell tumors. |
| Contraindications | Pregnancy, renal insufficiency. |
| Complications | Renal insufficiency. |
| Special points | Very sensitive (>80%) for identifying primary tumors. |
| Cost effectiveness | Cost is $1,500. |
Bone scan
| Standard procedure | Radiolabeled technetium to identify bony metastases. |
| Contraindications | Pregnancy. |
| Complications | None. |
| Special points | Most sensitive imaging modality for the detection of bone metastases. |
| Cost effectiveness | Cost is $600. |
Surgery
Once imaging studies have successfully localized the gastrinoma tumor, resection in patients with the sporadic form of ZES without evidence of metastases is warranted. Surgical cure of gastrinoma can be attained for up to 60% of patients at 5 years. The development of liver metastases is dependent on whether surgical resection is performed, with 20% of patients who have not undergone surgery developing metastatic disease compared to less than 5% in patients who have had surgery performed for cure. In patients with combined ZES and MEN I, surgical cure is not possible, and therefore surgical resection is not generally recommended in these patients [31–33].
Surgical exploration, excision of gastrinoma
| Standard procedure | Kocher maneuver to mobilize duodenum. Endoscopic transillumination of duodenum. Intraoperative ultrasonography. Manual palpation of pancreas and duodenum. |
| Contraindications | Widely metastatic disease (liver and bone). MEN I. Significant cardiopulmonary disease. |
| Complications | Pancreatic fistulas, bowel obstruction, pancreatitis, bowel leak, anastomotic ulcer. |
| Special points | Surgery should be performed by a surgeon with significant experience in intraoperative localization methods for islet cell tumors [33]. Perioperative gastric acid control is important for reducing the complications of gastric acid hypersecretion. |
| Cost effectiveness | The curative resection of gastrinoma tumors in nearly 60% of patients is significant for long-term prognosis of these patients [33]. Following curative gastrinoma resection, more than half of the patients will require long-term gastric acid secretory control because parietal cell mass remains increased despite curative resection [30,33]. |
Surgery for reduction of gastric acid secretion
| Standard procedure | Gastrectomy, Vagotomy and pyloroplasty. Antrectomy. Billroth I or II. |
| Contraindications | Poor medical condition. Patient is a candidate for curative gastrinoma resection. |
| Complications | Gastric outlet obstruction, anastomotic ulcer, gastric motility disorders, dumping syndrome. |
| Special points | Gastric acid reducing surgery, once the mainstay of therapy for ZES, has been largely replaced by curative gastrinoma resection. Gastric reducing surgery should be considered in the setting of an acute GI bleed where endoscopic therapy and antisecretory medications are unable to adequately manage peptic ulcer complications. |
| Cost effectiveness | Surgery reduces the dose requirement of antisecretory medications. Given the adequacy by which PPIs control gastric acid secretion, and the potential complications with surgery, gastric acid-reducing surgery cannot be generally recommended. |
Emerging therapies
Disseminated malignancy can occur and mainly involves spread to liver, lymph nodes and to bone. With the improved control of gastric acid hypersecretion, metastatic spread is now the principal determinant of early death [34]. Chemotherapy has been reserved primarily for those ZES patients with rapidly enlarging liver metastases. In addition, these patients may also be candidates for transcatheter arterial embolization (TAE). The majority of studies have focused on the use of streptozocin, 5-fluorouracil and doxorubicin (Adriamycin) or combinations of these agents [35–39]. However, because of a lack in tumor response, the toxicity associated with these agents and the short-lasting response, the use of these agents should be reserved for those patients with aggressive forms of the disease [40–44]. These studies are summarized in Table 1.
Studies have been performed by our group as well as others to investigate the efficacy of hormonal therapies for the management of metastatic gastrinomas. In one study, interferon alpha was shown to reduce tumor growth in 40% of patients treated with doses of 5 million units daily for at least 6 months (44). In European studies, interferon alpha had little to no effect on the progression of metastatic gastrinomas. The somatostatin analogue, Sandostatin, has been used in other studies and shown to have a minimal response when used alone. A summary of the studies investigating objective tumor responses for these agents is shown in Table 1.
More recently, studies are underway to investigate the response to combination therapy with octreotide and interferon alpha. The combination of these two agents is currently under study at UCLA (unpublished). Although SRS has been used for diagnosis of tumor location, the use of high-dose Indium-radioloabelled analogues of somatostatin to target and locally irradiate metastatic islet cell tumors is also a new area of recent investigation to treat metastatic disease [45, 46].
Emerging therapies include : radiolabeled somatostatin for therapeutic use; combination Interferon and Somatostatin; IV Pantoprazole for gastric acid control; and hepatic transplantation for metastatic gastrinoma.
Table 1.
Chemotherapy of Metastic Gastrinoma
| Author | Agent | Number | Objective Resopnse (%) |
|---|---|---|---|
| Moertel | STZ+DOX | 36 | 25 (69%) |
| Von Schrenk | STZ+5_FU+DOX | 10 | 4 (40%) |
| Pisegna | Interferon | 11 | 0 (0%) |
| Maton | Octreotide | 21 | 3 (14%) |
Abbreveations: STZ: streptozotcin; DOX: doxorubicin
Opinion statement.
The first goal of therapy is the control of gastric acid hypersecretion using PPIs or high-dose H2R antagonists.
The diagnosis of Multiple Endocrine Neoplasia (MEN I) should be established early in the disease.
Localization of gastrinoma tumor should be performed using a combination of endoscopic ultrasonography (EUS), somatostatin receptor scintigraphy (SRS), and computerized tomography (CT), or Magnetic Resonance Imaging (MRI).
Surgical resection in sporadic ZES should be performed to attempt cure of tumor.
Surgery, hormonal, chemotherapy, embolization therapy or therapeutic OctreoScan should be considered in patients with metastatic tumor.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1.Vinayek R: Zollinger-Ellison syndrome. Recent advances in the management of the gastrinoma. Gas-troenterol Clin North Am 1990, 19:197. [PubMed] [Google Scholar]
- 2.Berg CL: Zollinger-Ellison syndrome. Med Clin North Am 1991, 75:903. [DOI] [PubMed] [Google Scholar]
- 3.van Heerden JA: Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type I. Surgery 1986, 100:971. [PubMed] [Google Scholar]
- 4.Day JP, Richter JE: Medical and surgical conditions predisposing to gastroesophageal reflux disease. Gastroent Clin N Am 1990, 19(3):587–607. [PubMed] [Google Scholar]
- 5.Thompson JC: The role of surgery in the Zollinger-Ellison syndrome. Ann Surg 1983, 197:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinik AI: Controversies in the management of Zollinger-Ellison syndrome. Ann Intern Med 1986, 105:956. [DOI] [PubMed] [Google Scholar]
- 7.Rappaport KD, White JW: 45-year-old man with dermatitis and weight loss. J Mayo Clin Proc 1995, 70(8):785–788. [DOI] [PubMed] [Google Scholar]
- 8.Mozell E, Stenzel P, Woltering EA, et al. : Functional endocrine tumors of the pancreas: clinical presentation, diagnosis, and treatment. Curr Probl Surg 1990, 27(6):301–386. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekharappa SC, Guru SC, Manickam P, et al. : Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 1997, 276(5311):404–407. [DOI] [PubMed] [Google Scholar]
- 10.Guru SC, Goldsmith PK, Burns AL, et al. : Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci USA 1998, 95(4):1630–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess JR, Greenaway TM, Shepherd JJ: Expression of the MEN-1 gene in a large kindred with multiple endocrine neoplasia type 1. J Intern Med 1998, 243(6):465–470. [DOI] [PubMed] [Google Scholar]
- 12.Shan L, Nakamura Y, Nakamura M, et al. : Somatic mutations of multiple endocrine neoplasia type 1 gene in the sporadic endocrine tumors. Lab Invest 1998, 78(4):471–475. [PubMed] [Google Scholar]
- 13.Hersey SJ, Sachs G: Gastric Acid Secretion. Physiol Rev 1995, 75:155–218. [DOI] [PubMed] [Google Scholar]
- 14.Pisegna JR, deWeerth A, Huppi K, Wank SA: Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Comm 1992, 189:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanagisawa K, Yang H, Walsh JH, Tache Y: Role of acetylcholine, histamine and gastrin in the acid response to intracisternal injection of TRH analog, RX 77368, in the rat. Regul Pept 1990, 27:161–170. [DOI] [PubMed] [Google Scholar]
- 16.Prinz C, Kajimura M, Scott D, et al. : Histamine secretion from rat enterochromaffinlike cells. Gastroenterology 1993, 105:449–461. [DOI] [PubMed] [Google Scholar]
- 17.Zeng N, Kang T, Wong H, et al. : The pituitary adenylate cyclase activating polypeptide type 1 receptor (PAC1-R) is expressed on gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann N Y Acad Sci 1998, 865:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenberg A: Testing for Helicobacter pylori in the diagnosis of Zollinger-Ellison syndrome. Am J Gastroenterol 1991, 86:606. [PubMed] [Google Scholar]
- 19.Weber HC, Venzon DJ, Jensen RT, et al. : Studies on the interrelation between Zollinger-Ellison syndrome, Helicobacter pylori, and proton pump inhibitor therapy. Gastroenterology 1997, 112:84–91. [DOI] [PubMed] [Google Scholar]
- 20.Pisegna JR, Norton JA, Slimak GG, et al. : Effects of curative gastrinoma resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology 1992, 102(3):767–778. [DOI] [PubMed] [Google Scholar]
- 21.Fass R, Rosen HR, Walsh JH: Zollinger-Ellison syndrome: diagnosis and management. Hosp Pract 1995, 15: 73–76, 30(11):79–80. [DOI] [PubMed] [Google Scholar]
- 22.Stewart CA, Termanini B, Sutliff VE, et al. : Management of the Zollinger-Ellison syndrome in pregnancy. Am J Obstet Gynecol 1997, 176:224–233. [DOI] [PubMed] [Google Scholar]
- 23.Maton PN: Zollinger-Ellison syndrome. Recognition and management of acid hypersecretion. Drugs 1996, 52(1):33–44. [DOI] [PubMed] [Google Scholar]
- 24.Frucht H: Use of omeprazole in patients with Zollinger-Ellison syndrome. Dig Dis Sci 1991, 36:394. [DOI] [PubMed] [Google Scholar]
- 25.Metz DC: Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology 1992, 103:1498. [DOI] [PubMed] [Google Scholar]
- 26.Maton PN: Role of acid suppressants in patients with Zollinger-Ellison syndrome. Aliment Pharmacol Ther 1991, 5(Suppl 1):25. [DOI] [PubMed] [Google Scholar]
- 27.Metz DC, Pisegna JR, Fishbeyn VA, et al. : Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology 1992, 103:1498–1508. [DOI] [PubMed] [Google Scholar]
- 28.Metz DC, Lew E, Forsmark CE, et al. : Gastroenterology 1998, 114(4):G0926. [Google Scholar]
- 29.Pisegna JR, Huang M, Asvar C, et al. : Gastroenterology 1998, 114(4):G1065. [Google Scholar]
- 30.••.Hirschowitz BI: Zollinger-Ellison syndrome: pathogenesis, diagnosis, and management. Am J Gastroenterol 1997, 92(4 Suppl):44S–50S.This review provides an excellent overview on the diagnosis, medical and surgical treatments available. The review covers the use of proton pump inhibitors in the management of gastric acid hypersecretion.
- 31.••.Jensen RT: Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med 1998, 243(6):477–488.This article provides significant insights in the approach and management of patients with Zollinger-Ellison Syndrome and Multiple Endocrine Neoplasia Type I (MEN I). Issues such as the natural history of these two conditions are discussed. The role of surgical resection is explored. An extensive database of patients at the National Institutes of Health is used to provide the analysis of the best approaches to disease management.
- 32.••.Mignon M, Cadiot GJ: Diagnostic and therapeutic criteria in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Intern Med 1998, 243(6):489–494.This is an excellent review that provides a straight-forward approach to the management of those subsets of patients presenting with both Zollinger-Ellison Syndrome and Multiple Endocrine Neoplasia Type I (MEN I).
- 33.••.Norton JA: Gastrinoma: advances in localization and treatment. Surg Oncol Clin N Am 1998, 7(4):845–861.The author is a leading surgical expert in the surgical management of gastrinomas that produce the Zollinger-Ellison Syndrome. The review provides state of the art information on the management of the duodenal and pancreatic tumors involved in this syndrome.
- 34.Jensen RT: Should the 1996 citation for Zollinger-Ellison syndrome read: “Acid-reducing surgery in, aggressive resection out”? Am J Gastroenterol 1996, 91(6):1067–1070. [PubMed] [Google Scholar]
- 35.Moertel CG: Phase II trial of doxorubicin therapy for advanced islet cell carcinoma. Cancer Treat Rep 1982, 66:1567. [PubMed] [Google Scholar]
- 36.Moertel CG: Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992, 326:519. [DOI] [PubMed] [Google Scholar]
- 37.Moertel CG: The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med 1994, 120:302. [DOI] [PubMed] [Google Scholar]
- 38.Thompson W: Malignant islet-cell tumors of the pancreas. World J Surg 1984, 8:940. [DOI] [PubMed] [Google Scholar]
- 39.Eckhauser FE: Nonfunctioning malignant neuroendocrine tumors of the pancreas. Surgery 1986, 100:978. [PubMed] [Google Scholar]
- 40.Eastman RC: Adriamycin therapy for advanced insulinoma. J Clin Endocrinol Metab 1977, 44:142. [DOI] [PubMed] [Google Scholar]
- 41.Moertel CG: Phase II trial of doxorubicin therapy for advanced islet cell carcinoma. Cancer Treat Rep 1982, 66:1567. [PubMed] [Google Scholar]
- 42.Mignon M: Current approach to the management of tumoral process in patients with gastrinoma. World J Surg 1986, 10:703. [DOI] [PubMed] [Google Scholar]
- 43.Howard JM: Gastroenterology 1983, 84:1192. [PubMed] [Google Scholar]
- 44.Pisegna JR, Slimak GG, Metz DC, et al. : An evaluation of human recombinant alpha interferon in patients with metastatic gastrinoma. Gastroenterology 1993, 105:1179–1183. [DOI] [PubMed] [Google Scholar]
- 45.Ciaccia D, Gress FG: Somatostatin receptor scintigraphy in the Zollinger-Ellison syndrome. Ann Intern Med 1997, 126(9):741–742. [DOI] [PubMed] [Google Scholar]
- 46.Lebtahi R, Cadiot G, Sarda L, et al. : Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroentero-pancreatic tumors. Nucl Med 1997, 38(6):853–858. [PubMed] [Google Scholar]