Abstract
Myeloid‐derived suppressor cells (MDSCs) accumulate in tumors and the peripheral blood of cancer patients and demonstrate cancer‐promoting activity across multiple tumor types. A limited number of agents are known to impact MDSC activity. TLR8 is expressed in myeloid cells. We investigated expression of TLR8 on MDSC and the effect of a TLR8 agonist, motolimod, on MDSC survival and function. TLR8 was highly expressed in monocytic MDSC (mMDSC) but absent in granulocytic MDSC (gMDSC). Treatment of human PBMC with motolimod reduced the levels of mMDSC in volunteers and cancer donors versus control (P < 0.001). Motolimod did not impact levels of gMDSC. The reduction of mMDSC was due to induced cell death by TLR8 ligation. Pretreatment of PBMC with a FAS neutralizing antibody inhibited motolimod‐induced reduction of mMDSC (P < 0.001). Finally, we demonstrated that mMDSC impeded IL‐2 secretion by CD3/CD28‐activated T cells; IL‐2 secretion was partially restored when cells were cocultured with motolimod (142 ± 36 pg/ml vs. 59 ± 13 pg/ml; P = 0.03). There is increasing evidence that MDSCs contribute to the progression of cancer by inhibiting tumor‐directed T cells. TLR8 agonists may synergize with cancer immunotherapeutic approaches to enhance the antitumor effects of the adaptive immune response.
Keywords: apoptosis, cancers, MDSC, T cells, TLR8, TLR8 agonist
Abbreviations
- FL
fluorescence
- gMDSC
granulocytic MDSC
- Lin
Lineage
- mDC
myeloid DC
- MDSC
myeloid‐derived suppressor cell
- mMDSC
monocytic MDSC
- pDC
plasmacytoid DC
1. INTRODUCTION
Myeloid‐derived suppressor cells (MDSCs) demonstrate a variety of functions that promote tumor growth as well as inhibit immune cell activity. Specific subtypes of MDSCs, monocytic MDSC (mMDSC) and granulocytic MDSC (gMDSC), can prevent the egress of activated CD8 T cells into the tumor,1 downregulate TCR‐zeta chain thus limiting T‐cell activation,2 and impede T‐cell differentiation.3 Elevated levels of MDSC indicate a poor prognosis or predict disease progression in many types of cancers.4, 5, 6 High levels of MDSC in the peripheral blood of cancer patients prior to therapy is associated with a lack of or incomplete response to treatment for several cancers.7, 8 Elevated blood MDSC has also been shown to be associated with a lack of clinical response to immune therapy in cancer patients9, 10, 11 There are few agents known that impair MDSC function or survival, which could be used in the treatment of cancer.
MDSCs modulate the adaptive immune response and can be activated via TLRs, including TLR2 and TLR4.12, 13, 14 Signaling through TLR4, for example, causes MDSC to secrete IL‐10 and promote a Th2 immune phenotype.14 TLR signaling in MDSC is an important pathway resulting in control of inflammatory processes.15
Motolimod is a small molecule selective TLR8 agonist and has been shown to stimulate type I cytokine production from antigen‐presenting cells resulting in T‐cell activation.16 We questioned whether TLR8 was expressed in MDSCs and, if so, what effects TLR8 ligation would have on MDSC differentiation and function.
2. MATERIALS AND METHODS
2.1. Peripheral blood mononuclear cells
Frozen PBMCs from volunteer donors were obtained from Astarte Biologics (Seattle, WA).17 Patient PBMCs (melanoma, prostate, and colon cancers treated to complete remission) were obtained under an IRB‐approved protocol.18 Frozen PBMCs were thawed and washed before use.19
2.2. Cell subset isolation
PBMCs were stained with fluorochrome‐conjugated monoclonal antibodies. Cell subtypes were isolated by BD FACS Aria II cell sorter. Myeloid DCs (mDCs; Lineage (Lin)‐HLA‐DR+, CD11C+) and plasmacytoid DCs (pDCs; Lin‐HLA‐DR+, CD11c–, CD123+) and mMDSC (HLA‐DR–CD14+) and gMDSC (Lin‐HLA‐DR–CD33+) were isolated. mMDSC, monocytes (HLA‐DR+CD14+) and CD3+ T cells were also isolated.
2.3. TLR mRNA expression analysis
Total RNA was extracted from FACS‐sorted mDC, pDC, mMDSC, and gMDSC. mRNA expression of TLR7, TLR8, and TLR9 was analyzed and normalized to b‐actin/HPRT.20
2.4. Cell culture and treatment with TLR agonists
PBMCs (2 × 106/ml) were cultured with 167 or 500 nM motolimod (VentiRx Pharmaceuticals, Seattle, WA, USA) or PBS for 24 h. In some assays, the cells were cultured with 0.5 μM and 2 μM of TLR7/8 agonist CL‐075, 10 μM and 50 μM of TLR7 agonist Imiquimod, and 50 nM and 100 nM of TLR 9 agonist CpG ODN2006 with PBS control (all Invivogen, San Diego, CA). Doses were optimized as previously reported.16
2.5. Flow cytometry analysis
Cells were stained with antibodies and analyzed as percentage of mMDSC and gMDSC in PBMC. Cells were also stained with an annexin V PE, 7‐AAD apoptosis detection kit (BD Biosciences, San Jose, CA). All annexin V+ and 7‐AAD+ cells were counted as dead. Cell surface death receptor FAS (CD95) was added and mean fluorescence (FL) of FAS expression was compared between control and treated mMDSC. CD69+ and FAS‐L+ cells percentage of CD3+CD4+ and CD3+CD8+ cells was compared between groups.
2.6. MACS and labeling of mMDSC
HLA‐DR–CD14+ cells were enriched using microbeads (Miltenyl Biotec, Auburn, CA) and labeled with 3 μM CFSE for 15 min at 37°C using a Vybrant CFDA SE Cell Tracer Kit (Invitrogen, Carlsbad, CA).21 Labeled mMDSCs were mixed with donor PBMC and cultured with PBS and 500 nM motolimod for 24 h. Both HLA‐DR–and HLA‐DR+CD14+ cells percentage of CFSE‐labeled cells was analyzed.
2.7. FAS blocking
PBMCs were cultured with 2 μg/ml of a FAS neutralizing antibody (clone ZB4; EMDMillipore, Billerica, MA) and isotype control for 1 h. Cultured cells were incubated with or without 500 nM motolimod for 20 h before analysis.
2.8. Cytokine analysis
FACS‐sorted CD3+ cells (2.5 × 106/ml) were stimulated with CD3/CD28 beads (Life Technologies, Carlsbad, CA, USA) at a 5:1 ratio. FACS‐sorted mMDSCs were added to the activated CD3 T cells at 1:1 ratio ± 750 nM motolimod. Both CD3+ T cells and mMDSCs alone and with motolimod were used as control. FACS‐sorted HLA‐DR+CD14+ monocytes were also cultured with or without motolimod for 20 h and supernatants harvested. Quantification of secreted cytokines was performed using a cytokine human magnetic kit (Life Technologies) by Luminex (Qiagen) as per manufacturer's instructions and reported as mean of picogram per milliliter of duplicated measurements.22
2.9. Statistical analyses
GraphPad Prism version 6 was used (GraphPad Software, La Jolla, CA). Paired t test compares the differences between control and treated cells. Differences in cytokine levels among groups were analyzed using a Mann‐Whitney U test. P value < 0.05 was considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. mMDSCs, but not gMDSCs, express TLR8 mRNA
We have previously shown that TLR8 is expressed on monocytes and mDCs and the TLR8 agonist motolimod induces these cells to produce IL‐12 and TNF‐α.16 We questioned whether TLR8 was expressed on MDSC, a heterogeneic population of cells that contain at least two subsets, monocytic and granulocytic, both of which have variable immunosuppressive effects.3 We found that TLR8 was highly expressed on the mMDSC subpopulation (HLA‐DR–CD14+) but absent on gMDSC (Lin‐HLA‐DR–CD33+) (Fig. 1A). Further, the levels of TLR8 expression were higher in mMDSC than in mDC. mMDSCs, as compared to gMDSCs, are uniquely capable of inhibiting T cells that have become activated. mMDSCs prevent CD44 expression on activated T cells and limit the movement of T cells from the blood to tissues.23 mMDSCs also suppress expression of CD162 reducing T‐cell adherence.23 We evaluated the expression of TLR7 and TLR9 on MDSC in the same donors. We found that TLR7 mRNA was not expressed on either mMDSC or gMDSC (Fig. 1B). The expression of TLR9 on mMDSC and gMDSC was variable among donors (Fig. 1C).
Figure 1.
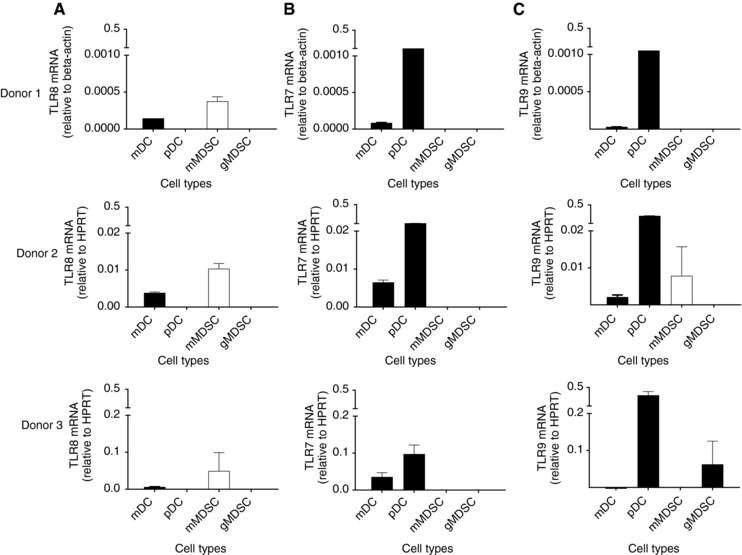
mMDSCs, but not gMDSCs, express higher levels of TLR8 mRNA. mRNA expression of TLR8 (A), TLR7 (B), and TLR9 (C) on mDC, pDC, mMDSC, and gMDSC in three donors. Columns and bars represents mean (±SE) of duplicate measurements. mMDSC, white column; other cell types, black columns
3.2. Motolimod treatment reduces the levels of mMDSC in PBMC
TLR8 is expressed on mDCs, which are some of the most effective antigen‐presenting cells in the tumor microenvironment and capable of activating cancer‐specific T cells.24 TLR8 ligation stimulates mDC to markedly upregulate costimulatory and MHC molecules, secrete type I cytokines such as TNF‐α and IL‐12, and can even restore defective type I cytokine secretion in the DC of patients with HIV.16, 25, 26 In contrast to TLR8 effects on DC, we found in vitro treatment of PBMC with varying doses of motolimod significantly decreased the number of mMDSCs in a dose‐dependent manner; 167 nM motolimod reduced mMDSCs to 42.9 ± 10.3% (mean ± SE: n = 15) of PBS treatment. When the cells were treated with 500 nM motolimod, the mMDSC fraction was further decreased to 23.1 ± 4.2% (Table 1). The majority of mMDSCs coexpress CD33. The HLA‐DR–CD14+CD33+ mMDSC in PBMC was also significantly decreased to 44.6 ± 14.8% of control (mean ± SE, n = 15) in cells treated with 167 nM and 16.3 ± 3.7% in cells treated with 500 nM motolimod (Table 1). Neither dose of motolimod affected Lin‐HLA‐DR–CD33+ gMDSC. The majority of gMDSCs coexpressed CD11b. The Lin‐HLA‐DR–CD33+CD11b+ gMDSC was not impacted by the treatment (Table 1). Representative flow cytometric dot plots are shown in Supplementary Fig. 1. We next questioned whether we would observe similar effects in the PBMC of cancer patients. PBMCs derived from two patients with colon cancer, one with prostate cancer, and one with melanoma were similarly treated. The mMDSC percentage in PBMC was decreased in all patients treated with motolimod (Fig. 2A).
Table 1.
Motolimod treatment reduces the levels of mMDSC in PBMC
| 167 nM Motolimod | 500 nM Motolimod | |
|---|---|---|
| Mean ± SE | Mean ± SE | |
| (n = 15) | (n = 15) | |
| HLA‐DR–CD14+ mMDSC % among PBMCs | 42.9 ± 10.3 | 23.1 ± 4.2 |
| HLA‐DR–CD14+ CD33+ mMDSC % among PBMCs | 44.6 ± 14.8 | 16.3 ± 3.7 |
| Lin‐HLA‐DR–CD33+ gMDSC % among PBMCs | 75.5 ± 27.0 | 57.6 ± 12.6 |
| Lin‐HLA‐DR–CD33+ CD11b+ gMDSC % among PBMCs | 84.1 ± 29.3 | 57.0 ± 15.0 |
The numbers above show the percentage reduction of MDSC among PBMCs as compared to PBS control.
Figure 2.
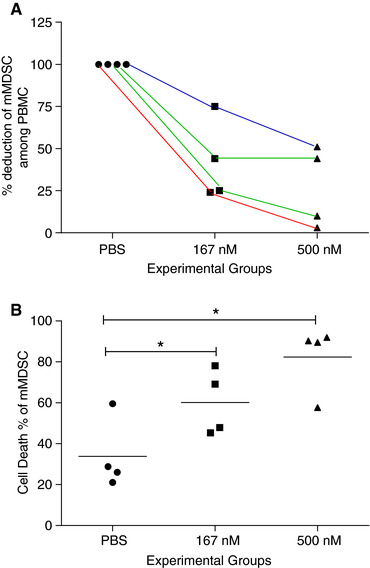
Motolimod treatment initiates apoptosis of mMDSC in cancer patients. (A) Percentage deduction of mMDSC among PBMCs in patients treated with 167 nM and 500 nM motolimod as compared to PBS control. Each line represents data from the same patient at varying doses. Red line: melanoma; green lines: colon cancer; blue line, prostate cancer. (B) Cell death percentage of mMDSC from above 4 patients. Lines are the means of each experimental group. *P < 0.05
3.3. The reduction in mMDSC was specific for treatment with a TLR8 agonist
We examined the effects of a TLR7/8 agonist CL075, a TLR7 agonist Imiquimod, and a TLR9 agonist ODN2006 on mMDSC. Similar to motolimod, the HLA‐DR–CD14+ mMDSC in PBMC was significantly decreased in cells treated with 0.5 μM CL075 (mean ± SE: 33.4 ± 7.1% of PBS control p = 0.0000) and 2 μM CL075 (22.8 ± 5.8%; p = 0.0000; n = 10; Fig. 3A). However, both doses of Imiquimod (P > 0.05, Fig. 3B) and ODN2006 (P > 0.05, Fig. 3C) did not impact the level of the mMDSC population.
Figure 3.
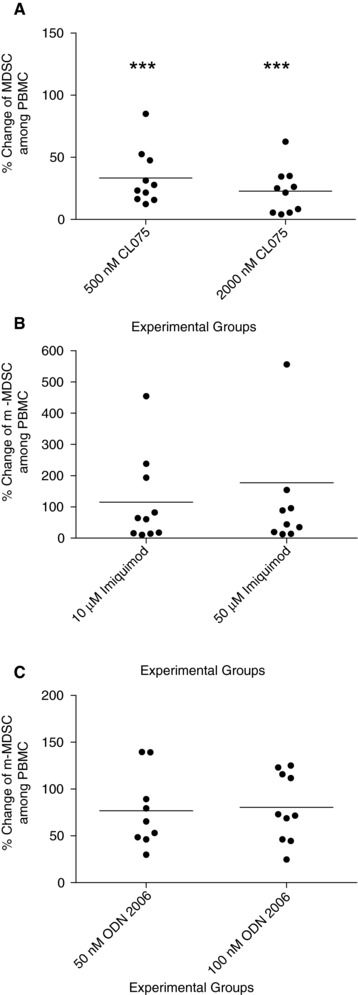
Reduction in mMDSC is specific for treatment with a TLR8 agonist. Percentage change of mMDSC among PBMCs in 0.5 μM and 2 μM CL075 (A), 10 μM and 500 μM Imiquimod (B), and 50 nM and 100 nM ODN 2006 (C) treated PBMC versus PBS controls. Lines are the means of the experimental groups (n = 10 volunteer donors). ***P < 0.000
Our results were unexpected as recent studies suggested incubation of mMDSC with a TLR7/TLR8 agonist could elicit significant IL‐12 secretion and promote the differentiation of the cells into M1 macrophage over a 3‐day period.27 We did find that motolimod treatment could promote limited type I cytokine secretion in mMDSC (Supplementary Fig. 2A and B) but without any increase in HLA‐DR+ cells (P > 0.05), which would indicate a shift in phenotype (P > 0.05; Supplementary Fig. 2C). These varying effects may be due to distinct functional differences between TLR8 and TLR7 agonists.28 TLR8 is present only in mDCs while TLR7 agonists will activate both mDCs and pDCs and B cells, which may result in differential stimulation of T cells. Moreover, studies demonstrate that type I cytokine levels produced by monocytes stimulated with a specific TLR8 agonist are 100 times greater than levels induced by a TLR7 agonist.28 Type I cytokine release mediated by motolimod could lead to direct activation of both CD4 and CD8 T cells resulting in upregulation of CD69 as well as FASL on the T‐cell surface.
3.4. Motolimod induces apoptosis of mMDSC, in part, through FAS ligation
FAS+ MDSCs are susceptible to FAS‐mediated killing by activated T cells.29 Investigations in mice have demonstrated that activated FASL+ T cells induce apoptosis in FAS‐expressing MDSCs.30, 31 We questioned whether the reduction of mMDSC in human PBMC was secondary to the same phenomenon—induced cell death. The induction of cell death of mMDSC within PBMC was significantly increased in cells treated with 167 nM motolimod (mean ± SE of cell death percentage of mMDSC: PBS, 45 ± 7%; 167 nM, 71 ± 9%; P = 0.0111). When PBMCs were treated with 500 nM motolimod, mMDSC death was further increased (84 ± 6%, vs. PBS, P = 0.0001; vs. 167 nM, P = 0.0455; Fig. 4A). In contrast, cell death among total PBMC was no different from the control with both doses of motolimod (P > 0.05; Fig. 4B; representative staining, Supplementary Fig. 3A and B). Motolimod treatment induced similar levels of cell death of the mMDSC in cancer patients (Fig. 2B).
Figure 4.
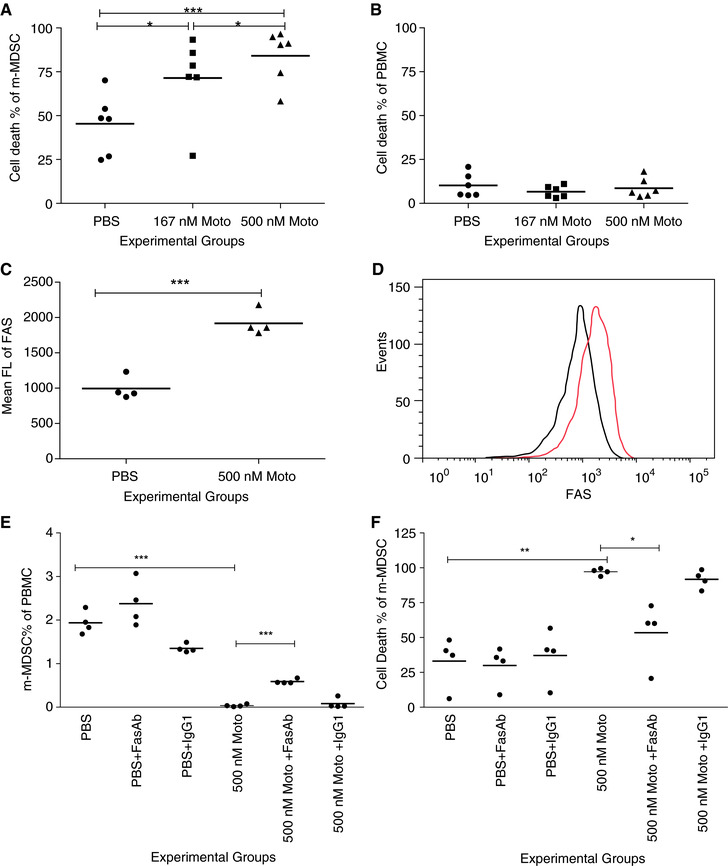
Motolimod induces apoptosis of mMDSC, in part through FAS ligation. (A and B) Cell death percentage of mMDSC (A) and PBMCs (B) following treatment with PBS or motolimod in doses of 167 and 500 nM (n = 6 volunteer donors). (C and D) FAS expression in mMDSC population following treatment with PBS or 500 nM motolimod; (C) mean FL of FAS, n = 4 volunteer donors, and (D) representative overlay FLOW histograms of FAS expression (black, PBS; red, motolimod). (E and F) mMDSC percentage of PBMC (E) and cell death percentage of mMDSC (F) in PBS alone, with FAS blocking Ab or IgG1 isotype control, and in 500 nM motolimod alone, with FAS blocking Ab or IgG1 isotype control. Lines are the means of the experimental groups. *P < 0.05; **P < 0.01; ***P < 0.001
We have previously shown that motolimod does not directly activate T cells.16 Treatment with the agent, however, indirectly activated T cells resulting in significant expression of CD69 on both CD4 (P = 0.0148) and CD8 (P = 0.0044) T cells compared to cells treated with PBS (Supplementary Fig. 4A and B). FASL was also significantly upregulated on CD4 and CD8 T cells with motolimod treatment (P = 0.0056 and 0.0257, respectively, as compared to PBS; Supplementary Fig. 4C and D). Murine MDSCs express the death receptor FAS and induce apoptosis upon exposure to activated T cells.30 We measured the FAS (CD95) expression on human mMDSC after motolimod treatment. The mean FL of FAS on mMDSCs was significantly elevated after the treatment (mean ± SE of mean FL: PBS, 994 ± 81%; motolimod, 1917 ± 88, P = 0.0003; Fig. 4C; representative histograms, Fig. 4D). The mMDSC population among PBMC was not affected by FAS‐neutralizing antibody or isotype control alone as compared to the PBS control (P > 0.05). The motolimod‐dependent reduction of the mMDSC population could be inhibited by pretreatment of the cells with FAS antibody (Fig. 4E) (mean ± SE of mMDSC percentage PBMC: motolimod, 0.04 ± 0.01; with FAS antibody, 0.59 ± 0.03; P = 0.0007) and not inhibited with the isotype control (P > 0.05). Representative analyses are shown in Supplementary Fig. 3C for the various culture conditions. Similarly, death of mMDSC induced by motolimod was significantly reduced by FAS antibody (mean ± SE of cell death percentage of mMDSC: motolimod: 94.1 ± 1.1%; with FAS antibody, 53.5 ± 10.1%, P = 0.0354) but not by the isotype control (P > 0.05, Fig. 4F).
3.5. Motolimod‐dependent depletion of mMDSC partially restores T‐cell activation
As shown in Fig. 5, mMDSC significantly inhibited IL‐2 secretion by CD3+ cells stimulated with CD3/CD28 antibody (mean ± SE of IL‐2 (pg/ml): CD3/CD28 antibody alone, 3213 ± 1172 pg/ml; with mMDSC, 59 ± 13 pg/ml; P = 0.029). IL‐2 secretion was partially restored when the cells were cocultured with motolimod (142 ± 36; P = 0.03). Motolimod incubation did not directly stimulate IL‐2 secretion from CD3+ cells or mMDSCs (Fig. 5).
Figure 5.
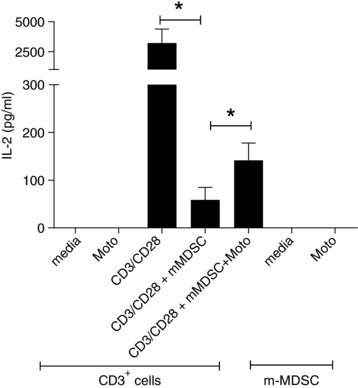
Motolimod‐induced depletion of mMDSC partially restores T‐cell activation. IL‐2 secretion from CD3+ cells treated with media, motolimod alone; and CD3+ cells stimulated with CD3/CD28, mMDSC, and mMDSC and motolimod; and mMDSC treated with media and motolimod. Columns and bars represent means (±SE) of 4 donors. *P < 0.05
4. CONCLUSIONS
Data presented here demonstrate that TLR8 is expressed in mMDSCs and that selective TLR8 ligation can significantly reduce the levels of mMDSCs in the peripheral blood via the induction of apoptosis. Reduction in the number of mMDSCs in PBMC, mediated by TLR8 agonist treatment, will significantly enhance type I cytokine secretion by activated T cells. There is increasing evidence that MDSC contribute to the progression of cancer by inhibiting tumor‐directed T cells and producing mediators that promote the growth and survival of tumor cells.12, 32, 33 Motolimod has a favorable safety profile when used as an immunotherapeutic in patients with cancer demonstrating limited toxicity and no evidence of cytokine storm.34 One strategy would be to precondition patients with systemic infusion of motolimod to deplete MDSC prior to therapy, especially an immune‐based therapy. Reducing MDSC, while activating T cells, may synergize with cancer immunotherapeutic approaches and enhance the antitumor effects of the adaptive immune response.
Supporting information
Supplemental Figure 1.
Supplemental Figure 2.
Supplemental Figure 3.
Supplemental Figure 4.
AUTHORSHIP
Y.D. and Z.J.R performed experiments, and analyzed and interpreted data. H.L. and Y.Y. performed experiments and analyzed data. G.D. and R.H. designed research and interpreted data. M.L.D. obtained the funding, designed, and supervised the study. Y.D. and M.L.D wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by a commercial research grant from VentiRX Pharmaceutical (M.L.D.). M.L.D. is also supported by a Komen Leadership Grant as well as the Athena Distinguished Professorship for Breast Cancer Research. Human samples from cancer patients were collected through the Clinical Research Center Facility at the University of Washington (NIH grant UL1TR000423).
DISCLOSURES
M.L.D. is a stockholder in Epithany and VentiRx and receives grant support from Celgene, EMD Serono, VentiRx, and Seattle Genetics. H.L. is an employee of Immune Design. R.H. is an employee and has ownership (shares) in VentiRX. The remaining authors have no conflict of interest.
Dang Y, Rutnam ZJ, Dietsch G, et al. TLR8 ligation induces apoptosis of monocytic myeloid‐derived suppressor cells. J Leukoc Biol 2018;103:157–164. 10.1002/JLB.5AB0217-070R
REFERENCES
- 1. Lesokhin AM, Hohl TM, Kitano S, et al. Monocytic CCR2(+) myeloid‐derived suppressor cells promote immune escape by limiting activated CD8 T‐cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyer C, Sevko A, Ramacher M, et al. Chronic inflammation promotes myeloid‐derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci USA. 2011;108:17111–17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schouppe E, Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Modulation of CD8(+) T‐cell activation events by monocytic and granulocytic myeloid‐derived suppressor cells. Immunobiology. 2013;218:1385–1391. [DOI] [PubMed] [Google Scholar]
- 4. Markowitz J, Brooks TR, Duggan MC, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid‐derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergenfelz C, Larsson AM, von Stedingk K, et al. Systemic monocytic‐MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS One. 2015;10:e0127028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirbod‐Mobarakeh A, Mirghorbani M, Hajiju F, Marvi M, Bashiri K, Rezaei N. Myeloid‐derived suppressor cells in gastrointestinal cancers: a systematic review. J Gastroenterol Hepatol. 2016;31:1246–1256. [DOI] [PubMed] [Google Scholar]
- 7. Koinis F, Vetsika EK, Aggouraki D, et al. Effect of first‐line treatment on myeloid‐derived suppressor cells' subpopulations in the peripheral blood of patients with non‐Small cell lung cancer. J Thorac Oncol. 2016;11:1263–1272. [DOI] [PubMed] [Google Scholar]
- 8. Meyer C, Cagnon L, Costa‐Nunes CM, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santegoets SJ, Stam AG, Lougheed SM, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer. 2014;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sade‐Feldman M, Kanterman J, Klieger Y, et al. Clinical significance of circulating CD33+CD11b+HLA‐DR‐ myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin Cancer Res. 2016;22:5661–5672. [DOI] [PubMed] [Google Scholar]
- 11. Kimura T, McKolanis JR, Dzubinski LA, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res. 2013;6:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Zhang L, Zhu X, Wang Y, Liu W, Gong W. Polysaccharide Agaricus blazei Murill stimulates myeloid derived suppressor cell differentiation from M2 to M1 type, which mediates inhibition of tumour immune‐evasion via the toll‐like receptor 2 pathway. Immunology. 2015;146:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand‐Rosenberg S. Inflammation enhances myeloid‐derived suppressor cell cross‐talk by signaling through toll‐like receptor 4. J Leukoc Biol. 2009;85:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ray A, Chakraborty K, Ray P. Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Front Cell Infect Microbiol. 2013;3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu H, Dietsch GN, Matthews MA, et al. VTX‐2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clinical Cancer Res. 2012;18:499–509. [DOI] [PubMed] [Google Scholar]
- 17. Dietsch GN, Lu H, Yang Y, et al. Coordinated activation of toll‐like receptor 8 (TLR8) and NLRP3 by the TLR8 agonist, VTX‐2337, ignites tumoricidal natural killer cell activity. PLoS One. 2016;11:e0148764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Disis ML, Dang Y, Coveler AL, et al. HER‐2/neu vaccine‐primed autologous T‐cell infusions for the treatment of advanced stage HER‐2/neu expressing cancers. Cancer Immunol Immunother. 2014;63:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Disis ML, dela Rosa C, Goodell V, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–18. [DOI] [PubMed] [Google Scholar]
- 20. Lu H, Knutson KL, Gad E, Disis ML. The tumor antigen repertoire identified in tumor‐bearing neu transgenic mice predicts human tumor antigens. Cancer Res. 2006;66:9754–9761. [DOI] [PubMed] [Google Scholar]
- 21. Dang Y, Wagner WM, Gad E, et al. Dendritic cell‐activating vaccine adjuvants differ in the ability to elicit antitumor immunity due to an adjuvant‐specific induction of immunosuppressive cells. Clin Cancer Res. 2012;18:3122–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phan‐Lai V, Dang Y, Gad E, Childs J, Disis ML. The antitumor efficacy of IL2/IL21‐cultured polyfunctional Neu‐specific T cells is TNF‐alpha/IL17 dependent. Clin Cancer Res. 2016;22:2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schouppe E, Mommer C, Movahedi K, et al. Tumor‐induced myeloid‐derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T‐cell activation events. Eur J Immunol. 2013;43:2930–2942. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt SV, Nino‐Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hackstein H, Knoche A, Nockher A, et al. The TLR7/8 ligand resiquimod targets monocyte‐derived dendritic cell differentiation via TLR8 and augments functional dendritic cell generation. Cell Immunol. 2011;271:401–412. [DOI] [PubMed] [Google Scholar]
- 26. Cardoso EC, Pereira NZ, Mitsunari GE, et al. TLR7/TLR8 activation restores defective cytokine secretion by myeloid dendritic cells but not by plasmacytoid dendritic cells in HIV‐infected pregnant women and newborns. PLoS One. 2013;8:e67036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J, Shirota Y, Bayik D, et al. Effect of TLR agonists on the differentiation and function of human monocytic myeloid‐derived suppressor cells. J Immunol. 2015;194:4215–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. [DOI] [PubMed] [Google Scholar]
- 29. Ostrand‐Rosenberg S, Sinha P, Chornoguz O, Ecker C. Regulating the suppressors: apoptosis and inflammation govern the survival of tumor‐induced myeloid‐derived suppressor cells (MDSC). Cancer Immunol Immunother. 2012;61:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand‐Rosenberg S. Myeloid‐derived suppressor cells express the death receptor Fas and apoptose in response to T cell‐expressed FasL. Blood. 2011;117:5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiss JM, Subleski JJ, Back T, et al. Regulatory T cells and myeloid‐derived suppressor cells in the tumor microenvironment undergo Fas‐dependent cell death during IL‐2/alphaCD40 therapy. J Immunol. 2014;192:5821–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen‐specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. [DOI] [PubMed] [Google Scholar]
- 33. Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid‐derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monk BJ, Brady MF, Aghajanian C, et al. A phase 2, randomized, double‐blind, placebo‐controlled study of chemo‐immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study. Ann Oncol. 2017;28:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.
Supplemental Figure 2.
Supplemental Figure 3.
Supplemental Figure 4.


