Abstract
Lansoprazole, a new substituted benzimidazole H+,K+-ATPase inhibitor, profoundly inhibits gastric acid secretion and has potential use in the management of diseases such as Zollinger-Ellison syndrome (ZES). In the present study we evaluated the efficacy and safety of lansoprazole in controlling acid hypersecretion in 20 patients with ZES. The starting dose was 60 mg once daily. Control of acid hypersecretion was defined as the dose required to reduce acid secretion to <10 meq/hr in the last hour before the next dose. Doses were adjusted upwards until effective control was achieved. Patients not controlled with 120 mg once daily were placed on twice daily lansoprazole. Most patients (90%) required lansoprazole once daily. During long-term follow-up (mean 18.5 months), 25% of patients required upward dose adjustments and 25% of patients required twice daily lansoprazole. Following cessation of therapy, the mean time for gastric acid output to reach half basal acid output was 39.1 hr. Lansoprazole was well-tolerated without side effects. Clinical chemistry and hematological studies were unchanged, and no gastric carcinoids developed. These results demonstrate that lansoprazole is a safe and effective inhibitor of gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Because it has a long duration of action, lansoprazole can be used to control gastric acid hypersecretion in most patients with Zollinger-Ellison syndrome using a once daily dosing schedule.
Keywords: gastrinoma; islet cell tumor; acid-peptic disease; H+,K+-ATPase inhibitor
Prior to the availability of histamine H2-receptor antagonists, patients with the Zollinger-Ellison syndrome required total gastrectomy for adequate management of gastric acid hypersecretion (1, 2). The histamine H2-receptor antagonists effectively inhibit gastric acid secretion in these patients with few side effects, but they have some limitations. High doses at frequent dosing intervals are required (3–9). At times, an anticholinergic drug, in addition to a histamine H2-receptor antagonist, is required to provide adequate inhibition of gastric acid secretion (9–13), and most patients require at least one dose increase per year (2, 7). In patients with ZollingerEllison syndrome and severe reflux esophagitis or previous partial gastrectomy, even higher doses are required to completely heal associated acid-peptic disease, and healing is not possible in all cases (2, 14–16).
The substituted benzimidazoles, such as omeprazole, act at the final common step in gastric acid production, where they inactivate the parietal cell H+,K+-ATPase (17, 18). Omeprazole, the first H+,K+-ATPase inhibitor available in the United States, has been shown to be very effective in the management of gastric acid hypersecretory states such as the Zollinger-Ellison syndrome (18–34). Omeprazole has a prolonged duration of action that permits less frequent administration than is required for histamine H2-receptor antagonists (22, 24, 33, 34). Numerous studies show that omeprazole is both safe and effective in the long-term control of gastric acid hypersecretion in patients with Zollinger-Ellison syndrome (19–26, 30). However, the use of omeprazole in some patients also has some limitations. Even with the use of high daily doses (ie, 120 mg/day), gastric acid secretion is not effectively controlled with once daily dosing in all patients (19, 21, 22, 25), and doses as high as 360 mg/day are occasionally needed (21). In some studies, up to 60% of patients require a twice daily dose (19, 21, 22, 25). Patients with Zollinger-Ellison syndrome complicated by severe gastroesophageal reflux disease or previous partial gastrectomy or those with multiple endocrine neoplasia type 1 (MEN-1) syndrome commonly fall into this category. Thus, although omeprazole currently is the drug of choice in the management of gastric acid hypersecretion in patients with Zollinger-Ellison syndrome (19, 21), in some patients a more potent, longer-acting proton pump inhibitor may provide a further improvement in the management of the gastric acid hypersecretion.
Lansoprazole (A 65006/AG 1749, TAP Pharmaceuticals Inc., Deerfield, Illinois) is a new, substituted benzimidazole, H+,K+-ATPase inhibitor that structurally resembles omeprazole (Figure 1) except that it has a trifluoroethoxy radical in position 4 of the pyridine ring and lacks a methyl and methoxy radical on the pyridine and benzimidazole rings, respectively (35, 36). Lansoprazole has a potency similar to that of omeprazole for inhibition of H+,K+-ATPase activity in isolated canine gastric microsomes (37, 38), either similar (37, 38) or twice the potency (39) of omeprazole in inhibiting histamine and dibutyryl cyclic AMP-stimulated acid formation by isolated canine gastric parietal cells, and similar potency for inhibition of gastric acid secretion in Heidenhain pouch dogs (37, 38). In contrast, lansoprazole has been shown to be 2–11 times more potent than omeprazole in inhibiting gastric acid secretion and in preventing both acute and chronic experimentally induced ulcers in rats (37, 38, 40).
Fig 1.
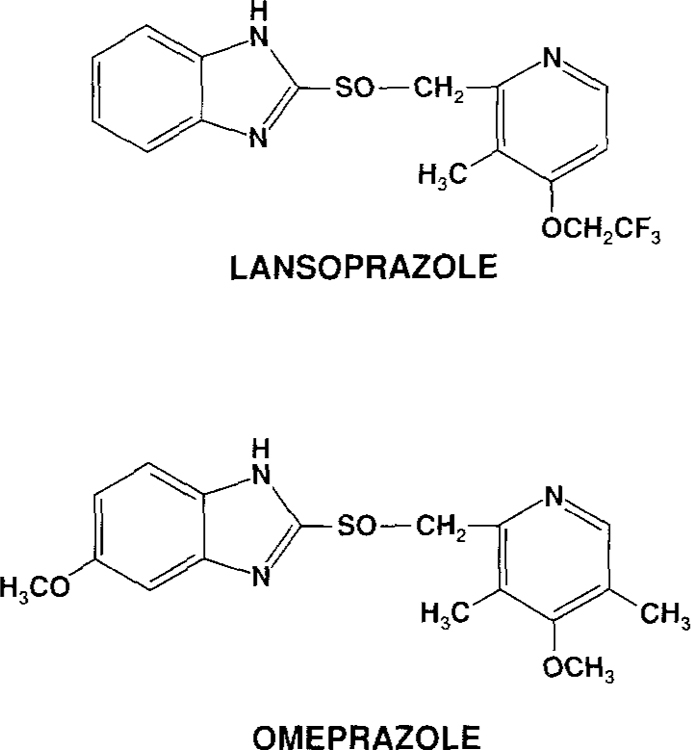
Structures of omeprazole and lansoprazole.
To date, the prolonged administration of lansoprazole in humans has not been studied. Preliminary human studies have shown lansoprazole to be an effective inhibitor of gastric acid secretion in normal volunteers (41–43), in patients with both inactive (44) and active (45) peptic ulcer disease, and in patients with refractory gastroesophageal reflux disease (46–50). Lansoprazole has also been shown to prevent aspirin-induced gastric lesions in healthy volunteers (51, 52). Initial reports have also shown that the use of lansoprazole in humans is well tolerated, safe, and without effect on standard laboratory determinations in the short term (50).
The short-term use of lansoprazole in the Zollinger-Ellison syndrome has been described in four patients who were previously treated with omeprazole (53). In this report, lansoprazole (administered for up to 30 days) was believed to demonstrate a comparable efficacy and safety to that of omeprazole, although the duration of action of lansoprazole was believed to be possibly slightly shorter than that of omeprazole. The purpose of the present study was to determine the possible usefulness of lansoprazole in gastric hypersecretory states by assessing the short- and long-term efficacy and safety of lansoprazole in patients with ZollingerEllison syndrome.
MATERIALS AND METHODS
Patients.
All patients had Zollinger-Ellison syndrome. The diagnostic criteria included a basal acid output (BAO) of > 15 meq/hr, an elevated fasting serum gastrin (>100 pg/ml) in the presence of gastric acid secretion, positive provocative testing with secretin stimulation (an increase of >200 pg/ml postinjection) or with calcium infusion (an increase of >395 pg/ml), a positive histological diagnosis of gastrinoma, or a combination of these as previously described (2, 8, 54). All patients provided written informed consent to a protocol approved by the Institutional Human Research Committee of the NIDDK. The study group included 17 patients with sporadic Zollinger-Ellison syndrome and three patients with Zollinger-Ellison syndrome associated with multiple endocrine neoplasia type 1 (MEN-1). Twelve patients had biopsy-proven disease and eight had confirmatory gastrin provocative testing but either a negative exploratory laparotomy (four patients) or no surgery had been performed (four patients).
Investigations.
Gastric acid secretion was determined by positioning a nasogastric tube in the stomach and continuously aspirating the gastric contents as previously described (55). Basal acid output (BAO) was measured after discontinuing all oral antisecretory medication for more than 30 hr (12 hr for intravenous antisecretory medication). Following an overnight fast, four consecutive 15-min samples were collected after emptying the stomach. Each sample was titrated to pH 7.0 with 0.01 N sodium hydroxide, and the BAO was determined by adding the number of milliequivalents of acid in the four samples. Maximal acid output (MAO) was determined by collecting four consecutive 15-min samples following the subcutaneous administration of pentagastrin (6 µg/kg). To provide an assessment of the inhibition of gastric acid secretion by lansoprazole, four consecutive 15-min samples were collected during the last hour before the next dose of drug. Adequate control was defined as less than 10 meq/hr prior to the next dose of antisecretory drug.
Upper gastrointestinal endoscopy was performed in the left lateral decubitis position using a Fujinon videoendoscopy system following sedation with meperidine (Demerol) and midazolam (Versed). Careful visual assessment of the esophagus, stomach, and duodenum was made. Forceps biopsies were taken if there were any nodules or masses seen or if there was any evidence of inflammation by gross inspection, especially in the esophagus. At least two gastric body biopsies from the region of the greater curvature were performed in all patients and these were examined histologically (Grimelius stains) for evidence of carcinoid tumors.
Routine bloodwork including hepatic transaminase values, serum lipids, assessment of thyroid and renal function, and complete blood count was done by the NIH clinical pathology department. Fasting serum gastrin values were determined by Bioscience Laboratories (New York, New York), and all samples were diluted into the normal range for accurate determination of higher values. Tumor extent was assessed in all patients using standard imaging criteria including ultrasound (56), CT scan (57), MRI scan (58), and bone scan as well as selective abdominal arteriography with secretin provocation and gastrin sampling in the hepatic veins as described previously (59).
Study Design.
Patients with Zollinger-Ellison syndrome were considered for the study provided they were over 18 years of age, had a BAO of greater than 15 meq/hr, had not undergone prior gastric surgery, and had not been previously treated with omeprazole. Pregnant females and patients with active peptic ulcer disease were excluded from the study.
The initial evaluation included a comprehensive history with special attention to symptoms of gastric acid hypersecretion including pyrosis, abdominal pain, nausea, vomiting, diarrhea, gastrointestinal bleeding, and overall well-being. Thereafter, a complete physical examination, upper gastrointestinal endoscopy, chest x-ray, electrocardiogram, and laboratory determinations of hematologic, hepatic, thyroid, lipid, and renal parameters as well as the assessment of stool for occult blood, urinalysis, and a pregnancy test (in females) were performed. The mean of three fasting serum gastrin determinations, drawn on consecutive days, was obtained prior to commencing treatment with lansoprazole.
Determination of Initial Lansoprazole Dose.
Lansoprazole was given once daily. The initial dose of the drug was established by measuring control of acid secretion following the initial starting dose of 60 mg in all patients. Reduction of gastric acid secretion to <10 meq/hr for the last hour prior to the next daily dose of lansoprazole was chosen as indicating satisfactory control because previous studies have shown that inhibition to this extent prevents recurrence of acid-peptic disease and heals peptic ulcers (55). Those who were acutely controlled with the first dose were reassessed 24 hr later thus providing two estimates of control before long-term follow-up was initiated. Patients who were not controlled on 60 mg had their lansoprazole dose titrated upwards by 15 mg daily with measurement of control the following day. On attaining an adequate control value, they also underwent a second measurement 24 hr later as above. Patients whose gastric acid hypersecretion could not be controlled with 120 mg lansoprazole daily had their daily dose split into two 12-hr 60-mg doses.
Determination of Long-Term Maintenance Dose and Assessment of Long-Term Safety.
Following the initial evaluation and the establishment of an initial dose requirement in each patient, patients were reevaluated three and six months later and every six months thereafter. At each subsequent evaluation a complete review of systems was obtained to evaluate any possible adverse effects of lansoprazole therapy and to assess clinical evidence of acid-peptic disease. All patients underwent a complete physical examination at each visit. Blood was taken for assessment of fasting serum gastrin, serum transaminases, lipids, thyroid and renal function, and a complete blood count and a urinalysis was obtained. In addition, all patients underwent gastric analysis to assess the control of gastric acid secretion and upper endoscopy with biopsy of the gastric body at each visit.
Unsatisfactory control of gastric acid secretion was defined as an acid output greater than 10 meq/hr in the last hour prior to the next dose of lansoprazole, endoscopic evidence of moderate to severe esophagitis, duodenitis or peptic ulceration, or the presence of persistent peptic symptoms. These patients had their lansoprazole dose titrated upwards as on the initial evaluation until their symptoms resolved or their control values decreased to below 10 meq/hr.
Duration of Action of Lansoprazole.
The duration of action of lansoprazole was examined in four patients who exhibited stable control of acid secretion on follow-up. In these patients, lansoprazole therapy was temporarily discontinued and gastric acid secretion was determined in the 24th hour after the last dose and every 8 hr thereafter for a further 48 hr or until their acid secretion reached 30 meq/hr or 50% of their individual pretreatment BAO measurement, whichever came first. This portion of the study was terminated by administering 150 mg of cimetidine intravenously followed by the reintroduction of lansoprazole at the previously effective dose.
RESULTS
The clinical and laboratory characteristics of the 20 patients with Zollinger-Ellison syndrome included in this study are shown in Table 1. They are representative of the larger cohort of patients with Zollinger-Ellison syndrome followed at the NIH since 1976 in terms of mean age, sex distribution, percentage with MEN-1 syndrome, basal and maximal acid secretory studies, fasting serum gastrin determination, disease duration, and tumor status. They were not representative of the larger cohort of patients at the NIH in terms of the number with previous gastric surgery, which in our population is 10%, since the protocol excluded this group because of the potential difficulties in obtaining reliable gastric acid secretion measurements. Assessment of tumor status was made at exploratory laparotomy (N = 16) or using imaging studies (N = 4). A pancreatic or duodenal primary tumor was identified in 35% (7/20) and 15% (3/20) of patients, respectively. Thirty percent of patients (6/20) had hepatic metastases. Prior to entering the study, no patient had a peptic ulcer or other mucosal abnormality on upper gastrointestinal endoscopy.
Table 1.
Patient Characteristics*
| Age (years) | |
| Mean ± 1 SEM | 49 ± 2.2 |
| Range | 27–69 |
| Sex (N), male/female | 10/10 |
| MEN-1 (N), present/absent | 3/17 |
| BAO (meq/hr) | |
| Mean ± 1 SEM | 38.7 ± 6.4 |
| Range | 16–143.9 |
| MAO (meq/hr) | |
| Mean ± 1 SEM | 58.7 ± 7.3 |
| Range | 24–143.9 |
| Fasting serum gastrin (pg/ml)† | |
| Mean ± 1 SEM | 866 ± 249 |
| Range | 162–5232 |
| Disease duration (months)‡ | |
| Mean ± 1 SEM | 83.5 ± 17.9 |
| Range | 6–278 |
| Previous gastric surgery | 0 |
| Tumor status | |
| Location of primary (N)§ | |
| Pancreas | 7 |
| Duodenum | 3 |
| Unknown | 10 |
| Liver metastases¶ | 6 |
Abbreviations: MEN-I, multiple endocrine neoplasia type-I; BAO, basal acid output; MAO, maximal acid output.
Mean of three consecutive daily determinations.
Onset of disease defined as the start of continuous peptic symptoms.
Sixteen patients had exploratory laparotomy. Four patients had no tumor found at surgery and two had only liver metastases identified at surgery. The four patients who did not have surgical exploration (2 MEN-I, l lost to follow-up, and 1 who was unfit for surgery) all had negative imaging studies.
Biopsy proven in 5 patients, suspected from imaging in one patient.
Determination of Initial Lansoprazole Dose.
The mean dose requirement for the effective control of acid secretion prior to the start of lansoprazole in the 19 patients taking ranitidine was 4. 1 g/day (range 1.2–7.2 g); the one patient taking cimetidine required 6.0 g/day (Table 2). The duration of histamine Hz-receptor antagonist therapy at the dose that the patients were taking just prior to starting therapy with lansoprazole ranged from 1 week to 21 months with a mean of 7.5 months. The duration of therapy with any dose of histamine H2-receptor antagonist ranged from 4 to 153 months with a mean of 56 months (Table 2).
Table 2.
Basal Acid Output, Dose, and Duration of Therapy with Lansoprazole and Histamine H2-Receptor Antagonist Prior to Starting Lansoprazole and Initial Acid Output Values on Lansoprazole*
| Histamine H2-receptor antagonist |
Lansoprazole |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | BAO (meq/hr)† | Drug | Dose (rag/day)‡ | Duration on this dose (months)§ | Total Duration—any doses¶ (months) | Dose (mg/day)** | Acid output (meq/hr)†† | Duration on this dose (months)‡‡ | Total Duration—any dose (months)§§ |
| 1 | 38.2 | Ran | 6000 | 9 | 19 | 60 | 5.2 | 6 | 29 |
| 2 | 62.9 | Ran | 7200 | 2 | 120 | 120¶¶ | 4.7 | 27 | 27 |
| 3 | 47.4 | Ran | 2400 | 2 | 14 | 60 | 0.4 | 27 | 27 |
| 4 | 16 | Ran | 4200 | 13 | 48 | 60 | 0.6 | 26 | 26 |
| 5 | 17.8 | Ran | 3600 | 12 | 18 | 60 | 5.8 | 25 | 25 |
| 6 | 17.8 | Ran | 2400 | 15 | 34 | 120 | 6.2 | 3 | 8*** |
| 7 | 29.1 | Ran | 6000 | 14 | 45 | 60 | 1.7 | 4 | 4*** |
| 8 | 38.8 | Ran | 3000 | 1 | 4 | 60 | 2.5 | 3 | 22 |
| 9 | 22.5 | Ran | 6000 | 12 | 86 | 60 | 6.1 | 12 | 21 |
| 10 | 63.3 | Ran | 3600 | 0.25 | 6 | 60 | 0.5 | 21 | 21 |
| 11 | 16.2 | Cim | 6000 | 4 | 96 | 60 | 0 | 20 | 20 |
| 12 | 55.2 | Ran | 7200 | 1 | 4 | 75 | 4.8 | 0.9 | 0.9*** |
| 13 | 43.5 | Ran | 3600 | 5 | 8 | 75 | 2.6 | 9.5 | 9.5*** |
| 14 | 143.9 | Ran | 4800 | 0.25 | 148 | 120¶¶ | 0.2 | 17 | 17 |
| 15 | 30.8 | Ran | 2400 | 12 | 21 | 60 | 0 | 15 | 15 |
| 16 | 24.6 | Ran | 4800 | 7 | 153 | 60 | 0.2 | 14 | 14 |
| 17 | 41.7 | Ran | 6000 | 14 | 135 | 60 | 0.2 | 13 | 13 |
| 18 | 34.4 | Ran | 1800 | 1 | 41 | 75 | 7.1 | 3 | 10 |
| 19 | 24 | Ran | 1200 | 4 | 96 | 60 | 0 | 5 | 5 |
| 20 | 34.9 | Ran | 3000 | 21 | 29 | 60 | 0.7 | 4 | 4 |
Abbreviations: BAO = basal acid output; Ran = ranitidine; Cim = cimetidine.
As defined in Materials and Methods section.
Last dose prior to starting lansoprazole.
Duration this dose was effective at controlling gastric acid secretion.
Total time of therapy with any H2-receptor antagonist.
Initial dose of lansoprazole to control gastric acid secretion acutely.
Acid output following effective upward titration of lansoprazole (higher of two values).
Duration initial dose was effective.
Total duration of therapy with lansoprazole. Patients with total duration at any dose higher than duration at initial dose required dose adjustment (see Table 3).
Split dosage of 60 mg twice daily.
Stopped taking lansoprazole: Pt. 6 discontinued treatment for personal reasons without side effects, Pt. 7 died of liver cirrhosis, Pt. 12 was removed from the protocol, and Pt. 13 was cured of Zollinger-Ellison syndrome following successful resection.
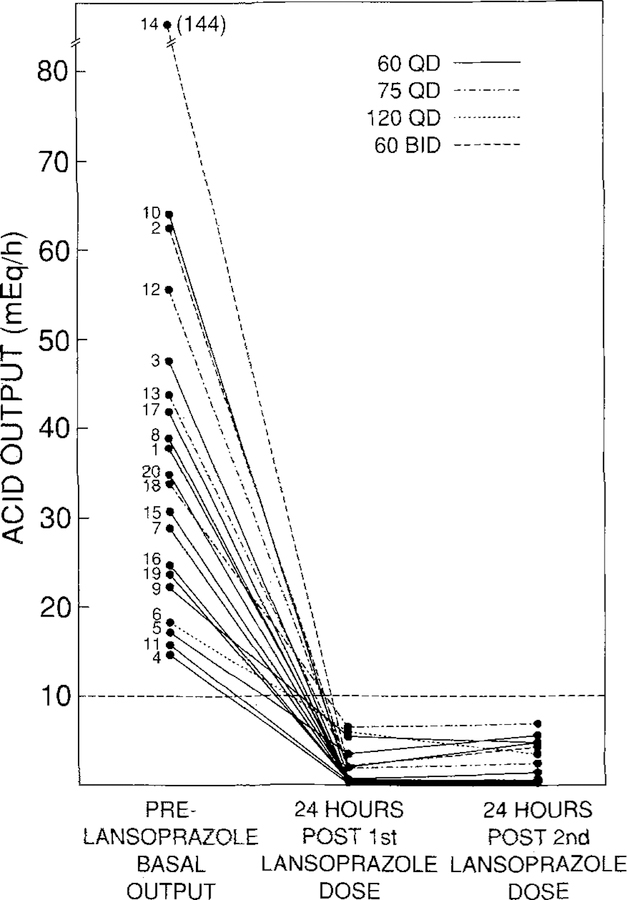
In the initial dosing study, 14 patients were controlled with a single daily dose of 60 mg of lansoprazole, three required 75 mg once daily, one required 120 mg once daily and two required a split dosage regimen of 60 mg twice daily (Figure 2). The mean initial lansoprazole dose requirement was 72 mg/day (range 60 mg daily to 60 mg twice daily). The efficacy of acid suppression after the first dose of drug was not less than that after the second dose. Specifically, acid control values after the second dose were higher in eight patients, lower in three patients, and unchanged (within 0.2 meq/hr) in the remaining nine patients as compared with acid control values after the first dose. There was no correlation between the initial dose requirements and BAO (Table 2, Figure 2) or MAO (data not shown). Similarly, there was no relationship between MEN-1 status and higher initial dosage requirements.
Fig 2.
Effect of the initial effective lansoprazole dose on gastric acid hypersecretion for the first two days of therapy. Numbers correspond with each patient in Table 2. Fourteen patients were controlled with 60 mg daily (—), 3 with 75 mg daily (–.–.–), one with 120 mg daily (– – – –), and two with 60 mg twice daily (– – –). The initial effective lansoprazole dose is the initial lansoprazole dose that decreases gastric acid secretion to ≤10 meq/hr.
Maintenance Therapy.
The total duration of therapy with lansoprazole at any dose ranged from 0.9 months to 29 months with a mean of 16 months (Table 2). The duration of therapy of the initial effective dose of lansoprazole ranged from 0.9 months to 27 months with a mean of 13 months (Table 2). The initial dose continued to be effective in controlling acid secretion in all but five patients who required an upward dose adjustment and who are discussed in more detail below. Sixteen patients are still taking lansoprazole with effective control of acid secretion (duration of follow-up 4–29 months with a mean of 18.5 months). A single daily dose of 60 mg lansoprazole has remained effective for control of gastric acid hypersecretion in 10/16 patients (63%) with 4/16 (25%) requiring a split dose of 60 mg twice daily. One patient each (6%) required single daily doses of 75 mg or 120 mg of lansoprazole daily. Four patients are no longer taking lansoprazole. One patient discontinued treatment for personal reasons, one patient died of liver cirrhosis, one patient was removed from the protocol for noncompliance, and one patient no longer required lansoprazole after curative gastrinoma resection. None of these patients discontinued lansoprazole because they developed side effects or because gastric acid secretion was not effectively controlled (Table 2). Of the 13 patients who have been followed for more than 12 months, 60 mg of lansoprazole once daily provided effective control of gastric acid hypersecretion in eight patients (62%), one patient (7%) required 75 mg once daily, and four patients (31%) required a split dose of 60 mg twice daily.
Dosage Adjustments.
The data on the five patients (25%) requiring upward dose adjustments of lansoprazole therapy are listed in Table 3. Upward dose adjustments were required in two patients (10%) on the basis of acid-peptic symptoms alone, in two patients (10%) on the basis of elevated acid outputs alone, and in one patient (5%) on the basis of both symptoms and an elevated acid output. No patients required upward dose adjustment because of endoscopic findings. Except for the three patients (patients 1, 6, and 18—Table 3) requiring upward dose adjustments because of peptic symptoms, all patients remained asymptomatic during follow-up. No patient developed endoscopic abnormalities on long-term treatment.
Table 3.
Patients Requiring Upward Lansoprazole Dose Adjustments*
| Prior to increasing dose |
Increased effective dose |
|||||
|---|---|---|---|---|---|---|
| Patient | Dose ( mg)† | Acid output (meq/hr)‡ | Time taking lansoprazole (months)§ | Reason for increase¶ | Dose (mg)** | Acid output (meq/hr)†† |
| 1 | 60QD | 12.4 | 6 | Poor control, peptic symptoms | 75QD | 3.9 |
| 6 | 120QD | 7.8 | 3 | Peptic symptoms | 60BID | 4.1 |
| 8‡‡ | 60QD | 10.0 | 3 | Poor control | 60BID | 6.8 |
| 9§§ | 60QD | 10.0 | 12 | Poor control | 60BID | 2.1 |
| 18¶¶ | 75QD | 4.8–18 | 3 | Peptic symptoms | 120QD | 6.1 |
Abbreviations: QD = 24 hourly; BID = 12 hourly.
Effective dose at prior evaluation.
Acid output immediately prior to requiting upward adjustment of lansoprazole dose.
Duration of therapy with lansoprazole at time of requiring upward adjustment.
Reason dose adjusted upwards as per study design.
Effective dose following upward titration of lansoprazole.
Acid output following effective upward titration (higher of two values).
Patient with widely metastatic disease and hemorrhagic liver metastases who continued to have peptic symptoms that were finally controlled with a 60-rag BID dose.
Patient with MEN-I syndrome who required multiple incremental increases in dose for effective acid control in spite of lack of symptoms.
patient who required multiple increases over a two-week period postoperatively with continued symptoms and repeat acid output controls on 90–105 mg QD of up to 18.1 meq/hr.
On analyzing the data from the 16 patients still taking lansoprazole at completion of the study, we could still find no correlation between long-term maintenance dose requirements for lansoprazole and BAO or MAO (data not shown). There was also no correlation between maintenance requirements and acid output if the patients with rapidly progressive disease and increasing tumor bulk were excluded from this analysis (data not shown). However, at completion of the study, two of the three MEN-1 syndrome patients were taking 60 mg lansoprazole twice daily for effective acid control and the third was taking 60 mg once daily.
Duration of Action of Lansoprazole.
The duration of action of the effective maintenance dose of lansoprazole in patients with Zollinger-Ellison syndrome was studied by discontinuing therapy in four patients (patients 1, 15, 18, and 20 taking 75, 60, 120, and 60 mg once daily and with stable dose requirements for 23, 15, 10, and 4 months, respectively). Inhibition of gastric acid secretion persisted for up to 48 hr (Figure 3). The mean BAO value prior to commencing therapy with lansoprazole in these four patients was 35 ± 1 meq/hr (mean ± 1 SEM), and the mean time to reach half their individual BAO values was 39.1 ± 3.7 hr (mean ± SEM). Basal acid secretion in the four patients studied was inhibited by more than 80% 24 hr after the last dose of lansoprazole. Two patients (patients 15 and 18) reached their pretreatment BAO values by 48 hr, one patient (patient 1) reached his pretreatment BAO value by 56 hr and one patient (patient 20) had not yet reached pretreatment BAO levels by 56 hr after discontinuing lansoprazole.
Fig 3.
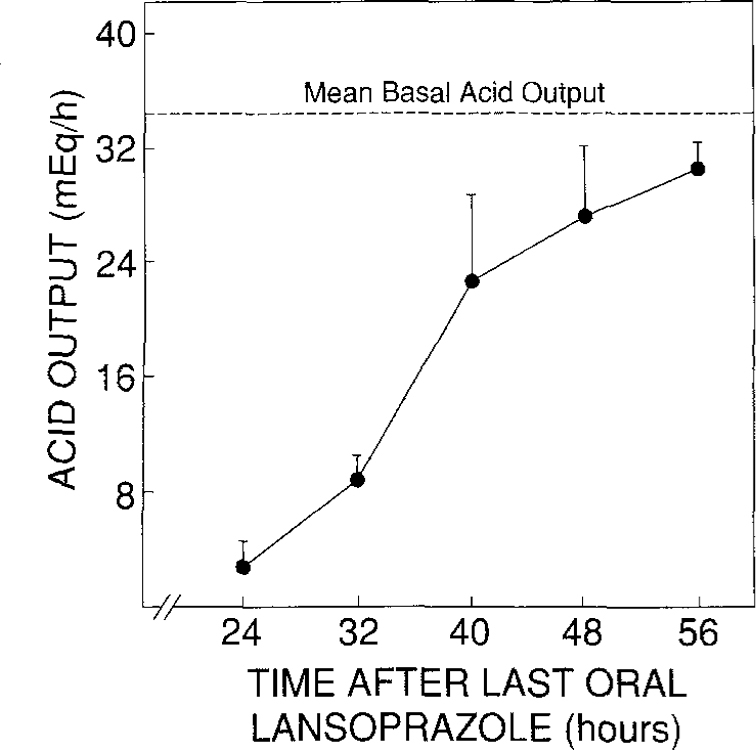
Duration of action of lansoprazole. Data are from four patients (patients 1, 15, 18, and 20, Table 2). Mean BAO (dashed line) is the mean gastric acid secretion (in meq/hr) with no antisecretory mediations measured prior to the study. Results are the mean acid outputs at each time point following the last dose of lansoprazole. Vertical bars represent 1 SEM.
Long-Term Safety.
Lansoprazole was well tolerated by all patients. There were no adverse effects attributable to lansoprazole therapy noted during the study period. Most patients preferred the convenience of once or twice daily administration compared with the more frequent dosing requirements for histamine H2-receptor antagonist therapy. In all patients, lansoprazole provided effective control of acid-peptic disease as assessed by both subjective (symptoms) and objective (endoscopy and acid control data) criteria.
Random biopsies of the gastric body were obtained from all patients prior to starting lansoprazole therapy. One of the patients with the MEN-1 syndrome (patient 14) had carcinoid tumors of the stomach and duodenal bulb on initial biopsy prior to starting lansoprazole. On follow-up biopsy, no additional carcinoid tumors have been found in any patient including patient 14 who has been followed for 12 months thus far.
Changes in fasting serum gastrin, serum hepatic transaminases (SGOT and SGPT), and total white cell count (WBC) during the first 18 months of continuous therapy with lansoprazole were analyzed in all patients. The effect of lansoprazole on fasting serum gastrin values was analyzed in 12 patients in whom fasting serum gastrin values were not influenced by other factors such as an increasing tumor bulk (six patients with liver metastases) or a reduction in tumor bulk (two patients with gastrinoma resections) (Figure 4). There was an upward trend in fasting serum gastrin values. The increase attained statistical significance at 6 and 18 months after starting therapy, with P values at 3 and 12 months of 0.16 and 0.10, respectively. Acid output measurements in these 12 patients after 3, 6, 12, and 18 months of therapy revealed that 18, 22, 42, and 66% of patients had been rendered achlorhydric.
Fig 4.
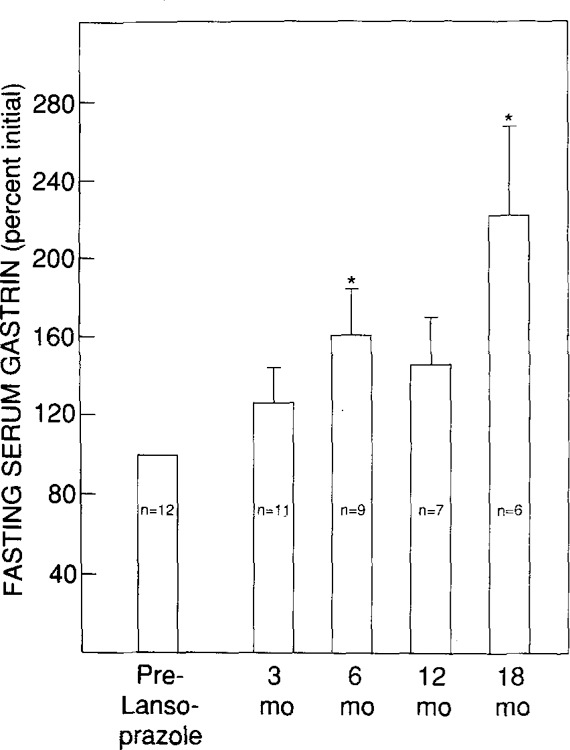
Effect of lansoprazole on fasting serum gastrin levels. Only patients with stable tumor bulk are included in the analysis (12 patients). Numbers within vertical columns depict the number of patients with measurements at each time point. Values are expressed as percent of initial mean. Vertical bars represent 1 SEM. *P < 0.05 by Student’s paired t test.
Tumor progression was seen in two patients (10%) during follow-up. In each case of tumor progression, the patient had extensive metastatic disease that was progressive before beginning lansoprazole and there was no evidence that lansoprazole increased this rate of growth.
The effect of lansoprazole on serum transaminase levels was analyzed in 14 patients in whom transaminase values were not elevated at any time prior to the start of lansoprazole therapy (Figure 5). Serum transaminase levels were not significantly altered during 18 months of therapy with lansoprazole, although minimally elevated values were noted on one occasion in each of two patients (patients 3 and 6). We do not believe that either abnormality was due to lansoprazole, since patient 3 has a long history of alcohol abuse and patient 6 has persistent, minimally elevated enzymes more than one year after discontinuing lansoprazole. Of the five patients with abnormal serum transaminases prior to starting lansoprazole, three had extensive liver metastases, one had chronic hepatitis B with cirrhosis and died from liver disease four months into therapy, and one most likely has chronic hepatitis In three of these patients, there was no change in transaminases with lansoprazole therapy, in one there was a minimal increase, and in only one of these five patients did serum transaminases rise higher than three times the upper limit of normal. In the latter patient (patient 8) the elevated values, which developed 12 months after starting lansoprazole therapy, are probably due to progressively enlarging metastatic disease of the liver.
Fig 5.
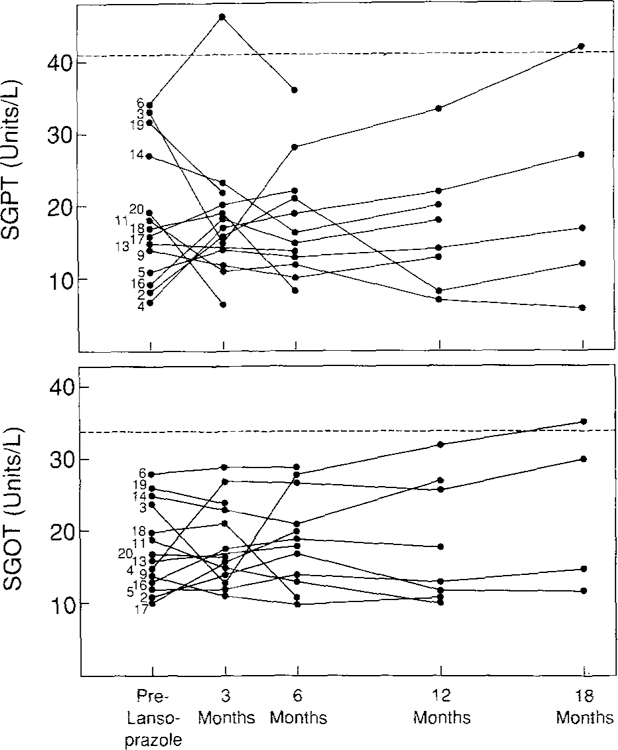
Effect of lansoprazole on serum hepatic transaminase levels (SGOT and SGPT). Only patients with normal transaminase levels before starting lansoprazole are included in the analysis (14 patients). Numbers correspond to individual patient numbers in Table 2. Dashed line represents the upper limit of normal.
No patient developed an increase or decrease in total white cell count that extended beyond the normal range for our laboratory throughout the duration of follow-up. Electrocardiograms, chest radiographs, urinalyses, and routine serum chemistry measurements including electrolytes, lipids, creatinine clearance, and thyroid function tests have remained unchanged throughout the period of treatment with lansoprazole.
DISCUSSION
The purpose of the present study was to evaluate the potential usefulness of lansoprazole in patients with Zollinger-Ellison syndrome by assessing its short- and long-term efficacy and by assessing its safety during prolonged administration. We found lansoprazole to be both safe and effective in patients with Zollinger-Ellison syndrome. Acid output is inhibited both acutely and for up to 29 months of treatment.
The 20 patients enrolled in the present study were effectively controlled with histamine H2-receptor antagonists immediately prior to beginning lansoprazole therapy. The mean ranitidine or ranitidine equivalent dose (5) required was 3.6 g/day. We have previously shown that histamine H2-receptor antagonist dose requirements as high as these are commonly required in patients with Zollinger-Ellison syndrome (3, 5, 6, 10). In addition, we have also shown that the histamine H2-receptor antagonist dose requirements correlate closely with measurements of gastric acid secretion but not with serum gastrin levels (3–5, 10). It is therefore apparent that the patients in the present study are representative of our larger cohort of patients with ZollingerEllison syndrome in their acid antisecretory drug requirements and that the results for the present study can most likely be extrapolated to our larger cohort of patients.
A single initial lansoprazole dose of 60 mg daily effectively controlled gastric acid output in 70% of patients and 90% were acutely controlled with a single dose of 120 mg daily or less. Two patients (10%) required a split dose of 60 mg twice daily for acute control of acid output. In comparison, using the same criteria for control of gastric acid secretion in 40 patients with Zollinger-Ellison syndrome, we previously showed (22) that omeprazole will acutely control gastric acid output in 38% of patients following a single oral dose of up to 60 mg daily and in 80% of patients following a single dose of 120 mg daily. Twenty percent of patients require a split dose of omeprazole (22).
For effective long-term control of gastric acid secretion in patients with Zollinger-Ellison syndrome, approximately two in three patients will be well controlled with 60 mg of lansoprazole as a once daily dose. If the highest dose for a once daily administration is arbitrarily set at 120 mg, approximately one in four patients will require a split dose of 60 mg twice daily. The present study indicates that once an effective starting dose of lansoprazole has been determined, upward dose adjustments are required in approximately 25% of patients during prolonged therapy. Furthermore, approximately 15% of patients who are initially controlled with a once daily dose will require a split dose of lansoprazole during prolonged therapy. In comparison, patients treated long-term with omeprazole require upward dose adjustments in 23% of cases, with a further 20% requiring dose adjustments in both an upward and downward direction (19, 21–23). Split dose regimens are required in 20–60% of patients treated long-term with omeprazole (19, 21–23). These results suggest that lansoprazole resembles omeprazole in providing stable control of gastric acid secretion in patients with Zollinger-Ellison syndrome.
Both lansoprazole and omeprazole have been shown to have improved inhibition of gastric acid secretion over the first few days of therapy (18, 41, 42). In the present study we did not find that the efficacy of lansoprazole improved after the second dose of drug compared with the first dose. It is unclear why we were unable to demonstrate a more profound inhibition of acid production on the second day of administration. This may have been due to the dose of lansoprazole employed in the present study. Our results suggest that repeated gastric analyses on two consecutive days to confirm efficacy of acid inhibition by lansoprazole at the time of initiation of therapy is not required and that one gastric analysis will suffice.
In a previous study (22) involving a large number of patients, a correlation was reported between the BAO, the MAO, the initial dose of omeprazole required to control acid secretion and the previous dose of histamine H2-receptor antagonist. In the present study with lansoprazole no such correlation was found. Because the studies were performed in an identical manner with omeprazole and lansoprazole in a similar patient population, it is likely this difference is due to the fact this study was too small to detect a significant correlation.
A number of studies have shown that both lansoprazole and omeprazole have a prolonged duration of action, providing an advantage over histamine H2-receptor antagonists in the management of gastric acid hypersecretory states (20–24, 33, 34, 53). In the present study, lansoprazole was also shown to have a prolonged duration of action. The mean time for acid output to reach half BAO value after cessation of therapy was 39 hr. This reflects a duration of action much longer than that demonstrated with any of the histamine H2-receptor antagonists. Using similar criteria for effective control of gastric acid secretion as in the present study, we previously demonstrated that the time for gastric acid secretion to recover to half the mean BAO after equipotent doses of cimetidine, ranitidine, and famotidine was 9.2, 10.5, and 12.5 hr, respectively (5). In contrast, the duration of action of lansoprazole is similar to that of omeprazole since, using the same criteria for the determination of the duration of action, we previously demonstrated that after cessation of therapy with omeprazole, acid output recovered to 45% of BAO by 40 hr (24). In another study using omeprazole at a standard dose of 80 mg orally in all patients, we found that the mean time for acid output to reach half the mean BAO value was 34 ± 6 hr (mean ± SEM) (34). These results suggest that lansoprazole and omeprazole have a similar duration of action, which is in contrast to the brief report by Hochlaf et al (53) in which it was suggested that lansoprazole may have a slightly shorter duration of action than does omeprazole in patients with Zollinger-Ellison syndrome.
Long-term lansoprazole was found to be safe and well tolerated. No patient had to be withdrawn from the study due to clinical side effects of therapy. The data from the present study reflect exposure to lansoprazole at higher doses and for a longer duration than previously studied in humans and confirm that lansoprazole, like omeprazole, does not affect serum transaminases, white cell count, and other routine clinical blood measurements (50).
Prolonged omeprazole can increase fasting serum gastrin concentrations in patients with idiopathic peptic ulcer disease (60, 61). However, some (21, 22, 62) but not others (25) have shown that omeprazole does not cause a further rise in gastrin values in patients with Zollinger-Ellison syndrome. Fasting serum gastrin elevation in Zollinger-Ellison syndrome is due to the gastrinoma (2, 8), and an additional increase in the presence of omeprazole therapy can be due to changes in the tumor itself or the induction of achlorhydria. In a study by Lehy et al (25), prolonged omeprazole treatment of patients with Zollinger-Ellison syndrome was associated with increased fasting serum gastrin values. It is likely that the further elevation in serum gastrin values seen in that study (25) and in ours with lansoprazole was due to the induction of achlorhydria. In the present study, a provision for dose reduction was not included because the long duration of action of lansoprazole would have required repeated dose adjustments to be made at weekly intervals. This was not logistically possible in this initial study. As a result, patients were maintained on a minimum dose of 60 mg of lansoprazole once daily. During follow-up, 3, 6, 12, and 18 months after starting therapy with lansoprazole, 18, 22, 42, and 66% of patients had been rendered achlorhydric. These data suggest that patients in the present study could likely have been maintained on lower doses of lansoprazole. Therefore, the most likely explanation for the trend towards a further increase in fasting serum gastrin values seen in the present study is the induction of achlorhydria.
These results allow a number of practical points to be made about the use of lansoprazole in patients with Zollinger-Ellison syndrome. As is the case with omeprazole (19, 24), lansoprazole should be started at a dose of 60 mg/day, with gastric acid secretion being determined the following day 1 hr before the next dose of drug. The dose should then be adjusted upward once per day until acid secretion is <10 meq/hr. It is recommended that this method be used initially rather than starting with a lower dose because of the importance of controlling gastric acid hypersecretion rapidly, because of the inability to predict final dosing in a given patient, and because this method has been shown to be safe in all patients treated with either lansoprazole in this study or with omeprazole in other studies (19, 24). Our results suggest that a number of patients could likely be maintained on lower lansoprazole doses. Because of its long duration of action, similar to that of omeprazole (24, 34), acute dose reductions are not interpretable. Therefore, it is recommended that once the initial maintenance dose for a given patient is established, the lansoprazole dose be decreased one to two weeks prior to each clinic visit. During this period clinical symptoms should be carefully monitored and acid secretion should ·be measured at the time of the visit. Because of its long duration of action, lansoprazole dose reductions should not be attempted more often than once every one to two weeks.
In conclusion, our study demonstrates that lansoprazole is at least as potent as omeprazole for inhibition of gastric acid secretion. It has a prolonged duration of action that is similar to that of omeprazole, and it is at least as effective in the short- and long-term management of the gastric acid hypersecretion associated with Zollinger-Ellison syndrome. Furthermore, prolonged administration of lansoprazole in these patients is safe. These results suggest that lansoprazole, like omeprazole, will be effective and useful in patients with Zollinger-Ellison syndrome.
REFERENCES
- 1.Zollinger RM, Ellison EH: Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg 142:709–728, 1955 [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen RT, Maton PN: Zollinger-Ellison syndrome. In The Stomach Gustavsson S, Kumar D, Graham DY (eds). London, Churchill Livingstone, 1991, pp 341–374 [Google Scholar]
- 3.Collen MJ, Howard JM, McArthur KE, Raufman J-P, Cornelius MJ, Ciarleglio CA, Gardner JD, Jensen RT: Comparison of ranitidine and cimetidine in the treatment of gastric hypersecretion. Ann Intern Med 100:52–58, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Jensen RT, Collen MJ, McArthur KE, Howard JM, Maton PN, Cherner JA, Gardner JD: Comparison of the effectiveness of ranitidine and cimetidine in inhibiting acid secretion in patients with gastric hypersecretory states. Am J Med 77:90–105, 1984 [PubMed] [Google Scholar]
- 5.Howard JM, Chremos AN, Collen MJ, McArthur KE, Cherner JA, Maton PN, Ciarleglio CA, Cornelius MJ, Gardner JD, Jensen RT: Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: Comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology 88:1026–1033, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Vinayek R, Howard JM, Maton PN, Wank SA, Slaff JL, Gardner JD, Jensen RT: Famotidine in the therapy of gastric hypersecretory states. Am J Med 81(suppl 4B):49–59, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Maton PN, Gardner JD, Jensen RT: Recent advances in the management of gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterol Clin North Am 18:847–863, 1989 [PubMed] [Google Scholar]
- 8.Jensen RT, Doppman JL, Gardner JD: Gastrinoma. In The Exocrine Pancreas: Biology, Pathobiology, and Diseases Go VLW, Gardner JD, Brooks FP, Lebenthal E, Dimagno EP, Scheele G (eds). New York, Raven Press, 1986, pp 727–745 [Google Scholar]
- 9.Jensen RT, Gardner JD, Raufman JP, Pandol SJ, Doppman JL, Collen MJ: Zollinger-Ellison syndrome. NIH Combined Clinical Staff Conference. RT Jensen (moderator). Ann Intern Med 98:59–75, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Jensen RT: Basis for failure of cimetidine in patients with Zollinger-Ellison syndrome. Dig Dis Sci 29:363–366, 1984 [DOI] [PubMed] [Google Scholar]
- 11.McCarthy DM: Report on the United States experience with cimetidine in Zollinger-Ellison syndrome and other hypersecretory states. Gastroenterology 74:453–458, 1978 [PubMed] [Google Scholar]
- 12.McCarthy DM, Hyman PE: Effect of isopropamide on response to oral cimetidine in patients with Zollinger-Ellison syndrome. Dig Dis Sci 27:353–359, 1982 [DOI] [PubMed] [Google Scholar]
- 13.Richardson CT, Walsh JH: The value of histamine H2-receptor antagonists in management of patients with Zollinger-Ellison syndrome. N Engl J Med 294:131–135, 1976 [DOI] [PubMed] [Google Scholar]
- 14.Maton PN, Frucht H, Vinayek R, Wank SA, Gardner JD, Jensen RT: Medical management of patients with ZollingerEllison syndrome who have had previous gastric surgery: A prospective study. Gastroenterology 94:294–299, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Miller LS, Vinayek H, Frucht H, Gardner JD, Jensen RT, Maton PN: Reflux esophagitis in patients with ZollingerEllison syndrome. Gastroenterology 98:341–346, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Jensen RT, Gardner JD: Zollinger-Ellison syndrome. Clinical presentation, pathology, diagnosis and treatment. In Peptic Ulcer and Other Acid-Related Diseases Dannenberg A, Zakim D (eds). New York, Academic Research Assoc., 1991, pp 117–212 [Google Scholar]
- 17.Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjostrand SE, Wallmark B: Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+/K+) ATPase. Nature 290:159–61, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Clissold SP, Campoli-Richards DM: Omeprazole. Drugs 32:15–47, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Frucht H, Maton PN, Jensen RT: Use of omeprazole in patients with Zollinger-Ellison syndrome. Dig Dis Sci 36:394–404, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Lamers CBHW, Lind T, Moberg S, Jansen JBMJ, Olbe L: Omeprazole in Zollinger-Ellison syndrome. Effects of a single dose and of long-term treatment in patients resistant to histamine Hz-receptor antagonists. N Engl J Med 310:758–761, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Davies KA, Rutgersson K, Solvell L: Omeprazole in the treatment of Zollinger-Ellison syndrome: A 4-year international study. Aliment Pharmacol Ther 2:13–32, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Maton PN, Vinayek R, Frucht H, McArthur KA, Miller LS, Saeed ZA, Gardner JD, Jensen RT: Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: A prospective study. Gastroenterology 97:827–836, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Hirschowitz BI, Deren J, Raufman JP, LaMont B, Berman R, Humphries T: A multicenter US study of omeprazole treatment of Zollinger-Ellison syndrome (ZES). Gastroenterology 94:A188, 1988 [Google Scholar]
- 24.McArthur KE, Collen MJ, Maton PN, Cherner JA, Howard JM, Ciarleglio CA, Cornelius MJ, Jensen RT, Gardner JD: Omeprazole: Effective, convenient therapy for ZollingerEllison syndrome. Gastroenterology 88:939–944, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Lehy T, Mignon M, Cadiot G, Elouaer-Blanc L, Ruszniewski P, Levin MJM, Bonfils S: Gastric endocrine cell behavior in Zollinger-Ellison patients upon long-term antisecretory treatment. Gastroenterology 96: 1029–1040, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Blanchi A, Delchier JC, Soule JC, Payen D, Bader JP: Control of acute Zollinger-Ellison syndrome with intravenous omeprazole. Lancet 2:1223–1224, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Oberg K, Lindstrom H: Reduction of gastric hypersecretion in Zollinger-Ellison syndrome with omeprazole. Lancet 1:66–67, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Vezzadini P, Tomassetti P, Toni R, Bonora G, Labo G: Omeprazole in the medical treatment of Zollinger-Ellison syndrome. Curr Ther Res 35:772–776, 1984 [Google Scholar]
- 29.Delchier JC, Soule JC, Mignon M, Goldfain D, Cortot A, Travers B, Isal JP, Bader JP: Effectiveness of omeprazole in seven patients with Zollinger-Ellison syndrome resistant to histamine H2-receptor antagonists. Dig Dis Sci 31:693–699, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Bardram L, Stadil F: Omeprazole in the Zollinger-Ellison syndrome. Scand J Gastroenterol 21:374–378, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Corleto J, Puoti M, Annibale B, Saggioro A, D’Ambra G, DiPaola M, Della Fave G: Loss of efficacy of famotidine (FMT) in the control of gastric acid secretion in patients with Zollinger-Ellison syndrome (ZES) reversed by omeprazole (OMP). Gastroenterology 94:A79, 1988 [Google Scholar]
- 32.Vezzadini P, Tomassetti P, Marrano D, Labo G: Life threatening gastrointestinal hemorrhage with omeprazole. Dig Dis Sci 33:766–767, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Vinayek R, Frucht H, London JF, Miller LS, Stark HA, Norton JA, Cederberg C, Jensen RT, Gardner JD, Maton PN: Intravenous omeprazole in patients with ZollingerEllison syndrome undergoing surgery. Gastroenterology 99:10–16, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Vinayek R, Amantea MA, Maton PN, Frucht H, Gardner JD, Jensen RT: Pharmacokinetics of oral and intravenous omeprazole in patients with Zollinger-Ellison syndrome. Gastroenterology 101:138–147, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Kubo K, Oda K, Kaneko T, Satoh H, Nohara A: Synthesis of 2-{[(4-fiuoroalkoxy-2-pyridyl)methyl]sulfinyl}-lH-benzimidazoles as antiulcer agents. Chem Pharm Bull (Tokyo) 38:2853–2858, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Iwahi T, Satoh H, Nakao M, Iwasaki T, Yamazaki T, Kubo K, Tamura T, Imada A: Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against helicobacter pylori. Antimicrobiol Agents Chemother 35:490–496, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh H, Inatomi N, Nagaya H, Inada I, Nohara A, Maki Y: Antisecretory activity of AG1749, a proton pump inhibitor. Jpn J Pharmacol 40(suppl):226P, 1986 [Google Scholar]
- 38.Satoh H, Inatomi N, Nagaya H, Inada I, Nohara A, Nakamura N, Maki Y: Antisecretory and antiulcer activities of a novel proton pump inhibitor AG-1749 in dogs and rats. J Pharmacol Exp Ther 248:806–815, 1989 [PubMed] [Google Scholar]
- 39.Nagaya H, Satoh H, Maki Y: Possible mechanism for the inhibition of acid formation by the proton pump inhibitor AG 1749 in isolated canine parietal cells. J Pharmacol Exper Ther 252:1289–1295, 1990 [PubMed] [Google Scholar]
- 40.Inatomi N, Satoh H, Nagaya H, Inada I, Sino A, Maki Y: Antiulcer activity of AG-1749, a proton pump inhibitor. Jpn J Pharmacol 43(suppl):233P, 1987 [Google Scholar]
- 41.Müller P, Dammann HG, Leucht U, Simon B: Human gastric acid secretion following repeated doses of AG-1749. Aliment Pharmacol Ther 3:193–198, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Dammann HG, Simon B, Müller P: 24 hour intragastric acidity under AG 1749—a newly developed proton pump inhibitor. Gastroenterology 96:Al08, 1989 [Google Scholar]
- 43.Sanders SW, Tolman KG, Sikora PA, Jennings DE, Hoyos PA, Page JG: Comparison of gastric pH following multiple oral AM and PM dosing of a new H+,K+-ATPase inhibitor, further evidence for diurnal variation in gastric acid secretion. Pharmacotherapy 8:141, 1988 [Google Scholar]
- 44.Kihara K, Yoshida Y, Hirose M, Kasano T, Sato Y, Kimura K: A study of the inhibition of gastric pH after the morning and evening administration of AG 1749. Nissho Shi 85:2693, 1988. (in Japanese) [Google Scholar]
- 45.Hawkey CJ, Bardhan KD, Long RG, Wormsley KG, Cochran RM, Christian J, Moules I: Improved symptom relief and duodenal ulcer healing with lansoprazole compared to ranitidine. Gastroenterology 100:A80, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson MG, Campbell DR, Sontag S, Sabesin SM, Greski PA, Jennings DE, Guy JM: Lansoprazole heals H2 resistant erosive reflux esophagitis. Gastroenterology 98:A113, 1990 [Google Scholar]
- 47.Stanescu L, Soule J-C, Robaskiewicz M, Goveroo H, Lemaire M: Effect of lansoprazole (L) and ranitidine (R) on esophageal acid over 24 hour periods in patients with symptomatic esophagitis. Gastroenterol Clin Biol 14:Al08, 1990 [Google Scholar]
- 48.Petite JP, Salducci J, Evreux M, Lemaire M: Lansoprazole (L) vs ranitidine (R) in the treatment of peptic esophagitis. Gastroenterol Clin Biol 14:Al08, 1990 [Google Scholar]
- 49.Antonson CW, Robinson MG, Hawkins TM, McIntosh DL, Campbell DR: High doses of histamine antagonists do not prevent relapses of peptic esophagitis following therapy with a proton pump inhibitor. Gastroenterology 98:Al6, 1990 [Google Scholar]
- 50.Bardhan KD, Long R, Hawkey CJ, Wormsley KG, Brocklebank D; Moules I: Lansoprazole, a new proton pump blocker, vs ranitidine in the treatment of reflux erosive esophagitis. Gastroenterology 100:A30, 1991 [Google Scholar]
- 51.Sharma HK, Daneshmend TK, Hawthorne AB, Bhaskar NK, Hawkey CJ: Human gastric mucosal protection by AG 1749, a new potent proton pump inhibitor. Gastroenterology 96:A464, 1989 [Google Scholar]
- 52.Bigard MA, Joubert M, DeMeynard C: Complete prevention by lansoprazole of aspirin induced gastric lesions in healthy subjects. Gastroenterology 100:A34, 1991 [Google Scholar]
- 53.Hochlaf S, Vatier J, Ruszniewski CH, Poiterin MJM, Mignon M: Is lansoprazole as effective as omeprazole in patients with Zollinger-Ellison syndrome (ZES)? Gastroenterology 100:A24, 1991 [Google Scholar]
- 54.Frucht H, Howard JM, Slaff JI, McCarthy DM, Maton PN, Wank SA, Vinayek R, Gardner JD, Jensen RT: Secretin and calcium provocative tests in Zollinger-Ellison syndrome. Ann Intern Med 111:713–722, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Raufman J-P, Collins SM, Pandol SJ, Korman LY, Collen MJ, Cornelius MJ, Feld MK, McCarthy DM, Gardner JD, Jensen RT: Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology 84:108–113, 1983 [PubMed] [Google Scholar]
- 56.London JF, Shawker TH, Doppman JL, Miller DL, Frucht H, Vinayek R, Stark HA, Miller LS, Norton JA, Jensen RT, Gardner JD, Maton PN: Prospective assessment of abdominal ultrasound in patients with Zollinger-Ellison syndrome. Radiology 178:763–767, 1991 [DOI] [PubMed] [Google Scholar]
- 57.Wank SA, Doppman JL, Miller DL, Collen MJ, Maton PN, Vinayek R, Norton JA, Gardner JD, Jensen RT: Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology 92:905–912, 1987 [DOI] [PubMed] [Google Scholar]
- 58.Frucht H, Doppman JL, Norton JA, Miller DL, Dwyer AJ, Frank JA, Vinayek R, Maton PN, Jensen RT: Gastrinomas: Comparison of MR imaging with CT, angiography and US. Radiology 171:713–717, 1989 [DOI] [PubMed] [Google Scholar]
- 59.Doppman JL, Miller DL, Chang R, Maton PN, London JF, Frucht H, Gardner JD, Jensen RT, Norton JA: Gastrinomas: Localization by means of selective intraarterial injection of secretin. Radiology 174:25–29, 1990 [DOI] [PubMed] [Google Scholar]
- 60.Lanzon-Miller S, Pounder RE, Hamilton MR, Ball S, Chronos NAF, Olausson FRM, Cederberg C: Twenty-four hour intragastric acidity and plasma gastrin concentrations before and during treatment with either ranitidine or omeprazole. Aliment Pharmacol Ther 1:239–251, 1987 [DOI] [PubMed] [Google Scholar]
- 61.Valenzuela JE, Berlin RG, Snape WJ, Johnson TL, Hirschowitz BI, Colon-Pagan J, Morse RS, ṕetrozza J, Van Deventer GM, Cagliola A, Whipple J, Berman R, Humphries TJ, and the Omeprazole DU Comparative Study Group: US experience with omeprazole in duodenal ulcer—multicenter double-blind comparative study with ranitidine. Dig Dis Sci 36:761–768, 1991 [DOI] [PubMed] [Google Scholar]
- 62.Maton PN, Lack EE, Collen MJ, Cornelius MJ, David E, Gardner JD, Jensen RT: The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology 99:943–950, 1990 [DOI] [PubMed] [Google Scholar]