Abstract
Large-conductance calcium-activated potassium (BK) channels are ubiquitously expressed in most cell types where they regulate many cellular, organ, and organismal functions. Although BK currents have been recorded specifically in activated murine and human microglia, it is not yet clear whether and how the function of this channel is related to microglia activation. Here, using patch-clamping, Griess reaction, ELISA, immunocytochemistry, and immunoblotting approaches, we show that specific inhibition of the BK channel with paxilline (10 μm) or siRNA-mediated knockdown of its expression significantly suppresses lipopolysaccharide (LPS)-induced (100 ng/ml) BV-2 and primary mouse microglial cell activation. We found that membrane BK current is activated by LPS at a very early stage through Toll-like receptor 4 (TLR4), leading to nuclear translocation of NF-κB and to production of inflammatory cytokines. Furthermore, we noted that BK channels are also expressed intracellularly, and their nuclear expression significantly increases in late stages of LPS-mediated microglia activation, possibly contributing to production of nitric oxide, tumor necrosis factor-α, and interleukin-6. Of note, a specific TLR4 inhibitor suppressed BK channel expression, whereas an NF-κB inhibitor did not. Taken together, our findings indicate that BK channels participate in both the early and the late stages of LPS-stimulated murine microglia activation involving both membrane-associated and nuclear BK channels.
Keywords: lipopolysaccharide (LPS), microglia, neuroinflammation, NF-κB (NF-KB), Toll-like receptor 4 (TLR4), BK channel, cytokine, innate immunity
Introduction
Large-conductance calcium-activated potassium (BK)3 channels play pivotal roles in many physiological and pathological processes by coupling cell membrane potential to intracellular calcium concentration (1–5). Abnormal BK channel function has been associated with a number of central nervous system disorders such as epilepsy, ataxia, mental retardation, and chronic pain (5). Until now, the manner in which BK channel function is related to these disorders has mainly been studied in neurons. Little is yet known about the role of the BK channel in microglia, which are the resident macrophages in brain and are considered important target cells for therapeutic intervention in the above neurologic diseases (6, 7). Some potassium channels, mainly voltage-gated potassium channel (KV1.3) and intermediate-conductance calcium-activated potassium channel (KCa3.1), have been reported to regulate a variety of microglia functions, including proliferation, migration, cytokine release, and reactive oxygen species production. Both channels are suggested as therapeutic targets for the suppression of detrimental microglia functions (8). The elucidation of whether and how BK channels affect microglia function could guide the development of new therapies for these neurologic disorders.
In the 1990s, BK currents were recorded in excised inside-out patches from cultured resting bovine and human microglia; however, these currents were not activated under normal whole-cell recording conditions (9, 10). These observations indicate a minor role for BK channel function in resting microglia. Later, Bordey and Spencer (11) found that activated microglia in hippocampal slices from seven patients who had undergone surgery for pharmacoresistant epilepsy expressed BK currents that could be enhanced 3.3-fold by chemokine macrophage inflammatory protein 1-α in whole-cell patch recordings. Schilling and Eder (12, 13) detected BK currents in microglia from brain slices of juvenile, young adult, and aged mice. BK currents were exclusively recorded in activated microglia of brain slices from juvenile mice and were up-regulated in microglia of brain slices from young adult and aged mice, whereas no significant difference was found in the mean BK current density of microglia between the young adult and aged mice (12, 13). These observations suggest a possible role for BK channels in microglia activation and indicate that the BK channel might not affect aging-related phenotype changes in microglia. Hayashi et al. (14) proposed blockade of BK channels in spinal microglia as the underlying mechanism of suppression of nerve injury-induced hyperactivation and migration of spinal microglia by S-ketamine, an important analgesic. Furthermore, the specific BK channel activator NS1619 could activate spinal microglia in naïve mice, whereas pharmacological blockade or the genetic deletion of BK channels in microglia prevented morphine-induced production of inflammatory factors (15). These results implied that BK channels are involved in microglia activation; however, the mechanisms underlying the contribution of microglial BK channels to cellular activation has remained unclear.
Previously, we observed that either BK knockout or inhibition affects murine microglia function (16). This study was designed to investigate the possible role and underlying mechanism of BK channels in microglia activation with lipopolysaccharide (LPS) as the pro-inflammatory stimulus. Both plasma membrane (PM) and nuclear BK channels were found to participate in microglia activation, although at different stages and through different pathways.
Results
BK channels are involved in LPS-induced activation of BV-2 cells
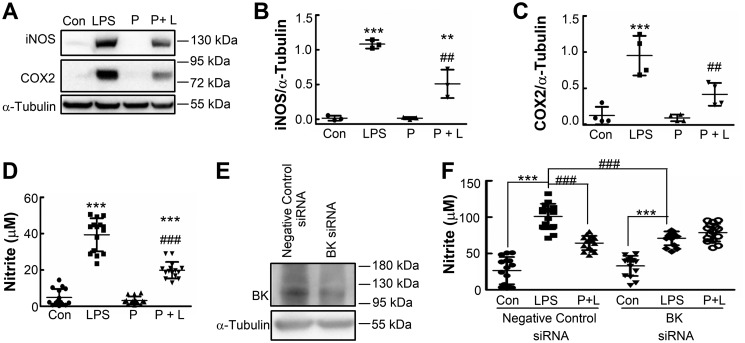
To test whether BK channels are involved in microglia activation, we first investigated the effect of the specific BK channel blocker paxilline on NO production and inducible nitric-oxide synthase (iNOS) and cyclooxygenase (COX2) expression in BV-2 cells with or without LPS stimulation (Fig. 1, A–D). Paxilline was applied 30 min prior to LPS treatment, which significantly inhibited NO production and iNOS and COX2 expression in BV-2 cells without affecting cell viability (data not shown). However, paxilline itself had no effect on resting BV-2 cells. We further knocked down BK channels using a BK-targeted siRNA oligonucleotide (Fig. 1E) and measured NO production induced by LPS in the absence or presence of paxilline. Compared with BV-2 cells transfected with negative control (NC) siRNA, BK knockdown BV-2 cells showed similar basal NO synthesis (33.0 ± 13.8 μm compared with 26.5 ± 18.7 μm, p > 0.05) and significantly decreased NO production (71.0 ± 9.5 μm compared with 101.0 ± 17.6 μm, p < 0.001) upon treatment with LPS. Preincubation with paxilline significantly inhibited LPS-induced NO production in BV-2 cells transfected with NC siRNA but did not inhibit LPS-induced NO production in BV-2 cells transfected with BK siRNA (Fig. 1F). These results suggest that BK channels are involved in LPS-induced microglia activation. We observed that basal NO levels in transfected BV-2 cells were much higher than those in nontransfected cells (26.5 ± 18.7 μm compared with 4.8 ± 4.7 μm), which might have been due to the transfection procedure.
Figure 1.
Blockade or knockdown of BK channels inhibits LPS-induced activation of BV-2 cells. A–C, BV-2 cells were left untreated (Con) or were treated with LPS (LPS, 100 ng/ml), with paxilline (P, 10 μm) for 24 h, or with paxilline (10 μm) 30 min prior to LPS (100 ng/ml) for 24 h (P + L); cell lysates were immunoblotted with antibodies against iNOS or COX2 (A). Quantification was performed by densitometric analysis of protein bands and normalized to α-tubulin levels. Data are presented as scatter plots; bars represent mean ± S.D. of three (iNOS, B) or 4 (COX2, C) independent experiments, each performed once. Differences among the groups were analyzed by two-way ANOVA with Bonferroni's multiple comparisons post-hoc test (B and C). F(1,8) = 155.61 (p < 0.0001) for LPS; F(1,8) = 20.86 (p = 0.0018) for paxilline; F(1,8) = 20.78 (p = 0.0019) for interaction (B); F(1,12) = 45.61 (p < 0.0001) for LPS; F(1,12) = 11.02 (p = 0.0061) for paxilline; F(1,12) = 8.67 (p = 0.0123) for interaction (C). **, p < 0.01; ***, p < 0.001 compared with the control group; ##, p < 0.01 compared with the LPS group. D, BV-2 cells were treated in the same manner as in A, and supernatants were analyzed for nitrites. Data are presented as a scatter plot; bars represent mean ± S.D. of three independent experiments, each performed in quintuplicate. Differences among the groups were analyzed by two-way ANOVA with Bonferroni's multiple comparisons post-hoc test (F(1,56) = 305.83 (p < 0.0001) for LPS; F(1,56) = 51.99 (p < 0.0001) for paxilline; F(1,56) = 37.21 (p < 0.0001) for interaction). ***, p < 0.001 compared with the control group; ###, p < 0.001 compared with the LPS group. E and F, BV-2 cells were transfected for 24 h with either negative control siRNA oligonucleotides or siRNA oligonucleotides targeting BK mRNA. Transfected cells were either harvested to prepare protein extracts for immunoblotting with anti-BK antibody or anti-α-tubulin antibody (E), or they were further untreated (Con) or treated with LPS (LPS, 100 ng/ml) for 24 h or paxilline (10 μm) 30 min prior to LPS (100 ng/ml) for 24 h (P + L), and then supernatants were analyzed for nitrites (F). Data are presented as a scatter plot; bars represent mean ± S.D. of three independent experiments, each performed in quintuplicate. Differences among the groups were analyzed by two-way ANOVA with Bonferroni's multiple comparisons post hoc test (F). F(2,84) = 129.74 (p < 0.0001) for the type of drug (Con, LPS, P + L); F(1,84) = 1.047 (p = 0.309) for the type of siRNA; F(2,84) = 21.33 (p < 0.0001) for interaction between drug and siRNA. ***, p < 0.001 compared with the control group; ###, p < 0.001 compared with the LPS group.
Membrane BK current is stimulated by LPS to facilitate NF-κB translocation in BV-2 cells
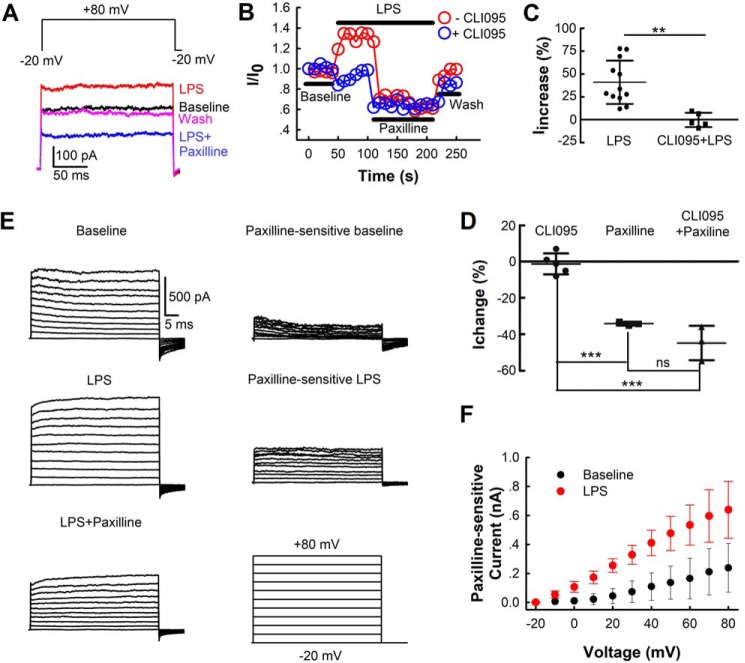
To confirm whether LPS could activate BK currents in BV-2 cells, we next examined the effects of LPS on membrane currents. A whole-cell patch clamp configuration was used to record membrane currents of BV-2 cells. The cells were held at −20 mV and depolarized to +80 mV for 200 ms to stimulate BK currents. The +80-mV pulse was repeated every 10 s, and membrane currents were recorded. The resting membrane potential of BV-2 cells is −71 ± 5 mV (n = 43 cells), which is not affected by LPS (Fig. S1). LPS (100 ng/ml) significantly enhanced membrane current (41.0 ± 23.7%), and its effect reached a plateau within 1 min. The subsequent application of paxilline completely inhibited LPS-induced membrane current and further reduced the current to 41.6 ± 22.9% lower than the initial baseline current, indicating that LPS activates the membrane BK current of BV-2 cells (Fig. 2, A–C). Because the membrane-spanning Toll-like receptor 4 (TLR4) is essential for LPS-initiated transmembrane signaling and microglia activation, we then measured the effect of LPS on BV-2 cells in the presence of the TLR4 antagonist CLI095 (2 μm). We found that LPS failed to increase membrane current under CLI095 treatment (Fig. 2, B and C), while CLI095 itself had no effect on membrane currents (Fig. 2D). Furthermore, we measured whole-cell membrane currents at voltages ranging from −20 to +80 mV and used paxilline-sensitive fractions as BK currents (Fig. 2, E and F). The paxilline-sensitive baseline currents showed a similar current-voltage (IV) relation with BK currents of microglia in juvenile mice hippocampal slices (12). LPS leftward-shifted the IV curve of BK channels in BV-2 cells (Fig. 2F).
Figure 2.
Effects of LPS on membrane BK currents in BV-2 cells. A, exemplar whole-cell current traces recorded before (baseline) and during application of LPS (100 ng/ml), LPS (100 ng/ml) + paxilline (10 μm), and washing out (wash). Currents were elicited by voltage of +80 mV for 200 ms. The prepulse potential was −20 mV for 20 ms (only the last 0.5 ms are shown), and the repolarization potential was −20 mV. B, time courses of activation of whole-cell current by LPS in the absence or presence of the TLR4 inhibitor CLI095 (2 μm) at +80 mV from two individual whole-cell patches. The steady-state currents before (baseline) and during application of LPS (100 ng/ml), LPS (100 ng/ml) + paxilline (10 μm), and washing out (wash) normalized to the steady-state current of initial baseline (I/I0) were used to plot the traces. Currents were recorded every 10 s. C and D, percentage of whole-cell steady-state currents changed upon various treatments at +80 mV (LPS (C, n = 12 cells from five independent experiments), CLI095 + LPS (C, n = 5 cells from three independent experiments), CLI095 (D, n = 5 cells from two independent experiments), paxilline (D, n = 3 cells from three independent experiments), and CLI095 + paxilline (D, n = 3 cells from two independent experiments)). The steady-state current of average baseline was used to calculate the percentages of current change. Data are presented as means ± S.D. Differences among the groups were analyzed by two-tailed unpaired Student's t tests (C) or one-way ANOVA with Bonferroni's multiple comparisons post hoc test (F(2,8) = 53.13 (p < 0.0001)) (D). **, p < 0.01; ***, p < 0.001; ns = not significant. E left, macroscopic current traces from one whole-cell patch recorded before (baseline) and after LPS (100 ng/ml), LPS (100 ng/ml) + paxilline (10 μm) treatment. Currents were elicited by voltages ranging from −20 to +80 mV for 50 ms with 10-mV increments. The prepulse potential was −20 mV for 20 ms (only the last 0.5 ms shown), and the repolarization potential was −20 mV. Right, paxilline-sensitive baseline (baseline − (LPS + paxilline)) and paxilline-sensitive LPS (LPS − (LPS + paxilline)) currents. F, I-V relations of paxilline-sensitive membrane currents in BV-2 cells before (baseline) and after LPS application. The means of steady-state currents from four individual whole-cell patches were plotted against relative voltages. Bars represent S.D.
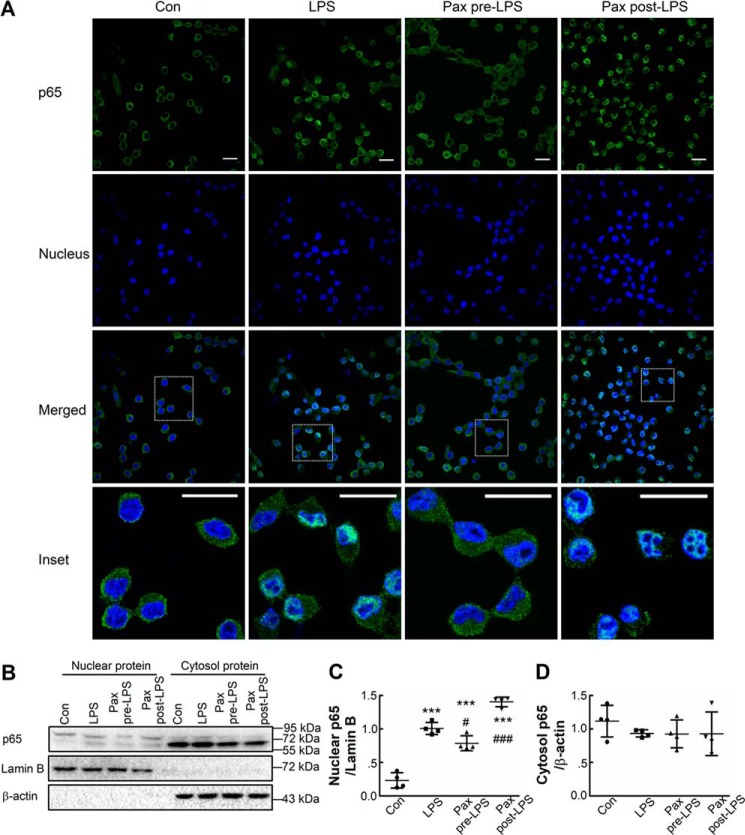
Because the key event in LPS-induced microglia activation is NF-κB translocation, we wondered whether activation of the membrane BK channel regulates NF-κB translocation. BV-2 cells were left untreated or were treated with LPS in the absence or presence of paxilline for 1 h before staining. Incubation for 1 h was chosen because almost all BV-2 cells show strong nuclear p65 staining at this time point (Fig. 3A). In control cells, green p65 staining only appeared in cytosolic compartments, and in cells treated with LPS, p65 staining was observed in condensed nuclei. The application of paxilline 30 min prior to LPS treatment significantly inhibited p65 translocation; cells under this condition showed much less nuclear p65 staining compared with cells treated with LPS alone. However, paxilline failed to prevent p65 translocation if applied 30 min after LPS treatment (Fig. 3A), indicating that membrane BK channel activation promotes NF-κB translocation. These results were further confirmed by immunoblot experiments. As shown in Fig. 3, LPS induced higher nuclear p65 quantity in BV-2 cells and application of paxilline preincubation rather than postincubation inhibited this LPS-induced elevation of nuclear p65 (Fig. 3, B and C). However, there appeared to be no significant differences in cytosolic p65 levels among these four treatment groups (Fig. 3, B and D).
Figure 3.
Pre- rather than post-inhibition of BK channels prevents LPS-induced NF-κB translocation in BV-2 cells. A, BV-2 cells were untreated (Con) or treated with LPS (LPS, 100 ng/ml) for 1 h or with paxilline (10 μm) 30 min prior to (Pax pre-LPS) or 30 min after (Pax post-LPS) LPS (100 ng/ml) for 1 h and were then fixed and stained with anti-p65 antibody (green, p65) and Hoechst 33258 (blue, nucleus), and cells were analyzed using confocal fluorescence microscopy. Insets are enlarged views of the selected rectangle areas. Scale bar, 20 μm. B–D, BV-2 cells were treated in the same manner as in A. Nuclear and cytosolic proteins were extracted and immunoblotted with antibodies against p65, lamin B, or β-actin (B). Quantification was performed by densitometric analysis of protein bands normalized to either lamin B (nuclear p65, C) or β-actin levels (cytosol p65, D). Data are presented as scatter plots; bars represent mean ± S.D. of four independent experiments, each performed once. Differences among the groups were analyzed by one-way ANOVA with Bonferroni's multiple comparisons post hoc test (C and D). F(3,12) = 98.14 (p < 0.0001) for C; F(3,12) = 0.6778 (p = 0.5822) for D.,***, p < 0.001 compared with the control group. #, p < 0.05; ###, p < 0.001 compared with the LPS group.
Blockade of BK channels after NF-κB translocation also represses BV-2 cell activation
To test whether the NF-κB translocation induced by membrane BK channel activation is the primary mechanism by which BK channels participate in microglia activation, we further examined the effect of paxilline on LPS-induced NO production in BV-2 cells at different time points. We found that 30 min of preincubation in paxilline decreased NO production due to LPS treatment by ∼50% (from 47.5 ± 7.0 to 22.1 ± 3.1 μm, p < 0.001). With application of paxilline at 30 min (Pax post-LPS 30 min), 1 h (Pax post-LPS 1 h), and even 3 h (Pax post-LPS 3 h) after LPS application and while it did not reverse NF-κB translocation, paxilline could still significantly inhibit NO production by ∼30%, from 47.5 ± 7.0 μm in LPS (no paxilline) to 33.3 ± 4.6 μm in Pax post-LPS 30 min (p < 0.001), 33.3 ± 4.5 μm in Pax post-LPS 1 h (p < 0.001), and 35.2 ± 2.8 μm in Pax post-LPS 3 h (p < 0.01). Paxilline had no effect after 6 h of LPS treatment (Fig. 4A). Moreover, when applied 30 min after LPS incubation, paxilline also significantly suppressed both tumor necrosis factor-α (TNFα) and interleukin-6 (IL-6) release, although to a slightly lesser extent than upon preincubation with paxilline (Fig. 4, C and D). These results suggest that BK channels can also modulate cytokine production through an NF-κB–independent pathway that might perform a more important role.
Figure 4.
Pre- or post-block of BK channels suppresses LPS-induced activation of BV-2 cells. A and B, BV-2 cells were untreated (Con) or treated with LPS (LPS, 100 ng/ml) for 24 h; paxilline (10 μm) for 30 min prior to (Pax Pre-LPS); or at 30 min (Pax post-LPS 30 min), 1 h (Pax post-LPS 1 h), 3 h (Pax post-LPS 3 h), 6 h (Pax post-LPS 6 h), or 12 h (Pax post-LPS 12 h) after LPS (100 ng/ml) during the 24-h LPS incubation. The supernatants were analyzed for nitrites (A), and cells were analyzed for viability using the MTT assay (B). Data are presented as scatter plots. Each point represents one determination, and the bars represent mean ± S.D. n = 6 from two independent experiments (each performed in triplicate) for control (Con) and LPS; n = 7 from two independent experiments (one performed in triplicate and the other in quadruplicate) for Pax pre-LPS and Pax post-LPS. Differences among the groups were analyzed by one-way ANOVA with Bonferroni's multiple comparisons post hoc test (A and B). F(7,46) = 44.02 (p < 0.0001) for A; F(7,46) = 2.182 (p = 0.0534) for B. ***, p < 0.001 compared with the control group. ##, p < 0.01; ###, p < 0.001 compared with the LPS group. $$, p < 0.01; $$$, p < 0.001 compared with the Pax Pre-LPS group. C and D, BV-2 cells were untreated (Con) or treated with LPS (LPS, 100 ng/ml) for 24 h, paxilline (10 μm) 30 min prior to (Pax Pre-LPS), or 30 min after (Pax post-LPS) LPS (100 ng/ml) for 24 h. The supernatants were analyzed for TNFα (C) and IL-6 (D). Data are presented as scatter plots. Each point represents one determination, and the bars represent mean ± S.D. of two independent experiments, each performed in duplicate. Differences among the groups were analyzed by one-way ANOVA with Bonferroni's multiple comparisons post hoc test (C) or one-way ANOVA with Newman-Keuls' multiple comparisons post hoc test (D). F(3,12) = 381.7 (p < 0.0001) for C; F(3,12) = 56.72 (p < 0.0001) for D. ***, p < 0.001 compared with the control group. ###, p < 0.001 compared with the LPS group. $, p < 0.05 compared with the Pax Pre-LPS group.
Nuclear BK channel expression is induced by LPS in late stages of BV-2 cell activation
Next, we explored the expression and distribution of BK channels in BV-2 cells stimulated with LPS. We observed that BK channels were expressed not only on cell membranes but were also expressed in intracellular compartments, especially in late stages of LPS treatment. Fig. 5A showed that the expression of BK channels increased with the increased duration of LPS treatment. BK channels resided mainly on the cell membrane in control cells but showed strong nuclear localization after 12 h of LPS treatment. Fig. 5, B and C, showed that the expression of BK channels in whole cells increased with the duration of LPS treatment and that nuclear BK channel expression increased significantly after 12 h of LPS treatment. Moreover, the observed increase in BK channel expression was not inhibited by the specific NF-κB inhibitor Bay11-7082 but was inhibited by the TLR4 inhibitor CLI-095 (2 μm, Fig. 5D). This result suggests that the enhanced expression of nuclear BK channels is not a downstream event of NF-κB activation but is independent of the activation of membrane BK channels and might contribute to NO and cytokine production in the late stages of microglia activation.
Figure 5.
Nuclear BK channel expression is induced by LPS in BV-2 cells. A, BV-2 cells were treated with LPS (100 ng/ml) for 0, 6, 12, or 24 h, fixed, and immunostained with anti-BK antibody (green, BK) and Hoechst 33258 (blue, nucleus), and then were analyzed by confocal fluorescence microscopy. Scale bar, 40 μm. Insets are enlarged views of the selected rectangle areas, and scale bar represents 20 μm. B and C, nuclear, cytosolic, and whole-cell proteins were extracted and immunoblotted. Quantification performed by densitometric analysis of protein bands was normalized to either lamin B (nuclear BK, C left) or β-actin levels (cytosol BK, C middle; whole-cell BK, C right). D, BV-2 cells were untreated (control) or treated with LPS (100 ng/ml) for 12 h in the absence or presence of either Bay11-7082 (20 μm, specific NF-κB inhibitor) or CLI095 (2 μm, specific TLR4 inhibitor). Quantification performed by densitometric analysis of protein bands was normalized to β-actin levels. Data are presented as scatter plots; bars represent mean ± S.D. of five (Nuclear BK, C left; cytosol BK, C middle; and whole BK, D right) or three (whole BK, C right) independent experiments, each performed once. Differences among the groups were analyzed by one-way ANOVA with Newman-Keuls' multiple comparisons post hoc test (C and D). F(3,16) = 13.15 (p = 0.0001) for C left, Nuclear BK; F(3,16) = 1.694 (p = 0.2082) for C middle, Cytosol BK; F(3,8) = 6.723 (p = 0.0141) for C right, whole BK; F(3,16) = 4.894 (p = 0.0134) for D. *, p < 0.05; ***, p < 0.001 compared with the control group. #, p < 0.05 compared with the LPS group.
BK channels also modulate activation of primary mouse microglia by LPS
We further investigated the role of BK channels in primary microglia activation. Primary mouse microglia were isolated and were either untreated, treated with paxilline before or after LPS stimulation, or treated with paxilline alone. As shown in Fig. 6, A–C, the inhibition of BK channels by paxilline significantly repressed NO, IL-6, and TNFα release, whether paxilline was applied before or after primary microglia activation by LPS, but paxilline did not affect the basal level of these cytokines. Notably, the inhibitory effect of paxilline on cytokine production was slightly stronger when it was applied before rather than after LPS treatment, similar to what has been shown in BV-2 cells. Immunocytochemistry results showed that in the control group p65 is located in the cytosol of primary mouse microglia (Fig. 6D, see positive staining with ionized calcium-binding adapter molecule 1 (Iba1), red, Con) and that p65 is translocated to the nucleus after 1 h of LPS incubation (Fig. 6D, LPS). Pre-application of paxilline inhibited translocation of p65 to the nucleus in ∼60% of primary mouse microglia (32 microglia counted in total from three different slides in two independent experiments, Fig. 6D, Pax pre-LPS). Addition of paxilline after treatment with LPS did not prevent p65 translocation (Fig. 6D, Pax post-LPS). As in BV-2 cells, BK channels are also broadly expressed in primary mouse microglia. Nuclear BK channel expression increased with increased duration of LPS treatment and reached a peak around 12 h of treatment (Fig. 6E).
Figure 6.
BK channel modulates primary mouse microglia activation by LPS. A–C, primary mouse microglia were untreated (Con) or treated with LPS (LPS, 100 ng/ml), or paxilline (Pax, 10 μm) for 24 h, or with paxilline (10 μm) 30 min prior to (Pax pre-LPS) or 30 min after (Pax post-LPS) LPS (100 ng/ml) for 24 h. The supernatants were analyzed for nitrites (A), IL-6 (B), and TNFα (C). Data are presented as a scatter plot; bars represent mean ± S.D. of three (A) or two (B and C) independent experiments, each performed in quadruplicate. Differences among the groups were analyzed by one-way ANOVA with Bonferroni's multiple comparisons post hoc test (A–C). F(4,55) = 45.22 (p < 0.0001) for A; F(4,35) = 66.05 (p < 0.0001) for B; F(4,35) = 15.32 (p < 0.0001) for C. ***, p < 0.001 compared with the control group. ##, p < 0.01; ###, p < 0.001 compared with the LPS group. D, primary mouse microglia were untreated (Con) or treated with LPS (LPS, 100 ng/ml) for 1 h, or with paxilline (10 μm) 30 min prior to (Pax pre-LPS) or 30 min after (Pax post-LPS) LPS (100 ng/ml) for 1 h, and were then fixed and stained with anti-p65 antibody (green, p65), anti-Iba1 antibody (red, Iba1), and Hoechst 33258 (blue, nucleus) and were analyzed by confocal fluorescence microscopy. Insets are enlarged views of the selected rectangle areas. Scale bar, 20 μm. E, primary mouse microglia were treated with LPS (100 ng/ml) for 0, 6, 12, or 24 h and then were fixed and immunostained with anti-BK antibody (green, BK), anti-Iba1 antibody (red, Iba1), and Hoechst 33258 (blue, nucleus) and were analyzed by confocal fluorescence microscopy. Insets are enlarged views of the selected rectangle areas. Scale bar, 20 μm.
Discussion
In this study, we demonstrate for the first time, to our knowledge, that PM and nuclear BK channels sequentially participate in LPS-induced microglia activation. The PM BK channel is activated by LPS via TLR4 at a very early stage of microglia activation, which then leads to translocation of NF-κB to the nucleus to initiate transcription of inflammatory cytokines. The expression of the nuclear BK channel is induced by LPS to facilitate the production of both cytokines and NO at late stages of microglia activation through a TLR4-dependent pathway (as depicted in Fig. 7).
Figure 7.

Models of the possible mechanisms underlying BK channel participation in LPS-induced microglia activation. In one scenario, at an early stage of microglia activation, LPS activates membrane BK channels through TLR4 to stimulate NF-κB translocation to the nucleus, which then leads to increased NF-κB-dependent gene transcription in microglia (blue arrows). In another scenario, LPS induces elevation of nuclear BK channel expression through TLR4 in later stages of microglia activation (pink arrows). Both scenarios result in increased cytokine production in microglia (red arrows).
Although little is known about the role of the BK channel in microglia activation, it is well-studied in macrophages. BK channel activation is associated with the proinflammatory effect of macrophages induced by various stimuli, including LPS (17–20), heparin sulfate (21), and uropathogenic Escherichia coli (20), and it facilitates LPS and interleukin-1β-induced adhesion of monocytes to endothelial cells (22, 23). BK channel inhibition significantly reduced interleukin-1β production in LPS-primed mouse Schwann cells and THP-1 macrophage (24, 25), and it might cause impaired inflammasome activation in human and mouse macrophage and dendritic cells (25). BK channel blockers were able to suppress myelin phagocytosis of LPS-activated rat macrophages (26) and abrogate the elevations of some inflammatory biomarkers (chemokines and cytokines) in urine specimens of mice whose bladders were inoculated with LPS or uropathogenic E. coli (20). During an early step in macrophage activation, the PM BK channel is activated by LPS (17, 27), which is essential for LPS-induced NF-κB activation and cytokine production (18). This study shows that a similar mechanism operates in LPS-induced microglia activation. However, regulation of NF-κB translocation might not be the primary function of BK channels in activation of microglia, particularly primary microglia, because blockade of the BK channel after NF-κB translocation can still suppress the release of most of the downstream inflammatory factors.
LPS has been reported to rapidly and directly activate BK channels at quite high concentrations (10–100 μg/ml) and with intracellular application in murine vascular smooth muscle cells (28, 29). Our results showed that in murine microglia LPS activates BK current at 100–1000-fold lower concentrations, and from the extracellular side, this effect can be inhibited by TLR4 antagonist CLI095, implying that LPS activates BK channels via TLR4. The mechanism underlying LPS activation of the PM BK channel through TLR4 in microglia will require further exploration. Scheel et al. (19) found that the BK channel and TLR4 both translocated to similar cellular compartments after LPS stimulation when exogenously expressed in HEK293 cells, suggesting that TLR4 might directly activate BK channels in microglia. Alternatively, stimulation of microglia with LPS has been shown to induce an increase in intracellular Ca2+ concentration (30–32); thus, TLR4 might activate BK channels by elevating intracellular Ca2+. However, determination of the exact mechanism will require further investigation.
The presence of BK channels in BV-2 cells had not been demonstrated previously. A previous study (33) failed to detect BK currents in both resting and lysophosphatidic acid–induced BV-2 cells using a ramp pulse from −120 to +30 mV with a holding potential of −60 mV. In that study, an intracellular pipette solution contained a high concentration (10 mm) of BAPTA (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), a fast Ca2+ chelator, which could inhibit local and global intracellular Ca2+ elevation during BV-2 activation. The basal level of expression of BK channels in BV-2 cells is quite low (Figs. 1E and 5, B and D), and the open probability of the channel at voltages ≤30 mV and low Ca2+ concentrations is also very low. All these conditions might have led to the previous inability to detect BK currents in BV-2 cells.
Paxilline is a specific BK channel blocker. Its IC50 is around 10 nm when the channel open probability (Po) is low but shifts to near 10 μm when the channel is fully opened (34). It has been reported that 1 μm paxilline had no effect on KV1.3 channel-regulated β-amyloid–induced BV-2 cell priming (35); 5 μm paxilline had no effect on KCa3.1 current in BV-2 cells induced by lysophosphatidic acid (33). To ensure complete inhibition of BK channel activity, we used 10 μm paxilline in this study. Although we cannot totally rule out the effect of 10 μm paxilline on other ion channels or proteins, we did not see any impact of 10 μm paxilline on cell viability, NO production, or iNOS, COX2 expression under basal conditions. Furthermore, with 100 ng/ml LPS treatment, 10 μm paxilline showed a similar effect with BK siRNA, suggesting that BK channel inhibition is the main effect of paxilline at this concentration in this study.
BK channels are expressed in the nucleus, mitochondria, lysosomes, and endoplasmic reticulum in various cell types (36, 37), but their functional roles are still largely unknown. Based on limited reports, the function of intracellular BK channels is mainly related to the regulation of Ca2+ concentrations in subcellular organelles. Mitochondrial BK channels are localized at the inner mitochondrial membrane, where they modulate the opening of mitochondrial permeability transition pores by affecting Ca2+ concentrations and mitochondrial reactive oxygen species production in cardiomyocytes to prevent cell death (37–39). Lysosomal BK channels, which mediate lysosomal Ca2+ release (40) or reuptake (41), are present in almost all cells; therefore, the loss of BK channels causes abnormal lysosomal degradation and trafficking. Nuclear BK channels have been shown to regulate nuclear Ca2+ signaling and gene expression in both rodent hippocampal neurons and the cerebral endothelial cells of Yorkshire piglets, but in opposite ways. Although the elevations of nuclear Ca2+ concentrations and gene transcription are induced by blocking nuclear BK channels in rodent hippocampal neurons, these processes are induced by activating nuclear BK channels in porcine cerebral endothelial cells (42, 43). We detected for the first time that BK channels were intracellularly expressed in microglia as well. Also, we observed that the expression of the nuclear BK channel increased after 6 h of stimulation with LPS but that inhibition of these channels by paxilline significantly suppressed cytokine and NO production in microglia, all of which implies that the nuclear BK channel is involved in microglia activation. Although the mechanism by which the nuclear BK channel regulates microglia activation requires further study, the Ca2+ and K+ fluxes in the nucleus modulated by nuclear BK channels might be part of the underlying mechanism.
BK channels have been found to be expressed in the majority of microglia cells from both young adult and aged mice but with no difference between the two age groups (13). This conclusion was based on data collected by a patch-clamp technique that can only detect channel activity on the cell membrane, while our results showed that nuclear BK channels also play important roles in microglia activation. To exclude the possible role of BK channels in aged microglia, the intracellular expression level of this channel should be examined as well. Interestingly, in astrocytes of rat brain slices, BK channels were not evenly distributed but were specifically localized in astrocytic endfeet (44). It is worth studying whether the expression of microglia BK channels in vivo is also polarized, which might provide additional knowledge about the role of this channel in microglia pathophysiology. The BK channel has been reported to neither affect β-amyloid–induced BV-2 cell priming (35) nor lysophosphatidic acid–induced BV-2 cell migration (33), indicating that the BK channel specifically gets involved in BV-2 cell activation, mainly impacting cytokine release. The BK channel was also observed to participate in microglia activation in another immortalized mouse microglia cell line MG6 and primary mouse microglia (this study and see Refs. 14, 15). Inhibition of the microglia BK channel can relieve the nerve injury-induced neuropathic pain (14) and morphine-induced hyperalgesia (15). These data suggest that the BK channel is a potential target for neuroinflammation treatment, and specific BK channel blockers might be served as anti-inflammation drugs. Although the roles of BK channels in both health and disease have been well-known, no BK channel activator or blocker has been successfully used in clinical practice so far (45, 46). BK channels are ubiquitously expressed in the body; however, they show different functions in different tissues or cells. Great efforts have been made to improve the potency and selectivity of the activators and blockers themselves. It is also very important to find effective ways for specific tissue or cell delivery, and this might bring some new chances for BK-targeted drug development.
Although the correlation of BK channel-regulated microglia activation and neuroinflammation-associated diseases remains largely unclear, another calcium-dependent potassium channel, KCa3.1, which also mediates microglia activation, has been proposed to be a promising therapeutic target for Alzheimer's disease (47–49). Studies showed that KCa3.1 channel was involved in microglia oxidative burst, nitric oxide production, and β-amyloid oligomer (AβO)-induced microglia activation (47, 50, 51). The KCa3.1 blockers reduced neuroinflammation and prevented microglia-mediated neurotoxicity induced by AβO both in vitro and in vivo (47, 49). Inhibition or genetic deletion of KCa3.1 significantly reduced infarct areas day 7 or 8 after middle cerebral artery occlusion and improved neurological deficit in mouse and rat models of ischemia/reperfusion stroke (52, 53).
In summary, BK channels regulate LPS-induced activation of murine microglia. Both PM- and nuclear-localized BK channels are involved in this activation. These results provide important new insights into the mechanism of microglia activation and extend our understanding of the functional roles of BK channels in neuroinflammation.
Experimental procedures
Animals
C57BL/6 postnatal day 1–2 (P1–2, either sex) mouse pups were purchased from the animal facility of Soochow University and were used each time upon arrival. All animal protocols were approved by the Animal Care and Use Committee of Soochow University.
Cell culture and transfection
Murine microglial BV-2 cells from female mice were purchased from the National Infrastructure of Cell Line Resource (Beijing, China). According to the vendor's protocol, the BV-2 cells were cultured under a humidified atmosphere of 5% CO2 at 37 °C in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/ml penicillin G, and 100 μg/ml streptomycin (Thermo Fisher Scientific, Shanghai, China). For siRNA oligonucleotides' (GenePharma, Shanghai, China) transfection, BV-2 cells were plated in 96- or 6-well plates at a density of 1 × 104 or 2 × 105 cells/well, grown for 24 h at 37 °C, and transfected with either NC siRNA (5′-UUC UCC GAA CGU GUC ACG UTT-3′) or BK siRNA (5′-GCA CUU ACG UAC UGG GAA UTT-3′) using Lipofectamine 3000 (Thermo Fisher Scientific). Cells were used for Western blot analysis at 24 h after transfection.
Primary mouse microglia culture
Primary microglia were prepared as described previously (54, 55) with some modifications. In brief, C57BL/6 P1–2 (either sex) mouse brains were harvested, and their meninges were removed in ice-cold DME/F-12 nutrient media (GE Healthcare, Beijing, China). Chopped brain tissues were then incubated in 0.25% trypsin for 30 min with gentle agitation. The trypsin reaction was stopped by adding an equal volume of DME/F-12 complete medium (supplemented with 10% heat-inactivated FBS, 100 IU/ml penicillin G, and 100 μg/ml streptomycin), and the brain tissues were washed three times. Tissues were triturated gently and then passed through a 70-μm nylon mesh cell strainer. Cells were resuspended in DME/F-12 complete medium and seeded into T-75 flasks, which were then maintained in a humidified atmosphere of 5% CO2 at 37 °C. The medium was changed on the 2nd day and then was changed every 3 days. Microglia were harvested by shaking (180 rpm, 2 h) after 2 weeks in culture. The purity of the isolated microglia was >95%, as determined by immunocytochemistry with antibodies against Iba1 (1:100, Santa Cruz Biotechnology catalog no. sc-32725, RRID:AB_667733, Shanghai, China). Microglia were allowed to recover for at least 48 h before further treatment.
Analysis of nitrites, TNFα, and IL-6
BV-2 cells or primary mouse microglia were plated in 96-well plates at a density of 1.2 × 104 cells/well, grown for 24 h at 37 °C, and either untreated or treated with different drugs for 24 h, as specified under “Results.” The culture supernatants were then collected for later cytokine analysis.
The production of NO by iNOS was measured indirectly by assaying nitrites in the culture supernatant using the Griess reaction (56). In brief, 50 μl of supernatant was incubated with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine in 2% phosphoric acid solution), and the absorbance was then read at 550 nm at room temperature.
TNFα and IL-6 in supernatants were quantified by ELISA performed following the manufacturer's instructions (Boster Biological Technology, Wuhan, China).
MTT assay
BV-2 cells or primary mouse microglia were plated in 96-well plates at a density of 1.2 × 104 cells/well, grown for 24 h at 37 °C, and either untreated or treated with different drugs for 24 h, as specified under the “Results.” At the end of each treatment, the supernatant was collected to use for other tests and 30 μl of MTT solution (5 mg/ml in PBS) was added to each well. The plate was further incubated for another 2 h at 37 °C. The supernatant was then discarded, and 100 μl of DMSO was added to thoroughly dissolve the dark blue crystal formazan. Absorbance at 570 nm was determined using a spectrophotometer.
Western blot analysis
BV-2 cells were plated in 6-well plates at a density of 3 × 105 cells/well (for whole-cell lysate preparations) or in 10-cm diameter plates at a density of 2 × 106 cells/plate (for subcellular lysate preparations), grown for 24 h at 37 °C, and were then either untreated or treated with different drugs for 1–24 h at 37 °C, as specified under the “Results.” Cells were then washed with ice-cold PBS without calcium and magnesium. Whole-cell lysates for extraction of both NF-κB and BK proteins were prepared in chilled RIPA lysis buffer (Cell Signaling Technology, Boston) containing 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% NonidetTM P-40, 1% sodium deoxycholate, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, and 1 μg/ml leupeptin. Before use, 1 mm phenylmethylsulfonyl fluoride was added, and cells were left on ice for 30 min with vortexing for 5 s every 10 min. Then, cell lysates for extraction of BK protein were further sonicated 10 times at 1 s each to facilitate BK protein extraction. After incubation or sonication, lysates were centrifuged at 14,000 × g for 10 min at 4 °C, and the supernatant was used as whole-cell lysates. Nuclear and cytosolic lysates were prepared using a nuclear–cytosol extraction kit following the manufacturer's instructions (Applygen polyacrylamide gels and were transferred onto polyvinylidene difluoride (PVDF) membranes (0.2 μm, Merck, Darmstadt, Germany). PVDF membranes were then incubated with primary antibodies against the following: iNOS (1:5000, Abcam catalog no. ab15323, RRID:AB_301857, Cambridge, UK); COX2 (1:1000, Abcam catalog no. ab62331, RRID:AB_942121); NF-κB p65 (1:1000, Cell Signaling Technology catalog no. 8242, RRID:AB_10859369); BK (1:1000, UC Davis/National Institutes of Health NeuroMab Facility catalog no. 75-022, RRID:AB_2249538, Davis, CA); lamin B1 (1:1000, Proteintech Group catalog no. 12987-1-AP, RRID:AB_2136290, Rosemont, IL); α-tubulin (1:1000, Proteintech Group catalog no. 11224-1-AP, RRID:AB_2210206); or β-actin (1:20000, Proteintech Group catalog no. 66009-1-Ig, RRID:AB_2687938) and followed by incubation with horseradish peroxidase–conjugated secondary antibodies. Immunoreactivity was detected using the Immobilon Western Chemiluminescent HRP substrate (Merck). Densitometric analysis of the bands was carried out using ImageJ software (National Institutes of Health, Bethesda) or Image Lab software (Bio-Rad).
Electrophysiology
Whole-cell membrane currents were measured using an EPC-10 patch-clamp amplifier (HEKA). Series resistance was compensated by ∼80%. Patch pipettes were pulled from borosilicate glass capillaries with a resistance of 2–5 megohms. The electrodes were filled with a solution containing 145 mm KCl, 5 mm NaCl, 2.5 mm MgCl2, 10 mm HEPES, 0.1 mm EGTA, and 2 mm K2ATP (pH 7.4). The extracellular solution contained 145 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, and 10 mm HEPES (pH 7.4). High-resistance seals were formed in a bath solution of ND-96 containing 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 5 mm HEPES, and 2.5 mm Na pyruvate (pH 7.4). All recordings were performed at room temperature (22–25 °C). Whole-cell currents were filtered at 2.9 kHz, and no leak subtraction was implemented during recording. Drug or control/wash solutions were applied locally onto the cell via a pressurized superfusion system containing eight solution channels (AutoMate Scientific, Inc., Berkeley, CA). The 100-μm diameter perfusion tip was located ∼50 μm from the recorded cell to allow a rapid exchange of solutions. Resting membrane potentials were determined in the current-clamp mode directly after establishment of the whole-cell configuration in ND-96 bath solution with or without LPS or LPS + paxilline addition, while no holding current was applied. Analyses were performed using Igor Pro software (WaveMetrics, Lake Oswego, OR), Clampfit (Molecular Devices, San Jose, CA), and SigmaPlot (Systat Software, Inc., San Jose, CA). The steady-state currents at +80 mV were used to plot time courses of changes in whole-cell currents and to calculate the percentage of whole-cell currents increased upon LPS treatment.
Immunocytochemistry
BV-2 cells were seeded onto 6-mm round glass coverslips (Glaswarenfabrik Karl Hecht GmbH & Co. KG, Sondheim, Germany), and the primary mouse microglia were seeded onto 96-well confocal plates (JingAn Biological, Shanghai, China). The plated cells were grown for 24 h at 37 °C and were either untreated or treated with different drugs for 1–24 h at 37 °C, as specified under the “Results.” Samples were fixed with 4% paraformaldehyde in PBS at room temperature for 30 min. Cells were washed twice with PBS and treated with 100 mm glycine in PBS for 30 min. Cells were then washed twice with PBS and permeabilized with 0.5% TritonTM X-100 for 3 min and washed once with PBS. Samples were incubated in blocking buffer (5% milk (w/v) in PBS) for 1 h at 37 °C followed by a wash with PBS. Samples were then treated with primary antibody against NF-κB p65 (1:80, Cell Signaling Technology catalog no. 8242, RRID:AB_10859369), Iba1 (1:100, Santa Cruz Biotechnology catalog no. sc-32725, RRID:AB_667733), or BK (1:200, Alomone Labs catalog no. APC-021, RRID:AB_2313725, Jerusalem, Israel), and incubation was continued for 1 h at 37 °C. Samples were washed three times with PBS and stained with secondary antibodies (goat anti-rabbit Alexa Fluor 488 (1:1000, Molecular Probes catalog no. A-11008, RRID:AB_143165) or goat anti-mouse Alexa Fluor 594 (1:1000, Thermo Fisher Scientific catalog no. R37121, RRID:AB_2556549) plus Hoechst 33258 (0.5 μg/ml, Thermo Fisher Scientific) in blocking buffer for 1 h, and then washed three times with PBS. Samples were mounted using Immu-Mount (Thermo Fisher Scientific) (BV-2 cells) or were observed directly (primary mouse microglia). Microscopy was carried out using a Zeiss LSM 710 microscope with ZEN software (Carl Zeiss Microscopy GmbH, Jena, Germany) or Olympus FV1000 microscope with FLUOVIEW FV1000 software (Olympus Corp., Tokyo, Japan).
Materials
CLI095 was obtained from InvivoGen (San Diego). Lipopolysaccharide (catalog no. L4391) was purchased from Sigma. All of the other reagents were obtained from Sigma, except where indicated.
Statistical analysis
All experiments were performed at least two times. Statistical analyses were performed by one-way or two-way ANOVA with appropriate post hoc tests or two-tailed unpaired Student's t tests, using GraphPad Prism 5.0 software (GraphPad Software, Inc. La Jolla, CA) or SPSS 19 software (IBM, Armonk, NY). Scatter plots represent individual data points combined with means ± S.D. p values of < 0.05 were considered significant.
Author contributions
X. Y., G. W., T. C., L. Z., Y. M., S. J., and X. S. data curation; X. Y., G. W., T. C., L. Z., Y. M., S. J., X. T., and X. S. formal analysis; X. Y., G. W., T. C., L. Z., Y. M., S. J., and X. S. investigation; X. Y., G. W., T. C., X. T., and X. S. methodology; X. Y. and G. W. writing-original draft; T. C., L. Z., Y. M., S. J., X. T., and X. S. writing-review and editing; X. T. and X. S. supervision; X. T. and X. S. funding acquisition; X. T. and X. S. project administration; X. S. conceptualization; X. S. validation.
Supplementary Material
Acknowledgments
We thank Dr. Eleonora Zakharian for reading the manuscript and providing valuable comments. We also thank Dr. Liang Sun for help with the electrophysiology experiments.
This work was supported by National Natural Science Foundation of China Grants 31400924 (to X. S.) and 31401197 (to X. T.) and the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1.
- BK channel
- large-conductance calcium-activated potassium channel
- AβO
- amyloid-β oligomer
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- COX2
- cyclooxygenase
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- Iba1
- ionized calcium-binding adapter molecule 1
- IL-6
- interleukin-6
- iNOS
- inducible nitric-oxide synthase
- IV relation
- current-voltage relation
- KCa3.1
- intermediate-conductance calcium-activated potassium channel
- KV1.3
- voltage-gated potassium channel
- LPS
- lipopolysaccharide
- MIP
- macrophage inflammatory protein
- MTT
- thiazolyl blue tetrazolium bromide
- NC
- negative control
- NO
- nitric oxide
- PM
- plasma membrane
- PVDF
- polyvinylidene difluoride
- TLR4
- Toll-like receptor 4
- TNFα
- tumor necrosis factor-α
- ANOVA
- analysis of variance
- Pax
- paxilline.
References
- 1. Tao J., Lan Z., Wang Y., Hei H., Tian L., Pan W., Zhang X., and Peng W. (2016) Large-conductance calcium-activated potassium channels in glomerulus: from cell signal integration to disease. Front. Physiol. 7, 248 10.3389/fphys.2016.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pyott S. J., and Duncan R. K. (2016) BK channels in the vertebrate inner ear. Int. Rev. Neurobiol. 128, 369–399 10.1016/bs.irn.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 3. Krishnamoorthy-Natarajan G., and Koide M. (2016) BK channels in the vascular system. Int. Rev. Neurobiol. 128, 401–438 10.1016/bs.irn.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 4. Duncan P. J., and Shipston M. J. (2016) BK channels and the control of the pituitary. Int. Rev. Neurobiol. 128, 343–368 10.1016/bs.irn.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 5. Contet C., Goulding S. P., Kuljis D. A., and Barth A. L. (2016) BK channels in the central nervous system. Int. Rev. Neurobiol. 128, 281–342 10.1016/bs.irn.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kettenmann H., Hanisch U. K., Noda M., and Verkhratsky A. (2011) Physiology of microglia. Physiol. Rev. 91, 461–553 10.1152/physrev.00011.2010 [DOI] [PubMed] [Google Scholar]
- 7. Michell-Robinson M. A., Touil H., Healy L. M., Owen D. R., Durafourt B. A., Bar-Or A., Antel J. P., and Moore C. S. (2015) Roles of microglia in brain development, tissue maintenance and repair. Brain 138, 1138–1159 10.1093/brain/awv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feske S., Wulff H., and Skolnik E. Y. (2015) Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 33, 291–353 10.1146/annurev-immunol-032414-112212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLarnon J. G., Sawyer D., and Kim S. U. (1995) Cation and anion unitary ion channel currents in cultured bovine microglia. Brain Res. 693, 8–20 10.1016/0006-8993(95)00664-C [DOI] [PubMed] [Google Scholar]
- 10. McLarnon J. G., Xu R., Lee Y. B., and Kim S. U. (1997) Ion channels of human microglia in culture. Neuroscience 78, 1217–1228 10.1016/S0306-4522(96)00680-X [DOI] [PubMed] [Google Scholar]
- 11. Bordey A., and Spencer D. D. (2003) Chemokine modulation of high-conductance Ca2+-sensitive K+ currents in microglia from human hippocampi. Eur. J. Neurosci. 18, 2893–2898 10.1111/j.1460-9568.2003.03021.x [DOI] [PubMed] [Google Scholar]
- 12. Schilling T., and Eder C. (2007) Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Res. 1186, 21–28 10.1016/j.brainres.2007.10.027 [DOI] [PubMed] [Google Scholar]
- 13. Schilling T., and Eder C. (2015) Microglial K+ channel expression in young adult and aged mice. Glia 63, 664–672 10.1002/glia.22776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayashi Y., Kawaji K., Sun L., Zhang X., Koyano K., Yokoyama T., Kohsaka S., Inoue K., and Nakanishi H. (2011) Microglial Ca2+-activated K+ channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J. Neurosci. 31, 17370–17382 10.1523/JNEUROSCI.4152-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashi Y., Morinaga S., Zhang J., Satoh Y., Meredith A. L., Nakata T., Wu Z., Kohsaka S., Inoue K., and Nakanishi H. (2016) BK channels in microglia are required for morphine-induced hyperalgesia. Nat. Commun. 7, 11697 10.1038/ncomms11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang X., Zhao W., Zhang L., Cui J., Sun X., and Zhen X. (2017) The role of BK channel in microglia activation. Biophys. J. 112, 548–548 10.1016/j.bpj.2016.11.2960 [DOI] [Google Scholar]
- 17. Seydel U., Scheel O., Müller M., Brandenburg K., and Blunck R. (2001) A K+ channel is involved in LPS signaling. J. Endotoxin Res. 7, 243–247 10.1177/09680519010070030901 [DOI] [PubMed] [Google Scholar]
- 18. Papavlassopoulos M., Stamme C., Thon L., Adam D., Hillemann D., Seydel U., and Schromm A. B. (2006) MaxiK blockade selectively inhibits the lipopolysaccharide-induced IκB-α/NF-κB signaling pathway in macrophages. J. Immunol. 177, 4086–4093 10.4049/jimmunol.177.6.4086 [DOI] [PubMed] [Google Scholar]
- 19. Scheel O., Papavlassopoulos M., Blunck R., Gebert A., Hartung T., Zähringer U., Seydel U., and Schromm A. B. (2006) Cell activation by ligands of the toll-like receptor and interleukin-1 receptor family depends on the function of the large-conductance potassium channel MaxiK in human macrophages. Infect. Immun. 74, 4354–4356 10.1128/IAI.01783-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeh J., Lu M., Alvarez-Lugo L., and Chai T. C. (2019) Bladder urothelial BK channel activity is a critical mediator for innate immune response in urinary tract infection pathogenesis. Am. J. Physiol. Renal Physiol. 316, F617–F623 10.1152/ajprenal.00554.2018 [DOI] [PubMed] [Google Scholar]
- 21. Ren J. D., Fan L., Tian F. Z., Fan K. H., Yu B. T., Jin W. H., Tan Y. H., and Cheng L. (2014) Involvement of a membrane potassium channel in heparan sulphate-induced activation of macrophages. Immunology 141, 345–352 10.1111/imm.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erdogan A., Schaefer M. B., Kuhlmann C. R., Most A., Hartmann M., Mayer K., Renner F. C., Schaefer C., Abdallah Y., Hoelschermann H., and Schaefer C. A. (2007) Activation of Ca2+-activated potassium channels is involved in lysophosphatidylcholine-induced monocyte adhesion to endothelial cells. Atherosclerosis 190, 100–105 10.1016/j.atherosclerosis.2006.02.035 [DOI] [PubMed] [Google Scholar]
- 23. Burgazli K. M., Venker C. J., Mericliler M., Atmaca N., Parahuleva M., and Erdogan A. (2014) Importance of large conductance calcium-activated potassium channels (BKCa) in interleukin-1b-induced adhesion of monocytes to endothelial cells. Eur. Rev. Med. Pharmacol. Sci. 18, 646–656 [PubMed] [Google Scholar]
- 24. Colomar A., Marty V., Médina C., Combe C., Parnet P., and Amédée T. (2003) Maturation and release of interleukin-1β by lipopolysaccharide-primed mouse Schwann cells require the stimulation of P2X7 receptors. J. Biol. Chem. 278, 30732–30740 10.1074/jbc.M304534200 [DOI] [PubMed] [Google Scholar]
- 25. Eugenia Schroeder M., Russo S., Costa C., Hori J., Tiscornia I., Bollati-Fogolín M., Zamboni D. S., Ferreira G., Cairoli E., and Hill M. (2017) Pro-inflammatory Ca2+-activated K+ channels are inhibited by hydroxychloroquine. Sci. Rep. 7, 1892 10.1038/s41598-017-01836-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vanheel A., Daniels R., Plaisance S., Baeten K., Hendriks J. J., Leprince P., Dumont D., Robben J., Brône B., Stinissen P., Noben J. P., and Hellings N. (2012) Identification of protein networks involved in the disease course of experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. PLoS One 7, e35544 10.1371/journal.pone.0035544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Müller M., Scheel O., Lindner B., Gutsmann T., and Seydel U. (2003) The role of membrane-bound LBP, endotoxin aggregates, and the MaxiK channel in LPS-induced cell activation. J. Endotoxin Res. 9, 181–186 10.1179/096805103125001595 [DOI] [PubMed] [Google Scholar]
- 28. Hoang L. M., Chen C., and Mathers D. A. (1997) Lipopolysaccharide rapidly activates K+ channels at the intracellular membrane face of rat cerebral artery smooth muscle cells. Neurosci. Lett. 231, 25–28 10.1016/S0304-3940(97)00519-3 [DOI] [PubMed] [Google Scholar]
- 29. Yakubovich N., Eldstrom J. R., and Mathers D. A. (2001) Lipopolysaccharide can activate BK channels of arterial smooth muscle in the absence of iNOS expression. Biochim. Biophys. Acta 1514, 239–252 10.1016/S0005-2736(01)00378-9 [DOI] [PubMed] [Google Scholar]
- 30. Hoffmann A., Kann O., Ohlemeyer C., Hanisch U. K., and Kettenmann H. (2003) Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J. Neurosci. 23, 4410–4419 10.1523/JNEUROSCI.23-11-04410.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korvers L., de Andrade Costa A., Mersch M., Matyash V., Kettenmann H., and Semtner M. (2016) Spontaneous Ca2+ transients in mouse microglia. Cell Calcium 60, 396–406 10.1016/j.ceca.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 32. Bader M. F., Taupenot L., Ulrich G., Aunis D., and Ciesielski-Treska J. (1994) Bacterial endotoxin induces [Ca2+]i transients and changes the organization of actin in microglia. Glia 11, 336–344 10.1002/glia.440110406 [DOI] [PubMed] [Google Scholar]
- 33. Schilling T., Stock C., Schwab A., and Eder C. (2004) Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur. J. Neurosci. 19, 1469–1474 10.1111/j.1460-9568.2004.03265.x [DOI] [PubMed] [Google Scholar]
- 34. Zhou Y., and Lingle C. J. (2014) Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J. Gen. Physiol. 144, 415–440 10.1085/jgp.201411259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schilling T., and Eder C. (2011) Amyloid-β–induced reactive oxygen species production and priming are differentially regulated by ion channels in microglia. J. Cell. Physiol. 226, 3295–3302 10.1002/jcp.22675 [DOI] [PubMed] [Google Scholar]
- 36. Singh H., Stefani E., and Toro L. (2012) Intracellular BK(Ca) (iBK(Ca)) channels. J. Physiol. 590, 5937–5947 10.1113/jphysiol.2011.215533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li B., and Gao T. M. (2016) Functional role of mitochondrial and nuclear BK channels. Int. Rev. Neurobiol. 128, 163–191 10.1016/bs.irn.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 38. Balderas E., Zhang J., Stefani E., and Toro L. (2015) Mitochondrial BKCa channel. Front. Physiol. 6, 10.3389/fphys.2015.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krabbendam I. E., Honrath B., Culmsee C., and Dolga A. M. (2018) Mitochondrial Ca2+-activated K+ channels and their role in cell life and death pathways. Cell Calcium 69, 101–111 10.1016/j.ceca.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 40. Cao Q., Zhong X. Z., Zou Y., Zhang Z., Toro L., and Dong X. P. (2015) BK channels alleviate lysosomal storage diseases by providing positive feedback regulation of lysosomal Ca2+ release. Dev. Cell 33, 427–441 10.1016/j.devcel.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 41. Wang W., Zhang X., Gao Q., Lawas M., Yu L., Cheng X., Gu M., Sahoo N., Li X., Li P., Ireland S., Meredith A., and Xu H. (2017) A voltage-dependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores. J. Cell Biol. 216, 1715–1730 10.1083/jcb.201612123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li B., Jie W., Huang L., Wei P., Li S., Luo Z., Friedman A. K., Meredith A. L., Han M. H., Zhu X. H., and Gao T. M. (2014) Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling. Nat. Neurosci. 17, 1055–1063 10.1038/nn.3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gobeil F. Jr., Dumont I., Marrache A. M., Vazquez-Tello A., Bernier S. G., Abran D., Hou X., Beauchamp M. H., Quiniou C., Bouayad A., Choufani S., Bhattacharya M., Molotchnikoff S., Ribeiro-Da-Silva A., Varma D. R., et al. (2002) Regulation of eNOS expression in brain endothelial cells by perinuclear EP(3) receptors. Circ. Res. 90, 682–689 10.1161/01.RES.0000013303.17964.7A [DOI] [PubMed] [Google Scholar]
- 44. Price D. L., Ludwig J. W., Mi H., Schwarz T. L., and Ellisman M. H. (2002) Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 956, 183–193 10.1016/S0006-8993(02)03266-3 [DOI] [PubMed] [Google Scholar]
- 45. Bentzen B. H., Olesen S. P., Rønn L. C., and Grunnet M. (2014) BK channel activators and their therapeutic perspectives. Front. Physiol. 5, 389 10.3389/fphys.2014.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu M., Liu S. L., Sun P. B., Pan H., Tian C. L., and Zhang L. H. (2016) Peptide toxins and small-molecule blockers of BK channels. Acta Pharmacol. Sin. 37, 56–66 10.1038/aps.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maezawa I., Zimin P. I., Wulff H., and Jin L. W. (2011) Amyloid-β protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 286, 3693–3706 10.1074/jbc.M110.135244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maezawa I., Jenkins D. P., Jin B. E., and Wulff H. (2012) Microglial KCa3.1 channels as a potential therapeutic target for Alzheimer's disease. Int. J. Alzheimers Dis. 2012, 868972 10.1155/2012/868972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin L. W., Lucente J. D., Nguyen H. M., Singh V., Singh L., Chavez M., Bushong T., Wulff H., and Maezawa I. (2019) Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer's disease. Ann. Clin. Transl. Neurol. 6, 723–738 10.1002/acn3.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khanna R., Roy L., Zhu X., and Schlichter L. C. (2001) K+ channels and the microglial respiratory burst. Am. J. Physiol. Cell Physiol. 280, C796–806 10.1152/ajpcell.2001.280.4.C796 [DOI] [PubMed] [Google Scholar]
- 51. Kaushal V., Koeberle P. D., Wang Y., and Schlichter L. C. (2007) The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 27, 234–244 10.1523/JNEUROSCI.3593-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen Y. J., Nguyen H. M., Maezawa I., Grössinger E. M., Garing A. L., Köhler R., Jin L. W., and Wulff H. (2016) The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 36, 2146–2161 10.1177/0271678X15611434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Y. J., Raman G., Bodendiek S., O'Donnell M. E., and Wulff H. (2011) The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 31, 2363–2374 10.1038/jcbfm.2011.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sawada M., Suzumura A., Yamamoto H., and Marunouchi T. (1990) Activation and proliferation of the isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C. Brain Res. 509, 119–124 10.1016/0006-8993(90)90317-5 [DOI] [PubMed] [Google Scholar]
- 55. Suzumura A., Bhat S., Eccleston P. A., Lisak R. P., and Silberberg D. H. (1984) The isolation and long-term culture of oligodendrocytes from newborn mouse brain. Brain Res. 324, 379–383 10.1016/0006-8993(84)90054-4 [DOI] [PubMed] [Google Scholar]
- 56. Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., and Tannenbaum S. R. (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138 10.1016/0003-2697(82)90118-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.